Abstract
The tumor microenvironment (TME) is a complex ecosystem of diverse cell types whose interactions govern tumor growth and clinical outcome. While the TME’s impact on immunotherapy has been extensively studied, its role in chemotherapy response remains less explored. To address this, we developed DECODEM (DEcoupling Cell-type-specific Outcomes using DEconvolution and Machine learning), a generic computational framework leveraging cellular deconvolution of bulk transcriptomics to associate the gene expression of individual cell types in the TME with clinical response. Employing DECODEM to analyze the gene expression of breast cancer (BC) patients treated with neoadjuvant chemotherapy, we find that the gene expression of specific immune cells (myeloid, plasmablasts, B-cells) and stromal cells (endothelial, normal epithelial, CAFs) are highly predictive of chemotherapy response, going beyond that of the malignant cells. These findings are further tested and validated in a single-cell cohort of triple negative breast cancer. To investigate the possible role of immune cell-cell interactions (CCIs) in mediating chemotherapy response, we extended DECODEM to DECODEMi to identify such CCIs, validated in single-cell data. Our findings highlight the importance of active pre-treatment immune infiltration for chemotherapy success. The tools developed here are made publicly available and are applicable for studying the role of the TME in mediating response from readily available bulk tumor expression in a wide range of cancer treatments and indications.
INTRODUCTION
The tumor microenvironment (TME) is a complex and multifaceted ecosystem that encompasses a plethora of cell types including immune cells (such as T- and B-lymphocytes, macrophages, dendritic cells etc.), stromal cells (such as fibroblasts, endothelial cells etc.), and the extracellular matrix. It plays crucial roles in regulating tumor progression and response to therapy. Emerging evidence suggest the context-specific behavior of the TME as either tumor-suppressive or supportive, presenting an attractive target for therapeutic intervention [1][2]. Numerous studies have elucidated the critical role of TME in mediating response to immunotherapy, however, insights into how the TME is relevant to chemotherapy response remains limited. Here we focus on studying the influence of the TME on chemotherapy response in breast cancer (BC), where neoadjuvant chemotherapy (NAC) is used prior to primary surgery to enable tumor downstaging and increase the likelihood of breast-conserving surgery [3][4][5].
Recent studies have underscored the relevance of the BC TME in determining patient response to chemotherapy. For instance, chemotherapy can induce dynamic changes in the TME that lead to an increase in regulatory T-cells and myeloid-derived suppressor-like cells, calling for a balance in immune population for positive treatment outcomes [6]. Lymphocyte density and tumor infiltrating lymphocytes have consistently been associated with BC patients achieving pathological complete response (pCR) following NAC and anti-HER2 therapy [7][8][9][10][11]. Additionally, multiple studies have reported the active involvement of TME in therapy resistance [12][13][14], with macrophages, endothelial, and BC stem cells promoting chemoresistance [15][16][17], and B-cells and plasma cells displaying varying roles in NAC response [18][19][20]. However, to our knowledge, no study has yet attempted to quantify the contribution and predictive power of each individual cell type in the TME to chemotherapy response in a systematic and rigorous manner. We, therefore, aim to investigate three fundamental research questions: (1) Can we apply a rigorous computational approach to delineate the cell-type-specific transcriptome predictive power in the BC TME from bulk transcriptomics data? (2) Which are the cell types in the BC TME and the corresponding pathways whose gene expression are most predictive of chemotherapy response? (3) Does aggregating the inferred expression of multiple predictive cell types in the BC TME lead to a more accurate predictor?
Current advances in precision oncology have highlighted the utility of artificial intelligence [1][2][21][22][23][24] in guiding treatment using diverse data types such as genomics [1][25][26], transcriptomics [29][30][31][32][33], histopathology [34][35][36][37][38][39], or through multimodal approaches [11][27][28][40][41][42]. However, investigations into neoadjuvant therapy response often encounter limitations like small sample size, treatment heterogeneity, or inadequate capturing of the TME’s complexity, as highlighted by Sammut et al. [11]. In their pioneering work, Sammut et al. presented a novel multiomic predictor of neoadjuvant therapy response in BC [11]. They have leveraged machine learning (ML) to incorporate 34 features extracted from clinical, DNA, RNA, pathology, and treatment information to develop a single model to predict patient response across all BC subtypes and different treatment regimens with high accuracy. Expanding on their groundwork and addressing the limitations, our study aims to decouple the cell-type-specific effects to NAC response in the BC TME from transcriptomics alone by focusing on the HER2-negative patients, where NAC is recommended to increase the likelihood of achieving pCR following surgery, especially in triple-negative breast cancer (TNBC) [49].
To systematically explore the association between the TME and chemotherapy response, we developed a computational framework, DECODEM (DEcoupling Cell-type-specific Outcomes using DEconvolution and Machine learning). This framework leverages cellular deconvolution with machine learning to elucidate the association of gene expression of diverse cell types in the TME to patient response to a given treatment. Recognizing that these phenotype effects are intricately regulated by the interactions among relevant cell types within the TME, we further extended DECODEM to DECODEMi, where ‘i’ stands for interaction. DECODEMi incorporates the inferred cell-to-cell communications to pinpoint the key cell-cell interactions (CCIs) influencing treatment response. As mentioned, we demonstrate the utility of our tools in an example scenario of investigating the role of the BC TME in modulating response to NAC. A recent study by Kester et al. investigated the association of cellular compositions of the TME with patient survival in BC [43], but to our knowledge, the current study is the first to analyze the cell-type-specific transcriptomic profiles in the BC TME to objectively quantify their association with treatment response.
Through the application of DECODEM, we illustrate the markedly improved predictive capabilities of cell-type-specific transcriptome over bulk transcriptome, untangling the individual contributions of different cell types in treatment response. Through the application of DECODEMi, we further pinpoint the notable immune interactions among these cell types. Together all these findings point to the necessity of an active pre-treatment immune system to facilitate positive treatment outcome, as supported by Sammut et al. [11]. Therefore, from a methodological perspective, our study provides tools to systematically assess the role of individual cellular components within the TME in anticancer therapy response (DECODEM), and to identify the prominent cell-to-cell communications associated with this response (DECODEMi). These tools will be made publicly available upon publication.
RESULTS
Overview of the analysis
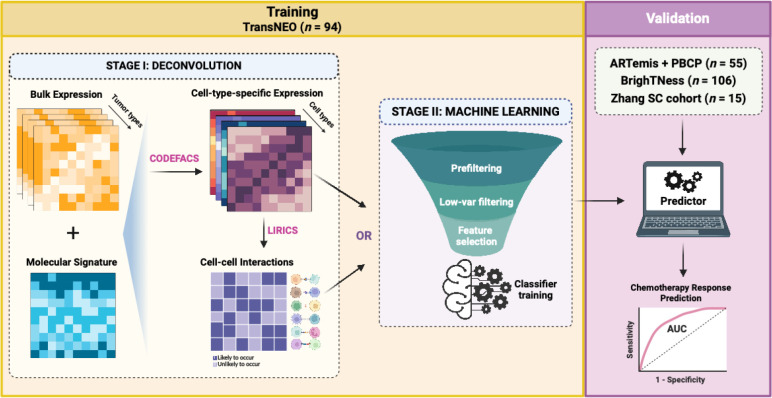
DECODEM and DECODEMi developed and presented here are new computational frameworks designed to explore the predictive powers of individual cell types and relevant cell-cell interactions, respectively, within the TME to response to therapy. They harness the prowess of CODEFACS and LIRICS, two recent tools developed in our laboratory [44]. CODEFACS facilitates the extraction of the cell-type-specific expression profiles from bulk gene expression data for each patient, while LIRICS discerns the activity of cell-type-specific ligand-receptor interactions within the patient’s TME. In essence, DECODEM takes the pre-treatment bulk gene expression as input and outputs the quantified predictive power (in terms of AUC and AP i.e., the area under the receiver operating characteristics (ROC) curve and average precision, equivalent to the area under the precision-recall curve, respectively) of each cell type to treatment response. DECODEMi then follows through to extract the most predictive cell-cell interactions among these cell types.
Specifically, DECODEM comprises of two sequential steps: First, for each bulk expression cohort, it generates nine cell-type-specific expression profiles (as annotated by Wu et al. [45] which were used for molecular signature generation; see details in METHODS: B-cells, Cancer-associated Fibroblasts (CAFs), Cancer Epithelial (malignant), Endothelial, Myeloid, Normal Epithelial, Plasmablasts, Perivascular-like Cells (PVL), and T-cells) in each sample using CODEFACS; Second, by leveraging these nine deconvolved expression profiles, it generates nine cell-type-specific predictors of clinical response (responders vs. non-responders e.g., pCR vs. residual disease) using a multi-stage ML pipeline (Figure 1). Within this pipeline, we performed rigorous feature selection to keep the top ‘m’ genes per cell type (2 ≤ m ≤ 25; the upper limit is chosen to avoid overfitting and facilitate faster runtime) before feeding their expression into an unweighted ensemble classifier consisting of four ML algorithms: regularized logistic regression, random forest, support vector machine, and XGBoost (see details in METHODS). The pipeline was trained with repeated three-fold cross-validation (CV) to optimize model hyperparameters. Additionally, we built a baseline predictor with bulk expression using the same ML pipeline to serve as a control, comparative rod.
Figure 1: The analysis pipeline, DECODEM and DECODEMi.
First, we apply CODEFACS on bulk expression to generate nine cell-type-specific expression profiles and further apply LIRICS to infer the cell-cell interactions (CCIs) present in the tumor microenvironment. Second, we train a multi-stage machine learning pipeline with either the nine cell-type-specific expression profiles to build nine cell-type-specific clinical response predictors (DECODEM) or the CCI profile to build a CCI-based predictor (DECODEMi). In validation, we deploy the abovementioned first stage to procure the cell-type-specific expression or CCIs and directly feed those as inputs to the trained predictors to evaluate model performance.
To assess the performance of DECODEM and evaluate the contribution of each cell type, we analyzed bulk transcriptomics data from three breast cancer cohorts with different ratios of responders to non-responders (R:NR) to NAC (Supplementary Figure 1) in two scenarios: (a) cross-validation with an additional five-fold CV on the TransNEO cohort (n = 94, R:NR = 22:72), recently published by Sammut et al. accompanied by a multiomic clinical response predictor [11], and (b) external validation on the ARTemis + PBCP (n = 55, R:NR = 8:47) and BrighTNess (n = 106, R:NR = 65:41) cohorts [46][47]. TransNEO and ARTemis + PBCP included all major BC subtypes, among which we focused on the HER2-negative patients [11][46] (Supplementary Figure 1B–C), whereas the BrighTNess cohort included only TNBC patients from arm B of the trial, treated with a combination of paclitaxel and carboplatin with a placebo for veliparib [47]. Moreover, we established DECODEM’s generalizability to single-cell transcriptomics through external validation on an independent cohort from Zhang et al, which consisted of 15 TNBC patients who received either paclitaxel alone (R:NR = 3:3) or in combination with atezolizumab, an anti-PD-L1 agent (R:NR = 4:5) [48]. Notably, we considered the Sammut et al. predictor built with 18 extracted features from clinical and RNA-seq data (e.g. not including the DNA-seq data) as a comparative rod, which achieved an AUC of 0.88 in CV and 0.89 in validation with ARTemis + PBCP [11]. This predictor outperformed a baseline predictor built with seven clinical features including ER status and HER2 status, which are routinely used to guide clinical decisions [49].
To investigate the effect of incorporating the expression profiles of multiple predictive cell types together, as indicated by our third question, we applied two extensions to DECODEM: (i) append the cell-type-specific expression profiles for two and three most predictive cell types together to train a multi-cell-ensemble ML pipeline, and (ii) extend to DECODEMi to incorporate the inferred CCIs to train a CCI-based ML pipeline (Figure 1; see details in METHODS). The former model maximizes the predictive performance by leveraging the complementary information from different cell types while the latter identifies the most predictive cell-to-cell communications for clinical response, thereby providing deeper insights to the underlying mechanism.
Gene expression of immune and stromal cells predicts response to neoadjuvant chemotherapy as accurately as the expression of malignant cells
To assess the impact of individual cell types on chemotherapy response, we applied DECODEM to TransNEO in a CV analysis. Our analysis demonstrated significant differences (one-tailed Wilcoxon rank-sum test p-value ≤ 0.001) in the resulting prediction scores between responders and non-responders across 91 patients (the patients with available predictions from Sammut et al.), with the identified cell types outperforming bulk and Sammut et al. predictors (Figure 2A). Seven out of nine cell types exhibited markedly enhanced predictive capabilities in identifying responders. Notably, immune cells (myeloid, plasmablasts, B-cells) contribute as prominently as the malignant (cancer epithelial) and stromal cells (endothelial, normal epithelial, CAFs) (Figure 2B). Our subsequent analyses, focused on these seven ‘prominent’ predictive cell types, whose abilities to accurately identify true responders to chemotherapy in terms of the odds ratio and sensitivity values are shown in Supplementary Figure 2A,C.
Figure 2: The prominent cell types mediating chemotherapy response in breast cancer tumor microenvironment.
A-B. Comparison of prediction scores (A) and model performance (B) in cross-validation with TransNEO (n = 91) for nine cell-type-specific, bulk, and Sammut et al. predictors. R and NR stand for responders and non-responders. The differences between the prediction scores were computed by using a one-tailed Wilcoxon rank-sum test. AUC and AP stand for the area under the receiver operating characteristics curve and average precision (equivalent to the area under precision-recall curve), respectively. Cell types are ranked by their AUC values in a descending order.
C. Comparison of model performance in external validation with ARTemis + PBCP (n = 55) for the seven prominent cell types, bulk, and Sammut et al. predictors. Cell types are ranked by their AUC values in a descending order.
D. Comparison of model performance in external validation with BrighTNess (n = 106) for the seven prominent cell types and bulk predictors. Cell types are ranked by their AUC values in a descending order.
These cell types have frequently been reported to influence clinical response in BC, particularly in the context of chemotherapy-induced immunogenic cell death as the proposed mechanism [51][11]. Changes in the immune population, such as an increase in regulatory T-cells and myeloid-derived suppressor-like cells, and a decrease in CD8+ T-cells, have been associated with achieving pCR following NAC [6]. Myeloid cells have been implicated in therapy response through interactions with the malignant and endothelial cells, inducing chemoresistance via the CXCL1/2 – S100A8/9 loop, whereas tumor-associated macrophages have been linked to adverse outcomes and chemoresistance through survival factor secretion, anti-apoptotic pathway activation, and modulation of signaling pathways [6][15][16]. VEGF-mediated upregulation of survivin in Endothelial cells was further implicated in playing central roles in chemoresistance [15]. Additionally, plasmablasts, as precursors of plasma cells, have been associated with better prognosis [19][20], while B-cells have shown both pro- and anti-tumor roles [18][19][20].
We further tested and validated the predictive power of these cell types independently in external validation using the ARTemis + PBCP (n = 55) and BrighTNess (n = 106) cohorts, observing consistent results (Figure 2C–D, Supplementary Figure 2A,C). In the ARTemis + PBCP cohort (dominated by ER-positive, HER2-negative BC), the prominent contributors to chemotherapy response were CAFs, normal epithelial, and cancer epithelial (Figure 2C), whereas in the TNBC-specific BrighTNess cohort, the key players were myeloid, plasmablasts, and cancer epithelial (Figure 2D). When stratifying TransNEO and ARTemis + PBCP cohorts by the available BC subtypes, our cell-type-specific models retain good performance across both subtypes (Supplementary Figure 3A–D). The models for ER-positive, HER2-negative BC were more predictive than TNBC, possibly due to sample size limitations as well as increased heterogeneity in TNBC. These findings support the prominent involvement of immune and stromal cells alongside the malignant cells in mediating patient response to chemotherapy in the TME, with variations observed likely due to tumor subtype composition and ER/HER2 status differences across cohorts.
Our analysis did not identify T-cells as a prominent predictive cell type, as their expression provide marginal to no enhancement in predictive power over bulk expression (Figure 2A–B). This lack of signal may stem from aggregating different T-cell subtypes together (due to limits on the resolution of the deconvolution, which breaks down for low-abundance cell types). To study this further, we performed an enrichment analysis to delineate the contributions of CD4+ and CD8+ T-cells to chemotherapy response. However, the expression of each of these cell types still exhibited limited stratification power in distinguishing responders from non-responders across the three bulk cohorts (Supplementary Figure 4A–D). Additionally, we complemented the expression-based analysis with an analysis of the association between cell abundances and clinical response. This revealed that the overall T-cell abundance exhibited predictive power similar to or even higher than CD4+ and CD8+ subtypes (Supplementary Figure 4E–G), albeit still significantly lower than that of bulk transcriptomics.
An ensemble model incorporating the expression of immune and stromal cells significantly boosts the predictive power of patient response
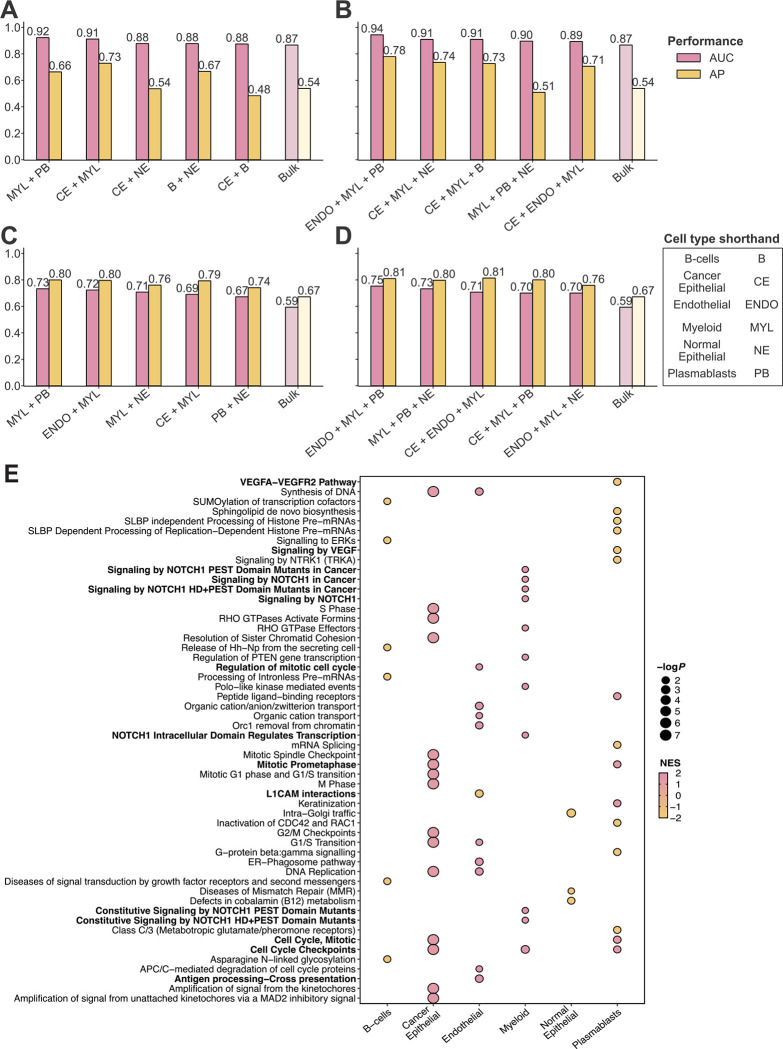
To further enhance DECODEM’s performance, we aggregated multiple individual cell types together by appending the corresponding expression profiles as the input features. We explored 35 combinations of two to three cell types exhaustively, This comprehensive analysis yielded a boost in predictive performance in both ARTemis + PBCP and BrighTNess cohorts, with significant overlaps observed in the top multi-cell-type combinations across cohorts (Figure 3A–D, Supplementary Figure 2B,D). Remarkably, the ensemble of immune and stromal cells, specifically the combination of the expressions of endothelial, myeloid, and plasmablast cells exhibited the strongest stratification capability in identifying chemotherapy responders (ARTemis + PBCP: AUC = 0.94, AP = 0.78, Figure 3B; BrighTNess: AUC = 0.75, AP = 0.81, Figure 3D). Additionally, the immune-cell-ensemble comprising myeloid and plasmablasts displayed high stratification (ARTemis + PBCP: AUC = 0.92, AP = 0.66, Figure 3A; BrighTNess: AUC = 0.73, AP = 0.80, Figure 3C). These findings testify to the complementary contributions of different cell types within the TME to chemotherapy response. However, the top ensemble for the BrighTNess cohort provided a similar performance to the top cell type, myeloid (AUC = 0.76, AP = 0.83, Figure 2D), suggesting that the interactions among different cell types are less prominent in this cohort compared to the other cohorts, illustrated further in Figure 4D. The subtype-specific stratification of ARTemis + PBCP reinforced the superior predictive capabilities of these ensembles across both available BC subtypes while recapitulating their diminished impact in TNBC (Supplementary Figure 3E–F).
Figure 3: The prominent multi-cell-type ensembles mediating chemotherapy response in breast cancer tumor microenvironment.
A-B. Validation performance with ARTemis + PBCP (n = 55) for the five most prominent two-cell-ensembles (A) and three-cell-ensembles (B) along with the bulk. AUC and AP stand for the area under the receiver operating characteristics curve and average precision (equivalent to the area under the precision-recall curve), respectively. Ensembles are ranked by their AUC values in a descending order.
C-D. Validation performance with BrighTNess (n = 106) for the five most prominent two-cell-ensembles (C) and three-cell-ensembles (D) along with the bulk. Ensembles are ranked by their AUC values in a descending order.
E. The enriched Reactome pathways (FDR-adjusted p-value ≤ 0.2) across the prominent cell types. For each cell type, only the top 10 relevant pathways are displayed. NES and logP stand for the normalized enrichment score from GSEA and log-scaled p-value, respectively.
Figure 4: The prominent cell-cell interactions (CCIs) mediating chemotherapy response in breast cancer tumor microenvironment.
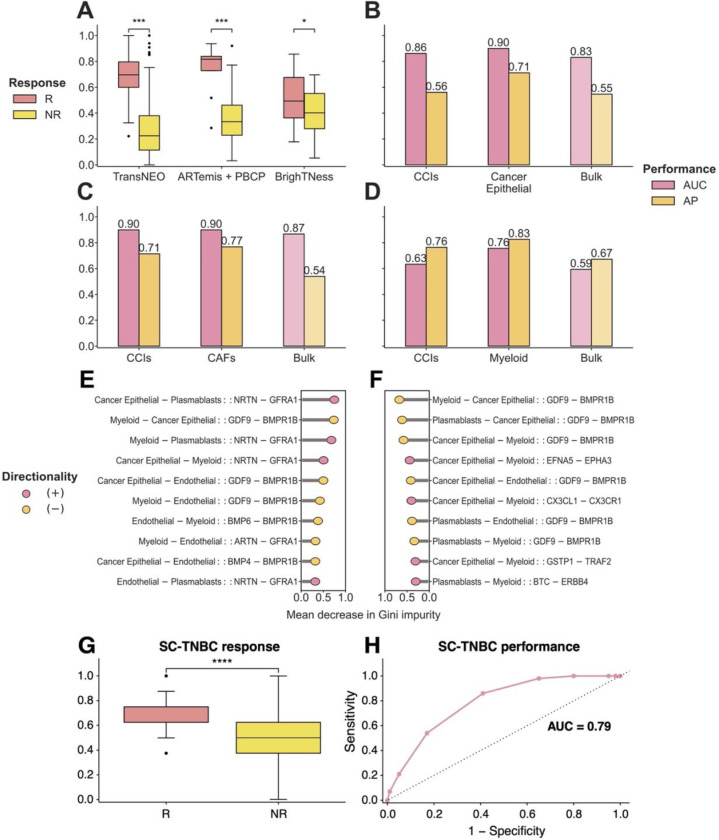
A. Prediction scores for DECODEMi in cross-validation with TransNEO (n = 94) and in external validation with ARTemis + PBCP (n = 55) and BrighTNess (n = 106). R and NR stand for responders and non-responders, respectively. The differences between the prediction scores were computed by using a one-tailed Wilcoxon rank-sum test.
B. Model performance in cross-validation with TransNEO for CCI-based, the top cell-type-specific, and bulk predictors. AUC and AP stand for the area under the receiver operating characteristics curve and average precision (equivalent to the area under precision-recall curve), respectively.
C-D. Model performance in external validation with ARTemis + PBCP (C) and BrighTNess (D) for CCI-based, the top cell-type-specific, and bulk predictors.
E-F. The 10 most predictive CCIs in external validation with ARTemis + PBCP (E) and BrighTNess (F). Each CCI is displayed as a quadruplet where the first pair containing the ligand and receptor cell types (separated by ‘−’) is separated by ‘::’ from the second pair containing the corresponding ligand and receptor genes (separated by ‘−’). Mean decrease in Gini impurity is the built-in feature importance measure in random forest where a higher value indicates a higher importance and vice versa [98]. Feature directionalities were computed using Fisher’s enrichment analysis.
G-H. Prediction scores (G) and AUC value (H) for chemotherapy response prediction in external validation with the SC-TNBC cohort (n = 200, sourced from Zhang et al. [48]) using the top 170 response-relevant CCIs (among B-cells, myeloid, and T-cells) identified in bulk by DECODEMi.
We conducted gene set enrichment analysis (GSEA) with Reactome pathways [52] to explore the functional contributions of these prominent cell types in chemotherapy response prediction. This analysis revealed the enrichment of cell cycle checkpoint and DNA replication pathways across different cell types (false discovery rate (FDR)-adjusted p-value ≤ 0.2; Figure 3E) as the most common functional contribution. Dysregulation of these pathways are frequent in BC and promotes tumor proliferation [53][54][55], making them valuable prognostic markers and therapeutic targets [56][57][58][59]. Immune cells exhibited enrichment in signal transduction pathways (e.g., VEGFA – VEGFR2 and VEGF signaling pathways in plasmablasts and multiple NOTCH1 signaling pathways in myeloid), implicated in malignant progression and therapy resistance, particularly in TNBC [60][61][62][63][64][65][66]. Stromal endothelial cells showed enrichment in development- and immune-related pathways (e.g., L1CAM interactions and antigen processing & presentation), contributing to aggressive BC progression and immune evasion with implications for both chemotherapy and immunotherapy [67][68][69][70][71][72][73].
To delve deeper into the underlying mechanism, we assessed DECODEM’s performance in light of the prevalent chemotherapy regimen within each cohort. TransNEO and ARTemis + PBCP predominantly employed T-FEC (n = 61 and 40, respectively; including docetaxel/taxotere, 5-flurouracil, epirubicin, and cyclophosphamide), while BrighTNess used a uniform regimen of paclitaxel and carboplatin (Supplementary Figure 1C). All six drugs have known clinical activities stimulating anticancer immunity via releasing immunostimulatory molecules from cancer cells (on-target effects) or promoting activation of effector cells and diminishing immunosuppressive cells (off-target effects) [73]. Remarkably, across all three cohorts spanning two BC subtypes and two regimens, DECODEM identified myeloid and plasmablasts to consistently stratify responders to immunogenic drug regimens, alongside malignant cells (Supplementary Figure 5A,B,D). For ARTemis + PBCP (dominated by ER-positive, HER2-negative BC), although the bulk mixture displayed comparable or superior predictive power than each of these two individual immune cells, their ensemble markedly boosted the performance, supporting the possibility that there may be prominent CCIs between these cell types that may be associated with the likelihood of response (Supplementary Figure 5C). Among the three-cell-ensemble models, the combination of endothelial, myeloid, and plasmablasts retains its highest performance, further underscoring the critical roles of the stromal – immune interactions. Additionally, for BrighTNess, the individual immune cell types showed notably strong predictive abilities, with less pronounced CCI effects (Supplementary Figure 5E, Figure 4D). Collectively, these findings corroborate the immunogenic effects mediated by chemotherapy, suggestive of immunogenic cell death [73] as suggested by Sammut et al. [11].
Identifying key cell-cell interactions associated with chemotherapy response
To investigate the impact of the presence of interactions among multiple cell types in modulating chemotherapy response, we use DECODEMi to analyze cell-cell interactions (inferred using LIRICS [44], starting with 2,422 ligand – receptor (L-R) pairs from Ramilowski et al. [50]; see details in METHODS) that are predictive of patient response. As before, we conducted two analyses: (a) cross-validation on TransNEO (n = 94), and (b) external validation on ARTemis + PBCP (n = 55) and BrighTNess (n = 106). We found that CCIs encompassing only the four most prominent cell types in the TME (cancer epithelial, endothelial, myeloid, and plasmablasts) exhibited a comparable classification power to all available CCIs across all three cohorts (one-tailed Wilcoxon rank-sum test p-value ≤ 0.05), further testifying to the influential roles of these specific cell types in patient response (Figure 4A–D, Supplementary Figure 6A–D). The CCIs involving these four cell types markedly improved the predictive power of CCI-based predictors in both TransNEO and ARTemis + PBCP (Figure 4A–C). However, the performance of CCIs was generally lower than the top predictive myeloid cells in BrighTNess cohort (Figure 4D). This discrepancy can again be attributed to cohort composition and treatment-specific variations. It is worth noting that this cohort included only TNBC patients treated with paclitaxel and carboplatin, for which very few samples were available in training (Supplementary Figure 1B–C). These results reinforced that leveraging information from multiple cell types can notably improve clinical response prediction and the inter-cellular crosstalk may involve mediating treatment response in the TME. To further assess the generalizability of DECODEMi beyond bulk cohorts, we assessed its effectiveness on an independent single-cell (SC) TNBC cohort without additional training. To overcome the limitation of small sample size (n = 6), we constructed a SC-TNBC cohort of 200 ‘pseudopatients’ treated with chemotherapy (R:NR = 100:100) by downsampling 28,209 cells across the six TNBC patients who received paclitaxel in Zhang et al.’s study [48]. We focused on the CCIs among the three cell types (encompassing B-cells, myeloid, and T-cells) present in both SC and deconvolved bulk data, and identified the 170 top predictive CCIs (covering 134 L-R pairs) in bulk TransNEO cohort. The prediction scores for the SC-TNBC cohort were computed by counting the number of activated CCIs within each pseudopatient (as inferred by Kumar et al. [103]; see details in METHODS). Our analysis demonstrated the significant and robust predictive capabilities of these top CCIs (one-tailed Wilcoxon rank-sum test p-value ≤ 0.001, Figure 4G–H), affirming DECODEMi’s ability to identify the CCIs predictive of patient response.
By computing feature importance from DECODEMi, we identified the most predictive cell-to-cell communications across the ARTemis + PBCP and BrighTNess cohorts, finding a significant overlap in top CCIs (Figure 4E–F). Remarkably, the interaction between ‘GDF9’ (growth differentiation factor 9) and ‘BMPR1B’ (bone morphogenetic protein receptor 1B), both members of the TGF-β superfamily (the corresponding protein-protein interaction (PPI) network, obtained from STRING [100], is provided in Supplementary Figure 6E) with roles in reproductive biology and signal transduction [74][75][76][77], emerged as a key CCI with the same directionality across multiple cell types. These genes have been implicated in BC prognosis and therapeutic outcomes [78][79][80], with BMPR1B exhibiting dual roles in chemotherapy response [81][82][83]. Another top CCI involved the renowned GDNF – RET signaling pathway interactions between ‘NRTN’ (neurturin), or its paralog ‘ARTN’ (artemin), and ‘GFRA1’ (GDNF family receptor alpha 1) in multiple cell types (Figure 4E–F; see PPI network in Supplementary Figure 6F), which are all members of the glial cell line-derived neurotrophic factor (GDNF) family within the TGF-β superfamily with roles in neuronal survival, growth, and differentiation [84]. The GDNF family exhibits diverse roles in cancer [85][86], including an association with therapy resistance in ER-positive BC, highlighting GFRA1 as a potential target [87][88][89][90]. Additionally, the well-studied chemokine interaction between ‘CX3CL1’ (C-X3-C chemokine ligand 1) and ‘CX3CR1’ (C-X3-C chemokine receptor 1) [91] is prominent in BrighTNess (Figure 4F; see PPI network in Supplementary Figure 6G), which is known to be involved in progression and metastasis across multiple cancers including BC, presenting a promising pan-cancer target [92][93][94][95][96][97].
Overall, DECODEMi application results in: (a) a simpler predictor leveraging CCIs that improves patient stratification compared to bulk transcriptomics, and (b) the identification of cell-to-cell communications predictive of treatment response, whose activation is associated with pro-cancerous roles, thus offering potential novel therapeutic targets.
DECODEM identifies responders to chemotherapy and anti-PD-L1 therapy from single-cell transcriptomics
To further assess the generalizability of DECODEM, we evaluated the predictive capabilities of the cell-type-specific models with single-cell transcriptomics. We analyzed a SC cohort containing the expression of B-cells, myeloid, and T-cells for 15 patients with triple negative breast cancer from Zhang et al., who were either treated with chemotherapy (paclitaxel) alone or combined with the anti-PD-L1 agent, atezolizumab [48]. We first stratified the patient cohort into those who received chemotherapy alone (n = 6) and those who underwent chemotherapy + immunotherapy (n = 9). For each subset, we applied our established DECODEM models for these three cell types which were trained with deconvolved TransNEO data alongside a baseline ‘pseudobulk’ model (analogous to bulk in previous scenarios; see details in METHODS). Our findings revealed that the expression of B-cells exhibited the highest predictive potential within this cohort (Figure 5A–B, Supplementary Figure 7A–D). Notably, this B-cells-specific predictor from DECODEM effectively distinguishes the responders from non-responders for both intervention types (one-tailed Wilcoxon rank-sum test p-value ≤ 0.05, Figure 5A–B). These findings further attest to the generalizability and robustness of DECODEM, highlighting its potential in capturing treatment-invariant properties of the BC TME to predict response to the combination of chemotherapy and immunotherapy through SC transcriptomics.
Figure 5: Generalizability of DECODEM to single-cell (SC) transcriptomics and for TCGA patient survival stratification.
A-B. Prediction scores (A) and the area under the receiver operating characteristics curve (AUC) values (B) in external validation with SC expression of TNBC patients (SC-TNBC) treated with chemotherapy alone (n = 6) or combined with immunotherapy (n = 9) from Zhang et al. [48]. R and NR stand for responders and non-responders, respectively. The differences between the prediction scores were computed by using a one-tailed Wilcoxon rank-sum test.
C-D. Kaplan-Meier plots depicting the overall survival (OS; C) and progression-free interval (PFI; D) of 705 BC patients from TCGA (TCGA-BRCA). Patients were stratified into low-risk, likely responder (n = 353) and high-risk, unlikely responder (n = 352) groups by their median DECODEM score computed using the expression of cancer epithelial cells, whereby the low-risk and high-risk groups comprised individuals with scores above and below the median, respectively. The differences between the two curves were computed by using a Log-rank test.
DECODEM effectively stratifies TCGA patients’ survival
We finally explored whether DECODEM can stratify the survival of 705 breast cancer patients in The Cancer Genome Atlas (TCGA-BRCA) from their deconvolved expression, obtained from Wang et al. [44]. Focusing on the expression of the prominent cell type of cancer epithelial (and endothelial) cells, DECODEM effectively stratified these patients into low-risk, likely responders (scores > median score, n = 353) and high-risk, unlikely responders (scores ≤ median score, n = 352), as displayed by their overall survival and progression-free interval (Log-rank test p-value ≤ 0.05, Figure 5C–D, Supplementary Figure 7E–F). Remarkably, this stratification was achieved regardless of treatment regimen, attesting that DECODEM captured the treatment-invariant properties of the BC TME that are predictive of patient survival.
DISCUSSION
Our study introduces DECODEM, a transcriptomics-based modeling framework employing CODEFACS, a cutting-edge deconvolution tool, with a multi-stage machine learning pipeline to systematically assess the cell-type-specific contributions to predicting clinical response to treatment. As a representative scenario, we apply it to build predictors of patient response to neoadjuvant chemotherapy in breast cancer. We further present DECODEMi, a companion tool that identifies the key cell-cell interactions associated with clinical response. A recent study by Kester et al. investigated the association of cellular compositions of the BC TME with patient survival [43], but to our knowledge, the current study is the first-of-its-kind to analyze the cell-type-specific transcriptomics profiles in the BC TME to quantify their respective contributions to treatment response prediction.
We applied DECODEM to investigate the predictive power of the expression of various cell types in the BC TME to patient response to NAC. Our findings highlight the prominence of immune cells (myeloid, plasmablasts, B-cells) in chemotherapy response, consistent with Kester et al. [43], elucidating the complementary roles of diverse cell types that allows for the incorporation of top predictive cell types to build accurate clinical response predictors, and showcase DECODEMi’s ability to identify key CCIs impacting response across HER2-negative BC subtypes. Furthermore, we illustrated DECODEM’s generalizability to single-cell transcriptomics where it captures treatment-invariant properties of the TME, enabling accurate classification of responders for chemotherapy and immunotherapy, and its application for patient survival stratification in TCGA.
Immune cells have a clear association with prognosis in BC, both without chemotherapy and after chemotherapy in the early-stage setting [104][30][31]. The International TILs Working Group was established for the express purpose of standardizing incorporation of tumor infiltrating lymphocyte pathological review for clinical use, and established an online prognosis calculator for patients with TNBC (https://www.tilsinbreastcancer.org/prognosis-tool/). Plasma cells and naïve B-cells have been associated with better pCR rates in ER-positive tumors, whereas regulatory T-cells have been associated with better pCR rates in TNBC [105]. In our analysis, immune cells such as plasmablasts and myeloid cells exhibit involvement in signal transduction, particularly through VEGF signaling and NOTCH signaling pathways, which are associated with tumor-associated angiogenesis, enrichment of BC stem cells and epithelial-to-mesenchymal transition, leading to poor prognosis and therapy resistance [60][61][64][66]. Stromal cells such as endothelial cells show enrichments in development- and immune-related pathways including L1CAM interactions and antigen processing & presentation, with L1CAM’s oncogenic role and defects in antigen processing leading to poor outcomes and therapy resistance [67][68][69][70][71]. Overall, our results paired with the existing clinical evidence reinforce the notion that the presence of an active immune infiltration within the TME prior to treatment, as indicated by the prominent contributions of immune cells, leads to favorable response to chemotherapy, as suggested by Sammut et al. [11].
It is also important to note that DECODEM and DECODEMi offer to extend upon the existing application of gene expression in the early-stage breast cancer setting. Currently, gene expression-based tests such as Oncotype DX and MammaPrint are used for prediction and prognostication in ER-positive BC patients, reporting if a subset should receive chemotherapy due to worse predicted outcomes [106]. These tests use quantitative RT-PCR or cDNA microarray to assess a known subset of genes, and largely apply only to a subset of early-stage ER-positive BC patients. DECODEM and DECODEMi provide complimentary context to these tools, expanding the applicable subtypes for chemotherapy response prediction as well as greater biological context.
Although we presented a comprehensive framework to assess the TME in a quantitative manner, certain limitations should be acknowledged and addressed in future studies. First, our predictors rely on data-driven algorithms involving deconvolution and ML, necessitating a large sample size to attain statistical robustness and avoid overfitting. Accordingly, the available datasets are limited in size, limiting our ability to perform robust subtype-specific analyses. Second, any limitations inherent to CODEFACS, such as discrepancies in RNA-seq processing and reliance on appropriate molecular signatures [44], will affect DECODEM in the downstream analysis. The same goes for DECODEMi and its dependence on LIRICS using an appropriate ligand – receptor database [43]. Third, our deconvolution analysis could not be performed separately for the CD4+ and CD8+ T-cell subsets. Alternatively, we provided an enrichment analysis assessing the predictive powers of CD4+ and CD8+ T-cells which corroborated our findings from using the overall T-cells.
Our work focused on examining the association of the BC TME, specifically in HER2-negative subtypes, to clinical response to NAC. However, both DECODEM and DECODEMi can be applied to investigate diverse treatment regimens in different cancer indications. Although both TransNEO and ARTemis + PBCP cohorts include different chemotherapy combinations, limited sample size prevented us from exploring further. Therefore, an intriguing direction could be to investigate the distinct role of the TME in different chemotherapy families with different mechanisms of actions (MOAs) e.g., taxanes, antimetabolites, platinum agents and so on. With DECODEMi, one could further pinpoint the family-specific significant cell-cell interactions to understand the underlying MOA and uncover novel CCIs with promising therapeutic implications. Additionally, expanding the investigation beyond the scope of cancer and examining the impact of the microenvironment in various indications such as aging-related disorders and autoimmune diseases could provide further valuable insights.
In summary, our study introduces two data-driven computational frameworks that enable systematic investigation of the roles of the microenvironment in various processes, including treatment response. These frameworks can utilize bulk transcriptomics from healthy and diseased conditions when single-cell information is not easily accessible, providing a valuable approach to study the TME in a comprehensive manner. Their application in breast cancer uncovers the considerable predictive power of diverse cell types within the TME and highlights key stromal – immune cell-cell interactions that are strongly predictive of patient response.
METHODS
Overview of the datasets
We analyzed three bulk transcriptomics datasets, encompassing a total of 255 breast cancer patients who received neoadjuvant chemotherapy (Supplementary Figure 1). The first dataset, recently published by Sammut et al., was the TransNEO cohort which included 94 HER2-negative breast tumors patients undergoing various NAC regimens [11]. Notably, Sammut et al. presented the complete TransNEO cohort, covering 168 patients with early and locally advanced BC from all major subtypes, and providing DNA-seq, RNA-seq, digital pathology, and treatment information for various subsets of patients [11]. The second dataset was the ARTemis + PBCP cohort, validated externally by Sammut et al., combining the control arm of the ARTemis trial (n = 38) received the combination therapy of FEC-T [46] and the HER2-negative cases from the Personalised Breast Cancer Programme (PBCP; n = 17) undergoing various NAC regimens [11] (Supplementary Figure 1B–C). The third dataset, the BrighTNess cohort, included 106 patients from arm B of the BrighTNess trial, a randomized, double-blind, placebo-controlled three-arm clinical study for veliparib in stage II/III TNBC [47]. Arm B included only patients who received the NAC combination of paclitaxel, carboplatin, and a placebo for veliparib (Supplementary Figure 1B–C). In all three cohorts, patient response was assessed at surgery using the residual cancer burden (RCB) index where patients achieving pathological complete response (pCR; RCB = 0) were considered as responders (R) and patients with residual diseases (RCB-I, II, III) as non-responders (NR) with different R:NR ratios (Supplementary Figure 1A).
Furthermore, we analyzed a recent single-cell cohort presented by Zhang et al. comprising 22 patients with advanced triple negative breast cancer. Half of the patients received paclitaxel alone, while the other half received paclitaxel combined with the anti-PD-L1 agent, atezolizumab [48]. This study included 78 tumor biopsies and blood samples collected at three time-points, including baseline. Zhang et al. provided the single-cell expression for four immune cell types (B-cells, T-cells, myeloid, and innate lymphoid cells) and patient response assessed using the Response Evaluation Criteria in Solid Tumors (RECIST) criteria [48]. We categorized the patients achieving complete or partial response as responders (R) and those with stable or progressive diseases as non-responders (NR). Our analysis focused solely on pre-treatment tissue samples, resulting in two treatment groups: (a) chemotherapy (n = 6, R:NR = 3:3) and (b) chemotherapy + immunotherapy (n = 9, R:NR = 4:5). To align with the output of CODEFACS, which provides the mean cell-type-specific expression profile per patient [44], we harmonized the cell-type-specific SC expression by averaging across all corresponding cells for each patient, while filtering for low-variance genes (threshold = 0.8). Additionally, we calculated the ‘pseudobulk’ expression as the mean expression across all cells encompassing the three cell types for each patient (post low-variance filtering) to generate a baseline expression profile similar to the bulk mixture.
Deconvolution with CODEFACS
The first step of DECODEM involves employing CODEFACS, a cellular deconvolution tool recently developed in our lab [44]. CODEFACS characterizes the TME by reconstructing the cell-type-specific expression in each sample from the input bulk expression, utilizing the corresponding cell type abundance or a cell-type-specific signature profile as another input. The output includes the cell-type-specific expression profiles, the corresponding cell type abundances, and a confidence score matrix that quantifies the confidence level (as a value between 0 and 1) of the estimated expression values for each gene in each cell type across all samples.
We derived a cell type signature matrix of 1,400 genes representing nine cell types from a single-cell RNA-seq (scRNA-seq) cohort from Wu et al. [45] using an in-house signature derivation tool based on the CIBERSORTx algorithm [101]. We then deconvolved the three bulk expression profiles with this signature into cell-type-specific expression profiles, encompassing the nine cell types: B-cells, Cancer-associated Fibroblasts (CAFs), Cancer Epithelial (malignant), Endothelial, Myeloid, Normal Epithelial, Plasmablasts, Perivascular-like Cells (PVL), and T-cells.
Extraction of cell-cell interactions with LIRICS
We extended DECODEM to DECODEMi by incorporating multi-cell-type information through cell-cell interactions. To this end, DECODEMi employs LIRICS [44] on the output of CODEFACS to infer the activity of the corresponding CCIs. LIRICS applies a two-step approach to infer the cell-type-specific ligand – receptor (L-R) pairs that are likely to be ‘active’ in each sample from deconvolved expression. The first step is to query a database of 49,397 plausible L-R interactions between each pair of cell types in BC TME (see below for details). The second step infers an L-R interaction to be active (indicated by ‘1’) in a pair of cell types in a given sample if the expressions of both ligand and receptor genes exceed their median cell-type-specific expression values or inactive (indicated by ‘0’) otherwise. We used this binary CCI activity matrix output from LIRICS to assess the predictive potential of cell-to-cell communications.
Curation of cell-type-specific ligand – receptor database for BC TME from single-cell transcriptomics
To infer the cell-cell interaction activity using LIRICS, we applied a two-step approach to construct a database of cell-type-specific ligand – receptor interactions (LRIs) to characterize all cell-to-cell communications that are likely to occur in the TME of BC patients. We first obtained a list of 2,422 potential L-R pairs, as annotated by Ramilowski et al. [50]. We next evaluated the potential of the L-R pairs occurring between the nine cell-types-of-interest using scRNA-seq data from Wu et al [45]. To discern the activity of the curated L-R list, we pooled together all 130,246 cells for the nine cell types from Wu et al. and adopted an empirical p-value based CCI inference approach following Kumar et al. [103], where an LRI with p-value ≤ 0.05 is categorized as ‘likely’ to occur in the TME of BC patients and ‘unlikely’ otherwise. We constructed a database of 49,397 cell-type-specific L-R pairs (i.e., CCI quadruplets) that are likely to occur in BC TME. This database composed the initial feature space for LIRICS to yield different numbers of CCIs across three bulk cohorts: TransNEO = 46,755, ARTemis + PBCP = 43,887, and BrighTNess = 38,285.
Machine learning pipeline with deconvolved expression and CCIs
In both DECODEM and DECODEMi, the second step involves building ML predictors of clinical response using cell-type-specific expression profiles and CCIs, respectively. For DECODEM, we built nine cell-type-specific predictors to classify responders and non-responders following treatment using a four-stage ML pipeline (Figure 1): (a) prefiltering to remove genes with CODEFACS confidence score < 0.99, (b) low-variance filtering to remove genes with variance < 0.1, (c) feature selection to select the top ‘m’ genes maximizing performance, where 2 ≤ m ≤ 25 with genes ranked by their ANOVA F-scores for association with response followed by standardization to scale each gene to zero mean and unit variance, and (d) classification with an unweighted ensemble classifier comprised of regularized logistic regression, random forest, support vector machine, and XGBoost (extreme gradient boosting) to predict the probabilities of achieving pCR. Here, the upper limit on the feature size is chosen to avoid model overfitting and longer runtimes (and evaluated to provide similar performance across different feature sizes in cross-validation). Furthermore, each prediction vector was rescaled between 0 and 1 to improve generalization for external validation. We trained this ML pipeline with three-fold cross-validation (CV) for hyperparameter tuning to maximize the AUC for each classifier separately. To avoid overfitting, we repeated the training procedure five times with five different CV splits and took the mean of the five prediction vectors as our final prediction. We analyzed the three bulk expression cohorts with this pipeline in two scenarios: (a) cross-validation with TransNEO (n = 94) where we applied an additional five-fold CV to estimate the performance, and (b) external validation on ARTemis + PBCP (n = 55) and BrighTNess (n = 106) using predictors trained on TransNEO. We further built a baseline predictor using bulk expression in ML pipeline directly and identified the ‘prominent’ cell types in CV that outperformed the bulk, subsequently validated in external validation.
To accommodate the sparse nature of SC transcriptomics for external validation with Zhang et al. [48], we slightly modified the pipeline to enable modeling for all available genes post filtering in step (c) i.e., 2 ≤ m ≤ ‘all’. Additionally, for CCI-based analysis with DECODEMi, we further updated the low-variance cut-off in step (b) to 0.08 and the classifier in step (d) to a random forest that is better suited for the binary nature of the CCI matrix and includes a built-in measure of feature importance, mean decrease in Gini impurity (similar performance to the permutation-based approaches) [98]. The corresponding CCI directionalities were computed using a two-tailed Fisher’s test (followed by false discovery rate (FDR) adjustment for multiple hypothesis correction i.e., FDR-adjusted p-value ≤ 0.05), where an odds ratio (OR) > 1 denotes an enrichment in responders and OR < 1 indicates enrichment in non-responders. Moreover, to filter CCIs for a subset of cell types, we only include the interactions involving the confident L-R genes (CODEFACS confidence level ≥ 0.99) in the corresponding cell type.
CD4+ / CD8+ T-cells enrichment analysis
To analyze the contribution of the individual T-cell subtypes to chemotherapy response, we performed a gene set variation analysis (GSVA) to summarize pathway activity per sample for each T-cell subtype. We employed two single-cell expression signatures derived from Wu et al. [45], collected via the Tumor Immune Single Cell Hub 2 (TISCH2) [102]. These signatures consisted of 207 and 424 differentially expressed genes (log-fold-change ≥ 0.5) for CD4+ and CD8+ T-cells, respectively (with an overlap of 151 genes). Subsequently, we evaluated the association of the GSVA enrichment scores (rescaled between 0 and 1) with clinical response across the three bulk cohorts through a one-tailed Wilcoxon rank-sum test (FDR-adjusted p-value ≤ 0.05), and also assessed the AUC and AP values independently.
Cell-type-specific enrichment analysis
We conducted gene set enrichment analysis (GSEA) to investigate the association of each cell type with different biological processes. For each cell type, we first ranked the 17,680 protein-coding genes available in TransNEO by their association with positive outcome (i.e., pCR), measured by the p-values from a one-tailed Wilcoxon rank-sum test, adjusted by CODEFACS confidence scores. We next performed a separate GSEA analysis using the Reactome pathways [52] for each prominent cell type (except CAFs). This resulted in six different sets of enriched pathways (FDR-adjusted p-value ≤ 0.2) across six prominent cell types. From these sets, we reported the top 10 relevant pathways for each cell type.
Single-cell validation of top cell-cell interactions
We validated the most predictive cell-cell interactions from DECODEMi using a single-cell cohort from Zhang et al. that includes six TNBC patients treated with chemotherapy [48]. We devised a three-step validation procedure: First, for consistency, we retrained DECODEMi using only the interactions among cell types available in both bulk and SC (namely B-cells, myeloid, and T-cells) in TransNEO. This resulted in the extraction of the top predictive CCIs (m = 170) involving a total of 134 unique L-R pairs. Second, to enhance the statistical power, we pooled the 28,209 cells encompassing these three cell types from the six chemotherapy patients in Zhang et al., and randomly downsampled 30% of the cells 100 times within each treatment group for each cell type. This procedure generated a profile of 200 ‘pseudopatients’ (R:NR = 100:100) with empirical p-values encompassing the significance levels of the 804 possible CCIs involving the 134 L-R pairs in three cell types (= 134 × 6) i.e., the ‘SC-TNBC cohort’. To identify the activation status of the 170 CCIs-of-interest in this cohort, we again adopted the CCI inference approach from Kumar et al. [103], where a CCI is denoted as ‘active’ if p-value ≤ 0.05 and ‘inactive’ otherwise. Third, for each pseudopatient, we predicted the chemotherapy response as the sum of the active CCIs, weighted by their directionalities (i.e., +1 or −1 when OR > 1 or < 1, FDR-adjusted p-value ≤ 0.05; and 0 otherwise), followed by a rescaling between 0 and 1 across all pesudopatients. We used these prediction scores to evaluate the predictive performance of these top CCIs learned from bulk on the SC-TNBC cohort through using a one-tailed Wilcoxon rank-sum test (FDR-adjusted p-value ≤ 0.05) and receiver operating characteristics (ROC) analysis.
Supplementary Material
ACKNOWLEDGEMENTS
This research is supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research. This work has utilized the computational resources of the NIH HPC Biowulf cluster (http://hpc.nih.gov). The results presented are in part based on the data generated by The Cancer Genome Atlas Research network (https://www.cancer.gov/tcga/). We would additionally like to acknowledge Dr. E. Michael Gertz and other members of the Cancer Data Science Laboratory for their help with data collection and constructive feedback on this work. Figure 1 has been created with http://biorender.com/.
Footnotes
COMPETING INTERESTS
E.R. is a co-founder of Medaware Ltd. (https://www.medaware.com/), Metabomed (https://www.metabomed.com/), and Pangea Biomed (https://pangeamedicine.com/). He has divested and serves as an unpaid scientific consultant to the latter company. The rest of the authors declare no conflicts of interest.
CODE AVAILABILITY
All relevant codes for reproducing the figures in this study will be made publicly available upon publication at https://github.com/ruppinlab/DECODEM/.
DATA AVAILABILITY
For TransNEO cohort, RNA-seq counts and clinical data were acquired from Sammut et al. (https://doi.org/10.1038/s41586-021-04278-5). RNA-seq counts were converted to TPM by using Ensembl gene annotations (GRCh37.87, http://ftp.ensembl.org/pub/grch37/release-87/gtf/homo_sapiens/). For ARTemis + PBCP cohort, clinical data were collected from Sammut et al. (https://doi.org/10.1038/s41586-021-04278-5) and RNA-seq TPM values were obtained directly from the authors. For BrighTNess cohort, RNA-seq FPKM values and clinical data were acquired from GEO (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE164458). The FPKM values were converted into TPM. For Zhang et al. cohort, scRNA-seq counts and clinical data were obtained from Zhang et al. (https://doi.org/10.1016/j.ccell.2021.09.010). For the single-cell signature for deconvolution, scRNA-seq counts and cell annotations were collected from Wu et al. (https://doi.org/10.1038/s41588-021-00911-1). The ligand-receptor list for LIRICS were obtained from Ramilowski et al. via GitHub (https://github.com/LewisLabUCSD/Ligand-Receptor-Pairs). The deconvolved expression and cell fraction data using CODEFACS on TCGA-BRCA patients were collected from Wang et al. (https://doi.org/10.1158%2F2159-8290.CD-21-0887). The corresponding TCGA-BRCA patients’ overall survival and progression-free interval (synonymous to progression-free survival) data were obtained from the UCSC Xena browser (https://xenabrowser.net). All data used in this study are either publicly available or available upon request to the original authors publishing the data. All deconvolved expression data generated in this study will be made publicly available upon publication.
REFERENCES
- [1].Tsimberidou A. M., Fountzilas E., Nikanjam M., & Kurzrock R. (2020). Review of precision cancer medicine: Evolution of the treatment paradigm. Cancer treatment reviews, 86, 102019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Huang K, Xiao C, Glass LM, Critchlow CM,. Machine learning applications for therapeutic tasks with genomics data. Patterns 2021; 2(10):100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fisher B., Brown A., Mamounas E., Wieand S., Robidoux A., Margolese R. G., … & Dimitrov N. V. (1997). Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. Journal of clinical oncology, 15(7), 2483–2493. [DOI] [PubMed] [Google Scholar]
- [4].Bear H. D., Anderson S., Brown A., Smith R., Mamounas E. P., Fisher B., ... & Wolmark N. (2003). The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. Journal of Clinical Oncology, 21(22), 4165–4174. [DOI] [PubMed] [Google Scholar]
- [5].Golshan M., Loibl S., Wong S. M., Huober J. B., O’Shaughnessy J., Rugo H. S., ... & Untch M. (2020). Breast conservation after neoadjuvant chemotherapy for triple-negative breast cancer: surgical results from the BrighTNess randomized clinical trial. JAMA surgery, 155(3), e195410–e195410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Urueña C., Lasso P., Bernal-Estevez D. et al. The breast cancer immune microenvironment is modified by neoadjuvant chemotherapy. Sci Rep 12, 7981 (2022). 10.1038/s41598-022-12108-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ali H. R., Dariush A., Provenzano E., Bardwell H., Abraham J. E., Iddawela M., ... & Caldas C. (2016). Computational pathology of pre-treatment biopsies identifies lymphocyte density as a predictor of response to neoadjuvant chemotherapy in breast cancer. Breast Cancer Research, 18(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ali H. R., Dariush A., Thomas J., Provenzano E., Dunn J., Hiller L., ... & Caldas C. (2017). Lymphocyte density determined by computational pathology validated as a predictor of response to neoadjuvant chemotherapy in breast cancer: secondary analysis of the ARTemis trial. Annals of Oncology, 28(8), 1832–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dieci M. V., Prat A., Tagliafico E., Paré L., Ficarra G., Bisagni G., ... & Guarneri V. (2016). Integrated evaluation of PAM50 subtypes and immune modulation of pCR in HER2-positive breast cancer patients treated with chemotherapy and HER2-targeted agents in the CherLOB trial. Annals of Oncology, 27(10), 1867–1873. [DOI] [PubMed] [Google Scholar]
- [10].Griguolo G., Serna G., Pascual T. et al. Immune microenvironment characterisation and dynamics during anti-HER2-based neoadjuvant treatment in HER2-positive breast cancer. npj Precis. Onc. 5, 23 (2021). HYPERLINK “https://doi.org/10.1038/s41698-021-00163-6. https://doi.org/10.1038/s41698-021-00163-63434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sammut SJ., Crispin-Ortuzar M., Chin Sf et al. Multi-omic machine learning predictor of breast cancer therapy response. Nature 601, 623–629 (2022). 10.1038/s41586-021-04278-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Klemm F, Joyce JA. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015. Apr;25(4):198–213. doi: 10.1016/j.tcb.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Son B, Lee S, Youn H, Kim E, Kim W, Youn B. The role of tumor microenvironment in therapeutic resistance. Oncotarget. 2017. Jan 17;8(3):3933–3945. doi: 10.18632/oncotarget.13907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wu P., Gao W., Su M., Nice E. C., Zhang W., Lin J., & Xie N. (2021). Adaptive mechanisms of tumor therapy resistance driven by tumor microenvironment. Frontiers in cell and developmental biology, 9, 641469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mehraj U., Dar A.H., Wani N.A. et al. Tumor microenvironment promotes breast cancer chemoresistance. Cancer Chemother Pharmacol 87, 147–158 (2021). 10.1007/s00280-020-04222-w [DOI] [PubMed] [Google Scholar]
- [16].Ruffell B., & Coussens L. M. (2015). Macrophages and therapeutic resistance in cancer. Cancer cell, 27(4), 462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Acharyya S., Oskarsson T., Vanharanta S., Malladi S., Kim J., Morris P. G., ... & Massagué J. (2012). A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell, 150(1), 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Li M., Quintana A., Alberts E., Hung M. S., Boulat V., Ripoll M. M., & Grigoriadis A. (2023). B Cells in Breast Cancer Pathology. Cancers, 15(5), 1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sakaguchi A., Horimoto Y., Onagi H. et al. Plasma cell infiltration and treatment effect in breast cancer patients treated with neoadjuvant chemotherapy. Breast Cancer Res 23, 99 (2021). 10.1186/s13058-021-01477-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yeong J., Lim J. C. T., Lee B., Li H., Chia N., Ong C. C. H., ... & Iqbal J. (2018). High densities of tumor-associated plasma cells predict improved prognosis in triple negative breast cancer. Frontiers in immunology, 9, 1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bhinder B., Gilvary C., Madhukar N. S., & Elemento O. (2021). Artificial intelligence in cancer research and precision medicine. Cancer Discovery, 11(4), 900–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Singla N., & Singla S. (2020). Harnessing big data with machine learning in precision oncology. Kidney cancer journal: official journal of the Kidney Cancer Association, 18(3), 83. [PMC free article] [PubMed] [Google Scholar]
- [23].Senft D., Leiserson M. D., Ruppin E., & Ze’ev A. R. (2017). Precision oncology: the road ahead. Trends in molecular medicine, 23(10), 874–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tsimberidou A. M., Fountzilas E., Bleris L., & Kurzrock R. (2020, September). Transcriptomics and solid tumors: The next frontier in precision cancer medicine. In Seminars in cancer biology. Academic Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Siravegna G., Marsoni S., Siena S., & Bardelli A. (2017). Integrating liquid biopsies into the management of cancer. Nature reviews Clinical oncology, 14(9), 531–548. [DOI] [PubMed] [Google Scholar]
- [26].Heitzer E., Haque I. S., Roberts C. E., & Speicher M. R. (2019). Current and future perspectives of liquid biopsies in genomics-driven oncology. Nature Reviews Genetics, 20(2), 71–88. [DOI] [PubMed] [Google Scholar]
- [27].Sawabata N. (2020). Circulating tumor cells: From the laboratory to the cancer clinic. Cancers, 12(10), 3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Beaubier N., Bontrager M., Huether R., Igartua C., Lau D., Tell R., ... & White K. P. (2019). Integrated genomic profiling expands clinical options for patients with cancer. Nature biotechnology, 37(11), 1351–1360. [DOI] [PubMed] [Google Scholar]
- [29].Hayashi H., Takiguchi Y., Minami H., Akiyoshi K., Segawa Y., Ueda H., ... & Nakagawa K. (2020). Site-specific and targeted therapy based on molecular profiling by next-generation sequencing for cancer of unknown primary site: a nonrandomized phase 2 clinical trial. JAMA oncology, 6(12), 1931–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Vaske O. M., Bjork I., Salama S. R., Beale H., Shah A. T., Sanders L., ... & Haussler D. (2019). Comparative tumor RNA sequencing analysis for difficult-to-treat pediatric and young adult patients with cancer. JAMA network open, 2(10), e1913968–e1913968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lee J. S., Nair N. U., Dinstag G., Chapman L., Chung Y., Wang K., ... & Ruppin E. (2021). Synthetic lethality-mediated precision oncology via the tumor transcriptome. Cell, 184(9), 2487–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sinha S., Dhruba S. R., Wu W., Kerr D. L., Stroganov O. V., Grishagin I., ... & Ruppin E. (2022). Predicting patient treatment response and resistance via single-cell transcriptomics of their tumors. bioRxiv. [Google Scholar]
- [33].Dinstag G., Shulman E. D., Elis E., Ben-Zvi D. S., Tirosh O., Maimon E., ... & Aharonov R. (2022). Clinically oriented prediction of patient response to targeted and immunotherapies from the tumor transcriptome. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fu Y., Jung A. W., Torne R. V., Gonzalez S., Vöhringer H., Shmatko A., ... & Gerstung M. (2020). Pan-cancer computational histopathology reveals mutations, tumor composition and prognosis. Nature Cancer, 1(8), 800–810. [DOI] [PubMed] [Google Scholar]
- [35].Noorbakhsh J., Farahmand S., Namburi S., Caruana D., Rimm D., Soltanieh-ha M., ... & Chuang J. H. (2020). Deep learning-based cross-classifications reveal conserved spatial behaviors within tumor histological images. Nature communications, 11(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yu K. H., Wang F., Berry G. J., Re C., Altman R. B., Snyder M., & Kohane I. S. (2020). Classifying non-small cell lung cancer types and transcriptomic subtypes using convolutional neural networks. Journal of the American Medical Informatics Association, 27(5), 757–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Couture H. D., Williams L. A., Geradts J., Nyante S. J., Butler E. N., Marron J. S., ... & Niethammer M. (2018). Image analysis with deep learning to predict breast cancer grade, ER status, histologic subtype, and intrinsic subtype. NPJ breast cancer, 4(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Qu H., Zhou M., Yan Z., Wang H., Rustgi V. K., Zhang S., ... & Metaxas D. N. (2021). Genetic mutation and biological pathway prediction based on whole slide images in breast carcinoma using deep learning. NPJ precision oncology, 5(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hoang D. T., Dinstag G., Hermida L. C., Ben-Zvi D. S., Elis E., Caley K., ... & Ruppin E. (2022). Synthetic lethality-based prediction of cancer treatment response from histopathology images. bioRxiv. [Google Scholar]
- [40].Tanioka M., Fan C., Parker J. S., Hoadley K. A., Hu Z., Li Y., ... & Perou C. M. (2018). Integrated Analysis of RNA and DNA from the Phase III Trial CALGB 40601 Identifies Predictors of Response to Trastuzumab-Based Neoadjuvant Chemotherapy in HER2-Positive Breast CancerIntegrated Response Models in HER2-Positive Breast Cancer. Clinical Cancer Research, 24(21), 5292–5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wong M., Mayoh C., Lau L., Khuong-Quang D. A., Pinese M., Kumar A., ... & Cowley M. J. (2020). Whole genome, transcriptome and methylome profiling enhances actionable target discovery in high-risk pediatric cancer. Nature medicine, 26(11), 1742–1753. [DOI] [PubMed] [Google Scholar]
- [42].Rodon J., Soria J. C., Berger R., Miller W. H., Rubin E., Kugel A., ... & Kurzrock R. (2019). Genomic and transcriptomic profiling expands precision cancer medicine: the WINTHER trial. Nature medicine, 25(5), 751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kester L., Seinstra D., van Rossum A. G., Vennin C., Hoogstraat M., van der Velden D., ... & van Rheenen J. (2022). Differential Survival and Therapy Benefit of Patients with Breast Cancer Are Characterized by Distinct Epithelial and Immune Cell Microenvironments Tumor Cellular Composition Predicts Benefit to Therapies. Clinical Cancer Research, OF1–OF12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wang K., Patkar S., Lee J.S., Gertz E.M., Robinson W., Schischlik F., Crawford D.R., Schäffer A.A. and Ruppin E. (2022). Deconvolving Clinically Relevant Cellular Immune Cross-talk from Bulk Gene Expression Using CODEFACS and LIRICS Stratifies Patients with Melanoma to Anti–PD-1 Therapy. Cancer discovery, 12(4), 1088–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wu S. Z., Al-Eryani G., Roden D. L., Junankar S., Harvey K., Andersson A., ... & Swarbrick A. (2021). A single-cell and spatially resolved atlas of human breast cancers. Nature genetics, 53(9), 1334–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Earl H. M., Hiller L., Dunn J. A., Blenkinsop C., Grybowicz L., Vallier A. L., ... & Hayward L. (2015). Efficacy of neoadjuvant bevacizumab added to docetaxel followed by fluorouracil, epirubicin, and cyclophosphamide, for women with HER2-negative early breast cancer (ARTemis): an open-label, randomised, phase 3 trial. The lancet oncology, 16(6), 656–666. [DOI] [PubMed] [Google Scholar]
- [47].Loibl S., O’Shaughnessy J., Untch M., Sikov W. M., Rugo H. S., McKee M. D., ... & Geyer C. E Jr. (2018). Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. The Lancet Oncology, 19(4), 497–509. [DOI] [PubMed] [Google Scholar]
- [48].Zhang Y., Chen H., Mo H., Hu X., Gao R., Zhao Y., ... & Liu Z. (2021). Single-cell analyses reveal key immune cell subsets associated with response to PD-L1 blockade in triple-negative breast cancer. Cancer Cell, 39(12), 1578–1593. [DOI] [PubMed] [Google Scholar]
- [49].Korde L. A., Somerfield M. R., Carey L. A., Crews J. R., Denduluri N., Hwang E. S., ... & Hershman D. L. (2021). Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. Journal of Clinical Oncology, 39(13), 1485–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ramilowski J., Goldberg T., Harshbarger J. et al. A draft network of ligand–receptor-mediated multicellular signalling in human. Nat Commun 6, 7866 (2015). 10.1038/ncomms8866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Green D. R., Ferguson T., Zitvogel L., & Kroemer G. (2009). Immunogenic and tolerogenic cell death. Nature Reviews Immunology, 9(5), 353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Fabregat A. et al. The Reactome Pathway Knowledgebase. Nucleic Acids Res. 46, D649–D655 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Molinari M. (2000). Cell cycle checkpoints and their inactivation in human cancer. Cell proliferation, 33(5), 261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bower J.J., Vance L.D., Psioda M. et al. Patterns of cell cycle checkpoint deregulation associated with intrinsic molecular subtypes of human breast cancer cells. npj Breast Cancer 3, 9 (2017). 10.1038/s41523-017-0009-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zhou K., Sun Y., Dong D. et al. EMP3 negatively modulates breast cancer cell DNA replication, DNA damage repair, and stem-like properties. Cell Death Dis 12, 844 (2021). 10.1038/s41419-021-04140-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Repo H., Löyttyniemi E., Kurki S. et al. A prognostic model based on cell-cycle control predicts outcome of breast cancer patients. BMC Cancer 20, 558 (2020). 10.1186/s12885-020-07045-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Vihervuori H., Korpinen K., Autere T. A., Repo H., Talvinen K., & Kronqvist P. (2022). Varying outcomes of triple-negative breast cancer in different age groups–prognostic value of clinical features and proliferation. Breast Cancer Research and Treatment, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Thu K. L., Soria-Bretones I., Mak T. W., & Cescon D. W. (2018). Targeting the cell cycle in breast cancer: towards the next phase. Cell Cycle, 17(15), 1871–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zhang J., Chan D. W., & Lin S. Y. (2022). Exploiting DNA Replication Stress as a Therapeutic Strategy for Breast Cancer. Biomedicines, 10(11), 2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhu X., & Zhou W. (2015). The emerging regulation of VEGFR-2 in triple-negative breast cancer. Frontiers in Endocrinology, 6, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ceci C., Atzori M. G., Lacal P. M., & Graziani G. (2020). Role of VEGFs/VEGFR-1 signaling and its inhibition in modulating tumor invasion: Experimental evidence in different metastatic cancer models. International journal of molecular sciences, 21(4), 1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Zhang Q., Lu S., Li T., Yu L., Zhang Y., Zeng H., ... & Lin Y. (2019). ACE2 inhibits breast cancer angiogenesis via suppressing the VEGFa/VEGFR2/ERK pathway. Journal of Experimental & Clinical Cancer Research, 38(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Edwards A., & Brennan K. (2021). Notch signalling in breast development and cancer. Frontiers in Cell and Developmental Biology, 9, 692173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zhou B., Lin W., Long Y. et al. Notch signaling pathway: architecture, disease, and therapeutics. Sig Transduct Target Ther 7, 95 (2022). 10.1038/s41392-022-00934-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Miao K., Lei J.H., Valecha M.V. et al. NOTCH1 activation compensates BRCA1 deficiency and promotes triple-negative breast cancer formation. Nat Commun 11, 3256 (2020). 10.1038/s41467-020-16936-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].BeLow M., & Osipo C. (2020). Notch signaling in breast cancer: a role in drug resistance. Cells, 9(10), 2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Li L., Wang X., Hu K. et al. ZNF133 is a potent suppressor in breast carcinogenesis through dampening L1CAM, a driver for tumor progression. Oncogene (2023). 10.1038/s41388-023-02731-5 [DOI] [PubMed] [Google Scholar]
- [68].van der Maten M., Reijnen C., Pijnenborg J. M., & Zegers M. M. (2019). L1 cell adhesion molecule in cancer, a systematic review on domain-specific functions. International journal of molecular sciences, 20(17), 4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Zhang J., Yang F., Ding Y., Zhen L., Han X., Jiao F., & Tang J. (2015). Overexpression of L1 cell adhesion molecule correlates with aggressive tumor progression of patients with breast cancer and promotes motility of breast cancer cells. International journal of clinical and experimental pathology, 8(8), 9240. [PMC free article] [PubMed] [Google Scholar]
- [70].Bandola-Simon J., & Roche P. A. (2019). Dysfunction of antigen processing and presentation by dendritic cells in cancer. Molecular immunology, 113, 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Dhatchinamoorthy K., Colbert J. D., & Rock K. L. (2021). Cancer immune evasion through loss of MHC class I antigen presentation. Frontiers in immunology, 12, 636568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].McDonnell A. M., Lesterhuis W. J., Khong A., Nowak A. K., Lake R. A., Currie A. J., & Robinson B. W. (2015). Tumor-infiltrating dendritic cells exhibit defective cross-presentation of tumor antigens, but is reversed by chemotherapy. European journal of immunology, 45(1), 49–59. [DOI] [PubMed] [Google Scholar]
- [73].Galluzzi L., Humeau J., Buqué A., Zitvogel L., & Kroemer G. (2020). Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nature reviews Clinical oncology, 17(12), 725–741. [DOI] [PubMed] [Google Scholar]
- [74].Li J., Li C., Liu X. et al. GDF9 concentration in embryo culture medium is linked to human embryo quality and viability. J Assist Reprod Genet 39, 117–125 (2022). 10.1007/s10815-021-02368-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Stocker W. A., Walton K. L., Richani D., Chan K. L., Beilby K. H., Finger B. J., ... & Harrison C. A. (2020). A variant of human growth differentiation factor-9 that improves oocyte developmental competence. Journal of Biological Chemistry, 295(23), 7981–7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Gomez-Puerto M. C., Iyengar P. V., García de Vinuesa A., Ten Dijke P., & Sanchez-Duffhues G. (2019). Bone morphogenetic protein receptor signal transduction in human disease. The journal of pathology, 247(1), 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Feary E. S., Juengel J. L., Smith P., French M. C., O’Connell A. R., Lawrence S. B., ... & McNatty K. P. (2007). Patterns of expression of messenger RNAs encoding GDF9, BMP15, TGFBR1, BMPR1B, and BMPR2 during follicular development and characterization of ovarian follicular populations in ewes carrying the Woodlands FecX2W mutation. Biology of reproduction, 77(6), 990–998. [DOI] [PubMed] [Google Scholar]
- [78].Hanavadi S., Martin T. A., Watkins G., Mansel R. E., & Jiang W. G. (2007). The role of growth differentiation factor-9 (GDF-9) and its analog, GDF-9b/BMP-15, in human breast cancer. Annals of surgical oncology, 14, 2159–2166. [DOI] [PubMed] [Google Scholar]
- [79].Harrath A. H., Jalouli M., Oueslati M. H., Farah M. A., Feriani A., Aldahmash W., ... & Alwasel S. (2021). The flavonoid, kaempferol-3-O-apiofuranosyl-7-O-rhamnopyranosyl, as a potential therapeutic agent for breast cancer with a promoting effect on ovarian function. Phytotherapy Research, 35(11), 6170–6180. [DOI] [PubMed] [Google Scholar]
- [80].Bach D. H., Park H. J., & Lee S. K. (2018). The dual role of bone morphogenetic proteins in cancer. Molecular Therapy-Oncolytics, 8, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Bokobza S. M., Ye L., Kynaston H. E., Mansel R. E., & Jiang W. G. (2009). Reduced expression of BMPR-IB correlates with poor prognosis and increased proliferation of breast cancer cells. Cancer genomics & proteomics, 6(2), 101–108. [PubMed] [Google Scholar]
- [82].Zheng Y., Jiang X., Wang M., Yang S., Deng Y., Li Y., ... & Chen L. (2022). BMPR1B Polymorphisms (rs1434536 and rs1970801) are Associated With Breast Cancer Susceptibility in Northwest Chinese Han Females: A Case-Control Study. Clinical Breast Cancer, 22(5), e641–e646. [DOI] [PubMed] [Google Scholar]
- [83].Dai K., Qin F., Zhang H., Liu X., Guo C., Zhang M., ... & Ma Y. (2016). Low expression of BMPRIB indicates poor prognosis of breast cancer and is insensitive to taxane-anthracycline chemotherapy. Oncotarget, 7(4), 4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Jing S., Wen D., Yu Y., Holst P. L., Luo Y., Fang M., ... & Fox G. M. (1996). GDNF–induced activation of the ret protein tyrosine kinase is mediated by GDNFR-α, a novel receptor for GDNF. Cell, 85(7), 1113–1124. [DOI] [PubMed] [Google Scholar]
- [85].Fielder G. C., Yang T. W. S., Razdan M., Li Y., Lu J., Perry J. K., ... & Liu D. X. (2018). The GDNF family: a role in cancer?. Neoplasia, 20(1), 99–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Li Q., Cao Z., & Zhao S. (2022). The Emerging Portrait of Glial Cell Line-derived Neurotrophic Factor Family Receptor Alpha (GFRα) in Cancers. International Journal of Medical Sciences, 19(4), 659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Pecar G., Liu S., Hooda J. et al. RET signaling in breast cancer therapeutic resistance and metastasis. Breast Cancer Res 25, 26 (2023). 10.1186/s13058-023-01622-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Horibata S., Rice E. J., Mukai C., Marks B. A., Sams K., Zheng H., ... & Danko C. G. (2018). ER-positive breast cancer cells are poised for RET-mediated endocrine resistance. PLoS One, 13(4), e0194023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Bhakta S., Crocker L. M., Chen Y., Hazen M., Schutten M. M., Li D., ... & Junutula J. R. (2018). An Anti-GDNF Family Receptor Alpha 1 (GFRA1) Antibody–Drug Conjugate for the Treatment of Hormone Receptor–Positive Breast Cancer Targeting Hormone-Positive Breast Cancer with a GFRA1 ADC. Molecular cancer therapeutics, 17(3), 638–649. [DOI] [PubMed] [Google Scholar]
- [90].Kim M., & Kim D. J. (2018). GFRA1: a novel molecular target for the prevention of osteosarcoma chemoresistance. International journal of molecular sciences, 19(4), 1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Liu H., Yang Z., Lu W., Chen Z., Chen L., Han S., ... & Cai Y. (2020). Chemokines and chemokine receptors: A new strategy for breast cancer therapy. Cancer medicine, 9(11), 3786–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Liang Y., Yi L., Liu P., Jiang L., Wang H., Hu A., ... & Dong J. (2018). CX3CL1 involves in breast cancer metastasizing to the spine via the Src/FAK signaling pathway. Journal of Cancer, 9(19), 3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Tardáguila M., Mira E., García-Cabezas M. A., Feijoo A. M., Quintela-Fandino M., Azcoitia I., ... & Manes S. (2013). CX3CL1 Promotes Breast Cancer via Transactivation of the EGF PathwayCX3CL1 Promotes Breast Cancer. Cancer research, 73(14), 4461–4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Jamieson-Gladney W.L., Zhang Y., Fong A.M. et al. The chemokine receptor CX3CR1 is directly involved in the arrest of breast cancer cells to the skeleton. Breast Cancer Res 13, R91 (2011). 10.1186/bcr3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Rivas-Fuentes S., Salgado-Aguayo A., Arratia-Quijada J., & Gorocica-Rosete P. (2021). Regulation and biological functions of the CX3CL1-CX3CR1 axis and its relevance in solid cancer: A mini-review. Journal of Cancer, 12(2), 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Yue Y., Zhang Q., & Sun Z. (2022). CX3CR1 acts as a protective biomarker in the tumor microenvironment of colorectal Cancer. Frontiers in Immunology, 12, 5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ishida Y., Kuninaka Y., Yamamoto Y., Nosaka M., Kimura A., Furukawa F., ... & Kondo T. (2020). Pivotal involvement of the CX3CL1-CX3CR1 axis for the recruitment of M2 tumor-associated macrophages in skin carcinogenesis. Journal of Investigative Dermatology, 140(10), 1951–1961. [DOI] [PubMed] [Google Scholar]
- [98].Breiman L. (2001). Random forests. Machine learning, 45, 5–32. [Google Scholar]
- [99].Michaud M., Martins I., Sukkurwala A. Q., Adjemian S., Ma Y., Pellegatti P., ... & Kroemer G. (2011). Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science, 334(6062), 1573–1577. [DOI] [PubMed] [Google Scholar]
- [100].Szklarczyk D., Gable A. L., Lyon D., Junge A., Wyder S., Huerta-Cepas J., ... & Mering C. V. (2019). STRING v11: protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic acids research, 47(D1), D607–D613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Newman A. M., Steen C. B., Liu C. L., Gentles A. J., Chaudhuri A. A., Scherer F., ... & Alizadeh A. A. (2019). Determining cell type abundance and expression from bulk tissues with digital cytometry. Nature biotechnology, 37(7), 773–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Han Y., Wang Y., Dong X., Sun D., Liu Z., Yue J., ... & Wang C. (2023). TISCH2: expanded datasets and new tools for single-cell transcriptome analyses of the tumor microenvironment. Nucleic acids research, 51(D1), D1425–D1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Kumar MP, Du J, Lagoudas G, Jiao Y, Sawyer A, Drummond DC, et al. Analysis of Single-Cell RNA-Seq Identifies Cell-Cell Communication Associated with Tumor Characteristics. Cell Rep [Internet]. 2018;25(6):1458–1468.e4. Available from: https://www.sciencedirect.com/science/article/pii/S221112471831636X [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Leon-Ferre R. A., Jonas S. F., Salgado R., Loi S., De Jong V., Carter J. M., ... & International Immuno-Oncology Biomarker Working Group. (2024). Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer. JAMA, 331(13), 1135–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Ali H. R., Chlon L., Pharoah P. D., Markowetz F., & Caldas C. (2016). Patterns of immune infiltration in breast cancer and their clinical implications: a gene-expression-based retrospective study. PLoS medicine, 13(12), e1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Chowdhury A., Pharoah P. D., & Rueda O. M. (2023). Evaluation and comparison of different breast cancer prognosis scores based on gene expression data. Breast Cancer Research, 25(1), 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For TransNEO cohort, RNA-seq counts and clinical data were acquired from Sammut et al. (https://doi.org/10.1038/s41586-021-04278-5). RNA-seq counts were converted to TPM by using Ensembl gene annotations (GRCh37.87, http://ftp.ensembl.org/pub/grch37/release-87/gtf/homo_sapiens/). For ARTemis + PBCP cohort, clinical data were collected from Sammut et al. (https://doi.org/10.1038/s41586-021-04278-5) and RNA-seq TPM values were obtained directly from the authors. For BrighTNess cohort, RNA-seq FPKM values and clinical data were acquired from GEO (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE164458). The FPKM values were converted into TPM. For Zhang et al. cohort, scRNA-seq counts and clinical data were obtained from Zhang et al. (https://doi.org/10.1016/j.ccell.2021.09.010). For the single-cell signature for deconvolution, scRNA-seq counts and cell annotations were collected from Wu et al. (https://doi.org/10.1038/s41588-021-00911-1). The ligand-receptor list for LIRICS were obtained from Ramilowski et al. via GitHub (https://github.com/LewisLabUCSD/Ligand-Receptor-Pairs). The deconvolved expression and cell fraction data using CODEFACS on TCGA-BRCA patients were collected from Wang et al. (https://doi.org/10.1158%2F2159-8290.CD-21-0887). The corresponding TCGA-BRCA patients’ overall survival and progression-free interval (synonymous to progression-free survival) data were obtained from the UCSC Xena browser (https://xenabrowser.net). All data used in this study are either publicly available or available upon request to the original authors publishing the data. All deconvolved expression data generated in this study will be made publicly available upon publication.