Abstract
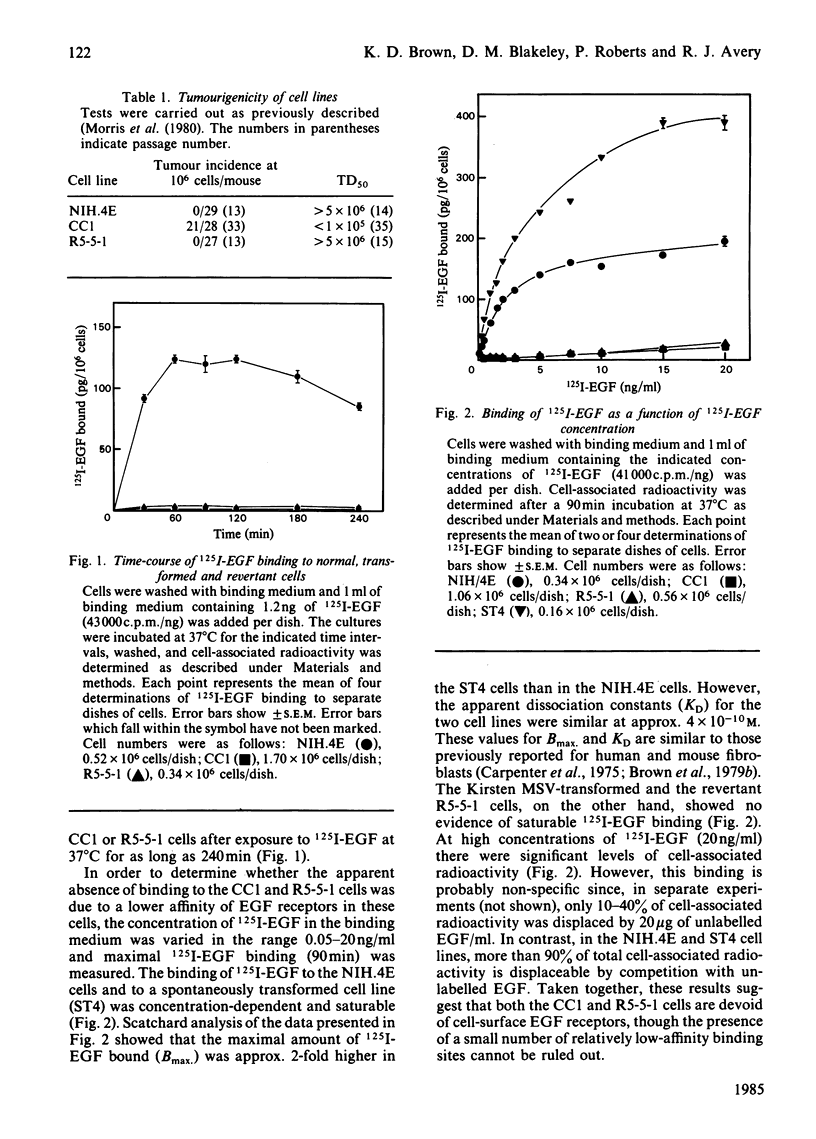
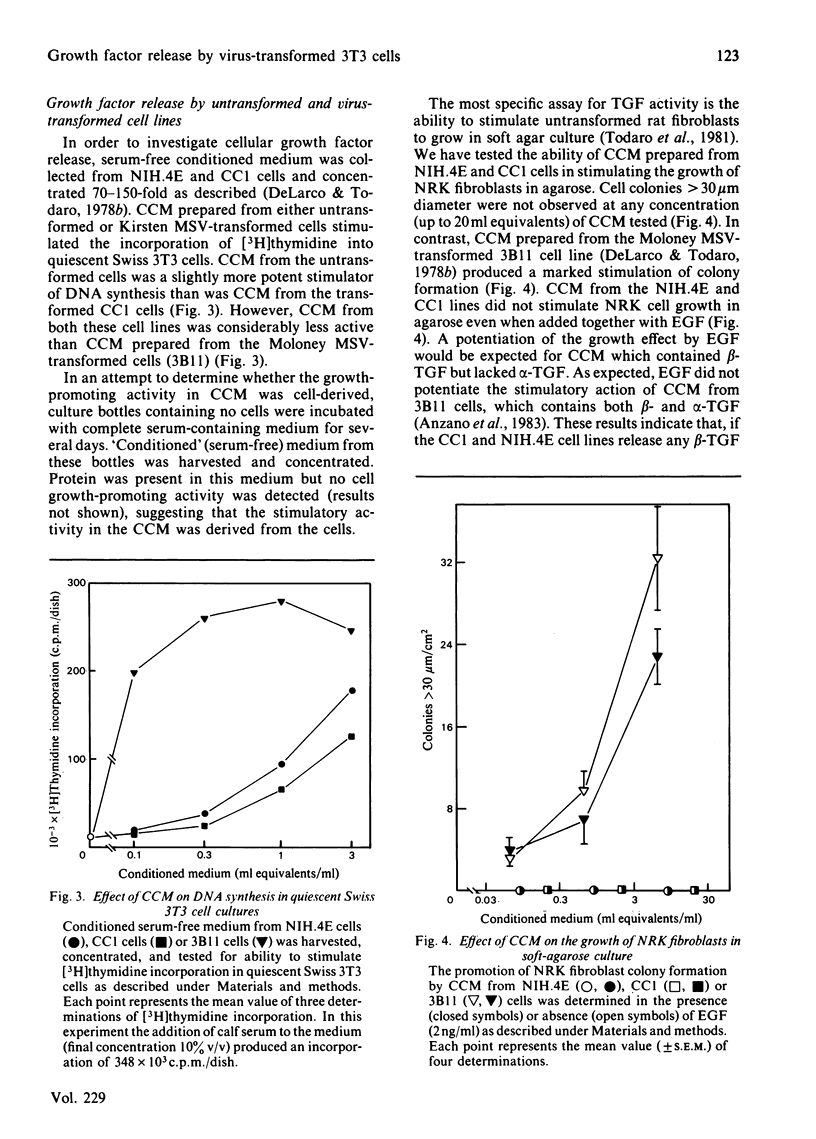
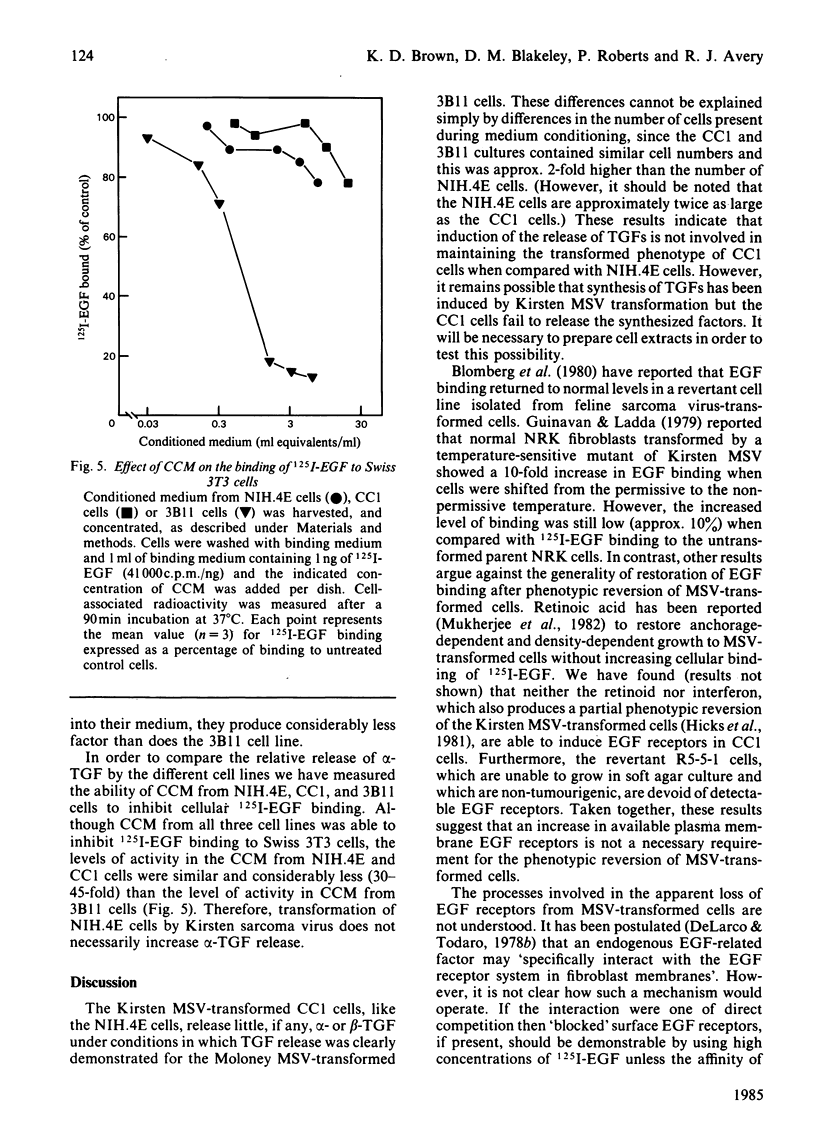
Transformation of NIH/3T3 cells by Kirsten murine sarcoma virus (MSV) caused a dramatic reduction in the number of cell-surface receptors for epidermal growth factor (EGF). However, the number of EGF receptors remained at a very low level in a non-tumourigenic revertant cell line isolated from the virus-transformed cells, indicating that an increase in EGF receptors is not a requirement for the phenotypic reversion of Kirsten MSV-transformed 3T3 cells. Serum-free conditioned medium from normal and virus-transformed cell lines contained similar amounts of cell growth-promoting activity as assayed by the ability to stimulate DNA synthesis in quiescent Swiss 3T3 cell cultures. However, the concentrated conditioned medium from these cell lines showed no evidence of beta-transforming growth factor (TGF) activity as assayed by promotion of anchorage-independent growth of untransformed normal rat kidney (NRK) fibroblasts in agarose. The cellular release of alpha-TGF activity was assayed by measuring the ability of concentrated conditioned medium to inhibit the binding of 125I-EGF to Swiss 3T3 cells. Conditioned medium protein from the virus-transformed cell line inhibited 125I-EGF binding but only to the same extent as conditioned medium protein prepared from the untransformed cell line. The alpha-TGF secretion by these cell lines was estimated to be 30-45-fold lower than the level of alpha-TGF released by a well-characterized alpha-TGF-producing cell line (3B11). These results suggest that the induction of TGF release is not a necessary event in the transformation of NIH/3T3 cells by Kirsten MSV.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anzano M. A., Roberts A. B., Meyers C. A., Komoriya A., Lamb L. C., Smith J. M., Sporn M. B. Synergistic interaction of two classes of transforming growth factors from murine sarcoma cells. Cancer Res. 1982 Nov;42(11):4776–4778. [PubMed] [Google Scholar]
- Anzano M. A., Roberts A. B., Smith J. M., Lamb L. C., Sporn M. B. Purification by reverse-phase high-performance liquid chromatography of an epidermal growth factor-dependent transforming growth factor. Anal Biochem. 1982 Sep 1;125(1):217–224. doi: 10.1016/0003-2697(82)90405-5. [DOI] [PubMed] [Google Scholar]
- Anzano M. A., Roberts A. B., Smith J. M., Sporn M. B., De Larco J. E. Sarcoma growth factor from conditioned medium of virally transformed cells is composed of both type alpha and type beta transforming growth factors. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6264–6268. doi: 10.1073/pnas.80.20.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg J., Reynolds F. H., Jr, Van de Ven W. J., Stephenson J. R. Abelson murine leukaemia virus transformation involves loss of epidermal growth factor-binding sites. Nature. 1980 Jul 31;286(5772):504–507. doi: 10.1038/286504a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Blakeley D. M., MacDonald M. Inhibition of epidermal growth factor binding to Swiss 3T3 cells by human platelet release-products. Biosci Rep. 1983 Jul;3(7):659–666. doi: 10.1007/BF01172876. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Blakeley D. M. Transforming growth factors: sources, properties and possible roles in normal and malignant cell growth control. Biochem Soc Trans. 1984 Apr;12(2):168–173. doi: 10.1042/bst0120168. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Dicker P., Rozengurt E. Inhibition of epidermal growth factor binding to surface receptors by tumor promotors. Biochem Biophys Res Commun. 1979 Feb 28;86(4):1037–1043. doi: 10.1016/0006-291x(79)90221-3. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Friedkin M., Rozengurt E. Colchicine inhibits epidermal growth factor degradation in 3T3 cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):480–484. doi: 10.1073/pnas.77.1.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D., Yeh Y. C., Holley R. W. Binding, internalization, and degradation of epidermal growth factor by balb 3T3 and BP3T3 cells: relationship to cell density and the stimulation of cell proliferation. J Cell Physiol. 1979 Aug;100(2):227–238. doi: 10.1002/jcp.1041000204. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Lembach K. J., Morrison M. M., Cohen S. Characterization of the binding of 125-I-labeled epidermal growth factor to human fibroblasts. J Biol Chem. 1975 Jun 10;250(11):4297–4304. [PubMed] [Google Scholar]
- Clewley J. P., Avery R. J. The virion RNA species of the Kirsten murine sarcoma-leukemia virus complex released from a clonally related series of mouse cells. Arch Virol. 1982;72(1-2):35–46. doi: 10.1007/BF01314448. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Scolnick E. M., Ozanne B., Hunter T. Epidermal growth factor receptor metabolism and protein kinase activity in human A431 cells infected with Snyder-Theilen feline sarcoma virus or harvey or Kirsten murine sarcoma virus. J Virol. 1983 Dec;48(3):752–764. doi: 10.1128/jvi.48.3.752-764.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Larco J. E., Preston Y. A., Todaro G. J. Properties of a sarcoma-growth-factor-like peptide from cells transformed by a temperature-sensitive sarcoma virus. J Cell Physiol. 1981 Oct;109(1):143–152. doi: 10.1002/jcp.1041090116. [DOI] [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Phorbol esters and vasopressin stimulate DNA synthesis by a common mechanism. Nature. 1980 Oct 16;287(5783):607–612. doi: 10.1038/287607a0. [DOI] [PubMed] [Google Scholar]
- Frolik C. A., Dart L. L., Meyers C. A., Smith D. M., Sporn M. B. Purification and initial characterization of a type beta transforming growth factor from human placenta. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3676–3680. doi: 10.1073/pnas.80.12.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinivan P., Ladda R. L. Decrease in epidermal growth factor receptor levels and production of material enhancing epidermal growth factor binding accompany the temperature-dependent changes from normal to transformed phenotype. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3377–3381. doi: 10.1073/pnas.76.7.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler H. T., Maxfield F. R., Willingham M. C., Pastan I. Dansylcadaverine inhibits internalization of 125I-epidermal growth factor in BALB 3T3 cells. J Biol Chem. 1980 Feb 25;255(4):1239–1241. [PubMed] [Google Scholar]
- Hicks N. J., Morris A. G., Burke D. C. Partial reversion of the transformed phenotype of murine sarcoma virus-transformed cells in the presence of interferon: a possible mechanism for the anti-tumour effect of interferon. J Cell Sci. 1981 Jun;49:225–236. doi: 10.1242/jcs.49.1.225. [DOI] [PubMed] [Google Scholar]
- Marquardt H., Hunkapiller M. W., Hood L. E., Todaro G. J. Rat transforming growth factor type 1: structure and relation to epidermal growth factor. Science. 1984 Mar 9;223(4640):1079–1082. doi: 10.1126/science.6320373. [DOI] [PubMed] [Google Scholar]
- Massagué J. Epidermal growth factor-like transforming growth factor. II. Interaction with epidermal growth factor receptors in human placenta membranes and A431 cells. J Biol Chem. 1983 Nov 25;258(22):13614–13620. [PubMed] [Google Scholar]
- Morris A., Clegg C., Jones J., Rodgers B., Avery R. J. The isolation and characterization of a clonally related series of murine retrovirus-infected mouse cells. J Gen Virol. 1980 Jul;49(1):105–113. doi: 10.1099/0022-1317-49-1-105. [DOI] [PubMed] [Google Scholar]
- Mukherjee B. B., Mobry P. M., Pena S. D. Retinoic acid induces anchorage- and density- dependent growth without restoring normal cytoskeleton, EGF binding, fibronectin content and ODC activity in a retrovirus- transformed mouse cell line. Exp Cell Res. 1982 Mar;138(1):95–107. doi: 10.1016/0014-4827(82)90095-7. [DOI] [PubMed] [Google Scholar]
- Neugut A. I., Weinstein I. B. The use of agarose in the determination of anchorage-independent growth. In Vitro. 1979 May;15(5):351–355. doi: 10.1007/BF02616141. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Meyers C. A., Wideman J., Blacher R., Pan Y. C., Stein S., Lehrman S. R., Smith J. M., Lamb L. C. Purification and properties of a type beta transforming growth factor from bovine kidney. Biochemistry. 1983 Dec 6;22(25):5692–5698. doi: 10.1021/bi00294a002. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Lamb L. C., Newton D. L., Sporn M. B., De Larco J. E., Todaro G. J. Transforming growth factors: isolation of polypeptides from virally and chemically transformed cells by acid/ethanol extraction. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3494–3498. doi: 10.1073/pnas.77.6.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Collins M., Brown K. D., Pettican P. Inhibition of epidermal growth factor binding to mouse cultured cells by fibroblast-derived growth factor. Evidence for an indirect mechanism. J Biol Chem. 1982 Apr 10;257(7):3680–3686. [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., De Larco J. E., Fryling C., Johnson P. A., Sporn M. B. Transforming growth factors (TGFs): properties and possible mechanisms of action. J Supramol Struct Cell Biochem. 1981;15(3):287–301. doi: 10.1002/jsscb.1981.380150306. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., De Larco J. E. Growth factors produced by sarcoma virus-transformed cells. Cancer Res. 1978 Nov;38(11 Pt 2):4147–4154. [PubMed] [Google Scholar]
- Twardzik D. R., Ranchalis J. E., Todaro G. J. Mouse embryonic transforming growth factors related to those isolated from tumor cells. Cancer Res. 1982 Feb;42(2):590–593. [PubMed] [Google Scholar]
- Twardzik D. R., Todaro G. J., Marquardt H., Reynolds F. H., Jr, Stephenson J. R. Transformation induced by Abelson murine leukemia virus involves production of a polypeptide growth factor. Science. 1982 May 21;216(4548):894–897. doi: 10.1126/science.6177040. [DOI] [PubMed] [Google Scholar]
- Twardzik D. R., Todaro G. J., Reynolds F. H., Jr, Stephenson J. R. Similar transforming growth factors (TGFs) produced by cells transformed by different isolates of feline sarcoma virus. Virology. 1983 Jan 15;124(1):201–207. doi: 10.1016/0042-6822(83)90307-0. [DOI] [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Epithelioid and fibroblastic rat kidney cell clones: epidermal growth factor (EGF) receptors and the effect of mouse sarcoma virus transformation. J Cell Physiol. 1978 Mar;94(3):335–342. doi: 10.1002/jcp.1040940311. [DOI] [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]


