Abstract
Dendritic cells (DCs) are pivotal antigen-presenting cells for regulating immune responses. A major focus of contemporary vaccine research is the genetic modification of DCs to express antigens or immunomodulatory molecules, utilizing a variety of viral and nonviral vectors, to induce antigen-specific immune responses that ameliorate disease states as diverse as malignancy, infection, autoimmunity, and allergy. The present study has evaluated adeno-associated virus (AAV) type 2 as a vector for ex vivo gene transfer to human peripheral blood monocyte (MO)-derived DCs. AAV is a nonpathogenic parvovirus that infects a wide variety of human cell lineages in vivo and in vitro, for long-term transgene expression without requirements for cell proliferation. The presented data demonstrate that recombinant AAV (rAAV) can efficiently transduce MOs as well as DCs generated by MO culture with granulocyte-macrophage colony-stimulating factor plus interleukin in vitro. rAAV transgene expression in MO-derived DCs could be enhanced by etoposide, previously reported to enhance AAV gene expression. rAAV transduction of freshly purified MO followed by 7 days of culture with cytokines to generate DCs, and subsequent sorting for coexpression of DC markers CD1a and CD40, showed robust transgene expression as well as evidence of nuclear localization of the rAAV genome in the DC population. Phenotypic analyses using multiple markers and functional assays of one-way allogeneic mixed leukocyte reactions indicated that rAAV-transduced MO-derived DCs were as equivalent to nontransduced DCs. These results support the utility of rAAV vectors for future human DC vaccine studies.
Dendritic cells (DCs) are potent antigen-presenting cells (APC) for initiating T-cell immunity, due to their ability to take up and process antigens for presentation by major histocompatibility complex (MHC) class I and class II molecules, migrate to T-cell areas of lymphoid tissues, and present antigen in conjunction with the appropriate T-cell costimulatory molecules and cytokines (reviewed in references 3, 4, 44, and 51). DCs can be manipulated ex vivo to express antigens in order to generate effective vaccines for a variety of immunotherapy applications. In the simpler approaches, DCs are pulsed by incubation with purified proteins, microbial or tumor cell lysates, synthetic MHC-binding peptides, or crude peptides eluted from tumor cells, all of which have shown promising results, although the persistence of antigens on pulsed DCs is of relatively short duration (4, 44, 51). Alternatively, the transfer of genes encoding antigens into DCs offers the advantages of sustained antigen expression and a broader spectrum of MHC peptide epitopes presented by DCs and allows modulation of DC receptors and cytokine secretion to further fine-tune the immune response (3, 4, 44, 51). Both nonviral and viral-vector-mediated gene transfer have been used for DC-based immunotherapy in animal models and human clinical trials, with the majority of viral-mediated DC transductions employing recombinant adenovirus or retroviral vectors (2, 12, 51).
Adeno-associated virus (AAV) is a nonpathogenic parvovirus that infects a wide variety of cells both in vitro and in vivo (30, 43). Recombinant AAV (rAAV) has been analyzed as a vector for direct in vivo genetic immunization (10, 28) and possesses several potential advantages for gene transfer into DCs. Mature DCs isolated from mouse spleen have been reported to be refractory to AAV infection (22), but more recent reports indicate modest transduction efficiencies for DCs generated from mouse spleen or bone marrow (56) and human peripheral blood (25). One advantage of rAAV is the ability to transduce both dividing and nondividing cells, which may allow transduction of DCs across a broad range of activation or maturation states. Another advantage of rAAV is the absence of viral coding sequences. Hence, rAAV-transduced DCs will not synthesize any viral proteins, which should minimize competition between transgene and viral peptides for MHC presentation and further diminish elimination of transduced DC by virus-specific cytolytic T cells (22, 34, 55).
A third potential advantage of rAAV is the capacity for persistent transgene expression, which could result in transgene expression for the life of the transduced cell (48). Current models of DC biology would suggest that longevity would not be a critical factor for DC-based vaccines, because antigen-primed DCs are considered to be very short-lived cells in vivo. They rapidly migrate to the regional lymph nodes, interact with T cells, and undergo apoptosis within about 2 days (20, 24). However, recent studies in mice suggest that DCs modified for increased life spans also exhibit enhanced immune adjuvant and vaccine activity in vivo (20, 35), allowing speculation that future clinical trials could incorporate strategies to prolong the life span of antigen-modified DC vaccines, in which case persistence of DC transgene expression might become a priority.
In the present study, we evaluated the efficiency of rAAV transduction in human monocyte (MO)-derived DCs, the type of DC used in many current vaccine clinical trials (13, 33, 46). We show that transduction of the MO progenitors with rAAV followed by 7 to 10 days of culture in IL-4 and GM-CSF resulted in robust transgene expression by the generated DCs. rAAV transduction of either MO precursors or DCs did not appear to compromise final DC viability, phenotype or immune function in vitro. These results support the potential application of rAAV-based vectors in gene-modified DC vaccines for immunotherapy.
MATERIALS AND METHODS
Cell lines, plasmids, viruses, and reagents.
The human embryonic kidney cell line 293 was obtained from American Type Culture Collection and maintained as described previously (37–39). The rAAV plasmid, pAAV-GFP, adenovirus (Ad) type 2 (Ad2), and the human megakaryocytic leukemia cell line M07e were kindly provided by Arun Srivastava, Indiana University School of Medicine, Indianapolis. M07e cells were maintained as previously described (40). Plasmid pSub201 containing the wild-type (wt) AAV genome was a kind gift of Jude Samulski (University of North Carolina, Chapel Hill), and a recombinant helper plasmid, pDG, was a kind gift of Jürgen Kleinschmidt (University of Heidelberg, Heidelberg, Germany). Clinical grade etoposide was from Bedford Laboratories.
Production of wt and rAAV.
For the production of wt AAV, plasmid pSub201 (45) was used. For rAAV encoding luciferase (luc) or green fluorescent protein (GFP), AAV plasmids containing the respective genes under the control of human cytomegalovirus immediate early gene promoter (CMV-P) were used as described previously (37). Packaging of mature virions was performed in 293 cells by calcium phosphate plasmid transfection (37–39). For the production of wt AAV, pSub201-transfected 293 cells were infected with Ad2 at 5 PFU/15-cm-diameter plate. For packaging of rAAV, cells were cotransfected with the respective rAAV plasmids and the helper plasmid, pDG, containing the wt AAV and Ad genes necessary for rAAV packaging (17). Cells were harvested 60 h posttransfection and lysed by three rounds of freezing and thawing. Further purification of AAV was done by two rounds of CsCl density gradient centrifugation (37–39). DNase I treatment of viral preparations was performed to digest unencapsidated DNA, and quantitative slot blot analysis was used to determine the particle titer (37, 38).
Human MO and MO-derived DC cultures and viral transduction.
Peripheral blood mononuclear cells were isolated from blood of healthy adult volunteers on Ficoll cushions (38), and MO were isolated by adherence to plastic culture dishes in serum-free Opti-MEM I medium (GIBCO-BRL, Gaithersburg, Md.) for 2 h at 37°C with 7% CO2, followed by vigorous washing to remove nonadherent cells. In some experiments, the freshly adherent MO were immediately infected by overlaying cells with rAAV in Opti-MEM I for 2 h at 37°C with 7% CO2. Unless otherwise specified, multiplicities of infection (MOI) of 100 were used to infect cells (1 MOI = 2,000 AAV particles/cell).
To generate DC, MO were cultured in RPMI 1640 medium with 2 mM l-glutamine (Mediatech), 10% fetal calf serum (HyClone), human recombinant interleukin 4 (IL-4) (17 ng/ml; R&D Systems), and 800 U of recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF) (Leukine, Immunex) per ml for up to 10 days, with partial cytokine medium replacement every 2 to 3 days. In some experiments, MO were cultured in complete RPMI medium without cytokines or in complete medium with GM-CSF alone. For infection of DC, the nonadherent and loosely adherent cells were harvested, washed once into Opti-MEM I medium, and incubated with 100 MOI of wt or rAAV for 2 h at 37C with 7% CO2 in a polypropylene tube (10 to 20 million cells/ml) with gentle agitation every 15 min. In AAV replication experiments, following AAV infection, cells were washed five times in 1× PBS (phosphate-buffered saline (PBS) (UAB Media Preparation Shared Facility) and incubated with wt Ad2 (10 PFU/cell) in serum-free Opti-MEM for an additional 2 h at 37°C with 7% CO2 as before. After infection, cells were washed three times and then cultured in complete RPMI medium with cytokines at 37°C with 7% CO2 until further analyses.
Flow cytometry.
Nonadherent and loosely adherent cells harvested from DC cultures were analyzed by two-color fluorescence flow cytometry to determine DC phenotype and purity using either fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated mouse monoclonal antibodies specific for human CD1a, CD80, CD83, CD86, CD40, CD3, CD19, and CD14 (all from BD Pharmingen) or with unconjugated monoclonal antibodies to human MHC class I/A,B,C and class II/DR (both from Leinco Tech., Inc.) followed by PE-labeled goat anti-mouse immunoglobulin G (IgG) (Southern Biotechnology Assoc.). One hundered thousand cells were incubated with antibodies at the concentrations recommended by the manufacturers plus aggregated human IgG (10 μg/ml) to block Fc receptors, in PBS containing 1% bovine serum albumin, on ice for 30 min. Cells were washed once with 10 volumes of PBS plus bovine serum albumin between incubations, and final cells were resuspended in PBS plus 1% paraformaldehyde prior to analysis. DCs transduced with rAAV-GFP were analyzed for percent GFP-positive cells by fluorescence flow cytometry at 490 nm. In some experiments, DCs were stained with CD1a-PE and CD40-fluorescein isothiocyanate and then sorted for double-positive and single-positive cells. All antibody staining mixtures contained 10 μg of of heat-aggregated human IgG per ml to block nonspecific binding to Fc receptors. Analyses and cell sorting were performed in the Flow Cytometry Core Facility of the UAB AIDS Center.
AAV replication assay.
Human MO-derived DC were infected with wt AAV with or without Ad2. As positive controls, 293 cells known to be permissive for both AAV and Ad2 infection were included. Forty-eight hours postinfection, low Mr DNA was isolated from the mock-infected and AAV- or Ad-infected cells by the method of Hirt (19) and analyzed on Southern blots using 32P-labeled AAV-specific or Ad2-specific DNA probes, as described earlier (36).
Luciferase assay.
Luciferase activity was determined using a commercial kit (Luciferase Assay System; Promega, Madison, Wis.). Briefly, the cells were washed two times with PBS, and the cell pellet was lysed with a buffer provided in the kit. After brief centrifugation at 4°C, the clarified supernatant was used to determine luciferase activity with a luminescent substrate in a Zylux Femtomaster FB12 luminometer. Data are expressed as relative light units (RLU) per microgram of protein, as determined by the method of Lowry et al. (26).
Fluorescence in situ hybridization (FISH).
Populations of mock-transduced and rAAV-transduced DCs positive for both CD1a and CD40 were sorted, and cell nuclei were isolated by standard methods (5). A digoxigenin-labeled luciferase probe was prepared by the nick translation method using the Nick Translation System (GIBCO-BRL). Digoxigenin-labeled dUTP (DID-11-dUTP) was purchased from Roche Molecular Biochemicals. Hybridization, amplification of the signal and counterstaining were performed with a hybridization kit (Oncor Inc., Gaithersburg, Md.) according to the manufacturer's instructions (Chromosome in situ hybridization manual). The stained nuclei were analyzed using an Olympus epifluorescence microscope.
MLR assay.
Cultured DCs were assayed for immunostimulatory activity by allogeneic one-way mixed lymphocyte reaction (MLR). Fresh Ficoll-purified peripheral blood mononuclear cells from a healthy donor (unrelated to the DC donor) were used as responders. Various numbers of irradiated (30 Gy) cultured DCs or DC-autologous fresh peripheral blood mononuclear cells, used as stimulators, were added to 105 allogeneic responders in quadruplicate wells of a 96-well culture plate in RPMI 1640 medium containing 2 mM l-glutamine and 10% human AB serum (Mediatech). After culture for 5 days at 37°C with 7% CO2, cells were pulsed with 1 μCi of tritiated thymidine (New England Nuclear) and then cultured for 18 to 20 h prior to harvesting with a Skatron Micro96 Harvester, and cell-incorporated tritium was assessed using a Packard Matrix 9600 Beta Counter. Results were expressed in counts per minute (mean ± standard deviation).
RESULTS
Replication of wt AAV in MO-derived DC.
AAV replication assays were performed as an initial evaluation of wt AAV infection of human MO-derived DCs. wt AAV requires the presence of specific helper virus proteins in addition to host DNA polymerases for efficient replication of the AAV genome, and in the absence of helper functions, the AAV genome remains latent (30, 47). Thus, Southern blot analysis of wt AAV genome structures in cells coinfected with Ad can be used to assess the efficiency of AAV infection, based on the presence of AAV replicative intermediates (38). Human MO-derived DCs on day 7 of IL-4 and GM-CSF culture were infected with wt AAV with or without Ad2. Characteristic AAV replicative intermediates were detected only after superinfection with Ad (Fig. 1B), demonstrating that the replicative intermediates were products of posttransduction events. Others have reported that wt Ad infection of DCs is relatively inefficient (2), which is consistent with results obtained using the Ad-specific probe (Fig. 1C and D). Nonetheless, these limitations did not affect the detection of wt AAV replication suggesting that low-level transduction and expression of Ad proteins was sufficient for AAV replication. These results clearly indicate that AAV transduces human MO-derived DCs in vitro.
FIG. 1.
Replication of wt AAV and Ad2 in 293 cells and human MO-derived DCs. Low- Mr DNA isolated from equal numbers of 293 cells and human DCs that were mock infected (lane 1), wtAAV infected (lane 2), Ad2 infected (lane 3), and wtAAV-plus Ad2 coinfected (lane 4) were analyzed on Southern blots with either wt AAV-specific (A and B) or Ad2-specific (C and D) 32P-labeled probes. Abbreviations: m and d, replicative monomeric and dimeric forms of wt AAV, respectively; Ad2, Ad2 replication.
rAAV transduction of human MO-derived DCs.
rAAV encoding either luciferase (rAAV-luc) or green fluorescent protein (rAAV-GFP) was used to analyze transduction of DCs by transgene expression. MO-derived DC from day 7 IL-4 and GM-CSF cultures were infected with either rAAV-luc or rAAV-GFP, and reporter gene activity was assessed after 48 h of additional culture in cytokines. Luciferase activity showed relatively efficient expression of the transgene in the human DCs, as compared to rAAV-luc-transduced 293 cells (Fig. 2). To estimate the percentage of transduced DCs, fluorescence flow cytometry of GFP reporter gene expression was used. We observed variations in the efficiency of transduction among DC cultures derived from different normal blood donors, varying between 2 and 55% based on GFP-positive DCs; an example of high-percentage transduction is presented in Fig. 3. Analogous donor-specific differences in the efficiency of rAAV transduction in human bone marrow-derived CD34+ primitive progenitor cells have been previously reported by us and others (11, 38).
FIG. 2.
Luciferase activity in MO7e, 293, and human DCs. Approximately 5 × 104 cells of each type were either mock infected or infected with rAAV-luc vector at an MOI of 100 in vitro, and luciferase activities were determined 48 h postinfection. Activity is expressed as RLU per microgram of protein from each cell lysate.
FIG. 3.
Expression of GFP in DCs following transduction by rAAV. Human DCs, derived from MO, were infected with rAAV (100 MOI) encoding GFP in vitro. Forty-eight hours postinfection, cells were analyzed by fluorescence-activated cell sorting for GFP fluorescence. GFP activity observed in rAAV-transduced DCs is shown as a shift towards the right from the untransduced control.
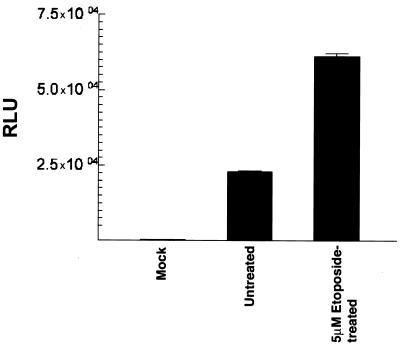
At MOI of less than 100, rAAV transduction of DCs was significantly reduced, indicating that higher vector dose is required to achieve optimal transduction of DCs (data not shown). However, MOI of less than 100 indicated higher transduction in 293 cells compared to DCs, which is probably due to a limited expression AAV receptor and/or coreceptors in the latter. As one means of enhancing DC expression of the transgene, we evaluated the topoisomerase inhibitor etoposide that has previously been reported to enhance AAV expression in both primary cells and cell lines (42). Prior to analyzing the transduction efficiency of rAAV following etoposide treatment, we conducted pilot experiments to determine if etoposide treatment affected either DC viability phenotype as compared to untreated DCs. MO-derived DCs from day 6 cultures with IL-4 and GM-CSF were treated with etoposide at concentrations of 0.5 to 50 μM for 16 h and then washed with PBS four times and cultured for an additional 48 h in complete medium with cytokines. Based on trypan blue dye exclusion, there was no evident cytopathic effects in DCs treated with up to 10 μM etoposide. A 50 μM concentration of etoposide, however, resulted in increased cytopathic effects. Subsequently, DCs were pretreated with 0 to 10 μM etoposide and either mock-transduced or transduced with rAAV-luc at an MOI of 100. After culture for an additional 48 h, the cells were harvested for luciferase assay. Results indicated an etoposide dose-dependent enhancement of luciferase activity, which appeared to be maximal at 5 μM etoposide (Fig. 4); pretreatment with 10 μM etoposide did not show significantly higher transgene expression than 5 μM (data not shown). Etoposide treatments followed by rAAV-luc infection did not appear to affect DC viability, based upon numbers of viable cells recovered at the end of the cultures, and cell surface phenotypes by as determined by flow cytometric analyses of multiple DC-associated markers were essentially identical between the etoposide-treated and untreated DC cultures (Fig. 5). Thus, it may be possible to pharmacologically augment rAAV transgene expression in DCs.
FIG. 4.
Luciferase expression in etoposide-treated DCs transduced with rAAV-luc. MO-derived DC cultures were either untreated or pretreated with 5 μM etoposide overnight and were subsequently infected with rAAV-luc (50 MOI). DCs were lysed 48 h later, and luciferase activity was determined. Activity is expressed as RLU per microgram of protein in each cell lysate.
FIG. 5.
Expression of surface markers in control and etoposide-treated DC. MO-derived DCs on day 7 of culture were either untreated or treated with 5 μM etoposide. The cultures were maintained for an additional 2 days in the presence of etoposide. DC cultures were analyzed by two-color flow cytometry for expression of CD1a and CD40 (A), CD86 and CD45 (B), CD80 and CD83 (C), CD58 and CD54 (D), MHC class I (E), and MHC class II (F). Results of untreated cells are on the left and etoposide-treated cells on the right in each panel.
rAAV transduction of monocytes prior to generation of MO-derived DCs.
Conversion of the single-stranded AAV genome to a double-stranded structure is a rate-limiting step for transcription of rAAV-encoded transgenes (15, 16). Optimal expression of AAV-transduced genes may require a few days to weeks, depending on cell lineage or metabolic state (14, 18, 48–50). In attempts to maximize rAAV expression, peripheral blood MO were infected with rAAV-luc immediately after adherence purification and subsequently cultured for various periods either without cytokines or with GM-CSF alone or with IL-4 plus GM-CSF prior to luciferase assay. There was an increase in the transgene activity with length of culture in all three types of culture conditions (Fig. 6). However, luciferase activity levels were consistently higher in the DC cultures (IL-4 plus GM-CSF) as compared to cultures with GM-CSF alone or with no added cytokines. Figure 6 presents representative results from independent experiments using four different donor MO cultures. These results suggest that the use of rAAV for DC transduction may be optimal if progenitors are infected prior to in vitro cytokine cultures to generate DCs.
FIG. 6.
Luciferase activity in control and rAAV-transduced MO cultures. Freshly isolated MO were either mock transduced or transduced with rAAV (100 MOI). Following transduction, monocytes were either cultured without cytokines (Mono) or with GM-CSF alone (GM) or with IL-4 plus GM-CSF (IL-4+GM). Analysis of luciferase activity (RLU) was performed 2, 4, and 7 days postinfection.
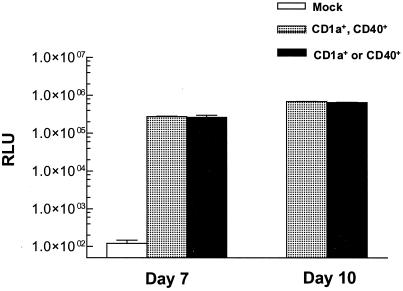
Because cultures of such MO-derived DCs were heterogeneous with respect to cell surface phenotypes, we assessed luciferase expression in cells sorted by flow cytometry into subpopulations coexpressing both CD1a and CD40 versus populations expressing only one of the markers. Results (Fig. 7) indicated equivalent amounts of luciferase expression in each sorted population on days 7 and 10 of cytokine culture, verifying that cells with specific DC phenotypes exhibited robust rAAV transgene expression. Further, the observation that luciferase activities were almost identical in both the subpopulations confirms the vector transduction of MO precursors and that the transgene expression was not affected by subsequent changes in the expression of DC markers.
FIG. 7.
Luciferase activity in fluorescence-activated cell-sorted CD40- and CD1a-positive DCs generated from MO transduced with rAAV-luc. Freshly adherent MO from human peripheral blood were transduced with rAAV-luc (100 MOI) and subsequently cultured with IL-4 plus GM-CSF for 7 or 10 days. Cells were then sorted using fluorescent antibodies to CD40 and CD1a into populations that were either double-positive (CD40+ CD1a+) or single-positive (CD1a+ or CD40+). Less than 5% of harvested cells were negative for both CD1a and CD40. Luciferase activity (RLU) was expressed per microgram protein content in each lysate.
Assay of rAAV-transduced DC function by MLR.
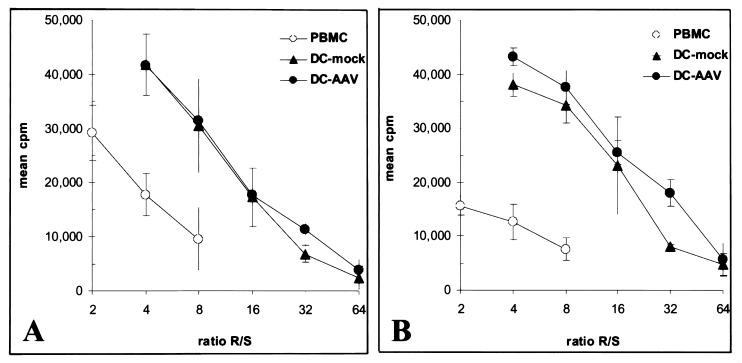
The allostimulatory capacity of DC after rAAV transduction was analyzed in one-way MLR assays, using responder peripheral blood lymphocytes from donors unrelated to the DC donors. For each experiment, fresh peripheral blood mononuclear cells autologous to the DCs were included as control stimulators. DC stimulators were either from day 6 cytokine cultures of monocytes transduced on day 0 or from DCs transduced on day 7 of culture and returned to cytokine culture for 3 additional days prior to MLR assay. The results show that, compared to mock-transduced DCs, there was no significant difference in the potency of rAAV-transduced DCs allostimulatory activity (Fig. 8). Similar results were obtained from DCs generated from different donors with variation in AAV transduction (data not shown). Thus, neither AAV infection nor transduction appeared to affect DC immunostimulatory activity under these conditions.
FIG. 8.
Allogeneic MLR assays of AAV-luc transduced DCs. MO transduced with AAV on day 0 and cultured for 6 days in IL-4 and GM-CSF to generate DCs (A) or DCs transduced with AAV after 7 days cytokine culture and recultured with cytokines for 3 additional days (B) were used as stimulators in allogenic MLR assays. In each experiment, control stimulators included mock-transduced DCs as well as fresh autologous peripheral blood mononuclear cells (PBMC). Stimulators were irradiated and added at various numbers to 96-well culture plates. Fresh peripheral blood lymphocytes from donors unrelated to the DC donors were added to the irradiated stimulators at 100,000 cells/well, incubated for 5 days, and then pulsed with 3H-thymidine overnight prior to harvesting.
FISH analysis of rAAV genome in transduced DCs.
rAAV vectors can persist as either episomal elements or by chromosomal integration in transduced cells (48). We performed FISH analysis in attempts to evaluate the AAV genome in transduced DCs. Freshly obtained peripheral blood monocytes were infected with rAAV-luc and subsequently cultured with IL-4 and GM-CSF for 10 days. The resultant DCs were subjected to two-color fluorescence-activated cell sorting flow cytometry using antibodies specific for CD40 and CD1a, as described above. Approximately 100 interphase nuclei were analyzed for the presence of proviral genome, and 21% showed the presence of the rAAV genome, in general agreement with above assessment of percent transduction by rAAV-GFP. Representative results from mock-transduced and rAAV-luc transduced cell nuclei is presented in Fig. 9. Attempts to obtain metaphase chromosomal spreads from the sorted DCs were not successful, in agreement with the general non-proliferative nature of such cultures (6). Thus, based on FISH analysis, we are unable to determine whether the proviral sequences observed in interphase nuclei of transduced DCs were episomal or integrated into the host genome. Nonetheless, the presence of vector genome in the infected DC nuclei confirms AAV transduction of the DCs.
FIG. 9.
FISH analyses of AAV genome in DCs derived in culture from rAAV-luc-transduced MO. Peripheral blood adherent MO were either mock transduced or transduced with AAV-luc and subsequently cultured with IL-4 and GM-CSF for 10 days. DCs were fluorescence-activated cell sorted for CD40 and CD1a expression, and double-positive cells were used to prepare nuclei for FISH analysis by staining with a digoxigenin-labeled luciferase probe. (A) Representative field from mock-transduced cells. (B) Representative field from rAAV-luc-transduced cells, with arrows indicating signal from AAV proviral genome. Magnification, ×100.
DISCUSSION
Immunotherapy strategies based on modified DCs are currently under extensive evaluation, both preclinically and in clinical trials, for the treatment of a variety of human diseases (4, 51). Most ongoing and proposed clinical trials utilize ex vivo culture of progenitor cells with cytokines to generate DCs, which are subsequently manipulated to express target antigens, or to overexpress regulatory molecules that enhance the desired immune response, or both (4, 51). Although multiple strategies to modify DCs have been successfully employed in preclinical analyses, those based upon genetic modification have the advantage of allowing antigen presentation by DC MHC class I and class II molecules irrespective of HLA genotype, in contrast to many MHC class I and class II peptide-pulsed DC strategies (6). Genetic modification also promises longer antigen expression than protein or peptide pulsing methods (52).
Multiple vectors have been evaluated for genetic modification of DCs in vitro, including polynucleotide transfection using plasmid DNA, mRNA or viral RNA (7, 8, 31), and viral transduction using recombinant retrovirus, Ad, vaccinia virus, poxvirus, AAV, and others (12, 21, 53, 54, 56). Despite reports from several studies on the potential utility of these vectors both in vitro and in vivo, it is unclear at this time if any of the currently tested vectors is superior for genetic modification of DCs. For optimal utility of a vector in DC-based immunotherapy, a combination of factors such as transduction efficiency, persistence of the transgene expression and the ability to retain or enhance antigen-presenting functions of DC are vital. In addition to these requirements, identifying optimal stages of DC culture for vector transduction is crucial to utilize the antigen expressing cells as potent effectors upon autologous transfer. Despite several reports that indicated successful application of gene-modified DCs as tumor vaccines in animal models, similar approaches in humans have so far produced only limited success (9). Thus, optimization of both DC transduction and conditions that promote antigen-presenting functions are important factors for current immunogene therapy.
In this study, we evaluated the potential utility of rAAV as a vector for in vitro gene modification of human peripheral blood MO-derived DCs, a type of DC that is readily generated for human clinical trials (13, 33, 46). Although some previous reports have suggested that rAAV transduces both mouse and human hematopoeitic cells with relatively good efficiency (32, 37, 38), inefficiency of rAAV transduction in mature mouse DCs has also been reported (22). Recent studies have suggested that progenitors of both mouse and human DCs can be transduced by rAAV (25, 56). The current study focused on evaluating rAAV transduction of human MO-DCs in more detail, with emphasis on transduction strategies useful for current clinical trials of ex vivo-modified DC vaccines.
Results suggest that MO progenitors of DCs, which are readily obtained and therefore commonly used in clinical trials, are transduced by rAAV with relatively good efficiency at a higher vector multiplicity. DCs generated by subsequent culture of rAAV-transduced MO for 6 to 10 days in the presence of IL-4 and GM-CSF demonstrated increased levels of reporter gene expression compared to MO cultured without cytokines or with GM-CSF alone, indicating that in vitro DC culture conditions are conducive for maintaining high levels of rAAV transgene expression. Further, results of the FACS analysis and MLR assays indicated that AAV-transduced DCs and DC precursors that were differentiated following transduction were as potent as unmodified DCs in both the expression of DC markers and functional immunoreactivity, indicating that the vector transduction and transgene expression did not impair cellular DC features required for vaccine therapy.
DCs activated for antigen presentation are believed to have life spans of only a few days in vivo (20, 24), and some studies have suggested that antigen expression in the context of host cell apoptosis or necrosis may enhance immune responses (29), leading to a hypothesis that short-lived DCs that undergo apoptosis after encountering T cells may exhibit optimal APC activity. In contrast, several studies have reported that DCs that are resistant to apoptosis exhibit enhanced immunostimulatory activity in vivo (23, 35). These include pretreatment of DCs with the tumor necrosis factor-related activation-induced cytokine (23), transduction of DCs with the Bcl-xL anti-apoptotic gene, and treatments with IL-12, which is known to enhance the hematopoietic cell survival (35). It is interesting that cDNA encoding these genes (i.e., those encoding TRANCE, Bcl-xL, and IL-12) are within the rAAV cloning capacity either individually or in tandem with tumor antigen genes or genes for additional cytokine or costimulatory molecules. Thus the potential of rAAV to stably transduce either DCs or DC precursors may be of particular advantage and relevance for vaccine strategies using DCs genetically modified for increased longevity as well as transgenic antigen presentation.
Another ex vivo approach that may be used to generate more potent DCs in vivo using rAAV vectors is to transduce DC-MO precursors with genes encoding IL-4 and GM-CSF followed by in vivo administration. In patients with metastatic solid tumors, administration of GM-CSF in combination with IL-4 resulted in enhancements of cell number and antigen-presenting activity of CD14+ and CD83+ circulating DCs (41). Thus, in vivo or intratumoral administration of genetically modified DC precursors expressing GM-CSF and IL-4 may lead to an enrichment of potent DC number and APC activity for enhanced antitumor responses. A recent study by Liu et al. reported that transduction of human peripheral blood-derived monocytes with an rAAV encoding GM-CSF followed by culture with IL-4 resulted in their differentiation into potent DC, suggesting the feasibility of such an approach (25).
It has been reported that modifications of culture conditions including pretreatment of target cells with certain transduction enhancing compounds have resulted in increased AAV vector transduction without compromising viability or function of the infected cells (1, 27, 42). Increasing AAV transgene expression by pretreatment with transduction enhancing compounds may particularly be beneficial in immunotherapy using ex vivo-modified DCs. In the present studies, we observed a modest increase in transgene expression following pretreatment with etoposide, a Food and Drug Administration-approved topoisomerase type II inhibitor that is routinely used as a chemotherapeutic drug for the treatment of certain malignancies, suggesting that it may be possible to modulate conditions to achieve further enhancements of DC transduction without compromising cell viability or antigen-presenting functions. Additional compounds, including hydroxyurea, and tyrphostin have been shown to increase AAV transgene expression in primary cells (1, 27, 42). The absence of a severalfold increase of AAV transgene expression in etoposide-treated DCs compared to results of earlier report (42) probably suggests that variations in the cell types and intracelluar events may account for the difference. Future studies to test these agents in cultured human DCs may have the potential to improve transduction efficiency and transgene expression.
At present, it is not clear whether higher levels of antigen expression by transduced DCs will be associated with greater vaccine potency in vivo. In this light, rAAV transduction of MO-DCs may be efficient enough without pharmacological enhancements. However, the ability to pharmacologically modulate levels of transgene expression, coupled with inherent durability of transgene expression, makes rAAV an attractive vector for DC-based immunotherapy.
ACKNOWLEDGMENTS
We express our gratitude to Albert F. LoBuglio for his constant encouragement and thank Connie Jenkins and Cherryl Basso for excellent technical assistance.
This work was supported by UAB Comprehensive Cancer Center American Cancer Society-Institutional Research grant 60-061-41 and a Career Development award (NIH-SPORE grant in Ovarian Cancer [5 P50-CA83591]) to S.P. and by a grant from the National Institutes of Health (R01 CA86881-01) to D.T.C.
REFERENCES
- 1.Alexander I E, Russell D W, Miller A D. DNA-damaging agents greatly increase the transduction of nondividing cells by adeno-associated virus vectors. J Virol. 1994;68:8282–8287. doi: 10.1128/jvi.68.12.8282-8287.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthur J F, Butterfield L H, Roth M D, Bui L A, Kiertscher S M, Lau R, Dubinett S, Glaspy J, McBride W H, Economou J S. A comparison of gene transfer methods in human dendritic cells. Cancer Gene Ther. 1997;4:17–25. [PubMed] [Google Scholar]
- 3.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu Y-J, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.Baurmann H, Cherif D, Berger R. Interphase cytogenetics by fluorescent in situ hybridization (FISH) for characterization of monosomy-7-associated myeloid disorders. Leukemia. 1993;7:384–391. [PubMed] [Google Scholar]
- 6.Bell D, Young J W, Banchereau J. Dendritic cells. Adv Immunol. 1999;72:255–324. doi: 10.1016/s0065-2776(08)60023-1. [DOI] [PubMed] [Google Scholar]
- 7.Berlyn K A, Ponniah S, Stass S A, Malone J G, Hamlin-Green G, Lim J K, Cottler-Fox M, Tricot G, Alexander R B, Mann D L, Malone R W. Developing dendritic cell polynucleotide vaccination for prostate cancer immunotherapy. J Biotechnol. 1999;73:155–179. doi: 10.1016/s0168-1656(99)00118-2. [DOI] [PubMed] [Google Scholar]
- 8.Boczkowski D, Nair S, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen presenting cells in vitro and in vivo. J Exp Med. 1996;184:465–472. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bodey B, Bodey B, Jr, Siegel S E, Kaiser H E. Failure of cancer vaccines: the significant limitations of this approach in immunotherapy. Anticancer Res. 2000;20:2665–2676. [PubMed] [Google Scholar]
- 10.Brockstedt D G, Podsakoff G M, Fong L, Kurtzman G, Mueller-Ruchholtz W, Engelman E G. Induction of immunity to antigens expressed by recombinant adeno-associated virus depends on the route of administration. Clin Immunol. 1999;92:67–75. doi: 10.1006/clim.1999.4724. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee S, Li W, Wong C A, Fisher-Adams G, Lu D, Guha M, Macer J A, Forman S J, Wong K K., Jr Transduction of primitive human marrow and cord blood-derived hematopoietic progenitor cells with adeno-associated virus vectors. Blood. 1999;93:1882–1894. [PubMed] [Google Scholar]
- 12.De Veerman M, Heirman C, Van Meirvenne S, Devos J, Corthals S, Moser M, Thielemans K. Retrovirally transduced bone marrow-derived dendritic cells require CD4+ T cell help to elicit protective and therapeutic antitumor immunity. J Immunol. 1999;162:144–151. [PubMed] [Google Scholar]
- 13.Dhodapkar M V, Steinman R M, Sapp M, Desai H, Fossella C, Krasovsky J, Donahue S M, Dunbar P R, Cerundolo V, Nixon D F, Bhardwaj N. Rapid generation of broad T-cell immunity in humans after a single injection of mature dendritic cells. J Clin Investig. 1999;104:173–180. doi: 10.1172/JCI6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duan D, Sharma P, Yang J, Yue Y, Dudus L, Zhang Y, Fisher K J, Englehardt J F. Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long-term episomal persistence in muscle tissue. J Virol. 1998;72:8568–8577. doi: 10.1128/jvi.72.11.8568-8577.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrari F K, Samulski T, Shenk T, Samulski R J. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher K J, Gao G P, Weitzman M D, DeMatteo R, Burda J F, Wilson J M. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimm D, Kern A, Rittner K, Kleinschmidt J A. Novel tools for production and purification of recombinant adeno associated virus vectors. Hum Gene Ther. 1998;10:2745–2760. doi: 10.1089/hum.1998.9.18-2745. [DOI] [PubMed] [Google Scholar]
- 18.Herzog R W, Hagstrom J N, Kung S H, Tai S J, Wilson J M, Fisher K J, High K A. Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus. Proc Natl Acad Sci USA. 1997;94:5804–5809. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967;26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- 20.Ingulli E, Mondino A, Khoruts A, Jenkins M K. In vivo detection of dendritic cell antigen presentation to CD4+ T cells. J Exp Med. 1988;185:2133–2141. doi: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenne L, Hauser C, Arrighi J F, Saurat J H, Hugin A W. Poxvirus as a vector to transduce human dendritic ells for immunotherapy: abortive infection but reduced APC function. Gene Ther. 2000;7:1575–1583. doi: 10.1038/sj.gt.3301287. [DOI] [PubMed] [Google Scholar]
- 22.Jooss K, Yang Y, Fisher K J, Wilson J M. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J Virol. 1998;72:4212–4223. doi: 10.1128/jvi.72.5.4212-4223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Josien R, Li H-L, Ingulli E, Sarma S, Wong B R, Vologodskaia M, Steinman R M, Choi Y. TRANCE, a tumor necrosis factor family member, enhances the longevity and adjuvant properties of dendritic cells in vivo. J Exp Med. 2000;191:495–502. doi: 10.1084/jem.191.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kupiec-Weglinski J W, Austyn J M, Morris P J. Migration patterns of dendritic cells in the mouse: traffic from the blood, and T cell-dependent and -independent entry to lymphoid tissues. J Exp Med. 1988;167:632–645. doi: 10.1084/jem.167.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Santin A D, Mane M, Chiriva-Internati M, Parham G P, Ravaggi A, Hermonat P L. Transduction and utility of the granulocyte-macrophage colony-stimulating factor gene into monocytes and dendritic cells by adeno-associated virus. J Interferon Cytokine Res. 2000;20:21–30. doi: 10.1089/107999000312702. [DOI] [PubMed] [Google Scholar]
- 26.Lowry O H, Rosebrough N J, Fair A L, Randall R J. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 27.Mah C, Qing K, Khuntirat B, Ponnazhagan S, Wang X S, Kube D M, Yoder M C, Srivastava A. Adeno-associated virus type 2-mediated gene transfer: role of epidermal growth factor receptor protein tyrosine kinase in transgene expression. J Virol. 1998;72:9835–9843. doi: 10.1128/jvi.72.12.9835-9843.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manning W C, Paliard X, Zhou S, Bland M P, Lee A Y, Hong K, Walker C M, Escobedo J A, Dwarki V. Genetic immunization with adeno-associated virus vectors expressing herpes simplex virus type 2 glycoproteins B and D. J Virol. 1997;71:7960–7962. doi: 10.1128/jvi.71.10.7960-7962.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matzinger P. An innate sense of danger. Semin Immunol. 1998;10:399–415. doi: 10.1006/smim.1998.0143. [DOI] [PubMed] [Google Scholar]
- 30.Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- 31.Nair S K, Boczkowski D, Morse M, Cumming R I, Lyerly H K, Gilboa E. Induction of primary carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes in vitro using human dendritic cells transfected with RNA. Nat Biotechnol. 1998;16:364–369. doi: 10.1038/nbt0498-364. [DOI] [PubMed] [Google Scholar]
- 32.Nathwani A C, Hanawa H, Vandergriff J, Kelly P, Vanin E F, Nienhuis A W. Efficient gene transfer into human cord blood CD34+ cells and the CD34+CD38− subset using highly purified recombinant adeno-associated viral vector preparations that are free of helper virus and wild-type AAV. Gene Ther. 2000;7:183–195. doi: 10.1038/sj.gt.3301068. [DOI] [PubMed] [Google Scholar]
- 33.Panelli M C, Wunderlich J, Jeffries J, Wang E, Mixon A, Rosenberg S A, Marincola F M. Phase 1 study in patients with metastatic melanoma of immunization with dendritic cells presenting epitopes derived from the melanoma-associated antigens MART-1 and gp100. J Immunother. 2000;23:487–498. doi: 10.1097/00002371-200007000-00013. [DOI] [PubMed] [Google Scholar]
- 34.Petrof B J, Lochmuller H, Massie L, Yang L, Macmillan C, Zhao J E, Nalbantoglu J, Karpati G. Impairment of force generation after adenovirus mediated gene transfer to muscle is alleviated by adenoviral gene inactivation and host CD8+T cell deficiency. Hum Gene Ther. 1996;7:1813–1826. doi: 10.1089/hum.1996.7.15-1813. [DOI] [PubMed] [Google Scholar]
- 35.Pirtskhalaishvili G, Shurin G V, Gambotto A, Esche C, Wahl M, Yurkovetsky Z R, Robbins P D, Shurin M R. Transduction of dendritic cells with Bcl-xL increases their resistance to prostate cancer-induced apoptosis and antitumor effect in mice. J Immunol. 2000;165:1956–1964. doi: 10.4049/jimmunol.165.4.1956. [DOI] [PubMed] [Google Scholar]
- 36.Ponnazhagan S, Erikson D, Kearns W G, Zhou S Z, Nahreini P, Wang X S, Srivastava A. Lack of site-specific integration of the recombinant adeno-associated virus 2 genomes in human cells. Hum Gene Ther. 1997;8:275–284. doi: 10.1089/hum.1997.8.3-275. [DOI] [PubMed] [Google Scholar]
- 37.Ponnazhagan S, Mukherjee P, Yoder M C, Wang X S, Zhou S Z, Kaplan J, Wardsworth S, Srivastava A. Adeno-associated virus 2-mediated gene transfer in vivo: organ-tropism and expression of transduced sequences in mice. Gene. 1997;190:203–210. doi: 10.1016/s0378-1119(96)00576-8. [DOI] [PubMed] [Google Scholar]
- 38.Ponnazhagan S, Mukherjee P, Wang X-S, Kurpad C, Qing K, Kube D, Mah C, Yoder M, Srour E F, Srivastava A. Adeno-associated virus 2-mediated transduction of primary human bone marrow derived CD34+ hematopoietic progenitor cells: donor variation and correlation of expression with cellular differentiation. J Virol. 1997;71:8262–8267. doi: 10.1128/jvi.71.11.8262-8267.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ponnazhagan S, Yoder M C, Srivastava A. Adeno-associated virus 2-mediated transduction of murine repopulating hematopoietic stem cells and long-term expression of a human globin gene in vivo. J Virol. 1997;71:3098–3104. doi: 10.1128/jvi.71.4.3098-3104.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ponnazhagan S, Wang X-S, Woody M J, Luo F, Kang L Y, Nallari M L, Munshi N C, Zhou S Z, Srivastava A. Differential expression in human cells from the p6 promoter of human parvovirus B19 following plasmid transfection and recombinant adeno-associated virus 2 (AAV) infection: human megakaryocytic leukemia cells are non-permissive for AAV infection. J Gen Virol. 1996;77:1111–1122. doi: 10.1099/0022-1317-77-6-1111. [DOI] [PubMed] [Google Scholar]
- 41.Roth M D, Gitlitz B J, Kiertscher S M, Park A N, Mendenhall M, Moldawer N, Figlin R A. Granulocyte macrophage colony-stimulating factor and interleukin 4 enhance the number and antigen-presenting activity of circulating CD14+ and CD83+ cells in cancer patients. Cancer Res. 2000;60:1934–1941. [PubMed] [Google Scholar]
- 42.Russell D W, Alexander I, Miller A D. DNA synthesis and topoisomerase inhibitors increase transduction by adeno-associated virus vectors. Proc Natl Acad Sci USA. 1995;92:5719–5723. doi: 10.1073/pnas.92.12.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russell D W, Kay M A. Adeno-associated virus vectors and hematology. Blood. 1999;94:864–874. [PMC free article] [PubMed] [Google Scholar]
- 44.Sallusto F, Lanzavecchia A. Mobilizing dendritic cells for tolerance, priming, and chronic inflammation. J Exp Med. 1999;189:611–614. doi: 10.1084/jem.189.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samulski R J, Chang L-S, Shenk T. A recombinant plasmid from which an infectious adeno-associated virus genome can be excised in vitro and its use to study viral replication. J Virol. 1987;61:3096–3101. doi: 10.1128/jvi.61.10.3096-3101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schuler-Thurner B, Dieckmann D, Keikavoussi P, Bender A, Maczek C, Jonuleit H, Roder C, Haendle I, Leisgang W, Dunbar R, Cerundolo V, von Den Driesch P, Knop J, Brocker E B, Enk A, Kampgen E, Schuler G. Mage-3 and influenza-matrix peptide-specific cytotoxic T cells are nducible in terminal stage HLA-A2.1+melanoma patients by mature monocyte-derived dendritic cells. J Immunol. 2000;165:3492–3496. doi: 10.4049/jimmunol.165.6.3492. [DOI] [PubMed] [Google Scholar]
- 47.Snyder R O. Adeno-associated virus-mediated gene delivery. J Gene Med. 1999;1:166–175. doi: 10.1002/(SICI)1521-2254(199905/06)1:3<166::AID-JGM34>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 48.Snyder R O, Miao C H, Patjin G A, Spratt S K, Danos O, Nagy D, Gown A M, Winther B, Meuse L, Cohen L K, Thompson A R, Kay M A. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet. 1997;16:270–276. doi: 10.1038/ng0797-270. [DOI] [PubMed] [Google Scholar]
- 49.Song S, Morgan M, Ellis T, Poirier A, Chesnut K, Wang J, Brantly M, Muzyczka N, Byrne B J, Atkinson M, Flotte T R. Sustained secretion of human alpha-1 antitrypsin from murine muscle transduced with adeno-associated virus vectors. Proc Natl Acad Sci USA. 1998;95:14384–14385. doi: 10.1073/pnas.95.24.14384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teramoto S, Bartlett J S, McCarty D, Xiao X, Samulski R J, Boucher R C. Factors influencing adeno-associated virus-mediated gene transfer to human cystic fibrosis airway epithelial cells: comparison with adenovirus vectors. J Virol. 1998;72:8904–8912. doi: 10.1128/jvi.72.11.8904-8912.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Timmerman J M, Levy R. Dendritic cell vaccines for cancer immunotherapy. Annu Rev Med. 1999;50:507–529. doi: 10.1146/annurev.med.50.1.507. [DOI] [PubMed] [Google Scholar]
- 52.Tüting T, Storkus W J, Lotze M T. Gene-based strategies for the immunotherapy of cancer. J Mol Med. 1997;75:478–491. doi: 10.1007/s001090050133. [DOI] [PubMed] [Google Scholar]
- 53.Wan Y, Bramson J, Carter R, Graham F, Gauldie J. Dendritic cells transduced with an adenoviral vector encoding a model tumor-associated antigen for tumor vaccination. Hum Gene Ther. 1997;8:1355–1363. doi: 10.1089/hum.1997.8.11-1355. [DOI] [PubMed] [Google Scholar]
- 54.Yang S, Kittlesen D, Slingluff C L, Jr, Vervaert C E, Seigler H F, Darrow T L. Dendritic cells infected with a vaccinia vector carrying the human gp100 gene simultaneously present multiple specificities and elicit high-affinity T cells reactive to multiple specifications and elicit high-affinity T cells reactive to multiple epitopes and restricted by HLA-A2 and -A3. J Immunol. 2000;164:4204–4211. doi: 10.4049/jimmunol.164.8.4204. [DOI] [PubMed] [Google Scholar]
- 55.Yang T, Su Q, Wilson J M. Role of viral antigens in destructive cellular immune responses to adenovirus vector-transduced cells in mouse lungs. J Virol. 1996;70:7209–7212. doi: 10.1128/jvi.70.10.7209-7212.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y, Chirmule N, Gao G P, Wilson J M. CD40 ligand-dependent activation of cytotoxic T lymphocytes by adeno-associated virus vectors in vivo: role of immature dendritic cells. J Virol. 2000;74:8003–8010. doi: 10.1128/jvi.74.17.8003-8010.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]