Abstract
Background
Malaria is more prevalent in rural areas due to fewer mosquito breeding habitats in urban settings. However, urban factors such as irrigated farming, open sewers, and discarded containers create mosquito breeding sites. This study investigates the diversity and distribution of larval habitats and the impact of physicochemical characteristics on the presence and density of Anopheles gambiae s.l. larvae in Accra, Ghana.
Methods
Larval surveys and collections were conducted at fifteen locations in Accra, divided into five categories: Irrigated Urban Farming (IUF), Lower Socioeconomic Status (LS), Middle Socioeconomic Status (MS), High Socioeconomic Status (HS), and Peri-urban (PU) areas. Physicochemical parameters were measured, and species identification was performed using morphological and molecular methods.
Results
A total of 727 breeding habitats were identified, with 65.34% (475/727) positive for Anopheles larvae. Drainage ditches were the most common habitat type (48.21%, 229/475). The highest abundance of An. gambiae s.l. was found in IUF sites (6,244/22,919), especially during the rainy season (77.01%, 17,650/22,919; R2 = 3.46, P = 0.000). Polluted habitats, including household effluents, had higher ammonium levels (3.4 mg/L NH -N) compared to unpolluted ones (1.3 mg/L NH -N). Other distinguishing parameters included dissolved oxygen (34% vs 52.9%), conductivity (5106 μS/cm vs 2049 μS/cm), and total dissolved solids (3181 mg/L vs 1255 mg/L). The predominant malaria vector was An. coluzzii(54.4%, 368/677). Additionally, the invasive An. stephensi, previously unreported in Ghana, was detected.
Conclusion
Malaria vectors breed in diverse and often polluted urban habitats, with high larval densities in urban agricultural areas. The detection of the invasive An. Stephensi highlights the need for continuous monitoring and vector control strategies in urban settings.
Keywords: Anopheles, larvae, urban, habitats, An. stephensi, Ghana, Irrigation, physicochemical, polluted
Background
Malaria, traditionally considered a rural disease, is less prevalent in urban areas primarily due to the scarcity of preferred breeding habitats for malaria vectors. However, certain urban factors, such as irrigated farming, create year-round breeding sites for mosquitoes (1)(2). Urban agriculture, a crucial socioeconomic activity that help to provide income and food security for urban residents(3)(4), but inadvertently supports mosquito breeding, thus posing a significant public health challenge.
Moreover, the existence of broken and open sewers, as well as discarded containers such as tin, can, plastic bowls, buckets, car tyres and other water impoundments, potentially serve as breeding habitats for mosquitoes in urban settings. Construction of roads, residential and other structures as a result of unplanned urbanization has also been implicated to contribute to the creation of breeding habitats (5).
In Africa, urbanization is increasing at an alarming rate (6). The African urban population is envisaged to escalate by 50% and 60% by the year 2030 and 2050 respectively (7). In Ghana, the high rate of infrastructural development is a reflection of what is observed in Africa, with the city of Accra recording the highest urbanization rate among other cities in the country (8). This rapid unplanned urbanization could have major implications on the transmission of malaria within the city as the dynamics of urbanization have been shown to be strongly associated with malaria vector abundance (9).
However, there is paucity of data on urban malaria transmission in the city of Accra, especially on the vectors involved (1)(10). The few studies conducted hitherto did not provide data on larval habitat types, and distribution and abundance of larval malaria vectors in the different urban areas of Accra(11)(12). This study, therefore, aims to investigate the larval habitat diversity and distribution, and the effect of their physicochemical characteristics on the presence and density of Anopheles gambiae s.l. larvae in Accra, Ghana.
Methodology
Study sites
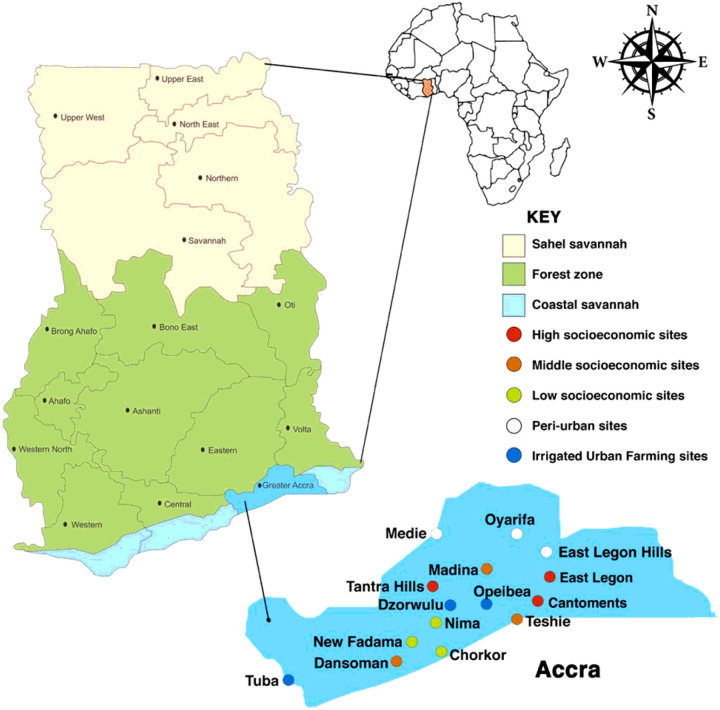
The study was undertaken in fifteen (15) sites within the city of Accra, the capital of Ghana, and peri-urban areas. Three (3) study sites were randomly selected in Accra, from areas that were categorized as low socio-economic (LS), Middle socioeconomic (MS), High socioeconomic (HS) and, Irrigated urban farming (IUF) areas. In comparison three (3) independent sites were also selected from the peri-urban areas surrounding Accra (Fig. 1). It is hypothesized that there are more mosquitoes in peri-urban and rural areas than urban areas.
Figure 1.
Map of Ghana and Accra showing location of the study sites.
In the irrigated urban farming categories, the sites included were Opeibea (5°35’52.8”N 0°10’48.2”W), Dzworwulu (5°36’53”N 0°12’03”W), and Tuba (5° 30’47”N 0° 23’ 16”W). Larval habitats are created all year round in these sites due to regular irrigation. Moreso, different agricultural chemicals that could contribute to insecticide resistance in mosquitoes are used by the farmers in these farms. Sites for the lower Socioeconomic (LS) consisted of Nima (5° 35’ 0” N, 0° 12’ 0” W), Chorkor (5°31’39”N 0°13’55”W), and New Fadama (5°35’54.1”N 0°14’52.2”W). These are slums, where the inhabitants reside in outmoded and overcrowded structures located in poorly demarcated plots of land. These areas have no well-designed drainage systems, with very poor sanitation systems. Madina (5°41’0”N 0°10’0”W), Dansoman (5° 33’ 0” N, 0° 16’ 0” W), and Teshie (5° 35’ 0” N, 0° 6’ 0” W) were selected as the Middle Socioeconomic (MS) sites. This category represents areas that have standard housing structures built on well-demarcated plots of land, with majority having access to either tarred or untarred road network. Areas within this category also have well-designed drainage and sanitation systems but poorly managed. East Legon (5°38’16.39”N 0°9’40.33”W), Cantonment (5° 35’ 10” N, 0° 10’ 35” W) and Tantra Hill (5° 34’ 44.0148”N 0°13’ 46.812”W) categorized as High Socioeconomic (HS) status sites have housing structures that are of the highest quality in Ghana. These sites have a well-planned environment with best-managed drainage and sanitation systems in the country. They have very good road network with huge sections of the roads tarred, hence have few pot-holes to serve as breeding habitats. The peri-urban areas consisted of Oyarifa (5° 46’ 14” N, 0° 10’ 50” W), Medie (5° 45’ 43”N 0° 19’ 20”W), and East Legon Hills (5° 41’ 28.5468”N 0° 6’ 0.018”W). These are areas just outside the city with many new settlements. Most of the roads and buildings are under construction with higher vegetation cover. During the rainy season numerous breeding habitats are created due to the nature of the land in these areas. The differences in landscape, drainage systems, sanitation, and land use (agriculture/non-agriculture) will help to compare and understand their impact on urban malaria transmission and vector resistance status to insecticides.
Accra which lies within the Greater Accra region, had a total population of about 2,557,000 in 2021, a population growth rate of 24.13% from 2010 to 2021 and a land area of 225.67 km2 (Ghana Population and Housing Census, 2021). The region has two rainy seasons with average annual rainfall and temperature of 730 mm and 27.6 °C respectively. The relative humidity is mostly high, ranging from 65% during the midafternoon to 95% at night. The rainfall pattern coupled with the poor drainage system supports the formation of stagnant waters, whereas the temperature and humidity create a favourable environment for mosquitoes.
Larval habitat characterization, abundance and measurement of densities
All larval habitats encountered were classified into natural or artificial. Natural habitats included natural ponds, swamps, and streams, while drainage ditches, hoof prints, footprints, car tyres, wells and furrows were categorized as man-made habitats. Land-use type was also classified based on the activities taking place on the land where larval habitat is located and natural vegetation. These included compounds with human settlements, farmland for cultivation sites, and roads and swamps. The habitat length and width were measured and recorded in metres. The vegetation cover was visually estimated as the percentage of vegetation covering the water surface. This was recorded as zero (0) where vegetation was not found on the surface of the habitat, ≤ 24%, 25–49%, 50–74%, and 75–100% surface coverage(13)(14).
Larvae were collected from all fifteen study sites in 2022 during the dry (from February to March) and rainy (from June to July) seasons. The WHO standard dipping method using the 350ml standard dipper was used to sample larvae from all potential larval habitats. Habitat sizes were categorized into ≤ 1, >1, > 2–5, >5–10, > 10–100, or > 100m and a maximum dip of 2, 4, 6, 10, 50, and 150 were taken respectively (depending on habitat size). For habitats with much smaller sizes such as footprints and hoofprints, a ladle was used to collect the samples. Anopheles larval instar stages collected were classified as (L1-L2) and (L3-L4), and the total number was estimated. Larvae and pupae collected were recorded, and larval density was calculated as the ratio of the number of larvae collected per number of dips taken(15)(2). The geographical coordinates of each larval habitat were recorded using a GPS device (Garmin eTrex 10 Worldwide Handheld GPS Navigator). Sampling usually started early in the morning (between 6:00 and 10:00 hours GMT) and early in the evening (between 15:30 and 18:00 hours GMT) to prevent distraction from the reflection of the sun on the water’s surface. Whenever there was heavy rain, larval sampling was halted and continued after three days.
Measurement of physical and chemical parameters of larval habitats
The physicochemical characteristics of the larval habitat were also measured using the PC60 Premium Multi-Parameter Tester (Apera Instruments, LLC). The recorded parameters included pH, temperature, salinity, specific conductivity (SPC), and total dissolved solids (TDS).
Additionally, some larval habitats, identified as either polluted or unpolluted (relatively clean visually) during larval collection, were selected for further physicochemical measurement. These habitats were located in Nima, Chorkor, Teshie and Madina. The YSI Pro Quatro Multiparameter Meter (YSI Incorporated, USA) was employed to measure temperature, pH, dissolved oxygen (DO), specific conductivity (SPC), salinity, total dissolved solids (TDS), volume/bulk resistivity (RES), ammonia (NH3-N), and ammonium (NH4-N) levels in these habitats.
Mosquito species identification
Samples (larvae and pupae) collected from the field were put into plastic containers and transported to the insectary of the Department of Medical Microbiology, University of Ghana, and raised into adults. Pupae were picked into pupal cups using Pasteur pipette and placed into mosquito cages daily. Larvae were fed with Tetramin® fish meal and emerged adults immediately fed on a 10% sugar solution soaked in a clean cotton wool ball. Adult mosquitoes were kept at a temperature and relative humidity of 27 ± 2°C and 72 ± 5% respectively. Emerged adults were aspirated into paper cups covered with muslin nets, and killed/knockdown using chloroformed. Mosquitoes were then identified morphologically under a stereomicroscope (Olympus, SZ60, Japan) using the keys developed by Maureen Coetzee(16). Sibling species of Anopheles gambiae s.l. were identified using rDNA polymerase chain reaction (PCR) (17). An. gambiae s.s. and An. coluzzii were further identified using PCR-restriction fragment length polymorphism (RFLP)(18).
Data analysis
Descriptive analyses were done to compare the abundance of the various larval breeding habitat types and larval densities in the different study sites and seasons. This was presented in the form of tables and graphs. The larval densities were calculated as the total number of larvae per dip. The total number of dips for smaller habitats such as foot and hoofprints were assumed as one dip. The density of Anopheles mosquito larvae was compared among the various breeding habitats and study site categories. The Mann-Whitney U test and the Kruskal-Wallis test were used to test the associations between continuous and categorical variables. The chi-square and Fisher’s exact tests were used to test the association between two categorical variables. Logistic regression using the glmer (from the lme4 package) was used to test the association between the habitat characteristics with categorical data and the presence of Anopheles larvae. The forward-backward stepwise method was used to select the best model based on Alkalie Information Criterion (AIC). Nested generalized linear mixed models using AD model builder with study sites nested within site class using the glmmADMB package was used to model the effect of habitat characteristics on larval densities. Correlations between physicochemical variables and larval densities were assessed using Pearson’s correlation analysis and principal component analysis (PCA). All statistical analyses were done in R 4.2.2 via RStudio (2022.12.0 + 353).
Results
Larval habitat type, distribution and abundance
A total of 727 breeding habitats were found in all the fifteen study sites, of which [63.41%, n = 461/727] were positive for Anopheles larvae (Table 2). Out of the total habitats that were positive for Anopheles larvae, [27.33%, n = 126/461] were found in the dry season and [72.67%, n = 335/461] in the rainy season (Table 2). Breeding habitats such as car tires, hooves prints and puddles were only found during the rainy season (Table 1). Overall, the most abundant larval habitat types that were encountered during the survey include drainage ditches [49.38%, n = 359/727], followed by tire tracks [14.17%, n = 103/727], swamps [11.55%, n = 84/727], furrows [6.74%, n = 49/727], artificial pond [5.64%, n = 41/727], foot imprint [4.26%, n = 31/727], puddles [2.89%, n = 21/727], well [2.48%, n = 18/727], natural ponds [1.38%, n = 10/727], car tires [0.69%, n = 5/727], hoofprints [0.55%, n = 4/727], and then pits [0.03%, n = 2/727], (Table 1).
Table 7.
Distribution of larval Anopheles species in study sites
| Categories | Study Site | An. coluzzii | An. gambiae s.s. | Hybrid | An. stephensi | Total |
|---|---|---|---|---|---|---|
| HS | Cantonment | 4 | 7 | 0 | 0 | 11 |
| East Legon | 10 | 38 | 2 | 0 | 50 | |
| Tantra Hills | 36 | 14 | 0 | 0 | 50 | |
| MS | Madina | 18 | 31 | 1 | 0 | 50 |
| Dansoman | 46 | 2 | 1 | 1 | 50 | |
| Teshie | 38 | 12 | 0 | 0 | 50 | |
| LS | Chorkor | 49 | 1 | 0 | 0 | 50 |
| Nima | 36 | 13 | 1 | 1 | 51 | |
| New Fadama | 42 | 8 | 0 | 0 | 50 | |
| IUF | Tuba | 36 | 9 | 5 | 1 | 51 |
| Dzorwulu | 11 | 5 | 0 | 0 | 16 | |
| Opeibea | 16 | 34 | 0 | 0 | 50 | |
| PU | E. Legon Hills | 15 | 28 | 7 | 0 | 50 |
| Medea | 4 | 46 | 0 | 0 | 50 | |
| Oyarifa | 11 | 37 | 2 | 0 | 50 | |
| Total | 372 | 285 | 19 | 3 | 679 |
HS = High socioeconomic, IUF = Irrigated urban farming, LS = Low socioeconomic, MS = Middle socioeconomic and PU = Peri-urban.
Table 2.
Mosquito larval abundance and distribution in all site categories
| Site Class | An. gambiae s.I. N (%) | Culex | Total | |
|---|---|---|---|---|
| Dry Season | Wet Season | |||
| High Socio-economic (HS) | 550 (10.44) | 3547 (20.10) | 1436 (18.8) | 5533 (18.11) |
| Irrigated Urban Agriculture (IUF) | 1835 (34.83) | 4409 (24.98) | 2151 (28.2) | 8395 (27.48) |
| Lower Socio-economic (LS) | 922 (17.50) | 3583 (20.30) | 1338 (17.5) | 5843 (19.12) |
| Middle Socio-economic (MS) | 1056 (20.04) | 3501 (19.84) | 1413 (18.5) | 5970 (19.54) |
| Peri-urban (PU) | 906 (17.01) | 2610 (14.79) | 1295 (16.9) | 4811 (15.75) |
| Total | 5269 (100) | 17650 (100) | 7633 (100) | 30552 (100) |
Table 1.
Larval habitat types and the presence of An. qambiae s.l during the dry and wet seasons
| Habitat type | Breeding habitats N (%) | Habitats with mosquito larvae present N (%) | Habitats with Anopheles species present N (%) | |||
|---|---|---|---|---|---|---|
| Dry | Wet | Dry | Wet | Dry | Wet | |
| Artificial pond | 25 (9.51) | 16 | 20 | 11 | 17 | 9 |
| Car tyre | 0 | 5 | 0 | 5 | 0 | 4 |
| Drainage ditch | 168 | 191 | 99 | 139 | 84 | 134 |
| Footprint | 1 | 30 | 1 | 25 | 1 | 24 |
| Furrow | 35 | 14 | 19 | 12 | 8 | 12 |
| Hoofprint | 0 | 4 | 0 | 3 | 0 | 3 |
| Natural pond | 7 | 3 | 7 | 2 | 4 | 2 |
| Pit | 2 | 0 | 2 | 0 | 2 | 0 |
| Puddle | 0 | 21 | 0 | 11 | 0 | 11 |
| Swamp | 9 | 75 | 8 | 54 | 5 | 52 |
| Tyre track | 4 | 99 | 3 | 79 | 3 | 78 |
| Well | 12 | 6 | 3 | 6 | 2 | 6 |
| Total | 263 | 464 | 162 | 347 | 126 | 335 |
A higher proportion of larval breeding sites were identified in the irrigated urban farming (IUF) site category (24.76%, 180/727), followed by middle socioeconomic (MS) 158 (21.73%), peri-urban (PU) 155 (21.32%), high socioeconomic (HS) 153 (21.05%), whereas the lowest socioeconomic (LS) category recorded the lowest (9.22%, 67/727) proportion of breeding sites. Furrows, natural and artificial ponds were only found in IUF sites. Drainage ditches were the most abundant habitat type encountered and was significantly associated with site category (χ2 = 228.13, df = 32, P< 0.001).
Seasonal distribution and densities of larval malaria vectors
A total of 30,552 mosquitoes belonging to two different genera were collected from all the study sites during the sampling period; HS [n = 5,533/30,552, 18.1%], PU [n = 4,811/30,552, 15.7%], MS [n = 5,970/30,552, 19.5%], LS [n = 5,843/30,552, 19.1%], IUF [n = 8,395/30,552, 27.5%]. Overall, 75.02%, (22,919/30,552) of mosquitoes sampled were Anopheles, whereas 24.98%, (7,633/30,552) were Culex.
Throughout the study period, the abundance of Anopheles was highest in IUF [6244/22,919], followed by MS [ 4,557/22,919], LS [4,505/22,919], HS [4,097/22,919], then PU [3516/22,919]. Overall, An. gambiae s.l. were more abundant in the rainy season (77.01%; 17,650/22,919) than in the dry season (22.99%; 5,269/22,919). The mean abundance of An. gambiae was significantly associated with season (R2 = 3.46, P= 0.000).
The site category with the most abundant culicine larvae sampled was from IUF = [28.2%, 2,151/7,633], followed by HS = [18.8%, 1,436/7,633], MS = [18.5%, 1,413/7,633], LS [17.5%, 1,338/7,633] and then PU = [16.9%, 1295/7,633]. Regression analysis indicated a significant productivity effect of Culex larvae in breeding habitats on the presence of Anopheles larvae (R2 = 2.78, P= 0.001). Similarly, generalized linear models analysis indicated that the presence of Culex larvae in breeding habitats had a significant effect (B = −0.46340, p= 0.001) on Anopheles larval density.
A high abundance of Anopheles larvae 8,560 (37.35%) were collected from drainage ditches whereas car tires recorded the lowest 78 (0.34%). There was a significant association between habitat type and the presence of Anopheles larvae (χ2 = 22.721, df = 8, P= 0.004).
The highest larval densities of 19.22 and 13.22 larvae/dip were recorded in swamps (MS) and tire track (IUF) respectively in the rainy season. However, in the dry season the highest larval density, 12 larvae/dip was recorded in tire track (IUF) (Table 3). Larval habitats with sizes less than 10 meters had significantly higher larval densities compared to those with sizes between 10 to 100 meters (X2 = 6.41, df= 1, P= 0.01). Anopheles gambiae s.l. larval density was significantly associated with season (t= 4.14, P= 0.00). The student T-test analysis indicated a significant association between Anopheles gambiae sl larval density and site category (t= 2.58, P= 0.01). Similarly, larval density was significantly associated with the presence of algae (Z= −2.19, P= 0.03), land use type (t =−1.93, P=0.053).
Table 3.
Anopheles larval density in the dry and rainy seasons
| Habitat type | Larval density (larvae/dip) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HS | IUF | LS | MS | PU | ||||||
| Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | |
| Artificial pond | 0 | 0 | 4.34 | 0.78 | 0 | 0 | 0 | 0 | 0 | 0 |
| Car tyre | 0 | 0.42 | 0 | 0 | 0 | 3.00 | 0 | 2.17 | 0 | 0 |
| Drainage ditch | 1.66 | 1.11 | 4.13 | 0.64 | 4.69 | 4.20 | 1.98 | 2.70 | 1.86 | 5.02 |
| Footprint | 0 | 0 | 0 | 2.78 | 0 | 2.51 | 0 | 4.75 | 0.27 | 0.67 |
| Furrow | 0 | 0 | 1.61 | 5.18 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hooves print | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.00 |
| Natural pond | 0 | 0 | 4.59 | 0.42 | 0 | 0 | 0 | 0 | 1.38 | 0.23 |
| Pit | 0 | 0 | 6.12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Puddle | 0 | 1.04 | 0 | 3.89 | 0 | 0 | 0 | 2.68 | 0 | 0.14 |
| Swamp | 0 | 0.87 | 4.24 | 0.88 | 0 | 2.60 | 0 | 5.04 | 6.17 | 1.37 |
| Tyre track | 0 | 1.64 | 12 | 8.77 | 0 | 3.97 | 0.61 | 2.72 | 0 | 1.56 |
| Well | 0 | 0.25 | 0.94 | 0 | 1.25 | 4.0 | 0 | 1.07 | 0 | 1.67 |
Physical and chemical properties of larval habitats
Overall, the pH of larval habitats in the study sites ranged from 7.0 to 9.02. The lowest pH was recorded from a drainage ditch (MS category) and the highest in a swamp (MS category) during the rainy season (Table 5b). A high (4940 ppm) (LS) turbidity was recorded in a swamp, whereas, the lowest (1.08 ppm) was recorded in a tyre track (IUF). The highest salinity (2.2ppt; dry season) (Table 4a) and conductivity (4033 mS/cm; rainy season) (Table 4b) were measured in natural pond (PU) and well (LS) respectively. The lowest salinity (0.07ppt) and conductivity (124.1 mS/cm) were both recorded in a natural pond respectively during the dry season in an IUF site category (Table 4a).
Table 5.
Correlation table showing relationship between physicochemical parameters and An. qambiae s.l. larval density.
| An. gambiae LD | pH | Temp | EC | Salinity | TDS | |
|---|---|---|---|---|---|---|
| An. gambiae LD | 1.00000000 | −0.05842815 | 0.09015693 | 0.21804142 | 0.22186362 | 0.22775660 |
| pH | −0.05842815 | 1.00000000 | 0.02301607 | −0.09329249 | −0.09390100 | −0.08588305 |
| Temp | 0.09015693 | 0.02301607 | 1.00000000 | 0.11895627 | 0.12155853 | 0.12887996 |
| EC | 0.21804142 | −0.09329249 | 0.11895627 | 1.00000000 | 0.90719215 | 0.88264834 |
| Salinity | 0.22186362 | −0.09390100 | 0.12155853 | 0.90719215 | 1.00000000 | 0.9082542 |
| TDS | 0.22775660 | −0.08588305 | 0.12887996 | 0.88264834 | 0.9082542 | 1.00000000 |
Table 4 a:
Physicochemical characteristics of larval habitat type and site categories during the dry season
| pH | Temperature (°C) | Salinity (ppt) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Habitat type | LS | MS | HS | PU | IUF | LS | MS | HS | PU | IUF | LS | MS | HS | PU | IUF | LS | MS | HS |
| Artificial pond | - | - | - | - | 7.48 | - | - | - | - | 28.05 | - | - | - | - | 0.37 | - | - | - |
| Natural pond | - | - | - | 7.51 | 7.65 | - | - | - | 26 | 26.80 | - | - | - | 0.05 | 0.07 | - | - | - |
| Pit | - | - | - | - | 7.5 | - | - | - | - | 29.45 | - | - | - | - | 0.59 | - | - | - |
| Drainage ditch | 7.2 | 7.34 | 7.31 | 7.33 | 7.48 | 27.2 | 26.5 | 26 | 26.1 | 25.22 | 1.36 | 0.32 | 0.31 | 0.35 | 0.30 | 956 | 418 | 419 |
| Swamp | - | - | - | 7.26 | 7.46 | - | - | - | 27 | 29.14 | - | - | - | 0.2 | 0.22 | - | - | - |
| Well | 7.08 | - | - | - | 7.34 | 29 | - | - | - | 29.50 | 2 | - | - | - | 0.18 | 285 | - | - |
| Footprint | - | - | - | 7.09 | - | - | - | - | 27 | - | - | - | - | 0.01 | - | - | - | - |
| Tyre track | - | 7.11 | - | - | 7.16 | - | 32 | - | - | 32.40 | - | 0.45 | - | - | 0.76 | - | 112 | - |
| Furrow | - | - | - | - | 7.31 | - | - | - | - | 31.19 | - | - | - | - | 0.31 | - | - | - |
HS = High socioeconomic, IUF = Irrigated urban farming, LS = Low socioeconomic, MS = Middle socioeconomic and PU = Peri-urban.
Table 4 b:
Physicochemical characteristics of larval habitat type and site categories during the rainy season
| pH | Temperature (°C) | Salinity (ppt) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Habitat type | LS | MS | HS | PU | IUF | LS | MS | HS | PU | IUF | LS | MS | HS | PU | IUF | LS | MS | HS | PU |
| Artificial pond | - | - | - | - | 7.5 | - | - | - | - | 26.4 | - | - | - | - | 0.2 | - | - | - | - |
| Natural pond | - | - | - | 7.6 | 7.7 | - | - | - | 30.3 | 27 | - | - | - | 2.2 | 0.3 | - | - | - | 933 |
| Drainage ditch | 7.5 | 7.0 | 7.8 | 7.6 | 7.5 | 28.3 | 27 | 27.1 | 27.5 | 26.3 | 0.9 | 1.6 | 0.6 | 0.6 | 0.3 | 1460 | 717 | 340 | 511.1 |
| Swamp | 8.0 | 9.2 | 8.3 | 7.7 | 7.7 | 25.6 | 27.7 | 28.4 | 28 | 26.1 | 0.2 | 0.9 | 0.5 | 0.4 | 0.3 | 4940 | 695 | 271.5 | 372.2 |
| Well | 7.3 | 9 | 7.2 | 8.7 | - | 29.7 | 26.3 | 27.3 | 26.6 | - | 1.5 | 0.6 | 0.6 | 1.2 | - | 219 | 898 | 887 | 220.2 |
| Footprint | 7.7 | 8.3 | - | 7.7 | 7.7 | 27 | 28.8 | - | 26.8 | 25.2 | 0.8 | 2 | - | 0.3 | 1.6 | 4760 | 0 | - | 317.4 |
| Tyre track | 7.6 | 8 | 8.3 | 7.8 | 7.7 | 26 | 28.7 | 27.3 | 27.8 | 27.1 | 0.4 | 1.4 | 0.6 | 0.5 | 0.9 | 900 | 637 | 573.6 | 1041 |
| Furrow | - | - | - | - | 8.5 | - | - | - | - | 28.7 | - | - | - | - | 1 | - | - | - | - |
| Car tyre | 7.5 | 7.9 | 7.7 | - | - | 29.5 | 27.4 | 28.3 | - | - | 0.28 | 0.7 | 0.5 | - | - | 395 | 372 | 211 | - |
| Puddle | - | 7.3 | 8.3 | 7.5 | 7.4 | - | 29.3 | 29.5 | 29.6 | 29.3 | - | 1.6 | 1.1 | 1.0 | 0.9 | - | 569 | 256 | 0.524 |
| Hooves print | - | - | - | 7.7 | - | - | - | - | 28.8 | - | - | - | - | 1.1 | - | - | - | - | 468 |
HS = High socioeconomic, IUF = Irrigated urban fa socioeconomic, PU = Peri-urban, and “-” = no larval habitat found.
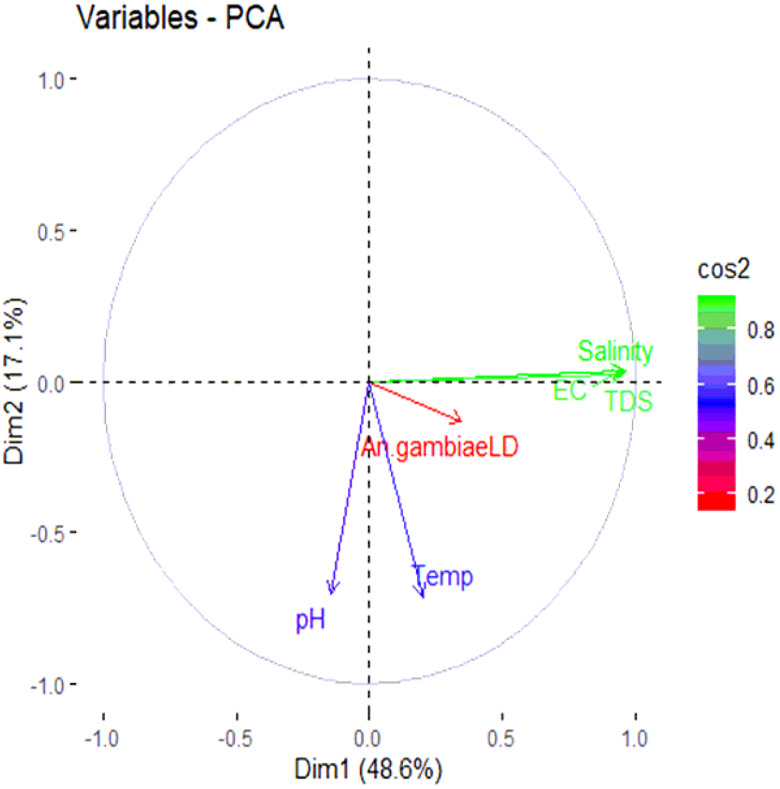
The relationship between the physicochemical variables and Anopheles larval productivity was determined using the principal component analysis. The projections showed that larval productivity was linked to the variation of some physicochemical parameters (Fig. 2). Correlation analysis (PCA) between the physicochemical parameters and distribution of Anopheles mosquitoes is represented in Table 5. The result shows a non-significant negative correlation (r= −0.058) between pH and Anopheles larval densities. Similarly, the abundance of An. gambiae s. l. showed no correlation (r= 0.090) to habitat temperature. However, there was a strong positive correlation between some of the chemical parameters measured in the larval habitats; EC and salinity (r = 0.907), EC and TDS (r = 0.883), and Salinity and TDS (r = 0.908) (Table 5).
Figure 2.
Types of habitats found during the study period. a furrows, b plastic containers, c puddle on cemented floor, d puddle in a compound, e concrete tank, f hoofprints, g base of water pumping machine, h car tyre, i soakaway cement tank, j puddle at construction site, k concrete drainage ditch, l artificial pond for irrigation, m puddle on cemented floor n drainage ditch and dumping site o puddle in cattle range.
Physical and chemical parameter levels in polluted and unpolluted habitats
The physical and chemical properties of polluted and unpolluted habitats were measured over a period of four (4) weeks. Polluted habitats had lower dissolved oxygen (1.4–3.6 ppm) and higher Total Dissolved Solids (1,026.5–4,567.0 mg/L) compared to unpolluted habitats (DO: 2.0–7.8 ppm; TDS: 751.0–1,780.2 mg/L). In polluted habitats, salinity (0.8–3.9 ppt) and specific conductance (1.6–8.0 mS/cm) were higher than in unpolluted habitats (salinity: 0.6–1.4ppt, specific conductance: 1.2–2.7 mS/cm). Polluted habitats also had elevated ammonium (0.1–11.9 mg/L) and ammonia (0–1.6 mg/L) levels and higher pH (8.3–9.1) values compared to the unpolluted habitats (ammonium: 0–4.9 mg/L), ammonia: 0–0.2 mg/L) pH (7.8–8.3) (Table 6).
Table 6.
Level of physicochemical parameters of polluted and unpolluted larval habitats
| Nima | Chorkor | Teshie | Madina | |||||
|---|---|---|---|---|---|---|---|---|
| Physicochemical Parameters | Polluted | Unpolluted | Polluted | Unpolluted | Polluted | Unpolluted | Polluted | Unpolluted |
| Temperature (°C) | 27.3 | 28.7 | 28.4 | 28.3 | 26.8 | 27.5 | 28 | 28 |
| Pressure (mmHg) | 757.7 | 759.0 | 762.2 | 762.3 | 760.9 | 760.5 | 756.2 | 757.7 |
| DO (%) | 17.6 | 26.1 | 36.95 | 51.4 | 46.3 | 100 | 34.2 | 34.1 |
| DO (ppm) | 1.4 | 2.0 | 2.9 | 3.96 | 3.6 | 7.8 | 2.7 | 2.7 |
| SPC (mS/cm) | 7.0 | 2.7 | 2.93 | 2.1 | 8.05 | 1.5 | 1.6 | 1.2 |
| SPC (uS/cm) | 7332.5 | 2932.0 | 3114.7 | 2237 | 8319.7 | 1807 | 1657.2 | 1219.80 |
| Salinity (ppt) | 3.9 | 1.4 | 1.5 | 1.1 | 4.4 | 0.9 | 0.8 | 0.6 |
| TDS (mg/L) | 4567.0 | 1780.2 | 1901.5 | 1366.8 | 5229.3 | 1120.7 | 1026.5 | 751 |
| Resistivity (ohm-cm) | 142.3 | 366.3 | 2750.4 | 475.7 | 124.3 | 580 | 704.3 | 876.3 |
| pH | 8.3 | 7.8 | 8.56 | 8.3 | 9.1 | 8.3 | 8.4 | 7.8 |
| NH4-N(mg/L) | 11.9 | 4.9 | 0.4 | 0 | 1.14 | 0 | 0.1 | 0.11 |
| NH3-N(mg/L) | 1.6 | 0.2 | 0.04 | 0 | 0.18 | 0 | 0 | 0 |
| Larval density | 2.6 | 1.2 | 6.6 | 4.5 | 4.7 | 1.9 | 3.1 | 1.7 |
Simple linear regressing analysis indicated that larval abundance had a significant relationship with polluted larval habitats: Nima (2.6 vs. 1.2), Chorkor (6.6 vs. 4.5), Teshie (4.7 vs. 1.9), and Madina (3.1 vs. 1.7). (B = 4.25, P= 0.002). Correlation analysis revealed a strong positive association between larval abundance and some physicochemical parameters: specific conductance (SPC) (R = 0.261), conductivity (COND) (R = 0.253), salinity (SAL) (R = 0.240), total dissolved solids (TDS) (R = 0.252), resistivity (RES) (R = 0.610), and pH (R = 0.710). Although ammonium (R = −0.4131, P = 0.309) and ammonia (R = −0.159, P = 0.706) showed non-significant negative correlations, a strong positive and significant correlation was found between larval abundance and pH (R = 0.710, P = 0.048).
Species discrimination in the Anopheles gambiae complex
A subsample of 679 of An. gambiae s.l. from all the study sites were randomly selected and used to discriminate the sibling species. An. Coluzzii 54.79% (372/677) was the most abundant species followed by An. gambiae s.s 42.97% (285/679) and An. gambiae/An. coluzzii Hybrid 2.81% (19/679). About 0.44% (3/679) were detected to be An. stephensi. According to the site category, An. coluzzii was the abundant species sampled in all the various categories, except in the HS = 111[An. gambiae s.s. = 59, An. Coluzzii = 50, hybrid = 2] and PU = 150 [An. gambiae s.s. = 111, An. coluzzii= 30, hybrid = 9] sites, where the species were dominated by the An. gambiae s.s. The new invasive species, An. stephensi was found in Dansoman, Tuba and Nima which are within the MS, IUF and LS category respectively (Table 7).
Discussion
Investigating malaria vector breeding habitat types, habitat characteristics and Anopheles larval abundance in urban settings is an essential element of urban malaria control (9). This can help in the elimination of urban malaria through effective larval source management. The present study assessed the larval habitat types, characteristics and their effect on the density of Anopheles gambiae s.l. in Accra, Ghana. Twelve (12) different larval habitat types, including drainage ditches, car tyres, and puddles were found. Irrigated urban farming (IUF) and Peri-urban (PU) sites had the highest frequency of larval habitats in dry and rainy season respectively. Drainage ditches were the most abundant and most productive habitat type for Anopheles mosquito larvae. Anopheles coluzzii is the most dominating malaria vector species found. This study identified the invasive malaria vector, An. Stephensi for the first time in Ghana.
The most abundant habitat type was drainage ditches which carried effluents from people’s homes. Other habitats such as car tires, hoof-prints and puddles were usually formed during the rainy season. Most of the mosquito breeding sites encountered during the survey in the different site categories resulted from anthropogenic activities, and their characteristics were related to factors associated with urbanization and agriculture. This emphasizes the importance of human activities through land-use in the creation of Anopheles breeding habitats and the impact they have on malaria transmission. Similar observation was reported in Accra and Takoradi (12), and in Cape Coast (19), Ghana, highlighting that man-made breeding habitats were the most abundant. The presence of numerous drainage ditches functioning as breeding habitats may stem from inadequate sanitation practices and insufficiently regulated urban development by both public authorities and residents. This results in the obstruction of drainage systems, allowing water to accumulate for extended periods and creating suitable conditions for mosquito breeding. In contrast, other studies highlighted puddles as the predominant breeding grounds for Anopheles mosquitoes in urban settings (12)(20).
Even though there was seasonal variation in larval habitat abundance with higher abundance recorded in the rainy season, breeding habitats were found throughout the dry and rainy seasons across all site categories. This could potentially contribute to malaria transmission all year round (21)(2). The IUF site category had the highest number of breeding habitats, likely attributable to the predominantly lowland nature of most IUF sites and their regular irrigation practices. This finding corroborates with that of Afrane et al. (22) and Klinkenberg et al. (11). Their study reported that irrigated fields generated large numbers of mosquitoes. These findings were similar to other studies in the town of Niono, Mali by Diuk-Wasser et al. (24) and in Dar es Salam, Tanzania by Dongus et al. (25) Ghana by Hinne et al. (2), they reported that irrigated farms contribute to high abundance of malaria mosquitoes. Furthermore, the IUF site category had the most diverse breeding habitat types, which included eight of the eleven habitat types encountered, coinciding with high abundance of An gambiae larvae reported in this study.
Throughout the entire sampling period, An. coluzzii emerged as the most predominant species, potentially influenced by the permanent nature of most of the breeding habitats. This finding aligns with previous reports by Kudom et al. (19) and Hinne et al. (2), indicating its preference to breeding in permanent larval habitats such as irrigated fields (26). Its abundance and distribution remain consistent irrespective of seasons or rainfall patterns, allowing for year-round breeding in various habitats. Similar findings were reported by Chabi et al. (27) in Ghana and Ossé et al. (28) in Benin.
Larval densities were observed to be higher during the rainy season in all sites, however, higher mean larval densities were observed in irrigated urban farm (IUF) site categories during both seasons. Additionally, the current study indicated that higher larval densities were significantly associated with habitat size, season of sampling, presence of algae in breeding habitats, land use type, and site category. Various physical, chemical, and biological parameters have the potential to influence habitat productivity and larval distribution in different breeding habitats. Previous studies, by Onchuru et al. (29), Hessou-Djossou et al (20) and Forson et al. (14), have reported a positive relationship between Anopheles larval density, and some physicochemical properties such as vegetation cover, habitat size, temperature, pH and conductivity. Muturi et al. (30) suggested that low water temperatures result in a decline in the growth of microorganisms that serve as food for mosquito larvae.
Findings from the current study showed that temperature and pH was negatively correlated with habitat preference of Anopheles mosquitoes in the study area. This conformed to the findings of Akeju et al. (31) and Getachew et al. (32); they both reported that pH of the habitat of immature stages of Anopheles mosquito was not significantly correlated with the density of Anopheles larvae in their respective location of study, though some other species of mosquito larvae have been reported to show a significant correlation with pH (33)(34).
More importantly, the data suggests that polluted Anopheles larval habitats in the study sites have higher levels of dissolved ions, salinity, total dissolved solids, and ammonia, as well as lower dissolved oxygen levels and resistivity compared to unpolluted habitats. These differences in physicochemical parameters coupled with the high abundance of Anopheles mosquito larvae could potentially influence the suitability of these habitats, hence affect Larval Source Management (LSM) and the dynamics of malaria transmission in urban settings.
Conclusion
Malaria vector species were identified breeding in diverse habitat types. Urban agricultural areas had the highest larval densities. Additionally, this study uncovered the presence of the new invasive malaria vector, An. stephensi. This discovery holds significant public health implications as this mosquito species demonstrates high resistance to chemical-based vector control and possesses highly invasive characteristics. To address these challenges, Larval Source Management (LSM) should be adapted to encompass all potential mosquito breeding sites in urban areas, especially those with agricultural activities.
Figure 3.
Figure 2: Contribution of physicochemical parameters to larval abundance
Acknowledgements
We are sincerely grateful to the communities that served as study sites for allowing us to conduct sampling within their communities and on their farms. We also extend our thanks to all the community field assistants for their help with the sampling process across various locations.
Funding
This study was funded by grants from the National Institutes of Health (R01 A1123074, R03AI186018 and D43 TW 011513). The funder had no role or influence in the study’s design, data collection, analysis, or interpretation, nor in the writing of this manuscript.
Abbreviations
- LS
Lower-socioeconomic status
- MS
Middle-socioeconomic status
- HS
High-socioeconomic status
- PU
Peri-urban
- IUF
Irrigated urban farming
- PCR
Polymerase chain reaction
- LSM
Larval source management
- RFLP
Restriction fragment length polymorphism
Funding Statement
This study was funded by grants from the National Institutes of Health (R01 A1123074, R03AI186018 and D43 TW 011513). The funder had no role or influence in the study’s design, data collection, analysis, or interpretation, nor in the writing of this manuscript.
Footnotes
Competing interests
The authors declare no competing interests.
Additional Declarations: No competing interests reported.
Contributor Information
Abdul Rahim Mohammed Sabtiu, University of Ghana.
Isaac Amankona Hinne, University of Nevada.
Isaac Kwame Sraku, University of Ghana.
Richard Tettey Doe, University of Ghana.
Simon Kwaku Attah, University of Ghana.
Fred Aboagye-Antwi, University of Ghana.
Yaw Asare Afrane, University of Ghana.
Availability of data and materials
The datasets utilized and analyzed in this study is available and can be obtained from the corresponding author upon request.
References
- 1.Klinkenberg E, Mccall PJ, Wilson MD, Akoto AO, Amerasinghe FP, Bates I, et al. Urban malaria and anaemia in children: a cross-sectional survey in two cities of Ghana. 2006;11(5):578–88. [DOI] [PubMed] [Google Scholar]
- 2.Hinne IA, Attah SK, Mensah BA, Forson AO, Afrane YA. Larval habitat diversity and Anopheles mosquito species distribution in different ecological zones in Ghana. Parasites and Vectors [Internet]. 2021;14(1):1–14. Available from: 10.1186/s13071-021-04701-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hampwaye G. Geoforum Benefits of urban agriculture: Reality or illusion ? Geoforum [Internet]. 2013;49:R7–8. Available from: 10.1016/j.geoforum.2013.03.008 [DOI] [Google Scholar]
- 4.Colson-Fearon B, Versey HS. Urban Agriculture as a Means to Food Sovereignty? A Case Study of Baltimore City Residents. Int J Environ Res Public Health. 2022;19(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilke ABB, Caban-Martinez AJ, Ajelli M, Vasquez C, Petrie W, Beier JC. Mosquito Adaptation to the Extreme Habitats of Urban Construction Sites. Trends Parasitol [Internet]. 2019;35(8):607–14. Available from: 10.1016/j.pt.2019.05.009 [DOI] [PubMed] [Google Scholar]
- 6.OECD/UNECA/AfDB. Africa’s Urbanisation Dynamics 2022 THE ECONOMIC POWER OF AFRICA’S CITIES. 2022. 1–205 p.
- 7.Guneralp B, Lwasa S, Masundire H, Parnell S, Seto KC. Urbanization in Africa: Challenges and opportunities for conservation. Environ Res Lett. 2018;13(1). [Google Scholar]
- 8.Cobbinah PB, Erdiaw-Kwasie MO. Urbanization in Ghana: Insights and implications for urban governance. E-Planning Collab Concepts, Methodol Tools, Appl. 2018;1–3(August):256–78. [Google Scholar]
- 9.Belisse D, Belisse PD, Kopya E, Ngadjeu CS, Chiana NS, Talipouo A, et al. Urban malaria in sub - Saharan Africa: dynamic of the vectorial system and the entomological inoculation rate. Malar J [Internet]. 2021;1–18. Available from: 10.1186/s12936-021-03891-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pwalia R, Joannides J, Iddrisu A, Addae C, Acquah-Baidoo D, Obuobi D, et al. High insecticide resistance intensity of Anopheles gambiae (s.l.) and low efficacy of pyrethroid LLINs in Accra, Ghana. Parasites and Vectors [Internet]. 2019;12(1):1–9. Available from: 10.1186/s13071-019-3556-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klinkenberg E, Mccall PJ, Wilson MD, Amerasinghe FP, Donnelly MJ. Impact of urban agriculture on malaria vectors in Accra, Ghana. 2008;9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mattah PAD, Futagbi G, Amekudzi LK, Mattah MM, Souza DK De, Kartey-attipoe WD, et al. Diversity in breeding sites and distribution of Anopheles mosquitoes in selected urban areas of southern Ghana. Parasit Vectors [Internet]. 2017;1–15. Available from: 10.1186/s13071-016-1941-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imbahale SS, Paaijmans KP, Mukabana WR, Van Lammeren R, Githeko AK, Takken W. A longitudinal study on Anopheles mosquito larval abundance in distinct geographical and environmental settings in western Kenya. Malar J. 2011;10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forson AO, Hinne IA, Sraku IK, Afrane YA. Larval habitat stability and productivity in two sites in Southern Ghana. Malar J [Internet]. 2023;1–13. Available from: 10.1186/s12936-023-04498-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kweka EJ, Munga S, Himeidan Y, Githeko AK, Yan G. Assessment of mosquito larval productivity among different land use types for targeted malaria vector control in the western Kenya highlands. Parasites and Vectors. 2015;8(356):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coetzee M. Key to the females of Afrotropical Anopheles mosquitoes (Diptera: Culicidae). Malar J [Internet]. 2020;1–20. Available from: 10.1186/s12936-020-3144-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49(4):520–9. [DOI] [PubMed] [Google Scholar]
- 18.Fanello C, Santolamazza F, Torre A della. Simultaneous identification of species and molecular forms of the Anopheles gambiae complex by PCR-RFLP Med Vet Entomol. 2002;16:461–4. [DOI] [PubMed] [Google Scholar]
- 19.Kudom AA. Larval ecology of Anopheles coluzzii in Cape Coast, Ghana : water quality, nature of habitat and implication for larval control. Malar J. 2015;1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hessou-Djossou D, Djegbe I, Ahadji-Dabla KM, Nonfodji OM, Tchigossou G, Djouaka R, et al. Diversity of larval habitats of Anopheles mosquitoes in urban areas of Benin and influence of their physicochemical and bacteriological characteristics on larval density. Parasites and Vectors [Internet]. 2022;15(1):1–17. Available from: 10.1186/s13071-022-05323-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olusi TA, Simon-Oke IA, Akeju A V. Composition, habitat preference and seasonal variation of malaria vector larval and pupa stage in Akure North Local Government Area of Ondo State, Nigeria. Bull Natl Res Cent [Internet]. 2021;45(1). Available from: 10.1186/s42269-021-00535-9 [DOI] [Google Scholar]
- 22.Asare Y, Klinkenberg E, Drechsel P. Does irrigated urban agriculture influence the transmission of malaria in the city of Kumasi, Ghana ? 2004;89:125–34. [DOI] [PubMed] [Google Scholar]
- 23.Klinkenberg E, McCall PJ, Wilson MD, Amerasinghe FP, Donnelly MJ. Impact of urban agriculture on malaria vectors in Accra, Ghana. Malar J. 2008;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diuk-Wasser MA, Toure MB, Dolo G, Bagayoko M, Sogoba N, Sissoko I, et al. Effect of rice cultivation patterns on malaria vector abundance in rice-growing villages in Mali. Am J Trop Med Hyg. 2007;76(5):869–74. [PMC free article] [PubMed] [Google Scholar]
- 25.Dongus S, Nyika D, Kannady K, Mtasiwa D, Mshinda H, Gosoniu L, et al. Urban agriculture and Anopheles habitats in Dar es Salaam, Tanzania. Geospat Health. 2009;3(2):189–210. [DOI] [PubMed] [Google Scholar]
- 26.Etang J, Mbida AM, Akono PN, Binyang J, Else C, Moukoko E, et al. Anopheles coluzzii larval habitat and insecticide resistance in the island area of. BMC Infect Dis [Internet]. 2016;1–11. Available from: 10.1186/s12879-016-1542-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chabi J, Baidoo PK, Datsomor AK, Okyere D, Ablorde A, Iddrisu A, et al. Insecticide susceptibility of natural populations of Anopheles coluzzii and Anopheles gambiae (sensu stricto) from Okyereko irrigation site, Ghana, West Africa. Parasites and Vectors [Internet]. 2016;9(1):1–8. Available from: 10.1186/s13071-016-1462-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osse RA, Bangana SB, Aikpon R. Larvae to Polluted Breeding Sites in Cotonou: A Strengthening in Urban Malaria Transmission in. 2019;(January).
- 29.Onchuru TO, Ajamma YU, Burugu M, Kaltenpoth M, Masiga D, Villinger J. Chemical parameters and bacterial communities associated with larval habitats of Anopheles, Culex and Aedes mosquitoes (Diptera : Culicidae) in western Kenya. 2016;(June).
- 30.Muturi EJ, Mwangangi J, Shililu J, Jacob BG, Mbogo C, Githure J, et al. Environmental factors associated with the distribution of Anopheles arabiensis and Culex quinquefasciatus in a rice agro-ecosystem in Mwea, Kenya Environmental factors associated with the distribution of Anopheles arabiensis and Culex quinquefasciatus in. 2008;33(1):56–63. [DOI] [PubMed] [Google Scholar]
- 31.Akeju AV, Olusi TA, Adepeju I, Oke S. Effect of physicochemical parameters on Anopheles mosquitoes larval composition in Akure North Local Government area of Ondo State, Nigeria. J Basic Appl Zool [Internet]. 2022; Available from: 10.1186/s41936-022-00298-3 [DOI] [Google Scholar]
- 32.Getachew D, Balkew M, Tekie H. Anopheles larval species composition and characterization of breeding habitats in two localities in the Ghibe River Basin, southwestern Ethiopia. Malar J [Internet]. 2020;19(1):1–13. Available from: 10.1186/s12936-020-3145-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dejenie T, Yohannes M, Assmelash T. ORIGINAL ARTICLE CHARACTERIZATION OF MOSQUITO BREEDING SITES IN. 1871;57–66. [DOI] [PMC free article] [PubMed]
- 34.Nikookar SH, Fazeli-dinan M, Azari-hamidian S, Mousavinasab N, Aarabi M, Ziapour SP et al. Correlation between mosquito larval density and their habitat physicochemical characteristics in Mazandaran Province, northern Iran. 2017;1–19. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets utilized and analyzed in this study is available and can be obtained from the corresponding author upon request.