An analysis of the complex structure obtained by crystallization of 5-nonanoyl-8-hydroxyquinoline and InCl3 in acetonitrile is reported.
Keywords: crystal structure, indium complex, quinoline, 8-hydroxyquinoline, hydrogen bonds, van der Waals interactions
Abstract
Crystallization of 5-nonanoyl-8-hydroxyquinoline in the presence of InCl3 in acetonitrile yields a dinuclear InIII complex crystallizing in the space group P . In this complex, [In2(C18H22NO2)2Cl4(H2O)2], each indium ion is sixfold coordinated by two chloride ions, one water molecule and two 8-quinolinolate ions. The crystal of the title complex is composed of two-dimensional supramolecular aggregates, resulting from the linkage of the Owater—H⋯O=C and Owater—H⋯Cl hydrogen bonds as well as bifurcated Carene—H⋯Cl contacts.
. In this complex, [In2(C18H22NO2)2Cl4(H2O)2], each indium ion is sixfold coordinated by two chloride ions, one water molecule and two 8-quinolinolate ions. The crystal of the title complex is composed of two-dimensional supramolecular aggregates, resulting from the linkage of the Owater—H⋯O=C and Owater—H⋯Cl hydrogen bonds as well as bifurcated Carene—H⋯Cl contacts.
1. Chemical context
As a result of the remarkable complexing properties of 8-hydroxyquinoline and its substituted derivatives towards various metal ions, their use as extracting agents for these ionic substrates has received much attention (for examples, see: Uhlemann et al., 1984 ▸; Filik et al., 1994 ▸; Gloe et al., 1996 ▸; Yamada et al., 2006 ▸). In addition, their application in the formation of luminescent coordination compounds has been the subject of intensive research (Matsumura et al., 1996 ▸; Montes et al., 2006 ▸; Feng et al., 2007 ▸, 2008 ▸). Furthermore, 8-hydroxyquinoline-based building blocks have been used to construct artificial receptors, such as carbohydrate receptors (Mazik et al., 2011 ▸; Geffert et al., 2013 ▸), and have formed the basis for the development of various supramolecular architectures (Albrecht et al., 2008 ▸).
Our previous studies on the extraction of indium ions by 8-hydroxyquinolines bearing alkanoyl or alkyl groups of different chain lengths showed that the 5-alkanoyl derivatives are more effective indium extractors than the analogues containing 5-alkyl-, 7-alkanoyl- or 7-alkyl substituents (Schulze et al., 2019 ▸). The derivative with the n-nonanoyl group at the 5-position proved to be a particularly effective extractor for indium ions, showing not only the best selectivity for indium over iron and zinc ions, but also the most favorable extraction kinetics under the chosen experimental conditions.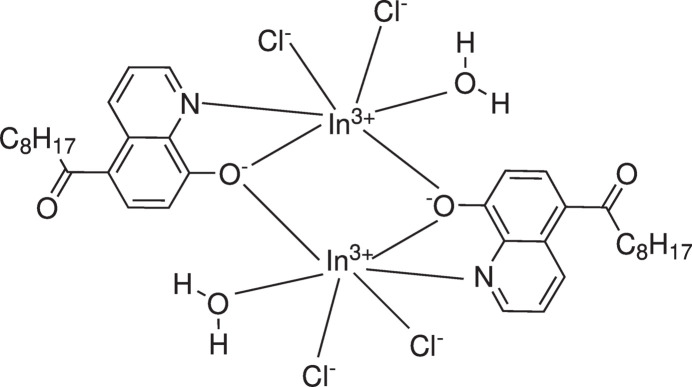
In this article we describe the crystal structure of a dinuclear InIII complex obtained by crystallization of 5-nonanoyl-8-hydroxyquinoline in the presence of InCl3 in acetonitrile.
2. Structural commentary
The title complex crystallizes in the centrosymmetric space group P with one half of the complex in the asymmetric unit of the cell. This structural motif is expanded by an inversion center to form a dinuclear complex as depicted in Fig. 1 ▸. Within the asymmetric unit, the indium ion is fivefold coordinated via one water molecule and two chloride ions as well as the atoms N1 and O1 of the bidentate quinolinolate ligand. The sixth coordination site of the metal center is occupied by the quinolinolate oxygen atom O1 of the inversion-related fragment of the complex, so that each InIII center adopts a distorted octahedral coordination geometry of the composition NO3Cl2. The In—Y bond lengths (Y = N, O, Cl) are listed in Table 1 ▸ and range between 2.17 and 2.43 Å. The nonanoyl fragment of the quinolinolate ligand exists in an elongated conformation. The complex structure is stabilized by intramolecular hydrogen bonds involving the water hydrogen atom H3B and the chloride ion Cl2 [d(H⋯Cl) = 2.27 (4) Å, O—H⋯Cl = 165 (4)°] as well as C—H⋯O contacts between the nonanoyl oxygen atom O2 and the arene hydrogen atom H3 [d(H⋯O) = 2.21 Å, C—H⋯O = 122°].
with one half of the complex in the asymmetric unit of the cell. This structural motif is expanded by an inversion center to form a dinuclear complex as depicted in Fig. 1 ▸. Within the asymmetric unit, the indium ion is fivefold coordinated via one water molecule and two chloride ions as well as the atoms N1 and O1 of the bidentate quinolinolate ligand. The sixth coordination site of the metal center is occupied by the quinolinolate oxygen atom O1 of the inversion-related fragment of the complex, so that each InIII center adopts a distorted octahedral coordination geometry of the composition NO3Cl2. The In—Y bond lengths (Y = N, O, Cl) are listed in Table 1 ▸ and range between 2.17 and 2.43 Å. The nonanoyl fragment of the quinolinolate ligand exists in an elongated conformation. The complex structure is stabilized by intramolecular hydrogen bonds involving the water hydrogen atom H3B and the chloride ion Cl2 [d(H⋯Cl) = 2.27 (4) Å, O—H⋯Cl = 165 (4)°] as well as C—H⋯O contacts between the nonanoyl oxygen atom O2 and the arene hydrogen atom H3 [d(H⋯O) = 2.21 Å, C—H⋯O = 122°].
Figure 1.
Perspective view of the molecular structure of the title complex including the labeling of atoms in the asymmetric unit. The ellipsoids correspond to the thermal displacement at 50% probability.
Table 1. Geometric data (Å, °) for short intra- and intermolecular interactions.
CgA is the centroid of the N1/C1–C4/C9 ring.
| In—Y | In–Y | |||
|---|---|---|---|---|
| In1—O1i | 2.166 (3) | In1—N1ii | 2.241 (4) | |
| In1—O1ii | 2.209 (3) | In1—Cl1ii | 2.382 (2) | |
| In1—O3ii | 2.227 (4) | In1—Cl2ii | 2.430 (2) | |
| D—H⋯A/Cg | D—H | H⋯A/Cg | D—A/Cg | D—H⋯A/Cg |
| O3—H3A⋯O2iii | 0.84 (4) | 1.84 (4) | 2.668 (4) | 168 (5) |
| O3—H3B⋯Cl2i | 0.92 (4) | 2.27 (4) | 3.165 (4) | 165 (4) |
| C1—H1⋯Cl1iv | 0.95 | 2.76 | 3.417 (6) | 127 |
| C2—H2⋯Cl1iv | 0.95 | 2.89 | 3.469 (5) | 120 |
| C3—H3⋯O2ii | 0.95 | 2.21 | 2.835 (6) | 122 |
| C15—H15A⋯CgAv | 0.99 | 2.94 | 3.766 (6) | 141 |
Symmetry codes: (i) x − 1, −y + 1, −z + 1; (ii) x, y, z; (iii) −x + 2, −y, −z + 1; (iv) −x + 1, −y, −z + 1; (v) x + 1, y, z.
3. Supramolecular features
Regarding the packing behavior of the dinuclear complexes, hydrogen bonds play an important role. On one hand, the observed interaction between the water hydrogen atom H3A and the carbonyl oxygen atom O2 [d =1.84 (4) Å, C—H⋯O = 168 (5)°; see Fig. 2 ▸] of adjacent molecules leads to the formation of infinite supramolecular chains in the [10 ] direction. On the other hand, weaker Carene—H⋯Cl hydrogen bonds with the chloride ion Cl2 acting as a bifurcated acceptor for H1 and H2 of the neighboring molecule (see Fig. 3 ▸a and Table 1 ▸) crosslink these chains along the b axis to form a two-dimensional supramolecular network.
] direction. On the other hand, weaker Carene—H⋯Cl hydrogen bonds with the chloride ion Cl2 acting as a bifurcated acceptor for H1 and H2 of the neighboring molecule (see Fig. 3 ▸a and Table 1 ▸) crosslink these chains along the b axis to form a two-dimensional supramolecular network.
Figure 2.
Supramolecular chain formed by strong hydrogen bonds; color code: N – blue, O – red, Cl – green, In – magenta, C/H – gray.
Figure 3.
(a) Chain-like association of complex molecules via C—H⋯Cl interactions; color code: N – blue, O – red, Cl – green, In – magenta, C/H – gray. (b) Excerpt of the packing structure showing two supramolecular networks assembled via hydrogen bonds (dashed lines). Their mutual interactions are largely restricted to dispersive forces between interlocking aliphatic moieties. (c) Graphical representation of weak interactions in which the aliphatic substituents participate.
The packing structure of the complex shown in Fig. 3 ▸b indicates that the parallel orientation of the aliphatic C8H17 units has a strong influence on the cohesion of the crystal structure by van der Waals forces. They are supported by C—H⋯π interactions between H15A and the heterocyclic subunit (A) of the quinoline scaffold (see Fig. 3 ▸c and Table 1 ▸). In addition, the interactions between H17B and C6 of the quinoline ring appear to have a stabilizing effect. Other contacts involving the aromatic rings are absent in the crystal, as the closest Cg⋯Cg distances between their centroids amount to about 4.2 Å.
4. Database survey
A search in the Cambridge Structural Database (CSD, Version 5.44, update of September 2023; Groom et al., 2016 ▸) for indium complexes with ligands based on 8-hydroxyquinoline yielded 15 hits. The quinolines often occur as individual ligands within the complexes, but sometimes they are also incorporated as a subunit of larger molecules.
Common to all complexes is that the 8-quinolinolate acts as a chelating ligand, complexing the indium ion via its oxygen and ring nitrogen atom. The reported indium complexes are predominantly mononuclear. However, three dinuclear complexes are also included in the database entries mentioned above. In the case of the dinuclear chelate complexes, the indium ions possess coordination numbers of six (ALESES; Alexander et al., 2021 ▸) or five (SOMYOL, SOMZEC; Kwak et al., 2019 ▸). The mononuclear complexes mostly have a coordination number of six, but occasionally the indium ion is coordinated five-, seven- or eightfold.
In the crystal structure with the reference code ALESES, the indium ion adopts a coordination environment of the composition N2O3Cl. Since this complex lacks a quinoline-bound keto group and no water molecule is involved, a strand-like association as in the crystal structure of the title complex cannot be observed. Instead, weak Caryl—H⋯Cl and Caryl—H⋯O hydrogen bonds as well as π⋯π contacts between the quinoline units of adjacent complexes lead to the formation of two-dimensional supramolecular networks. The packing structures of the complexes with the reference codes SOMYOL and SOMZEC, containing NO2C2-coordinated indium ions, consist of an infinite strand-like arrangement of molecules connected by π⋯π interactions similar to those mentioned above.
5. Synthesis and crystallization
5-Nonanoyl-8-hydroxyquinoline (50 mg, 0.18 mmol) and indium(III) chloride (116 mg, 0.52 mmol) were stirred in methanol (5 mL) for 30 min at room temperature and the solvent was removed under vacuum. Afterwards the residue was crystallized by slow evaporation from acetonitrile. 5-Nonanoyl-8-hydroxyquinoline was synthesized according to the literature procedure (Uhlemann et al., 1981 ▸).
6. Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. All non-hydrogen atoms were refined anisotropically. Both hydrogen atoms of the water molecule (H3A, H3B) were located in difference-Fourier maps and placed accordingly. The remaining hydrogen atoms were positioned geometrically and refined isotropically using a riding model, with C—H bond distances of 0.95 Å (aryl), 0.98 Å (methylene) and 0.99 Å (methyl). The thermal displacement ellipsoids of all hydrogen atoms were set to Uiso(H) = 1.2Ueq(C) and Uiso(H) = 1.5 Ueq(C/O), the latter applying to methyl and water moieties.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | [In2(C18H22NO2)2Cl4(H2O)2] |
| M r | 976.20 |
| Crystal system, space group | Triclinic, P
|
| Temperature (K) | 163 |
| a, b, c (Å) | 10.297 (4), 10.940 (4), 11.711 (5) |
| α, β, γ (°) | 63.57 (3), 72.47 (3), 62.92 (3) |
| V (Å3) | 1043.8 (8) |
| Z | 1 |
| Radiation type | Mo Kα |
| μ (mm−1) | 1.40 |
| Crystal size (mm) | 0.19 × 0.10 × 0.07 |
| Data collection | |
| Diffractometer | Stoe Stadivari |
| Absorption correction | Multi-scan (X-RED32; Stoe & Cie, 2002 ▸) |
| Tmin, Tmax | 0.766, 0.907 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 62899, 62899, 42801 |
| R int | 0.039 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.039, 0.061, 0.88 |
| No. of reflections | 62899 |
| No. of parameters | 234 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.94, −0.74 |
The crystal was refined as a two-component non-merohedral twin, whereby the main domain makes up 72% of the crystal. The two domains were identified and integrated simultaneously via the Recipe/Index/Refine and Integrate modules, respectively, of the X-AREA program suite, followed by absorption correction and scaling of the resulting HKLF5 dataset via the modules X-RED32 and LANA, respectively (Stoe & Cie, 2002 ▸). The reflection file employed in the subsequent refinement contained reflections from the two individual domains as well as reflections to which both domains contributed.
By recognizing twinning, the R-values as well as the maximum residual electron density (Table 2 ▸) improved drastically compared to the model based on untreated HKLF4 data (R1 = 7.24%, wR2 = 22.71%, maximum electron density = 3.94 e Å−3).
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S205698902400882X/jp2009sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S205698902400882X/jp2009Isup2.hkl
Supporting information file. DOI: 10.1107/S205698902400882X/jp2009Isup3.cdx
CCDC reference: 2382976
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
We would like to thank the Audi Stiftung für Umwelt for funding. Open Access Funding by the Publication Fund of the Technische Universität Bergakademie Freiberg is gratefully acknowledged.
supplementary crystallographic information
Bis(µ2-5-nonanoylquinolin-8-olato)bis[aquadichloridoindium(III)] . Crystal data
| [In2(C18H22NO2)2Cl4(H2O)2] | Z = 1 |
| Mr = 976.20 | F(000) = 492 |
| Triclinic, P1 | Dx = 1.553 Mg m−3 |
| a = 10.297 (4) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 10.940 (4) Å | Cell parameters from 24127 reflections |
| c = 11.711 (5) Å | θ = 2.2–28.7° |
| α = 63.57 (3)° | µ = 1.40 mm−1 |
| β = 72.47 (3)° | T = 163 K |
| γ = 62.92 (3)° | Plate, colourless |
| V = 1043.8 (8) Å3 | 0.19 × 0.10 × 0.07 mm |
Bis(µ2-5-nonanoylquinolin-8-olato)bis[aquadichloridoindium(III)] . Data collection
| Stoe Stadivari diffractometer | 62899 independent reflections |
| Radiation source: Primux 50 Mo | 42801 reflections with I > 2σ(I) |
| Graded multilayer mirror monochromator | Rint = 0.039 |
| Detector resolution: 5.81 pixels mm-1 | θmax = 27.5°, θmin = 2.2° |
| rotation method, ω scans | h = −13→13 |
| Absorption correction: multi-scan (X-Red32; Stoe & Cie, 2002) | k = −14→14 |
| Tmin = 0.766, Tmax = 0.907 | l = −15→15 |
| 62899 measured reflections |
Bis(µ2-5-nonanoylquinolin-8-olato)bis[aquadichloridoindium(III)] . Refinement
| Refinement on F2 | Primary atom site location: dual |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.039 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.061 | w = 1/[σ2(Fo2) + (0.0118P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 0.88 | (Δ/σ)max = 0.001 |
| 62899 reflections | Δρmax = 0.94 e Å−3 |
| 234 parameters | Δρmin = −0.74 e Å−3 |
| 0 restraints |
Bis(µ2-5-nonanoylquinolin-8-olato)bis[aquadichloridoindium(III)] . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refined as a 2-component twin. |
Bis(µ2-5-nonanoylquinolin-8-olato)bis[aquadichloridoindium(III)] . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.7219 (4) | 0.0957 (5) | 0.3966 (5) | 0.0270 (12) | |
| H1 | 0.6420 | 0.0662 | 0.4143 | 0.032* | |
| C2 | 0.8613 (4) | 0.0088 (5) | 0.3551 (4) | 0.0284 (12) | |
| H2 | 0.8760 | −0.0788 | 0.3459 | 0.034* | |
| C3 | 0.9763 (4) | 0.0515 (5) | 0.3279 (4) | 0.0260 (12) | |
| H3 | 1.0710 | −0.0055 | 0.2973 | 0.031* | |
| C4 | 0.9562 (4) | 0.1795 (5) | 0.3447 (4) | 0.0202 (11) | |
| C5 | 1.0678 (4) | 0.2360 (5) | 0.3187 (4) | 0.0226 (11) | |
| C6 | 1.0297 (4) | 0.3609 (5) | 0.3417 (5) | 0.0266 (12) | |
| H6 | 1.1039 | 0.3973 | 0.3255 | 0.032* | |
| C7 | 0.8868 (4) | 0.4380 (5) | 0.3880 (4) | 0.0260 (12) | |
| H7 | 0.8668 | 0.5223 | 0.4053 | 0.031* | |
| C8 | 0.7761 (4) | 0.3912 (5) | 0.4080 (4) | 0.0202 (11) | |
| C9 | 0.8106 (4) | 0.2605 (5) | 0.3875 (4) | 0.0202 (11) | |
| C10 | 1.2233 (4) | 0.1595 (5) | 0.2701 (5) | 0.0252 (12) | |
| C11 | 1.3194 (4) | 0.2486 (5) | 0.2001 (5) | 0.0274 (12) | |
| H11A | 1.3243 | 0.2894 | 0.2584 | 0.033* | |
| H11B | 1.2720 | 0.3328 | 0.1255 | 0.033* | |
| C12 | 1.4756 (4) | 0.1645 (5) | 0.1521 (5) | 0.0301 (13) | |
| H12A | 1.4734 | 0.1317 | 0.0866 | 0.036* | |
| H12B | 1.5228 | 0.0760 | 0.2244 | 0.036* | |
| C13 | 1.5642 (4) | 0.2633 (5) | 0.0937 (5) | 0.0312 (13) | |
| H13A | 1.5621 | 0.2980 | 0.1595 | 0.037* | |
| H13B | 1.5156 | 0.3510 | 0.0216 | 0.037* | |
| C14 | 1.7255 (4) | 0.1892 (5) | 0.0439 (5) | 0.0327 (13) | |
| H14A | 1.7693 | 0.0905 | 0.1089 | 0.039* | |
| H14B | 1.7296 | 0.1761 | −0.0356 | 0.039* | |
| C15 | 1.8144 (4) | 0.2809 (5) | 0.0162 (5) | 0.0340 (13) | |
| H15A | 1.8062 | 0.2969 | 0.0953 | 0.041* | |
| H15B | 1.7710 | 0.3785 | −0.0501 | 0.041* | |
| C16 | 1.9767 (4) | 0.2114 (5) | −0.0297 (5) | 0.0404 (14) | |
| H16A | 2.0183 | 0.1097 | 0.0320 | 0.048* | |
| H16B | 1.9861 | 0.2058 | −0.1140 | 0.048* | |
| C17 | 2.0644 (4) | 0.2983 (6) | −0.0424 (5) | 0.0453 (15) | |
| H17A | 2.0549 | 0.3039 | 0.0420 | 0.054* | |
| H17B | 2.0225 | 0.4001 | −0.1039 | 0.054* | |
| C18 | 2.2278 (4) | 0.2295 (6) | −0.0887 (5) | 0.066 (2) | |
| H18A | 2.2719 | 0.1317 | −0.0248 | 0.099* | |
| H18B | 2.2781 | 0.2924 | −0.1001 | 0.099* | |
| H18C | 2.2379 | 0.2203 | −0.1707 | 0.099* | |
| Cl1 | 0.34019 (11) | 0.21319 (13) | 0.53300 (14) | 0.0374 (4) | |
| Cl2 | 0.38785 (12) | 0.53711 (14) | 0.27233 (13) | 0.0403 (4) | |
| In1 | 0.47713 (3) | 0.36247 (4) | 0.47732 (4) | 0.02240 (9) | |
| N1 | 0.6970 (3) | 0.2161 (4) | 0.4119 (4) | 0.0220 (9) | |
| O1 | 0.6352 (2) | 0.4620 (3) | 0.4468 (3) | 0.0220 (8) | |
| O2 | 1.2707 (3) | 0.0302 (3) | 0.2842 (4) | 0.0403 (10) | |
| O3 | 0.5333 (3) | 0.2442 (3) | 0.6779 (3) | 0.0288 (9) | |
| H3A | 0.588 (4) | 0.154 (5) | 0.700 (5) | 0.043* | |
| H3B | 0.559 (4) | 0.294 (5) | 0.707 (4) | 0.043* |
Bis(µ2-5-nonanoylquinolin-8-olato)bis[aquadichloridoindium(III)] . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.026 (3) | 0.023 (3) | 0.035 (4) | −0.012 (2) | 0.000 (2) | −0.013 (3) |
| C2 | 0.029 (2) | 0.019 (3) | 0.040 (4) | −0.007 (2) | 0.000 (2) | −0.017 (3) |
| C3 | 0.023 (2) | 0.019 (3) | 0.030 (3) | −0.003 (2) | 0.001 (2) | −0.012 (3) |
| C4 | 0.021 (2) | 0.016 (3) | 0.021 (3) | −0.004 (2) | −0.002 (2) | −0.007 (2) |
| C5 | 0.018 (2) | 0.019 (3) | 0.029 (3) | −0.003 (2) | −0.003 (2) | −0.011 (2) |
| C6 | 0.016 (2) | 0.024 (3) | 0.039 (4) | −0.007 (2) | −0.001 (2) | −0.014 (3) |
| C7 | 0.023 (2) | 0.019 (3) | 0.039 (4) | −0.006 (2) | −0.001 (2) | −0.017 (3) |
| C8 | 0.019 (2) | 0.016 (3) | 0.021 (3) | −0.003 (2) | −0.002 (2) | −0.007 (2) |
| C9 | 0.019 (2) | 0.016 (3) | 0.025 (3) | −0.003 (2) | −0.006 (2) | −0.008 (2) |
| C10 | 0.021 (2) | 0.024 (3) | 0.030 (3) | −0.003 (2) | −0.007 (2) | −0.013 (3) |
| C11 | 0.021 (2) | 0.027 (3) | 0.035 (4) | −0.009 (2) | 0.002 (2) | −0.015 (3) |
| C12 | 0.018 (2) | 0.033 (3) | 0.039 (4) | −0.004 (2) | −0.001 (2) | −0.020 (3) |
| C13 | 0.023 (2) | 0.033 (3) | 0.037 (4) | −0.009 (2) | 0.000 (2) | −0.017 (3) |
| C14 | 0.022 (2) | 0.037 (3) | 0.037 (4) | −0.008 (2) | 0.000 (2) | −0.017 (3) |
| C15 | 0.026 (3) | 0.036 (3) | 0.038 (4) | −0.013 (2) | 0.000 (2) | −0.013 (3) |
| C16 | 0.026 (3) | 0.056 (4) | 0.040 (4) | −0.018 (3) | 0.004 (2) | −0.020 (3) |
| C17 | 0.032 (3) | 0.068 (4) | 0.036 (4) | −0.029 (3) | 0.003 (3) | −0.013 (3) |
| C18 | 0.033 (3) | 0.106 (6) | 0.065 (5) | −0.036 (3) | 0.008 (3) | −0.035 (4) |
| Cl1 | 0.0307 (6) | 0.0322 (8) | 0.0596 (11) | −0.0178 (6) | 0.0014 (6) | −0.0234 (8) |
| Cl2 | 0.0544 (8) | 0.0296 (8) | 0.0394 (10) | −0.0098 (7) | −0.0191 (7) | −0.0124 (7) |
| In1 | 0.01802 (15) | 0.01785 (17) | 0.0336 (2) | −0.00564 (12) | −0.00088 (14) | −0.01387 (16) |
| N1 | 0.0185 (18) | 0.018 (2) | 0.030 (3) | −0.0068 (17) | 0.0014 (17) | −0.011 (2) |
| O1 | 0.0158 (15) | 0.0197 (17) | 0.032 (2) | −0.0057 (13) | 0.0026 (13) | −0.0154 (17) |
| O2 | 0.0220 (17) | 0.022 (2) | 0.069 (3) | −0.0035 (15) | 0.0001 (17) | −0.018 (2) |
| O3 | 0.0322 (18) | 0.0194 (19) | 0.032 (2) | −0.0070 (15) | −0.0026 (16) | −0.0109 (18) |
Bis(µ2-5-nonanoylquinolin-8-olato)bis[aquadichloridoindium(III)] . Geometric parameters (Å, º)
| C1—N1 | 1.308 (5) | C13—H13A | 0.9900 |
| C1—C2 | 1.396 (5) | C13—H13B | 0.9900 |
| C1—H1 | 0.9500 | C14—C15 | 1.522 (5) |
| C2—C3 | 1.367 (5) | C14—H14A | 0.9900 |
| C2—H2 | 0.9500 | C14—H14B | 0.9900 |
| C3—C4 | 1.414 (5) | C15—C16 | 1.524 (5) |
| C3—H3 | 0.9500 | C15—H15A | 0.9900 |
| C4—C9 | 1.420 (5) | C15—H15B | 0.9900 |
| C4—C5 | 1.437 (5) | C16—C17 | 1.522 (6) |
| C5—C6 | 1.368 (6) | C16—H16A | 0.9900 |
| C5—C10 | 1.497 (5) | C16—H16B | 0.9900 |
| C6—C7 | 1.402 (5) | C17—C18 | 1.534 (5) |
| C6—H6 | 0.9500 | C17—H17A | 0.9900 |
| C7—C8 | 1.372 (5) | C17—H17B | 0.9900 |
| C7—H7 | 0.9500 | C18—H18A | 0.9800 |
| C8—O1 | 1.344 (4) | C18—H18B | 0.9800 |
| C8—C9 | 1.421 (6) | C18—H18C | 0.9800 |
| C9—N1 | 1.370 (5) | Cl1—In1 | 2.3825 (14) |
| C10—O2 | 1.215 (5) | Cl2—In1 | 2.4302 (19) |
| C10—C11 | 1.511 (5) | In1—O1i | 2.166 (3) |
| C11—C12 | 1.522 (5) | In1—O1 | 2.209 (3) |
| C11—H11A | 0.9900 | In1—O3 | 2.227 (4) |
| C11—H11B | 0.9900 | In1—N1 | 2.241 (3) |
| C12—C13 | 1.528 (5) | O1—In1i | 2.166 (3) |
| C12—H12A | 0.9900 | O3—H3A | 0.84 (4) |
| C12—H12B | 0.9900 | O3—H3B | 0.92 (4) |
| C13—C14 | 1.538 (5) | ||
| N1—C1—C2 | 122.5 (4) | C15—C14—H14B | 109.4 |
| N1—C1—H1 | 118.7 | C13—C14—H14B | 109.4 |
| C2—C1—H1 | 118.7 | H14A—C14—H14B | 108.0 |
| C3—C2—C1 | 119.0 (4) | C14—C15—C16 | 114.1 (4) |
| C3—C2—H2 | 120.5 | C14—C15—H15A | 108.7 |
| C1—C2—H2 | 120.5 | C16—C15—H15A | 108.7 |
| C2—C3—C4 | 120.8 (4) | C14—C15—H15B | 108.7 |
| C2—C3—H3 | 119.6 | C16—C15—H15B | 108.7 |
| C4—C3—H3 | 119.6 | H15A—C15—H15B | 107.6 |
| C3—C4—C9 | 116.0 (4) | C17—C16—C15 | 112.3 (4) |
| C3—C4—C5 | 125.9 (4) | C17—C16—H16A | 109.1 |
| C9—C4—C5 | 118.0 (4) | C15—C16—H16A | 109.1 |
| C6—C5—C4 | 118.3 (4) | C17—C16—H16B | 109.1 |
| C6—C5—C10 | 120.2 (4) | C15—C16—H16B | 109.1 |
| C4—C5—C10 | 121.4 (4) | H16A—C16—H16B | 107.9 |
| C5—C6—C7 | 123.5 (4) | C16—C17—C18 | 112.7 (4) |
| C5—C6—H6 | 118.2 | C16—C17—H17A | 109.0 |
| C7—C6—H6 | 118.2 | C18—C17—H17A | 109.0 |
| C8—C7—C6 | 119.7 (4) | C16—C17—H17B | 109.0 |
| C8—C7—H7 | 120.2 | C18—C17—H17B | 109.0 |
| C6—C7—H7 | 120.2 | H17A—C17—H17B | 107.8 |
| O1—C8—C7 | 123.6 (4) | C17—C18—H18A | 109.5 |
| O1—C8—C9 | 117.5 (3) | C17—C18—H18B | 109.5 |
| C7—C8—C9 | 118.9 (4) | H18A—C18—H18B | 109.5 |
| N1—C9—C4 | 121.8 (4) | C17—C18—H18C | 109.5 |
| N1—C9—C8 | 116.8 (4) | H18A—C18—H18C | 109.5 |
| C4—C9—C8 | 121.4 (4) | H18B—C18—H18C | 109.5 |
| O2—C10—C5 | 121.4 (4) | O1i—In1—O1 | 72.65 (11) |
| O2—C10—C11 | 120.6 (4) | O1i—In1—O3 | 78.60 (12) |
| C5—C10—C11 | 118.0 (4) | O1—In1—O3 | 84.69 (12) |
| C10—C11—C12 | 115.3 (4) | O1i—In1—N1 | 144.79 (11) |
| C10—C11—H11A | 108.5 | O1—In1—N1 | 73.40 (11) |
| C12—C11—H11A | 108.5 | O3—In1—N1 | 89.29 (13) |
| C10—C11—H11B | 108.5 | O1i—In1—Cl1 | 112.71 (8) |
| C12—C11—H11B | 108.5 | O1—In1—Cl1 | 169.15 (8) |
| H11A—C11—H11B | 107.5 | O3—In1—Cl1 | 87.16 (9) |
| C11—C12—C13 | 109.9 (3) | N1—In1—Cl1 | 99.38 (10) |
| C11—C12—H12A | 109.7 | O1i—In1—Cl2 | 89.09 (9) |
| C13—C12—H12A | 109.7 | O1—In1—Cl2 | 91.53 (9) |
| C11—C12—H12B | 109.7 | O3—In1—Cl2 | 167.69 (9) |
| C13—C12—H12B | 109.7 | N1—In1—Cl2 | 100.88 (11) |
| H12A—C12—H12B | 108.2 | Cl1—In1—Cl2 | 97.88 (6) |
| C12—C13—C14 | 114.7 (4) | C1—N1—C9 | 119.8 (3) |
| C12—C13—H13A | 108.6 | C1—N1—In1 | 124.9 (3) |
| C14—C13—H13A | 108.6 | C9—N1—In1 | 115.3 (3) |
| C12—C13—H13B | 108.6 | C8—O1—In1i | 134.6 (2) |
| C14—C13—H13B | 108.6 | C8—O1—In1 | 117.0 (2) |
| H13A—C13—H13B | 107.6 | In1i—O1—In1 | 107.35 (11) |
| C15—C14—C13 | 111.3 (4) | In1—O3—H3A | 118 (3) |
| C15—C14—H14A | 109.4 | In1—O3—H3B | 115 (3) |
| C13—C14—H14A | 109.4 | H3A—O3—H3B | 112 (4) |
| N1—C1—C2—C3 | −0.8 (7) | C4—C5—C10—O2 | −20.8 (7) |
| C1—C2—C3—C4 | 1.7 (7) | C6—C5—C10—C11 | −23.3 (6) |
| C2—C3—C4—C9 | −1.6 (7) | C4—C5—C10—C11 | 157.9 (4) |
| C2—C3—C4—C5 | −179.6 (4) | O2—C10—C11—C12 | −0.7 (7) |
| C3—C4—C5—C6 | −179.3 (5) | C5—C10—C11—C12 | −179.4 (4) |
| C9—C4—C5—C6 | 2.8 (7) | C10—C11—C12—C13 | −175.2 (4) |
| C3—C4—C5—C10 | −0.5 (7) | C11—C12—C13—C14 | 178.9 (4) |
| C9—C4—C5—C10 | −178.4 (4) | C12—C13—C14—C15 | −166.9 (4) |
| C4—C5—C6—C7 | −0.8 (7) | C13—C14—C15—C16 | 178.2 (4) |
| C10—C5—C6—C7 | −179.6 (4) | C14—C15—C16—C17 | −173.9 (4) |
| C5—C6—C7—C8 | −2.3 (7) | C15—C16—C17—C18 | −179.9 (4) |
| C6—C7—C8—O1 | −177.0 (4) | C2—C1—N1—C9 | −0.3 (7) |
| C6—C7—C8—C9 | 3.2 (7) | C2—C1—N1—In1 | 179.8 (3) |
| C3—C4—C9—N1 | 0.5 (6) | C4—C9—N1—C1 | 0.5 (7) |
| C5—C4—C9—N1 | 178.6 (4) | C8—C9—N1—C1 | −179.1 (4) |
| C3—C4—C9—C8 | 180.0 (4) | C4—C9—N1—In1 | −179.6 (3) |
| C5—C4—C9—C8 | −1.8 (7) | C8—C9—N1—In1 | 0.8 (5) |
| O1—C8—C9—N1 | −1.4 (6) | C7—C8—O1—In1i | −12.1 (7) |
| C7—C8—C9—N1 | 178.4 (4) | C9—C8—O1—In1i | 167.7 (3) |
| O1—C8—C9—C4 | 179.1 (4) | C7—C8—O1—In1 | −178.5 (4) |
| C7—C8—C9—C4 | −1.1 (7) | C9—C8—O1—In1 | 1.3 (5) |
| C6—C5—C10—O2 | 157.9 (5) |
Symmetry code: (i) −x+1, −y+1, −z+1.
Bis(µ2-5-nonanoylquinolin-8-olato)bis[aquadichloridoindium(III)] . Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O3—H3A···O2ii | 0.84 (4) | 1.84 (4) | 2.668 (4) | 168 (5) |
| O3—H3B···Cl2i | 0.92 (4) | 2.27 (4) | 3.165 (4) | 165 (4) |
Symmetry codes: (i) −x+1, −y+1, −z+1; (ii) −x+2, −y, −z+1.
References
- Albrecht, M., Fiege, M. & Osetska, O. (2008). Coord. Chem. Rev.252, 812–824.
- Alexander, O. T., Duvenhage, M. M., Kroon, R. E., Brink, A. & Visser, H. G. (2021). New J. Chem.45, 2132–2140.
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst.42, 339–341.
- Farrugia, L. J. (2012). J. Appl. Cryst.45, 849–854.
- Feng, L., Wang, X. & Chen, Z. (2008). Spectrochim. Acta A Mol. Biomol. Spectrosc.71, 312–316. [DOI] [PubMed]
- Feng, L., Wang, X., Zhao, S. & Chen, Z. (2007). Spectrochim. Acta A Mol. Biomol. Spectrosc.68, 646–650. [DOI] [PubMed]
- Filik, H. & Apak, R. (1994). Sep. Sci. Technol.29, 2047–2066.
- Geffert, C., Kuschel, M. & Mazik, M. (2013). J. Org. Chem.78, 292–300. [DOI] [PubMed]
- Gloe, K., Stephan, H., Krüger, T., Möckel, A., Woller, N., Subklew, G., Schwuger, M. J., Neumann, R. & Weber, E. (1996). Prog. Colloid Polym. Sci.101, 145–148.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Kwak, S. W., Kim, M. B., Shin, H., Lee, J. H., Hwang, H., Ryu, J. Y., Lee, J., Kim, M., Chung, Y., Choe, J. C., Kim, Y., Lee, K. M. & Park, M. H. (2019). Inorg. Chem.58, 8056–8063. [DOI] [PubMed]
- Matsumura, M. & Akai, T. (1996). Jpn. J. Appl. Phys.35, 5357–5360.
- Mazik, M. & Geffert, C. (2011). Org. Biomol. Chem.9, 2319–2326. [DOI] [PubMed]
- Montes, V. A., Pohl, R., Shinar, J. & Anzenbacher, P. (2006). Chem. Eur. J.12, 4523–4535. [DOI] [PubMed]
- Schulze, M., Löwe, R., Pollex, R. & Mazik, M. (2019). Monatsh. Chem.150, 983–990.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Stoe & Cie (2002). X-AREA, X-AREA Recipe, X-RED32 and LANA. Stoe & Cie, Darmstadt, Germany.
- Uhlemann, E., Mickler, W., Ludwig, E., Ludwig, E. & Klose, G. (1981). J. Prakt. Chem.323, 521–524.
- Uhlemann, E., Weber, W., Fischer, C. & Raab, M. (1984). Anal. Chim. Acta, 156, 201–206.
- Yamada, H., Hayashi, H. & Yasui, T. (2006). Anal. Sci.22, 371–376. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S205698902400882X/jp2009sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S205698902400882X/jp2009Isup2.hkl
Supporting information file. DOI: 10.1107/S205698902400882X/jp2009Isup3.cdx
CCDC reference: 2382976
Additional supporting information: crystallographic information; 3D view; checkCIF report





