Abstract
Purpose:
To study the clinical presentation and treatment outcomes of indocyanine green-enhanced transpupillary thermotherapy (ICG-TTT) for treatment-naïve juxtapapillary retinal capillary hemangioblastoma (JRCH).
Methods:
A prospective interventional case series. The technique involved ICG dye infusion 45 seconds prior to application of TTT. The main study outcomes were local tumor control, resolution of subretinal fluid (SRF), and improvement in best-corrected visual acuity (BCVA).
Results:
Eight eyes of seven patients (5 males and 2 females) were included. The mean age was 26 years (range: 5–56 years). Systemic evaluation revealed von-Hippel Lindau (VHL) disease in five patients. The most common location was the temporal aspect of the optic disc (5 eyes). The mean basal diameter was 2.9 mm (range: 1–8 mm), and tumor thickness was 1.4 mm (range: 1–4 mm). All eight eyes were treated with multiple sessions of ICG-TTT (mean: 3 sessions). Six eyes received adjuvant intravitreal injection of dexamethasone implant (4 eyes) and/or bevacizumab (4 eyes). Post treatment, six eyes (75%) had tumor regression with reduction of SRF. One eye had a partial response with persisting SRF, and one eye showed poor response to TTT for which external beam radiotherapy was performed. At the last follow-up (median: 11 months; range: 6–29 months), the BCVA remained stable in seven eyes and improved in one eye (hand motion to 20/40).
Conclusion:
Multiple ICG-TTT sessions can be considered as an alternative treatment option for JRCH with effective local tumor control and SRF resolution.
Keywords: Indocyanine green angiography, juxtapapillary retinal capillary hemangioblastoma, transpupillary thermotherapy, treatment
Retinal capillary hemangioblastomas (RCH) are benign retinal vascular tumors presenting as round, sessile orange-red vascular lesions in the mid-peripheral retina or rarely in the juxtapapillary area.[1,2] They can manifest either sporadically or as a part of von Hippel–Lindau (VHL) syndrome.[3] Juxtapapillary RCH (JRCH) are small nodular vascular hamartomas seen over the optic disc or located in the juxtapapillary region. Isolated RCH is usually stable and unassociated with vision-threatening complications and hence can be safely observed.[4] However, RCH associated with VHL is usually progressive and complicated by associated vitreous hemorrhage, secondary exudative retinal detachment due to tumor exudation, or tractional retinal detachment due to glial proliferation around the tumor.[3,5] Because of their location, JRCH are easily misdiagnosed before they exhibit endophytic growth protruding into the vitreous cavity, resulting in visual impairment secondary to macular exudation, subretinal fluid accumulation, macular edema, epiretinal membrane formation, and exudative or tractional retinal detachment.[6]
Various treatment modalities such as laser photocoagulation, photodynamic therapy, transpupillary thermotherapy (TTT), or a combination of these modalities along with the use of intravitreal agents have been tried for small tumors located at the posterior pole.[7,8,9,10,11] The treatment for JRCH is challenging due to collateral laser-induced damage to the optic nerve and surrounding retinal blood vessels. Photodynamic treatment with/without the use of intravitreal antivascular endothelial growth factor (VEGF) has been the standard of care for the treatment of JRCH with good anatomical and functional outcomes.[12,13,14] However, with the current unavailability of verteporfin dye in many countries, we explored the option of indocyanine green-enhanced TTT (ICG-TTT), which would enable a more selective vascular occlusion with less damage to the optic disc. ICG-TTT has been in use for treatment of various intraocular tumors such as RCH, choroidal hemangioma, retinoblastoma, and choroidal melanoma with good tumor control and dose-dependent decrease in TTT fluence threshold as compared to standard TTT.[10,15,16,17,18,19]
In this study, we aimed to report the clinical presentation and investigate the efficacy and safety of ICG-TTT in a series of patients diagnosed with JRCH.
Methods
Study patients
Institutional review board approval was obtained for this study. A prospective, interventional, treatment-naive case series of patients diagnosed with JRCH at a tertiary eye institute from May 2019 to October 2022 were included in this study. The diagnosis was based on a combination of past medical and/or family history of VHL disease, clinical examination, and use of ancillary imaging studies such as fundus photography, fluorescein angiography, optical coherence tomography, and ultrasonography.
Patient characteristics
The following baseline demographic features were recorded for all patients: age, gender, location of the tumor in relation to the optic disc, and presenting best-corrected Snellen’s visual acuity. Tumor dimensions, including the base diameter and height of the lesion, were estimated during clinical examination and confirmed using B-scan ultrasonography. Optical coherence tomography (OCT) scan was performed over the tumor to study its configuration, involvement of retinal layers, growth pattern (endophytic or exophytic), and presence of associated features such as subretinal fluid (SRF), macular edema, and tractional maculopathy. Fluorescein angiography was performed to demonstrate the leakage pattern of the lesion as well as detect any subclinical small lesions located at the posterior pole or in the periphery.
Treatment regimen
All the patients were treated with ICG-TTT along with anti-VEGF injection or dexamethasone implant depending on the tumor location and presence of macular SRF. The dosage was calculated based on 0.5 mg/kg of body weight. Under sterile conditions, 2-mL ICG dye (Aurogreen injection, Aurolabs, India) was injected 45 seconds prior to the application of TTT (Iris Medical OcuLight SLx; Iridex Corporation, Mountain View, CA, USA). Laser settings were adjusted to give retinal greying over the tumor area following 1-minute exposure time by using a spot size of 1.2 mm and power ranging 300–400 mW. The final endpoint was graded in terms of SRF resolution at the macula and reduction in tumor thickness. The patients were examined at 6–8 weeks intervals post laser for determining the tumor response, resolution of SRF, and need for additional interventions. Complete response was defined as a reduction in tumor thickness with or without scarring/fibrosis and absence of SRF; partial response was defined as the presence of SRF at the macula with minimal reduction in tumor thickness. At the last follow-up, final visual acuity and multimodal imaging using fundus photography and OCT were performed to document and grade the treatment response. Treatment-related complications such as an increase in tumor thickness, persistent or recurrent SRF, exudative retinal detachment, and foveal atrophy were noted at the last follow-up.
Outcome measures
The main outcome measures were local tumor control, SRF resolution, and final visual acuity at the last follow-up.
Results
Eight eyes of seven patients (5 males and 2 females) diagnosed to have JRCH were included in the study. The mean age at presentation was 26 years (median: 24 years; range: 5–56 years). Bilateral RCHs were seen in five patients (71%), of which one patient had bilateral JRCH, while in the other four eyes, one eye had small focal RCH and three eyes were pre-phthisical. A positive finding of VHL disease was seen in five patients (71%). The most common presenting complaint was diminution of vision (100%) and floaters (29%). Patient characteristics and clinical features are described in Table 1.
Table 1.
Demography and clinical presenatation of juxtapapillary retinal capillary hemangioblastoma (JRCH)
| Patient | Age (years) | Gender | Eye | VHL disease | Growth | Tumor dimensions (mm) | Optic disc involvement | Angiographic features | OCT features |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subretinal fluid | Macular edema | |||||||||||||||||||
| 1 | 34 | M | BE | Yes | RE-Endophytic LE-Exophytic |
RE- 3×2.5×2 LE- 2×1.5×1 |
RE-Inferotemporal LE-nasal | Early and late leak | Yes (BE) | Yes (BE) | ||||||||||
| 2 | 5 | F | LE | Yes | Exophytic | LE- 8×7 × 4 | >270-degree involvement | Peripapillary leakage | Yes | Yes | ||||||||||
| 3 | 11 | M | LE | No | Exophytic | 4×3.5×2.5 | >270-degree involvement |
Not done | Yes | Yes | ||||||||||
| 4 | 28 | M | LE | Yes | Endophytic | 2×2 × 1 | Temporal | Peripapillary leakage | Yes | Yes | ||||||||||
| 5 | 24 | F | LE | Yes | Exophytic | 1.5×1 × 1 | Temporal | Not done | Yes | No | ||||||||||
| 6 | 56 | M | RE | No | Endophytic | 3×2 × 2 | Superotemporal | Not done | Yes | Yes | ||||||||||
| 7 | 22 | M | LE | Yes | Endophytic | 1.5×1 × 1 | Inferotemporal | Peripapillary leakage | Yes | No | ||||||||||
M: male; F: female; BE: both eyes; RE: right eye; LE: left eye
The most common tumor location was on the temporal aspect of the optic disc (5 eyes), while in two eyes, the tumor extended >270° in the parapapillary area. Tumors exhibited endophytic growth in four eyes and exophytic growth in four eyes. The mean largest basal diameter was 2.9 mm (range: 1–8 mm) and tumor thickness was 1.4 mm (range: 1–4 mm). The best corrected visual acuity ranged from no perception of light (NPL) to 20/20. OCT demonstrated involvement of outer retinal layers in four eyes, with the presence of SRF involving the macula in all eight eyes (100%). Fluorescein angiography performed in four eyes showed extensive peripapillary leakage and pooling in the macular area in the late phases.
All eight eyes were treated with multiple sittings of ICG-TTT after a minimum interval of 6–8 weeks. A mean of three sessions of TTT was required during the study for tumor control (range: 1–7 sessions). Of the eight eyes, six eyes received adjuvant intravitreal injections, including anti-VEGF (bevacizumab) in four eyes and intravitreal dexamethasone implant in two eyes [Table 2]. Post treatment with multiple sittings of ICG-TTT, regression of the tumor with resolution of SRF was seen in six eyes (75%). In two patients (case no. 2 and 3), because of the larger size of the tumor and exudative fluid, there was a suboptimal treatment response with persistent SRF. At the last follow-up (median: 11 months; range: 6–29 months), the best corrected visual acuity remained stable in seven eyes and improved in one eye (hand motion to 20/40). The representative case illustrations showing the fundus photograph and OCT are highlighted in Figs. 1a-l, 2a-h, 3a-h, and 4a-h.
Table 2.
Treatment and visual outcomes
| Patient | Eye | TTT parameters | Number of ICG-TTT sessions | Adjuvant treatment: first line | Treatment response | At presentation BCVA | Final BCVA | Follow-up (months) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||||||||
| Power (mW) | Counters (1 min duration) | Tumor | OCT feature (SRF) | |||||||||||||||||
| 1 | BE | 350 | RE- 9 LE- 5 |
2 | Intravitreal Bevacizumab (BE) | Regressed (BE) | Reduced | RE- 20/60 LE- 20/20 |
RE- 20/60 LE- 20/25 |
6 | ||||||||||
| 2 | LE | 350 | 17 | 7 | Intravitreal dexamethasone implant | Partial | Persisting | LE- CF CF | LE- HM | 15 | ||||||||||
| 3 | LE | 400 | 15 | 2 | Intravitreal dexamethasone implant | Partial | Persisting | LE- CF CF | LE- CF CF | 11 | ||||||||||
| 4 | LE | 300 | 6 | 3 | Intravitreal dexamethasone implant | Regressed | Reduced | LE-20/20 | LE- 20/20 | 6 | ||||||||||
| 5 | LE | 300 | 8 | 1 | None | Regressed | Reduced | LE-20/20 | LE- 20/20 | 29 | ||||||||||
| 6 | RE | 300 | 9 | 2 | None | Regressed | Reduced | RE- HM | RE- 20/40 | 10 | ||||||||||
| 7 | LE | 400 | 2 | 2 | Intravitreal bevacizumab | Regressed | Reduced | LE- 20/20 | LE- 20/20 | 11 | ||||||||||
M: male; F: female; BE: both eyes; RE: right eye; LE: left eye; SRF: subretinal fluid; BVCA: best-corrected visual acuity; CF CF: countinf fingers close to face; HM: hand motions
Figure 1.
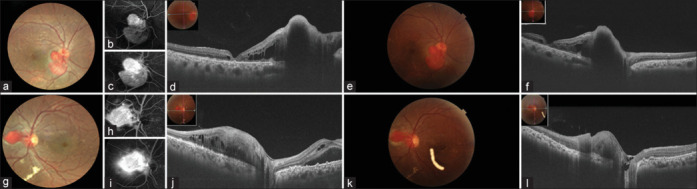
(a–l): A 34-year-old male presented with bilateral JRCH with macular edema with BCVA of 20/60 and 20/20 in OD and OS, respectively. Fluorescein angiography (FA) demonstrated early hyperfluorescence of the tumor vessels and progressive leakage in the late phase of the angiogram, as shown in Figs. 1(b), 1(c), 1(h), and 1(i). He underwent ICG-guided TTT with intravitreal bevacizumab in both eyes. At 6 weeks follow-up, SRF was persisting; hence, a repeat sitting of ICG-TTT with intravitreal dexamethasone implant was given. At 12 weeks, the BCVA was stable with complete resolution of SRF and tumor control
Figure 2.
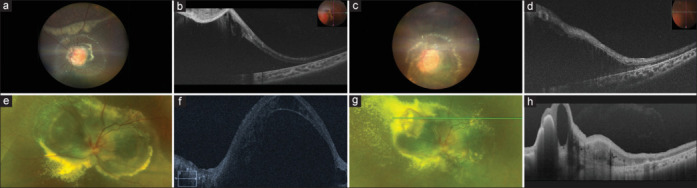
(a–d): A 5-year-old girl presented with small focal peripheral RCH in the right eye and a large JRCH involving >270° of parapapillary area with SRF. Her BCVA was 20/30 in the right eye and CF CF in the left eye. There was complete regression of lesion in the right eye following one sitting of TTT, while multiple ICG-TTT sessions (a total of 7) were required for the left eye every 8–10 weeks interval along with intravitreal injection of dexamethasone. At the last follow-up, the BCVA remained stable with partial regression of lesion and persisting SRF. (e–h): An 11-year-old boy presented with a large JRCH in the left eye. At presentation, his BCVA was CF CF in the left eye. He underwent one sitting of ICG-TTT with intravitreal bevacizumab, following which there was increase tumor exudation and massive inferior exudative retinal detachment. Considering the size, location, and inferior exudative RD, he was referred for external beam radiation treatment (EBRT). At 2 months post treatment, BVCA remained stable with resolving exudative RD and partial tumor regression
Figure 3.
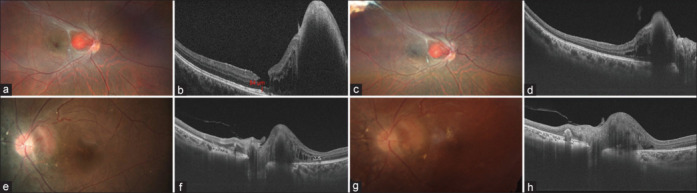
(a–d): A 28-year-old male presented with a small JRCH in the left eye. His BCVA was No PL in the right eye and 20/20 in the left eye. He underwent three sittings of ICG-TTT with an intravitreal dexamethasone implant in the left eye at an interval of 2 months. At the last follow-up, the tumor showed regression with resolution of SRF and stabilization of vision. (e–h): A 24-year-old female was diagnosed to have left eye JRCH with presence of SRF and intraretinal fluid. Her BVCA was No PL in the right eye and 20/20 in the left eye. She was treated with one sitting of ICG-TTT, following which the tumor remained stable with resolution of SRF and stable vision
Figure 4.
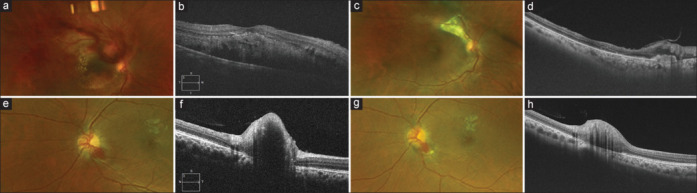
(a–d): A 56-year-old male presented with exudation and subretinal hemorrhage involving the macula in the right eye. A detailed evaluation showed the presence of an exophytic variant of JRCH with BCVA of hand motions in the right eye. The patient was treated with two sittings of ICG-TTT. At the last follow-up, BCVA improved to 20/40 with complete resolution of SRF and regression of the tumor. (e–h): A 22-year-old male presented with a small, solitary JRCH in the left eye involving the inferotemporal quadrant of the optic disc. He has lost vision in the right eye follwing a failed vitreoretinal surgery for RCH. His BCVA was No PL in the right eye and 20/20 in the left eye. He underwent two sittings of ICG-TTT with intravitreal bevacizumab in the left eye. At last follow-up, the the tumor regressed with stabilization of vision
Discussion
The longitudinal progression of retinal capillary hemangiomas over the course of the disease has been shown to result in progressive loss of vision, usually from exudation, macular edema, serous retinal detachment, or secondary glial proliferation leading to epiretinal membrane formation or tractional maculopathy.[5,6,20]
The prevalence of JRCHs has been reported to be between 15% and 20% among VHL patients, with a higher likelihood in younger patients.[6,21] In our series, of the eight eyes, four patients (5 eyes) with VHL disease had JRCH (71%), with one having bilateral JRCH. The mean age of presentation in our series was 26 years (median: 24 years), with the youngest patient being 5 years. Both patients 2 and 3 (aged 5 and 11 years, respectively) had a large JRCH involving more than 270° of the parapapillary retina. The majority of JRCH affects the temporal aspect of the optic nerve, thereby leading to serous macular detachment and progressive loss of vision.[1,6] In our series also, the most common tumor location was on the temporal aspect of the optic disc (63%), with associated retinal features of macular exudation and edema seen in seven eyes and serous detachment in all eight eyes. The tumor growth pattern was endophytic and exophytic in four eyes each with exophytic tumors associated with larger base diameter and thickness and exudative retinal detachment.
The treatment for JRCH remains challenging particularly due to its anatomic location close to the optic disc, macula, and major retinal blood vessels as well as less clearly defined efferent and afferent feeder vessels. Various treatment modalities such as observation, laser photocoagulation, TTT, photodynamic therapy, anti-VEGF, and intravitreal steroid injections in combination or alone have been tried with limited success. Laser photocoagulation is used to treat small tumors of up to 1.5 mm in the peripheral retina but carries additional risk for JRCH because of its proximity to the optic nerve and macula, leading to a large central scotoma in the visual field. Because of inadvertent collateral damage to surrounding structures, PDT due to its selective vascular occlusion of tumor vessels is the standard of care for treatment of JRCH. Studies have reported good anatomical and functional outcomes following the use of PDT for the treatment of JRCH.[8,12,13,22] However, since July 2021, a worldwide shortage of verteporfin (Visudyne®) occurred, resulting in a major impact on the care of ophthalmic patients across the world, thereby disrupting the treatment option of PDT.[23] As an ocular oncologist, we felt an urgent need to look for alternative treatment strategies for various intraocular tumors such as choroidal hemangioma, choroidal melanoma, and JRCH. TTT, a modified laser photocoagulation technique, has been tried in the past for the treatment of RCH by using a low-level heat and a modified diode laser.[10,11,24,25,26] TTT works on the principle of thermotherapy and not direct coagulation of tumor vessels, as seen with argon laser photocoagulation. The effect of thermotherapy leads to the dilation of capillaries and an increase in exudation, which is not a desirable effect; hence, very high power (500–1200 mW) is needed to prevent such complications. However, the high-power usage of TTT, especially for JRCH, would damage the sensory retina and increase retinal traction, thereby compromising final visual acuity. ICG-enhanced TTT has been tried in the past for the treatment of choroidal neovascularization and small choroidal melanomas with good efficacy and safe outcomes.[15,16,25,27] ICG increases the laser uptake within the tumor without increasing the laser intensity, thereby minimizing the diffuse heat generation from the RPE. In our study, we used a very low power (300–400 mW) and used the ICG dye as a photosensitizer to achieve the desired effect without causing any collateral damage to the surrounding structures. Costa et al.[28] studied the effects of photodynamic therapy by using ICG and 810-nm light irradiation on the choriocapillaris and retinal pigment epithelium at different threshold levels with a power of 230 mW/cm2 that caused laser-induced endothelium-bound intraluminal photothrombosis, with no obvious alteration in retinal and choroidal architecture and minimal loss of visual cells, whereas power density >630 mW/cm2 caused choriocapillaris occlusion and endothelial cell cytoplasmic alteration with disruption of retinal pigment epithelium cells and photoreceptor outer segment. Similarly, Peyman et al.[18] demonstrated that intravenous ICG pretreatment can reduce the TTT threshold fluence and irradiance needed to create angiographically visible lesions in normal rabbit choriocapillaris. Kim et al.[29] studied the effect of thermal injury to the optic nerve head by using TTT, which led to increased expression of heat shock proteins (Hsps), thereby demonstrating a neuroprotective effect of TTT on retinal ganglion cells.
In our series, six eyes (75%) showed tumor regression with resolution of SRF following a mean of three sessions of TTT. These results are comparable to PDT treatment, which has shown excellent tumor control and good visual outcomes.[8,13,14] A recent study of nine cases highlighted the use of PDT for the treatment of JRCH, wherein tumor control was achieved in seven of nine tumors (78%), with subretinal fluid resolution in six of nine eyes (67%) and stable or improved visual acuity in seven of nine eyes (78%).[12] In our series also, BCVA remained stable in seven eyes (88%) and improved in one eye till last follow-up. Recent studies have shown increased VEGF expression in patients with RCH, thereby exploring the use of adjuvant anti-VEGF injections in the treatment of RCH and its associated tumor-related sequelae such as macular edema, SRF, or exudation.[12,30,31,32,33] However, the long-term results have been variable with a transient reduction in SRF. In our series, six eyes received adjuvant intravitreal injections of either anti-VEGF (4 eyes) or sustained-release dexamethasone implant (4 eyes) at various time points, showing temporary reduction in the SRF and macular edema. Unfortunately, in two of our patients, there was a dramatic increase in SRF post ICG-TTT despite receiving adjuvant intravitreal injections. This has been reported previously related to an increase in intraocular tumor leakage (exudative response) following laser or PDT treatment.[12,34] One of our patients had massive exudative retinal detachment following two sessions of ICG-TTT and hence was referred for external beam radiation treatment (22.5 Gy dose delivered in 7.5 Gy/fraction). At 3 months follow-up, the tumor showed partial tumor regression with resolution of exudative detachment.
This study has several limitations, including a small sample size and surgeon-related variability of treatment combinations. TTT is known to cause damage to the optic nerve and macula structures; however, we have not performed optic nerve function tests such as HVF, which could have provided some insights related to ONH function, and we accept this as a limitation of the paper. Considering the rare nature and presentation of JRCH, this prospective interventional case series highlights the clinical presentation and treatment outcomes. Furthermore, our series provides a novel opportunity to study the efficacy of ICG-TTT in a prospective cohort of treatment-naïve patients.
Conclusion
In this prospective interventional case series, we analyzed the potential use of multiple sessions of ICG-TTT along with adjuvant anti-VEGF agents as an alternative treatment option for JRCH with effective local tumor control, SRF resolution, and vision stabilization. However, larger tumors associated with severe exudation and leakage have an overall poor prognosis. In the future, larger studies are necessary to study the long-term outcomes of this novel treatment.
Statement of ethics
Institutional Review Board approval was obtained at L V Prasad Eye Institute (LEC -BHR-P-10-21-762).
Financial support and sponsorship:
This work was supported by an unrestricted departmental grant from Hyderabad Eye Research Foundation, Hyderabad, India
Conflicts of interest:
There are no conflicts of interest.
References
- 1.Gass JD, Braunstein R. Sessile and exophytic capillary angiomas of the juxtapapillary retina and optic nerve head. Arch Ophthalmol. 1980;98:1790–7. doi: 10.1001/archopht.1980.01020040642011. [DOI] [PubMed] [Google Scholar]
- 2.Singh AD, Shields CL, Shields JA. von Hippel-Lindau disease. Surv Ophthalmol. 2001;46:117–42. doi: 10.1016/s0039-6257(01)00245-4. [DOI] [PubMed] [Google Scholar]
- 3.Webster AR, Maher ER, Moore AT. Clinical characteristics of ocular angiomatosis in von Hippel-Lindau disease and correlation with germline mutation. Arch Ophthalmol. 1999;117:371–8. doi: 10.1001/archopht.117.3.371. [DOI] [PubMed] [Google Scholar]
- 4.Singh AD, Nouri M, Shields CL, Shields JA, Perez N. Treatment of retinal capillary hemangioma. Ophthalmology. 2002;109:1799–806. doi: 10.1016/s0161-6420(02)01177-6. [DOI] [PubMed] [Google Scholar]
- 5.Kreusel KM, Bechrakis NE, Krause L, Neumann HP, Foerster MH. Retinal angiomatosis in von Hippel-Lindau disease: A longitudinal ophthalmologic study. Ophthalmology. 2006;113:1418–24. doi: 10.1016/j.ophtha.2006.02.059. [DOI] [PubMed] [Google Scholar]
- 6.McCabe CM, Flynn HW, Jr, Shields CL, Shields JA, Regillo CD, McDonald HR, et al. Juxtapapillary capillary hemangiomas. Clinical features and visual acuity outcomes. Ophthalmology. 2000;107:2240–8. doi: 10.1016/s0161-6420(00)00422-x. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt D, Natt E, Neumann HP. Long-term results of laser treatment for retinal angiomatosis in von Hippel-Lindau disease. Eur J Med Res. 2000;5:47–58. [PubMed] [Google Scholar]
- 8.Sachdeva R, Dadgostar H, Kaiser PK, Sears JE, Singh AD. Verteporfin photodynamic therapy of six eyes with retinal capillary haemangioma. Acta Ophthalmol. 2010;88:e334–40. doi: 10.1111/j.1755-3768.2010.02008.x. [DOI] [PubMed] [Google Scholar]
- 9.Welch RB. Von Hippel-Lindau disease: The recognition and treatment of early angiomatosis retinae and the use of cryosurgery as an adjunct to therapy. Trans Am Ophthalmol Soc. 1970;68:367–424. [PMC free article] [PubMed] [Google Scholar]
- 10.Parmar DN, Mireskandari K, McHugh D. Transpupillary thermotherapy for retinal capillary hemangioma in von Hippel-Lindau disease. Ophthalmic Surg Lasers. 2000;31:334–6. [PubMed] [Google Scholar]
- 11.Mochizuki Y, Noda Y, Enaida H, Hata Y, Ueno A, Yoshikawa H, et al. Retinal capillary hemangioma managed by transpupillary thermotherapy. Retina (Philadelphia, Pa) 2004;24:981–4. doi: 10.1097/00006982-200412000-00028. [DOI] [PubMed] [Google Scholar]
- 12.Di Nicola M, Williams BK, Jr, Hua J, Bekerman VP, Mashayekhi A, Shields JA, et al. Photodynamic therapy for retinal hemangioblastoma: Treatment outcomes of 17 consecutive patients. Ophthalmol Retina. 2022;6:80–8. doi: 10.1016/j.oret.2021.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Lorenzo D, Dias B, Caminal JM. Regression of a juxtapapillary retinal capillary hemangioma after verteporfin photodynamic therapy. J Fr Ophtalmol. 2021;44:600–1. doi: 10.1016/j.jfo.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Papastefanou VP, Pilli S, Stinghe A, Lotery AJ, Cohen VM. Photodynamic therapy for retinal capillary hemangioma. Eye (London, England) 2013;27:438–42. doi: 10.1038/eye.2012.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Potter P, Jamart J. Adjuvant indocyanine green in transpupillary thermotherapy for choroidal melanoma. Ophthalmology. 2003;110:406–13. doi: 10.1016/S0161-6420(02)01560-9. discussion 413-404. [DOI] [PubMed] [Google Scholar]
- 16.Hasanreisoglu M, Saktanasate J, Schwendeman R, Shields JA, Shields CL. Indocyanine green-enhanced transpupillary thermotherapy for retinoblastoma: Analysis of 42 tumors. J Pediatr Ophthalmol Strabismus. 2015;52:348–54. doi: 10.3928/01913913-20150929-17. [DOI] [PubMed] [Google Scholar]
- 17.Kamal A, Watts AR, Rennie IG. Indocyanine green enhanced transpupillary thermotherapy of circumscribed choroidal haemangioma. Eye (London, England) 2000;14:701–5. doi: 10.1038/eye.2000.187. [DOI] [PubMed] [Google Scholar]
- 18.Peyman GA, Genaidy M, Yoneya S, Men G, Ghahramani F, Kuo PC, et al. Transpupillary thermotherapy threshold parameters: Effect of indocyanine green pretreatment. Retina (Philadelphia, Pa) 2003;23:378–86. doi: 10.1097/00006982-200306000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Raval V, Tyagi M, Chhablani J, Kaliki S, Reddy R, Das T. Understanding the structural changes following photodynamic and transpupillary thermotherapy for choroidal hemangioma using optical coherence tomography and optical coherence tomography angiography. Indian J Ophthalmol. 2019;67:2023–8. doi: 10.4103/ijo.IJO_962_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toy BC, Agrón E, Nigam D, Chew EY, Wong WT. Longitudinal analysis of retinal hemangioblastomatosis and visual function in ocular von Hippel-Lindau disease. Ophthalmology. 2012;119:2622–30. doi: 10.1016/j.ophtha.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garg G., Finger P.T. Visual prognosis based staging for retinal capillary hemangioma. Russ Ophthalmol J. 2020;13:12–7. [Google Scholar]
- 22.Schmidt-Erfurth UM, Kusserow C, Barbazetto IA, Laqua H. Benefits and complications of photodynamic therapy of papillary capillary hemangiomas. Ophthalmology. 2002;109:1256–66. doi: 10.1016/s0161-6420(02)01059-x. [DOI] [PubMed] [Google Scholar]
- 23.Sirks MJ, van Dijk EHC, Rosenberg N, Hollak CEM, Aslanis S, Cheung CMG, et al. Clinical impact of the worldwide shortage of verteporfin (Visudyne®) on ophthalmic care. Acta Ophthalmol. 2022;100:e1522–32. doi: 10.1111/aos.15148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pochop P, Kodetova M, Dotrelova D. Treatment of retinal capillary hemangioma using 810 nm infrared laser. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2018 doi: 10.5507/bp.2018.019. doi: 10.5507/bp.2018.019. [DOI] [PubMed] [Google Scholar]
- 25.Costa RA, Meirelles RL, Cardillo JA, Abrantes ML, Farah ME. Retinal capillary hemangioma treatment by indocyanine green-mediated photothrombosis. Am J Ophthalmol. 2003;135:395–8. doi: 10.1016/s0002-9394(02)01966-9. [DOI] [PubMed] [Google Scholar]
- 26.Shields JA. Discussion by Jerry A. Shields, MD. Ophthalmology. 2000;107:54. [Google Scholar]
- 27.Kim JE, Shah KB, Han DP, Connor TB., Jr Transpupillary thermotherapy with indocyanine green dye enhancement for the treatment of occult subfoveal choroidal neovascularization in age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2006;37:272–7. doi: 10.3928/15428877-20060701-02. [DOI] [PubMed] [Google Scholar]
- 28.Costa RA, Farah ME, Freymüller E, Morales PH, Smith R, Cardillo JA. Choriocapillaris photodynamic therapy using indocyanine green. Am J Ophthalmol. 2001;132:557–65. doi: 10.1016/s0002-9394(01)01138-2. [DOI] [PubMed] [Google Scholar]
- 29.Kim SJ, Kim YJ, Park KH. Neuroprotective effect of transpupillary thermotherapy in the optic nerve crush model of the rat. Eye (London, England) 2009;23:727–33. doi: 10.1038/eye.2008.189. [DOI] [PubMed] [Google Scholar]
- 30.Liang X, Shen D, Huang Y, Yin C, Bojanowski CM, Zhuang Z, et al. Molecular pathology and CXCR4 expression in surgically excised retinal hemangioblastomas associated with von Hippel-Lindau disease. Ophthalmology. 2007;114:147–56. doi: 10.1016/j.ophtha.2006.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ach T, Thiemeyer D, Hoeh AE, Schaal KB, Dithmar S. Intravitreal bevacizumab for retinal capillary haemangioma: Longterm results. Acta Ophthalmol. 2010;88:e137–8. doi: 10.1111/j.1755-3768.2009.01594.x. [DOI] [PubMed] [Google Scholar]
- 32.Ziemssen F, Voelker M, Inhoffen W, Bartz-Schmidt KU, Gelisken F. Combined treatment of a juxtapapillary retinal capillary haemangioma with intravitreal bevacizumab and photodynamic therapy. Eye (London, England) 2007;21:1125–6. doi: 10.1038/sj.eye.6702896. [DOI] [PubMed] [Google Scholar]
- 33.Wong WT, Liang KJ, Hammel K, Coleman HR, Chew EY. Intravitreal ranibizumab therapy for retinal capillary hemangioblastoma related to von Hippel-Lindau disease. Ophthalmology. 2008;115:1957–64. doi: 10.1016/j.ophtha.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim HM, Park KH, Woo SJ. Massive exudative retinal detachment following photodynamic therapy and intravitreal bevacizumab injection in retinal capillary hemangioma. Korean J Ophthalmol. 2015;29:143–5. doi: 10.3341/kjo.2015.29.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]


