Abstract
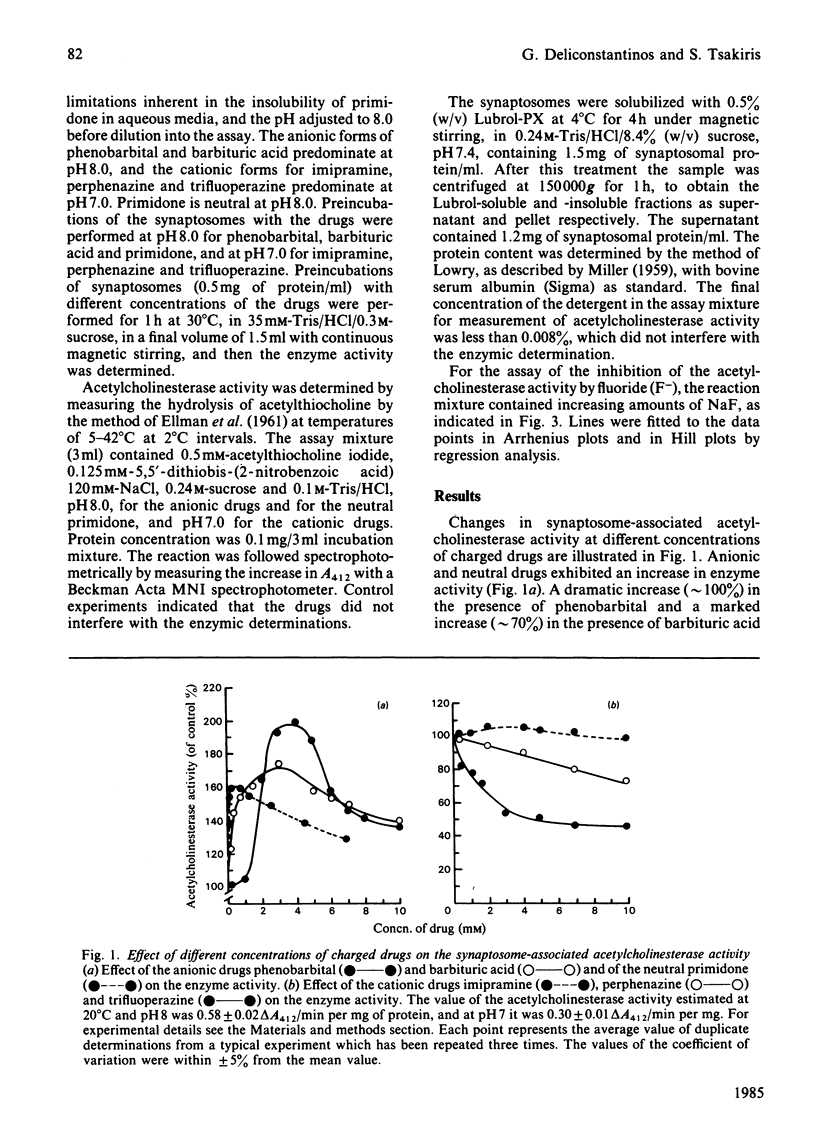
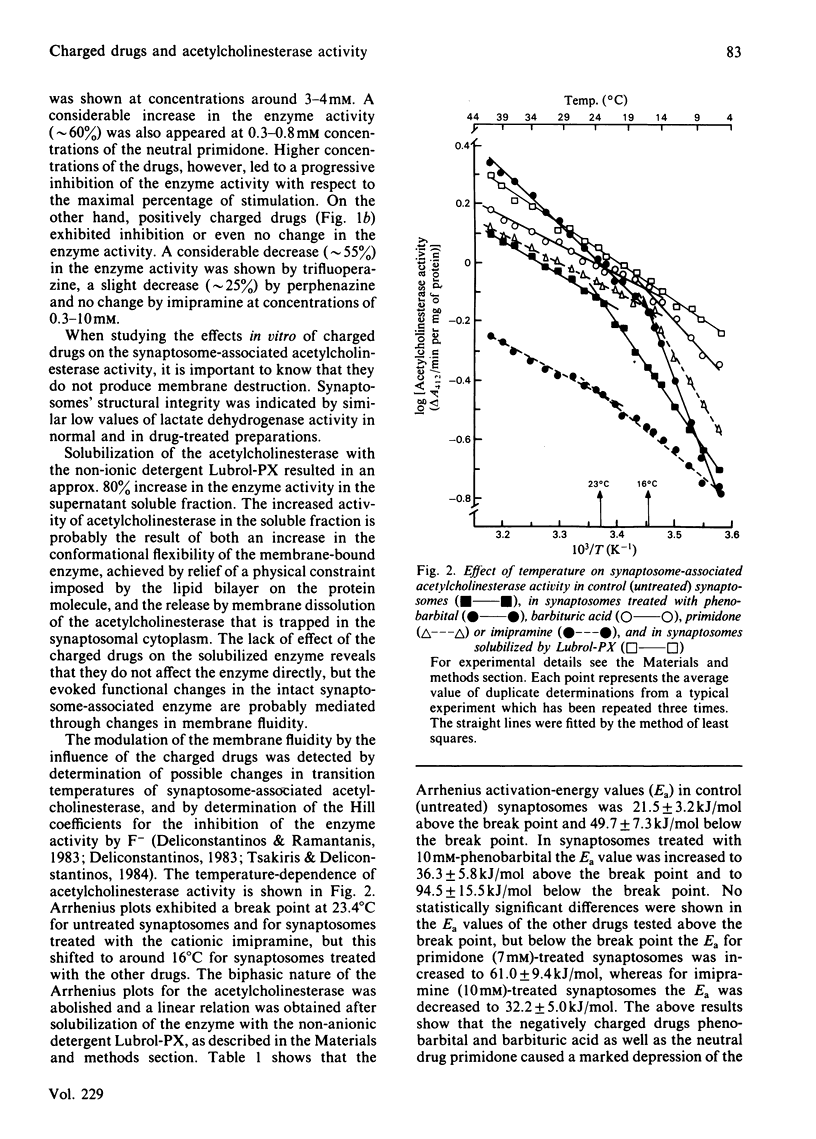
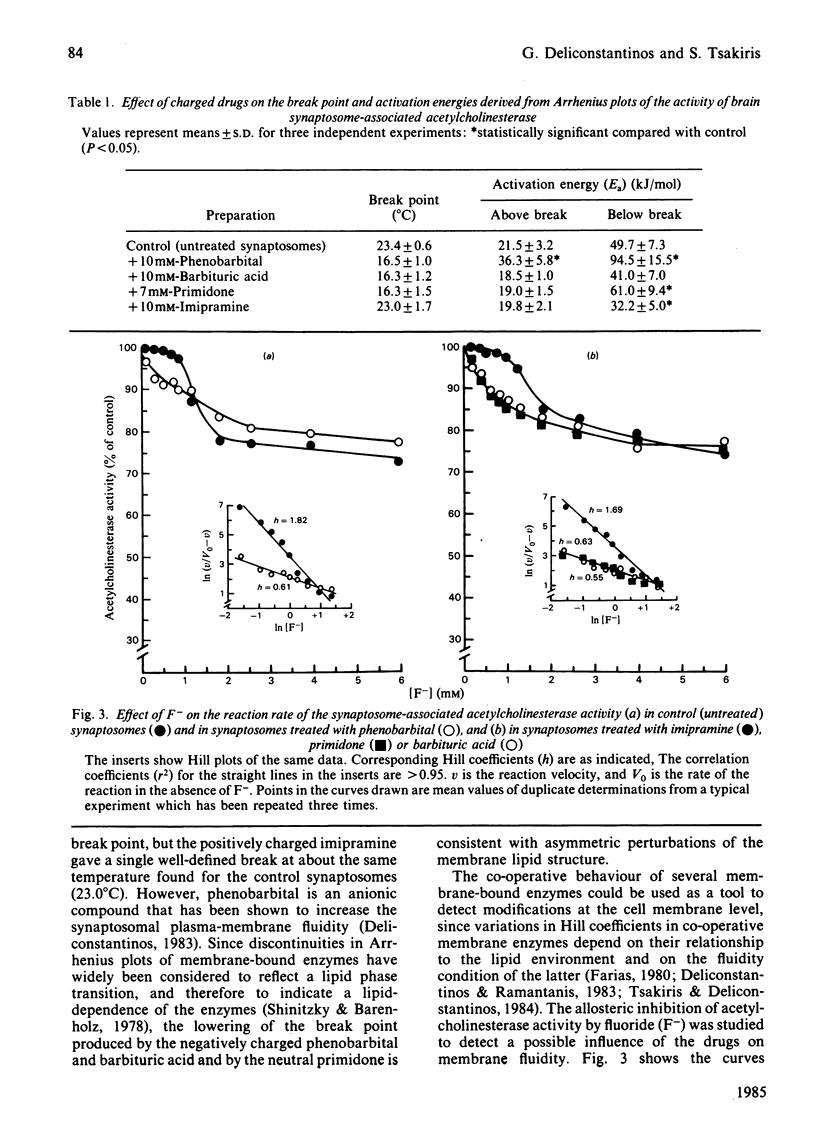
The evoked effects of the negatively charged drugs phenobarbital and barbituric acid, the positively charged imipramine, perphenazine and trifluoperazine, and the neutral primidone, on the synaptosome-associated acetylcholinesterase activity were studied. A marked increase in the enzyme activity was exhibited in the presence of low concentrations (up to 3 mM) of phenobarbital, barbituric acid and primidone. Higher concentrations (up to 10 mM), however, led to a progressive inhibition of the enzyme activity. However, the activity of the enzyme was not affected by imipramine, but it was decreased by perphenazine and trifluoperazine. Arrhenius plots of acetylcholinesterase activity exhibited a break point at 23.4 degrees C for the untreated (control) synaptosomes, which was shifted to around 16 degrees C in the synaptosomes treated with the charged drugs. The allosteric inhibition by F- of acetylcholinesterase was studied in control synaptosomes and in those treated with the charged drugs. Changes in the Hill coefficients in combination with changes in Arrhenius activation energy produced by the charged drugs would be expected if it is assumed that charged drugs 'fluidize' the synaptosomal plasma membranes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed M., Burton J. S., Hadgraft J., Kellaway I. W. Partitioning and efflux of phenothiazines from liposomes. Biochem Pharmacol. 1980 Sep 1;29(17):2361–2365. doi: 10.1016/0006-2952(80)90270-1. [DOI] [PubMed] [Google Scholar]
- Alivisatos S. G., Deliconstantinos G., Papaphilis A., Theodosiadis G. P. Cooperative nature of the binding of cholesterol on to synaptosomal plasma membranes of dog brain. Biochim Biophys Acta. 1981 May 20;643(3):642–649. doi: 10.1016/0005-2736(81)90360-6. [DOI] [PubMed] [Google Scholar]
- Alivisatos S. G., Deliconstantinos G., Theodosiadis G. P. Specificity of binding of cholesterol, steroid hormones and other compounds in synaptosomal plasma membranes, and their effect on ouabain-sensitive ATPase. Biochim Biophys Acta. 1981 May 20;643(3):650–658. doi: 10.1016/0005-2736(81)90361-8. [DOI] [PubMed] [Google Scholar]
- Deliconstantinos G., Anastasopoulou K., Karayiannakos P. Modulation of hepatic microsomal Ca2+-stimulated ATPase and drug oxidase activities of guinea pigs by dietary cholesterol. Biochem Pharmacol. 1983 Apr 1;32(7):1309–1312. doi: 10.1016/0006-2952(83)90287-3. [DOI] [PubMed] [Google Scholar]
- Deliconstantinos G. Phenobarbital modulates the (Na+, K+)-stimulated ATPase and Ca2+-stimulated ATPase activities by increasing the bilayer fluidity of dog brain synaptosomal plasma membranes. Neurochem Res. 1983 Sep;8(9):1143–1152. doi: 10.1007/BF00964928. [DOI] [PubMed] [Google Scholar]
- Deliconstantinos G., Ramantanis G. Alterations in the activities of hepatic plasma-membrane and microsomal enzymes during liver regeneration. Biochem J. 1983 May 15;212(2):445–452. doi: 10.1042/bj2120445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliconstantinos G. Temperature effect of cholesterol association with synaptosomal plasma membranes of rabbit brain. Biochem J. 1984 Sep 15;222(3):825–828. doi: 10.1042/bj2220825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipple I., Gordon L. M., Houslay M. D. The activity of 5'-nucleotidase in liver plasma membranes is affected by the increase in bilayer fluidity achieved by anionic drugs but not by cationic drugs. J Biol Chem. 1982 Feb 25;257(4):1811–1815. [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Farías R. N. Membrane cooperative enzymes as a tool for the investigation of membrane structure and related phenomena. Adv Lipid Res. 1980;17:251–282. doi: 10.1016/b978-0-12-024917-6.50012-4. [DOI] [PubMed] [Google Scholar]
- Grafius M. A., Bond H. E., Millar D. B. Acetylcholinesterase interaction with a lipoprotein matrix. Eur J Biochem. 1971 Oct 14;22(3):382–390. doi: 10.1111/j.1432-1033.1971.tb01555.x. [DOI] [PubMed] [Google Scholar]
- Higgins J. A., Evans W. H. Transverse organization of phospholipids across the bilayer of plasma-membrane subfractions of rat hepatocytes. Biochem J. 1978 Aug 15;174(2):563–567. doi: 10.1042/bj1740563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollunger E. G., Niklasson B. H. The release and molecular state of mammalian brain acetylcholinesterase. J Neurochem. 1973 Mar;20(3):821–836. doi: 10.1111/j.1471-4159.1973.tb00042.x. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Dipple I., Gordon L. M. Phenobarbital selectively modulates the glucagon-stimulated activity of adenylate cyclase by depressing the lipid phase separation occurring in the outer half of the bilayer of liver plasma membranes. Biochem J. 1981 Sep 1;197(3):675–681. doi: 10.1042/bj1970675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaphilis A., Deliconstantinos G. Modulation of serotonergic receptors by exogenous cholesterol in the dog synaptosomal plasma membrane. Biochem Pharmacol. 1980 Dec;29(24):3325–3327. doi: 10.1016/0006-2952(80)90311-1. [DOI] [PubMed] [Google Scholar]
- Pastuszko A. Action of barbiturates on activity of acetylcholinesterase from synaptosomal membranes. Neurochem Res. 1980 Jul;5(7):769–776. doi: 10.1007/BF00964714. [DOI] [PubMed] [Google Scholar]
- Reavill C. A., Wooster M. S., Plummer D. T. The interaction of purified acetylcholinesterase from pig brain with liposomes. Biochem J. 1978 Sep 1;173(3):851–856. doi: 10.1042/bj1730851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Lenard J. Membrane asymmetry. Science. 1977 Feb 25;195(4280):743–753. doi: 10.1126/science.402030. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Barenholz Y. Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim Biophys Acta. 1978 Dec 15;515(4):367–394. doi: 10.1016/0304-4157(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Tsakiris S., Deliconstantinos G. Influence of phosphatidylserine on (Na+ + K+)-stimulated ATPase and acetylcholinesterase activities of dog brain synaptosomal plasma membranes. Biochem J. 1984 May 15;220(1):301–307. doi: 10.1042/bj2200301. [DOI] [PMC free article] [PubMed] [Google Scholar]



