Abstract
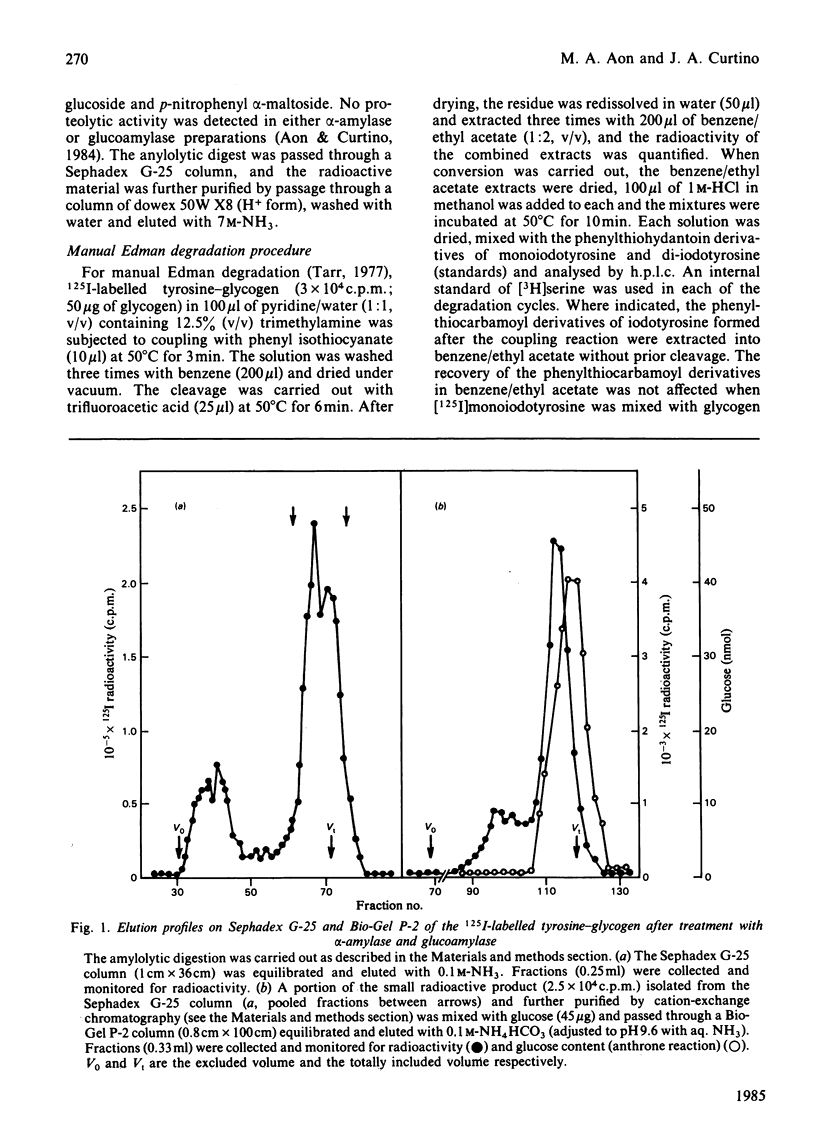
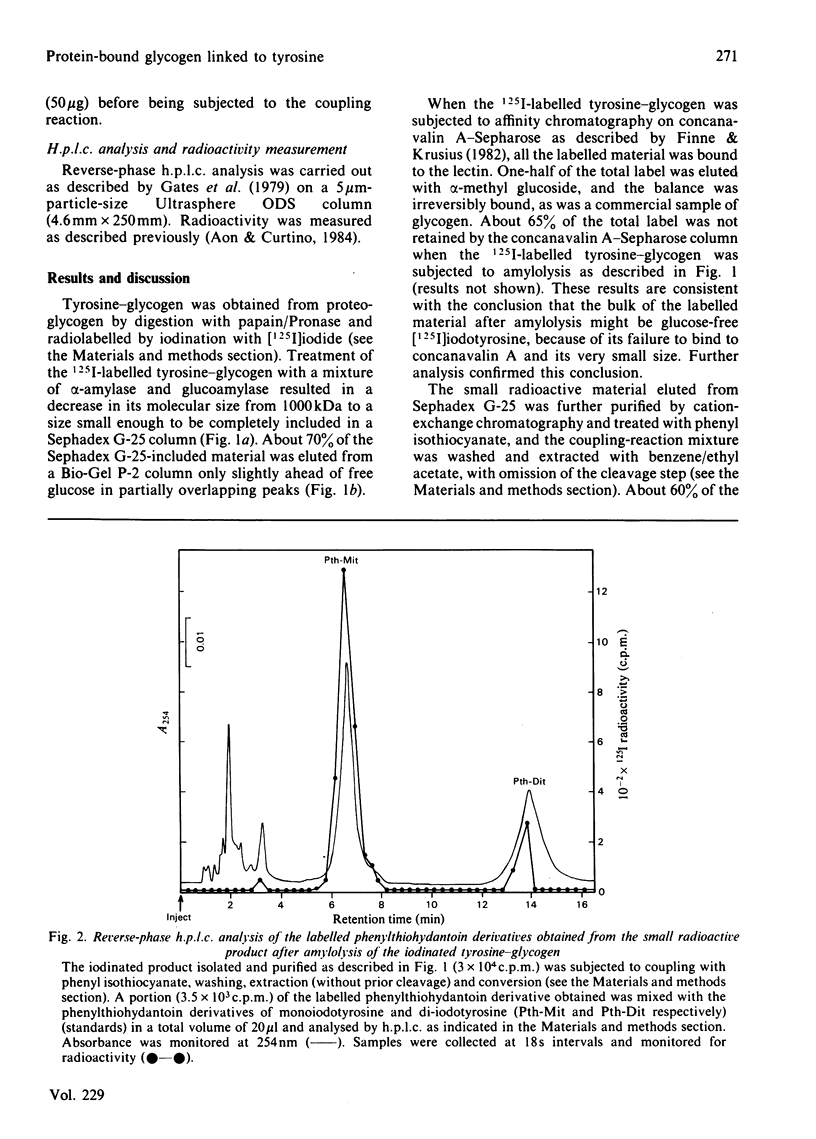
Tyrosine-glycogen obtained from retina proteoglycogen by exhaustive proteolytic digestion was radiolabelled with 125I. The 125I-labelled tyrosine-glycogen was degraded by amylolytic digestion to a very small radioactive product, which was identified as iodotyrosine by h.p.l.c. The amylolytic mixture used released glucose and maltose that were alpha-linked to the phenolic hydroxy group of p-nitrophenol. No free iodotyrosine was found before or after the intact [125I]iodotyrosine-glycogen was subjected to two cycles of the Edman degradation procedure. The linkage between protein and glycogen was alkali-stable. Therefore it is concluded that the protein-bound glycogen was O-glycosidically linked to the phenolic hydroxy group of tyrosine. The amino acid has not been heretofore found to be involved in the linkage of carbohydrates to proteins.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aon M. A., Curtino J. A. Evidence for the glycoprotein nature of retina glycogen. Eur J Biochem. 1984 May 2;140(3):557–566. doi: 10.1111/j.1432-1033.1984.tb08138.x. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of Vibrio cholerae enterotoxin with cell membranes. Biochemistry. 1973 Aug 28;12(18):3547–3558. doi: 10.1021/bi00742a031. [DOI] [PubMed] [Google Scholar]
- Finne J., Krusius T. Preparation and fractionation of glycopeptides. Methods Enzymol. 1982;83:269–277. doi: 10.1016/0076-6879(82)83020-6. [DOI] [PubMed] [Google Scholar]
- Gates F. T., 3rd, Coligan J. E., Kindt T. J. Complete amino acid sequence of rabbit beta 2-microglobulin. Biochemistry. 1979 May 29;18(11):2267–2272. doi: 10.1021/bi00578a021. [DOI] [PubMed] [Google Scholar]
- Mayberry W. E., Rall J. E., Bertoli D. Kinetics of iodination. IV. A comparison of the kinetics of iodination of L-tyrosine and some derivatives. Biochemistry. 1965 Dec;4(12):2606–2611. doi: 10.1021/bi00888a009. [DOI] [PubMed] [Google Scholar]
- Tarr G. E. Improved manual sequencing methods. Methods Enzymol. 1977;47:335–357. doi: 10.1016/0076-6879(77)47036-8. [DOI] [PubMed] [Google Scholar]
- Tomita M., Furthmayr H., Marchesi V. T. Primary structure of human erythrocyte glycophorin A. Isolation and characterization of peptides and complete amino acid sequence. Biochemistry. 1978 Oct 31;17(22):4756–4770. doi: 10.1021/bi00615a025. [DOI] [PubMed] [Google Scholar]


