Abstract
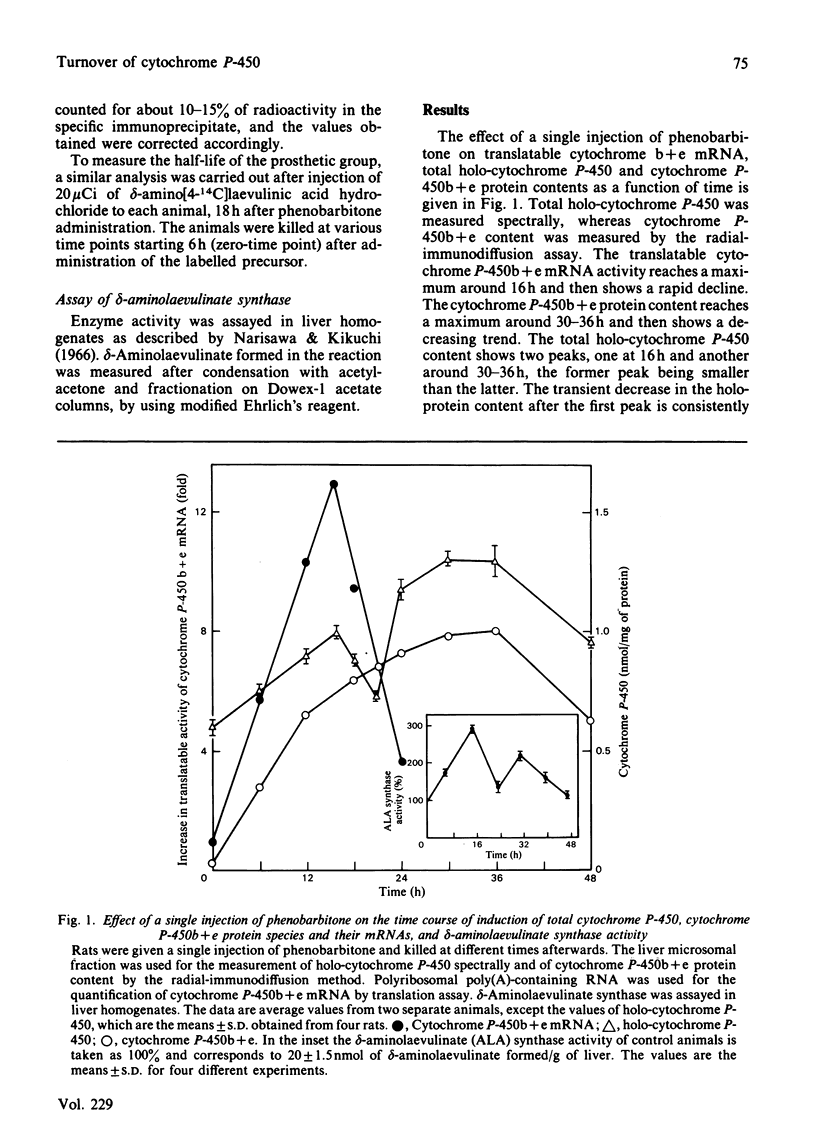
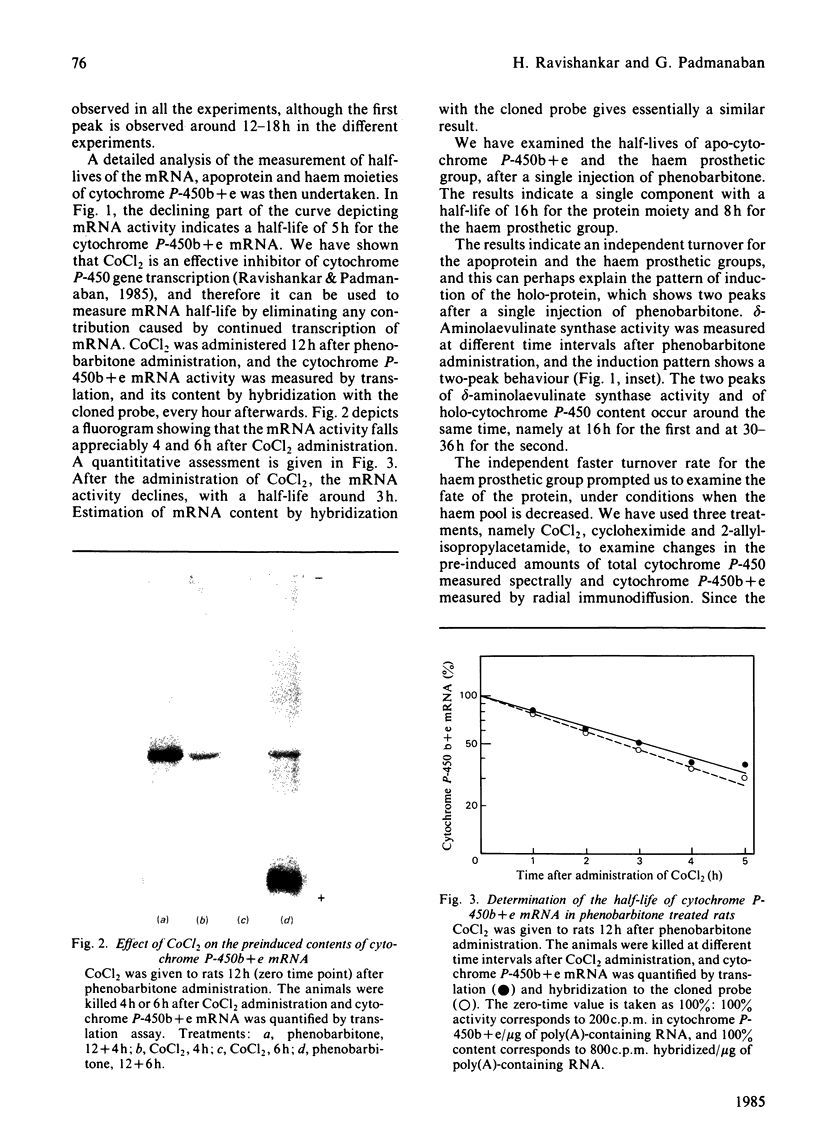
A single injection of phenobarbitone elicits asynchronous behaviour in the pattern of induction of mRNA, apoprotein and haem of cytochrome P-450b + e. The mRNA content reaches a maximum around 16h, the apoprotein content reaches a maximum around 30-36h, and the holoprotein content shows biphasic behaviour, with maxima around 16h and 30-36h. With the use of CoCl2 to block fresh transcription of cytochrome P-450b + e mRNA, the half-life of the preinduced mRNA was found to be 3h. The apoprotein and haem moieties of cytochrome P-450b + e turn over with half-lives of 16h and 8h respectively. The pattern of induction of delta-aminolaevulinate synthase shows biphasic behaviour, with maxima around 16h and 30-36h. The biphasic behaviour of the holoprotein content is thus due to differences in the extent of saturation of the apoprotein with haem, the process being regulated by the activity of the rate-limiting delta-aminolaevulinate synthase and the independent faster turnover rate of haem with respect to the apoprotein. Massive degradation of the haem moiety of preformed cytochrome P-450b + e results in the subsequent degradation of the apoprotein.
Full text
PDF




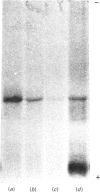
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Bar-Nun S., Maschio F., Zunich M., Lippman A., Bard E. Mechanism of induction of cytochrome P-450 by phenobarbital. J Biol Chem. 1981 Oct 25;256(20):10340–10345. [PubMed] [Google Scholar]
- Atchison M., Adesnik M. A cytochrome P-450 multigene family. Characterization of a gene activated by phenobarbital administration. J Biol Chem. 1983 Sep 25;258(18):11285–11295. [PubMed] [Google Scholar]
- Bhat K. S., Padmanaban G. Studies on the biosynthesis of cytochrome P-450 in rat liver--a probe with phenobarbital. Arch Biochem Biophys. 1979 Nov;198(1):110–116. doi: 10.1016/0003-9861(79)90400-4. [DOI] [PubMed] [Google Scholar]
- De Matteis F. Rapid loss of cytochrome P-450 and haem caused in the liver microsomes by the porphyrogenic agent 2-allyl-2-isopropylacetamide. FEBS Lett. 1970 Feb 25;6(4):343–345. doi: 10.1016/0014-5793(70)80094-1. [DOI] [PubMed] [Google Scholar]
- Dubois R. N., Waterman M. R. Effect of phenobarbital administration to rats on the level of the in vitro synthesis of cytochrome P-450 directed by total rat liver RNA. Biochem Biophys Res Commun. 1979 Sep 12;90(1):150–157. doi: 10.1016/0006-291x(79)91602-4. [DOI] [PubMed] [Google Scholar]
- Gasser R., Hauri H. P., Meyer U. A. The turnover of cytochrome P450b. FEBS Lett. 1982 Oct 18;147(2):239–242. doi: 10.1016/0014-5793(82)81050-8. [DOI] [PubMed] [Google Scholar]
- Hardwick J. P., Gonzalez F. J., Kasper C. B. Cloning of DNA complementary to cytochrome P-450 induced by pregnenolone-16 alpha-carbonitrile. Characterization of its mRNA, gene, and induction response. J Biol Chem. 1983 Aug 25;258(16):10182–10186. [PubMed] [Google Scholar]
- Hayashi N., Yoda B., Kikuchi G. Difference in molecular sizes of delta-aminolevulinate synthetases in the soluble and mitochondrial fractions of rat liver. J Biochem. 1970 Jun;67(6):859–861. doi: 10.1093/oxfordjournals.jbchem.a129319. [DOI] [PubMed] [Google Scholar]
- Krystosek A., Cawthon M. L., Kabat D. Improved methods for purification and assay of eukaryotic messenger ribonucleic acids and ribosomes. Quantitative analysis of their interaction in a fractionated reticulocyte cell-free system. J Biol Chem. 1975 Aug 10;250(15):6077–6084. [PubMed] [Google Scholar]
- Kumar A., Padmanaban G. Studies on the synthesis of cytochrome P-450 and cytochrome P-448 in rat liver. J Biol Chem. 1980 Jan 25;255(2):522–525. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lu A. Y., West S. B. Multiplicity of mammalian microsomal cytochromes P-45. Pharmacol Rev. 1979 Dec;31(4):277–295. [PubMed] [Google Scholar]
- Mizukami Y., Sogawa K., Suwa Y., Muramatsu M., Fujii-Kuriyama Y. Gene structure of a phenobarbital-inducible cytochrome P-450 in rat liver. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3958–3962. doi: 10.1073/pnas.80.13.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morville A. L., Thomas P., Levin W., Reik L., Ryan D. E., Raphael C., Adesnik M. The accumulation of distinct mRNAs for the immunochemically related cytochromes P-450c and P-450d in rat liver following 3-methylcholanthrene treatment. J Biol Chem. 1983 Mar 25;258(6):3901–3906. [PubMed] [Google Scholar]
- Narisawa K., Kikuchi G. Mechanism of allylisopropylacetamide-induced increase of delta-aminolevulinate synthetase in rat-liver mitochondria. Biochim Biophys Acta. 1966 Sep;123(3):596–605. doi: 10.1016/0005-2787(66)90226-7. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Parkinson A., Thomas P. E., Ryan D. E., Levin W. The in vivo turnover of rat liver microsomal epoxide hydrolase and both the apoprotein and heme moieties of specific cytochrome P-450 isozymes. Arch Biochem Biophys. 1983 Aug;225(1):216–236. doi: 10.1016/0003-9861(83)90025-5. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ravishankar H., Padmanaban G. Effect of cobalt chloride and 3-amino-1,2,4-triazole on the induction of cytochrome P-450 synthesis by phenobarbitone in rat liver. Arch Biochem Biophys. 1983 Aug;225(1):16–24. doi: 10.1016/0003-9861(83)90002-4. [DOI] [PubMed] [Google Scholar]
- Ravishankar H., Padmanaban G. Regulation of cytochrome P-450 gene expression. Studies with a cloned probe. J Biol Chem. 1985 Feb 10;260(3):1588–1592. [PubMed] [Google Scholar]
- Ryan D. E., Thomas P. E., Levin W. Purification of characterization of a minor form of hepatic microsomal cytochrome P-450 from rats treated with polychlorinated biphenyls. Arch Biochem Biophys. 1982 Jun;216(1):272–288. doi: 10.1016/0003-9861(82)90212-0. [DOI] [PubMed] [Google Scholar]
- Sadano H., Omura T. Turnover of two drug-inducible forms of microsomal cytochrome P-450 in rat liver. J Biochem. 1983 May;93(5):1375–1383. doi: 10.1093/oxfordjournals.jbchem.a134272. [DOI] [PubMed] [Google Scholar]
- Satyabhama S., Padmanaban G. Effect of thioacetamide on cytochrome P-450 synthesis in rat liver. Biochem J. 1984 Mar 1;218(2):371–377. doi: 10.1042/bj2180371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter B. A., Yoda B., Israels L. G. Cyclic oscillations in rat hepatic heme oxygenase and delta-aminolevulinic acid synthetase following intravenous heme administration. Arch Biochem Biophys. 1976 Mar;173(1):11–17. doi: 10.1016/0003-9861(76)90228-9. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Doyle D. Control of enzyme levels in animal tissues. Annu Rev Biochem. 1970;39:929–976. doi: 10.1146/annurev.bi.39.070170.004433. [DOI] [PubMed] [Google Scholar]
- Sinclair J. F., Sinclair P. R., Healey J. F., Smith E. L., Bonkowsky H. L. Decrease in hepatic cytochrome P-450 by cobalt. Evidence for a role of cobalt protoporphyrin. Biochem J. 1982 Apr 15;204(1):103–109. doi: 10.1042/bj2040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. E., Korzeniowski D., Ryan D., Levin W. Preparation of monospecific antibodies against two forms of rat liver cytochrome P-450 and quantitation of these antigens in microsomes. Arch Biochem Biophys. 1979 Feb;192(2):524–532. doi: 10.1016/0003-9861(79)90122-x. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman A. D., Collins A., Tschudy D. P. Oscillations of hepatic delta-aminolevulinic acid synthetase produced in vivo by heme. Biochem Biophys Res Commun. 1966 Sep 8;24(5):675–683. doi: 10.1016/0006-291x(66)90377-9. [DOI] [PubMed] [Google Scholar]
- West S. B., Huang M. T., Miwa G. T., Lu A. Y. A simple and rapid procedure for the purification of phenobarbital-inducible cytochrome P-450 from rat liver microsomes. Arch Biochem Biophys. 1979 Mar;193(1):42–50. doi: 10.1016/0003-9861(79)90006-7. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Kikuchi G. Reaction of the microsomal heme oxygenase with cobaltic protoporphyrin IX, and extremely poor substrate. J Biol Chem. 1978 Dec 10;253(23):8479–8482. [PubMed] [Google Scholar]
- Yuan P. M., Ryan D. E., Levin W., Shively J. E. Identification and localization of amino acid substitutions between two phenobarbital-inducible rat hepatic microsomal cytochromes P-450 by micro sequence analyses. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1169–1173. doi: 10.1073/pnas.80.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]