Abstract
The gene therapy approach for retinal disorders has been considered largely over the last decade owing to the favorable outcomes of the US Food and Drug Administration-approved commercial gene therapy, Luxturna. Technological advances in recent years, such as next-generation sequencing, research in molecular pathogenesis of retinal disorders, and precise correlations with their clinical phenotypes, have contributed to the progress of gene therapies for various diseases worldwide, and more recently in India as well. Thus, considerable research is being conducted for the right choice of vectors, transgene engineering, and accessible and cost-effective large-scale vector production. Many retinal disease-specific clinical trials are presently being conducted, thereby necessitating the collation of such information as a ready reference for the scientific and clinical community. In this article, we present an overview of existing gene therapy research, which is derived from an extensive search across PubMed, Google Scholar, and clinicaltrials.gov sources. This contributes to prime the understanding of basic aspects of this cutting-edge technology and information regarding current clinical trials across many different conditions. This information will provide a comprehensive evaluation of therapies in existing use/research for personalized treatment approaches in retinal disorders.
Keywords: Clinical trials, inherited retinal disorders, retinal gene therapy, viral vectors
The retina is an intricate structure that converts light into electrical signals that enable the perception of vision. Several genes playing roles in mechanisms such as retinal architecture, development, function, maintenance, and survival are implicated in retinal disorders.[1] Various retinal diseases and their subtypes [Fig. 1] compromise vision, often leading to partial or complete blindness.[2] Recent developments in personalized health care are being discussed as new avenues for a patient-prioritized treatment strategy. Gene therapy (GT) is used to treat genetic disorders of high severity and less prevalence.[3] It aims to treat genetic disorders by modifying faulty genes or replacing them with a healthy gene to mitigate pathologic effects with long-lasting, persistent benefits and does not require repeated interventions.[4] It can treat a broad range of genetic and acquired diseases,[5] including certain types of inherited retinal disorders (IRDs),[6] as well as age-related degenerative diseases.[7] Our review collates the status of the emerging trials in retinal GT.
Figure 1.
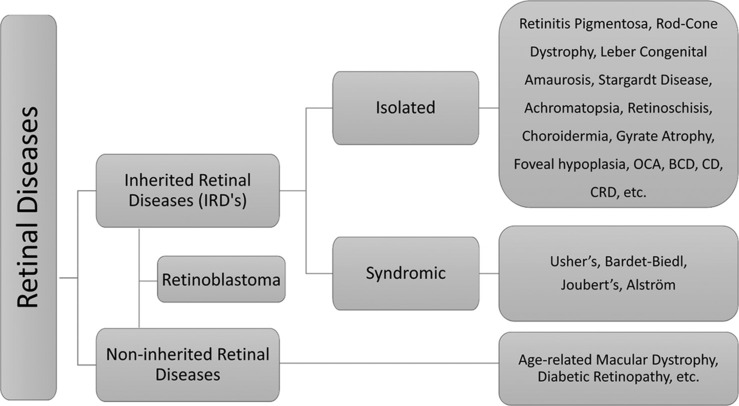
Categories of retinal diseases with examples. BCD = Bietti’s crystalline dystrophy, CD = cone dystrophy, CRD = cone–rod dystrophy, OCA = oculocutaneous albinism
Method of Literature Review
We conducted an extensive literature search for this article using PubMed, Google Scholar, and https://clinicaltrials.gov. We utilized keywords such as “retina,” “gene therapy,” “non-viral vectors”, “viral vectors,” “ and “retinal disorders” for finding relevant review articles, original research, book chapters, and web reports. We also considered references within these sources and did not restrict the search by publication year. Fig. 2 depicts the increasing trajectory of clinical trials dedicated to retinal gene therapy throughout the years.
Figure 2.
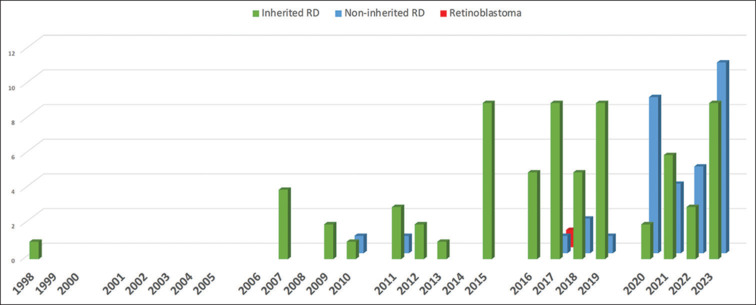
Bar graph representing increase in clinical trial numbers in retinal gene therapy over the years as listed in clinicaltrials.gov until September 2023
IRDs encompass rare genetic conditions that primarily affect the neurosensory retina, retinal pigment epithelium (RPE), and sometimes the choroid. Gene mutations affecting the retinal structure, function, and/or maintenance cause these conditions.[8] Mutations may be inherited via autosomal dominant or autosomal recessive or X-linked or mitochondrial mode.[9] These conditions can be stationary or progressive, and the latter are typically characterized by progressive retinal degeneration, leading to vision impairment or social blindness.[10] These are further classified into non-syndromic IRDs having exclusive ocular presentation and syndromic IRDs with ocular and extraocular manifestations. Non-inherited retinal disorders (NIRDs) include age-related macular degeneration (AMD), diabetic retinopathy (DR), etc., The following sections report and describe IRDs that have been considered for GT as per https://clinicaltrials.gov.
Non-syndromic IRDs
Retinitis pigmentosa
Retinitis pigmentosa (RP) causes photoreceptor (PR) degeneration starting in the mid-peripheral retina. It typically starts with night/dark vision problems with progressive narrowing of the field of vision. Common genes implicated are CERKL, EYS, and PROM1. GT trials for RP often target specific gene mutations that cause the disease. Promising clinical trials involving the RPE65 gene have shown improvements in patient vision. Thirty-three US Food and Drug Administration (FDA)-approved trials have been listed [Table S1 (219.7KB, pdf) ], and multiple ongoing trials aim to expand the purview of gene therapies for RP.[11]
Leber congenital amaurosis
Leber congenital amaurosis (LCA) is a severe IRD that affects infants and young children, resulting in blindness from birth. CEP290, RPE65, and GUCY2D gene mutations, among others, cause LCA.[12] Ground-breaking GT trials have demonstrated remarkable success in treating some LCA subtypes.[13] Among these Luxturna (Spark Therapeutics, Philadelphia, PA, United States) is an FDA-approved GT that targets the RPE65 gene and has shown considerable improvement offering hope for those affected by this debilitating condition.[12] Despite its excellent potential, Luxturna faces significant hurdles including its exorbitant price tag, approximately US$ 850,000 per eye, which raises concerns about affordability and accessibility.[6] Twenty-one approved LCA clinical trials are listed at https://clinicaltrials.gov [Table S1 (219.7KB, pdf) ].
Stargardt disease
Stargardt disease (STGD) is an IRD that mostly affects children and young adults. It leads to central vision loss caused by PR degeneration starting at the macula. The most commonly implicated genes are ABCA4 followed by PROM1 and ELOVL4.[14] Only one GT trial reported for STGD (https://clinicaltrials.gov) [Table S1 (219.7KB, pdf) ] aims to halt or reverse this degeneration. Several ongoing strategies investigate different gene editing techniques and vectors for the delivery of therapeutic genes.[15]
Achromatopsia
Achromatopsia (ACHM) is an IRD characterized by stationary cone dysfunction with severe loss of color vision. Common genes implicated are CNGB3, CNGA3, GNAT2, PDE6C, and PDE6H.[16] Six GT trials for ACHM have been listed so far [Table S1 (219.7KB, pdf) ].
Gyrate atrophy
Gyrate atrophy (GA) is an autosomal recessive condition wherein increase in ornithine levels in the plasma due to ornithine aminotransferase enzyme deficiency is caused by mutations in its gene (OAT).[17] GA occurs within the first two decades of life and involves chorioretinal atrophic patches starting from the mid-peripheral retina, spreading to the central retina, and presenting with nyctalopia, visual field constriction, and central vision diminution due to macular edema in most cases. The earliest clinical trial for GT for any retinal dystrophy was a phase-1 safety and efficacy study on ex-vivo transfer of keratinocytes to treat gyrate atrophy, listed in 1998 [Table S1 (219.7KB, pdf) ]. This approach involved taking a skin biopsy, gene transfer into the biopsy, and these cells with increased OAT protein are grafted back.
Bietti’s crystalline dystrophy
Bietti’s crystalline dystrophy (BCD) is an autosomal recessive IRD causing predominant retinal, sometimes corneal, degeneration, with classic crystalline deposits and sometimes sclerosis of choroidal vessels. The genetic cause for this disorder is mutations in CYP4V2 gene, This gene gives instructions to make the steroid and fatty acid metabolism-associated family of enzymes cytochrome P450.[18] Four GT clinical trials are listed for BCD [Table S1 (219.7KB, pdf) ].
Choroideremia
Choroideremia (CHM) is an X-linked rare IRD characterized by progressive chorioretinal degeneration causing symptoms like nyctalopia and loss of peripheral vision, with preservation of central vision up to late adulthood.[19] The gene CHM that codes Rab Escort Protein-1 (REP-1) causes CHM. Nine GT clinical trials have been listed for CHM.[20] Adeno-associated virus (AAV)-mediated GT for CHM is a feasible venture due to the gene’s small size (~1.9 kb) and macular region preservation in the advanced disease stages.
Leber hereditary optic neuropathy
Leber hereditary optic neuropathy (LHON) is a rare mitochondrially inherited ganglion cell and optic nerve disorder. It is caused by NADH dehydrogenase MT-ND1, MT-ND4, and MT-ND6 gene mutations. These genes play a major role in mitochondrial respiratory chain. The disorder primarily affects the retinal ganglion cells (RGCs) that are selectively vulnerable to mitochondrial dysfunction.[21] Two MT-ND4 clinical trials are listed so far [Table S1 (219.7KB, pdf) ].
Juvenile X-linked retinoschisis
Juvenile X-linked retinoschisis is typically an X-linked IRD affecting male children. In this condition, retinoschisin 1 (RS1) gene mutation causes unusual splitting of retinal layers, predominantly in the macula and sometimes in the peripheral retina.[22] RS1 provides lateral adhesion of cells. Three clinical trials are listed for retinoschisis so far.[22]
Syndromic IRDs
Usher syndrome
Usher syndrome (US) is characterized by auditory loss and retinal degeneration. It has several subtypes based on the genes involved and clinical presentation. Common genes implicated are USH2A and MYO7A. Clinical trials of GT explore strategies to restore both hearing and vision in affected individuals. Six GT trials are listed [Table S1 (219.7KB, pdf) ]. These trials often involve simultaneous targeting of associated genes.[23]
Bardet–Biedl syndrome
Bardet–Biedl syndrome, an autosomal recessively inherited IRD impacting multiple body systems, including the genitalia, brain, kidneys, and eye, is caused by mutations in genes involved in primary cilia function. Frequently implicated genes are BBS1, BBS10, and BBS2. The common feature in patients is a severe rod–cone dystrophy phenotype. Other associated systemic features include cognitive impairment, renal abnormalities, hypogonadism/genitourinary anomalies, truncal obesity, postaxial polydactyly, etc.[24] AXV101, the lead program developed by Axovia Therapeutics, is an AAV (AAV9)-based GT specifically targeting BBS1 gene mutation-carrying patients.[25]
Non-inherited retinal disorders
The following sections report and describe multifactorial retinal disorders that have been considered for GT as per https://clinicaltrials.gov.
Age-related macular degeneration
AMD causes vision loss in older adults. While treatment for wet AMD involves anti-vascular endothelial growth factor (anti-VEGF) drugs, dry AMD currently has no cure. GT clinical trials for AMD explore approaches to slow disease progression.[26] Twenty wet AMD and four dry AMD clinical trials listed [Table S1 (219.7KB, pdf) ] involve therapeutic gene delivery to retinal cells.[27] ADVM-022 is a GT trial that utilizes AAV2.7m8 equipped with a potent, widespread expression system containing a codon-optimized cDNA of the aflibercept protein. This offers the promise of addressing neovascularization, which occurs before vision impairment in individuals with wet AMD, making it a potential consideration for therapy.[28] Reid et al.[29] described a wet AMD GT approach involving recombinant AAV-based anti-VEGF treatment. This therapy aims to prevent choroidal neovascularization by inducing Eylea (aflibercept) overexpression. In another study, a breakthrough approach involved delivering an optimized NADH-ubiquinone oxidoreductase (NDI1) gene using AAV vector. This GT was orchestrated by a specific promoter VMD2, which is specific to the RPE cells. The results significantly enhanced mitochondrial function, with notable functional improvements in vivo. This stands as the first example of GT directly targeting mitochondrial function.[30]
Diabetic retinopathy
Diabetic retinopathy is a chronic and progressive condition characterized by the onset of microvascular alterations within retinal tissues, primarily induced by prolonged hyperglycemia.[31] In its advanced stages, DR can lead to severe impairment of vision or blindness. Early detection through regular ophthalmic examinations is imperative as timely interventions involving laser photocoagulation, intravitreal anti-VEGF therapy, or surgical procedures can effectively curtail disease progression.[32]
Diabetic macular edema (DME) is a pathologic condition stemming from diabetes-induced vascular damage.[33] Timely identification and intervention via therapies such as laser photocoagulation, anti-VEGF agent intravitreal injections, or surgical procedures are imperative to mitigate progressive vision impairment. Currently, eight clinical trials are listed for DR and 13 trials are reported for DME [Table S1 (219.7KB, pdf) ].
Retinoblastoma
Retinoblastoma is considered the most frequent childhood ocular malignancy. It is caused by RB1 gene mutations, causing inactivation of both its alleles, leading to an RB that is defective, further causing uncontrolled cell proliferation and impairment of the cell cycle.[34] An oncolytic adenovirus, VCN-01, was designed to selectively replicate in tumor cells. This is suggestive of a tumor-specific, chemotherapy-independent treatment.[35] A reported RB clinical trial began in 2017 and was completed in 2022.
Gene Transfer Modalities in Use for Retinal Diseases
Retinal GT is delivered in multiple ways using various vehicles as discussed below and illustrated in Fig. 3.[36,37] It must be noted that the genetic element of the treatment itself has three categories: gene augmentation, gene editing, and gene silencing.[38] The most common modality is gene augmentation, which is used for autosomal recessive conditions, X-linked conditions, and polygenic/multifactorial diseases. Here, the normal gene copy produces a protein that makes up for the functional loss. Gene editing and silencing are suitable for dominant conditions or gene mutations, where supplying a normal gene copy would not be useful since the dominant mutant protein’s effect needs to be nullified. Gene editing (including base and prime editing) is done by Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-Cas enzyme systems, which target a specific site on the DNA to make modifications/corrections/deletions, thereby removing the disease cause. Gene silencing and exon skipping methods can alter the expression status of mutant alleles or dominant causative molecular pathways to bring about the therapeutic effect. To deliver the genetic element, an effective vector is required. Finally, selecting the appropriate route of delivery of a GT vector and patient selection are the critical components of therapeutic success. These include subretinal, intravitreal, and suprachoroidal routes [Fig. 3]. Of these, the subretinal delivery route has been the choice for most of the IRD treatments.
Figure 3.
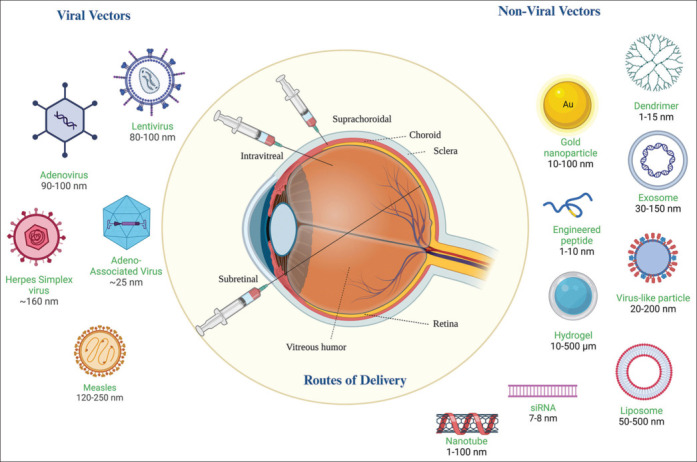
Classification of gene therapy vectors and different delivery routes; illustration created by Biorender.com
A suitable vector serves as a carrier to transport the genetic material safely and effectively without side effects.[39,40] The vector must also be nontoxic and should enable long-term gene expression in target tissues/cells. In the following sections, we describe different categories of vectors [Tables 1 and 2] currently used for therapeutic gene delivery:
Table 1.
Viral vectors used in retinal gene therapy clinical trials
| Viral vectors | Target disease | Gene | Ongoing clinical trials | |||
|---|---|---|---|---|---|---|
| Adenovirus | Retinoblastoma | RB1 | NCT03284268[104] | |||
| Lentivirus | Diseases of the retina, choroidal neovascularization (nAMD, DME, RVO-ME) | Cas9 mRNA and expression cassettes for a guide RNA targeting VEGF-A | NCT05099094[35,105] | |||
| AAV | wAMD, nAMD, DMR/DR, RP, X-linked retinoschisis, non-syndromic retinitis pigmentosa, X-linked retinitis pigmentosa | Anti-VEGF, aflibercept, RS1, PDE6B, RPGR | NCT05984927, NCT05536973, NCT05930561, NCT05748873, NCT05657301, NCT06066008, NCT05672121, NCT05197270, NCT03326336, NCT03328130, NCT03316560[28,106,107] | |||
| Retrovirus | GA | OAT | NCT00001735[108,109] | |||
| Herpes simplex virus | NA | NA | NA | |||
| Measles virus | NA | NA | NA | |||
| Vaccinia virus | NA | NA | NA |
AAV=Adeno-associated virus, DME=Diabetic macular edema, DR=Diabetic retinopathy, GA=Gyrate atrophy, RP=Retinitis pigmentosa, RVO-ME=Retinal vein occlusion – Macular edema, nAMD=Neovascular Age related macular degeneration
Table 2.
List of nonviral vectors used in retinal gene therapy
| Nonviral vectors | Target disease | Gene | Ongoing clinical trials | |||
|---|---|---|---|---|---|---|
| Liposomes | NA | NA | ||||
| Dendrimer | nAMD, DME | Aflibercept | NCT05387837, NCT05105607 | |||
| Exosomes | NA | NA | ||||
| CRISPR-Cas9 ribonucleoproteins | LCA | CEP290 | NCT03872479[110] | |||
| Hydrogels | NA | NA | ||||
| Nanotubes | NA | NA |
LCA=Leber congenital amaurosis
Viral vectors
Nonviral vectors.
Viral vectors
-
Adenovirus vectors:
Adenovirus vectors are promising tools in GT, offering unique advantages and challenges.[41] These nonenveloped, double-stranded DNA viruses with good transduction ability accommodate large DNA payloads (~40 kb) and can be administered in retinal cells.[42] In a study, recombinant adenovirus (rAd) carrying the MERTK gene to treat RPE defects showed increased PR count in the regions injected and enhanced PR function lasting for 1 month posttreatment. Adenoviruses can effectively infect dividing and nondividing cells, which is a crucial feature in the postmitotic retina. Another study showed that adenovirus vectors (Ad-ABCA1) were utilized for developing a targeted approach to treat glaucoma. Full-length human ABCA1 gene was delivered, following which the upregulation of ABCA1 resulted in a notable decline in the nuclear translocation of ANXA1 experimentally in vivo and in vitro. This reduction in ANXA1 nuclear localization was concomitant with a significant amelioration of retinal degeneration and RGCs’ preservation following ischemia–reperfusion injury.[43]
-
Retrovirus vectors:
These are the first viral vectors listed for in vivo GT. These RNA-carrying vectors perform reverse transcription of their genetic material to form double-stranded DNA, leading to host genome integration,[44] ensuring that the therapeutic gene becomes constitutive of the target cell’s genome.[45] In addition, retrovirus vectors show promise in targeting specific cells, such as PR or RPE cells, which is critical for addressing various retinal diseases. However, the integration process that makes retroviruses attractive for long-term expression also poses potential risks. Random integration could cause insertional mutagenesis, where insertion of the therapeutic gene disrupts the function of existing genes, potentially causing adverse effects.[46]
-
Lentivirus vectors:
Lentiviruses belong to the retrovirus group and are commonly applied for long-term GT. Lentivirus vectors are used in retinal GT because they target specific retinal cell types.[14] With advancements in vector design, these can be tailored to deliver genes to specific retinal cells[47] and achieve stable and long-term gene expression. A lentivirus enables the integration of the therapeutic gene into the host genome. This feature is useful for inherited diseases that require lifelong gene expression, such as RP. They can also deliver large and complex genetic payloads, which is significant for retinal GT as many retinal genetic disorders involve multiple genes or require the delivery of large therapeutic constructs.[47]
-
AAV vectors:
These are nonpathogenic viruses providing stable, long-term gene expression without integration into the host genome.[40] They are often used in clinical trials owing to their safety and efficacy. AAVs are a versatile and highly effective tool in GT.[48] These are ~25-nm, nonenveloped viruses of the parvovirus family and have garnered significant attention for their safety, efficiency, and targeted gene delivery capabilities. Phylogenetic analysis has classified AAV into 12 serotypes with >108 isolates known as serovars.[49,50] Serotypes 1, 3, 4, 7, 8, 10, and 11 were initially found in non-human primates, while serotypes 2, 5, 6, and 9 originated in humans. This classification aids in understanding the evolutionary relationships and is significant for applications like GT.[50] AAV capsids can also be engineered to selectively transduce specific cell types[51] such as PR or RPE cells.[52] This precision-focused strategy minimizes off-target effects, reduces immunogenic responses, and maximizes therapeutic efficacy.[53] However, some challenges are associated with AAV vectors in retinal GT. One of them is their limited packaging capacity (~4.7 kb) restricting the deliverable genetic payload size,[54] which may be a limitation in treating disorders caused by large genes or requiring complex genetic constructs.[55] Dual AAV or split gene vectors such as hybrid dual AAVs are well suited for delivering large therapeutic genes,[49] including genome-editing tools like CRISPR-Cas9.[56] Although toxic immune responses to AAVs are rare, cross-reactive resistance in individuals reduces the efficacy of these vectors.
Nonviral vectors
These are synthetic carriers of therapeutic nucleic acids into host cells. These vectors are designed to efficiently deliver genes, minimizing immunogenicity and viral vector–associated safety concerns.[57] They encompass lipids, polymeric nanoparticles (NPs) like poly (lactic) acid, and poly (lactide-co-glycolide) (PLGA) NPs gene transfer.[58] Similarly, in ARPE-19 cells, PLGA NPs improved the intracellular delivery of a VEGF antisense oligonucleotide, leading to a reduction in both VEGF secretion and mRNA expression.[59] Physical methods like electroporation, etc., are all aimed at improving controlled gene transfer for therapeutic purposes.[60]
Plasmid DNA: Circular DNA (plasmids) with the therapeutic transgene can directly be introduced into target cells through physical or chemical methods. In a previous paper, pure plasmid DNA (pDNA:pCMV-lacZ) expression was better than adenovirus (avMena-lacZ) and retrovirus (rvMoMULV-lacZ) injections in mature tibialis anterior muscles of mice, while the differences (>10%) were not significant in regenerative muscle.[61] Likewise, few more studies suggest the use of pDNA as a vector system for GT.[62]
RNA-based gene modulation
The types of gene modulation therapies explored based on RNA are single-stranded antisense oligonucleotides (AONs), double-stranded small interfering RNAs, mRNAs and aptamers.[63] AONs are therapeutic approaches for treatment of certain IRD subtypes. An advantage is that they act at the RNA level and do not alter the genome.[64] NCT05176717 is a clinical trial listed as an RNA-based therapy, QR-421 for treating Usher’s Syndrome has been considered a promising option for the future of clinical development.
Gene Editing Vectors
CRISPR-Cas9 Vectors: CRISPR and CRISPR-associated proteins (Cas) are used to perform precise gene editing by introducing, modifying, or deleting specific genomic sequences.[65] Studies show the achievement of efficient gene modification by delivering a CRISPR/SpCas9 construct in the retina through AAV7m8-mediated delivery.[66] CRISPR-Cas9 technology was used to rescue endoplasmic reticulum stress associated with mutant myocilin gene (MYOC), causing decreased intraocular pressure and prevention of glaucomatous damage in mice and in vitro trabecular meshwork cell and skin fibroblast models.[67]
Zinc finger nuclease (ZFN) vectors: ZFNs are engineered proteins that are applied to specifically target and edit DNA sequences.[68] In a previous study, designed ZFNs could drive site-specific incorporation of long stretches of DNA into a predetermined genomic locus. Segments of DNA between 12 base pairs and ~8 kilo bases of length were shown to integrate with high frequency.[69]
Transcription activator-like effector nuclease (TALEN) vectors: TALENs are custom-designed proteins that can be applied in targeted gene editing. They feature a central DNA-binding region with tandem repeats, typically consisting of 34 amino acids. The sequence within these repeats is close to identical, except in the case of variable amino acid residues at 12 and 13 positions. These are called repeat variable di-residues that dictate the sequential specificity of base recognition.[70]
Exosome-based vectors
These tiny vesicles, naturally secreted by cells, can carry specific genes or therapeutic molecules, allowing targeted delivery to cells or tissues.[71] In an exosome-based study (Exo-AAV2), the green fluorescent protein (GFP) gene showed deep penetration in the retina, primarily extending into the inner nuclear layer (INL) and the outer plexiform layer, with limited presence in the outer nuclear layer, establishing Exo-AAV2 as a reliable tool in the mouse retina.[72]
Dendrimers
Dendrimers are highly branched macromolecules that offer precise targeting by attaching ligands to target specific types of retinal cells, reducing off-target effects.[73] Previous reports suggest systemic delivery of hydroxyl-terminated polyamidoamine dendrimer–triamcinolone acetonide conjugate in rats, with selective uptake by injured microglia/macrophage and RPE.[74] Both intravitreal and systemic administration effectively target activated retinal microglia, demonstrating excellent biodistribution.[75] The potential use of dendrimer-based drug formulations for treating retinal diseases associated with microglial activation, such as AMD, DR, and retinal degenerations.[76] Recently, Ashvattha Therapeutics (Redwood City, CA, USA) has announced enrollment of the first patient in clinical trial (Phase 1/2). Its release highlights [18F] OP-801 as an imaging agent based on hydroxyl dendrimers, which have the unique capability of specifically targeting reactive macrophages and microglia.[75]
Lipid-based vectors
Liposomes have an internal aqueous compartment encapsulated by a phospholipid bilayer membrane, typically ranging from 10 to 100 nm in diameter. These spherical vesicles can deliver therapeutic genes, small molecules, proteins, etc.[77] Liposomes have been effectively delivered to the rabbit retina and validated in patients experiencing treatment-resistant macular edema.[78] In a previous study, liposomes of hydrogenated soybean phosphatidylcholine and cholesterol were surface modified by coating with Agm6-M-oleate, a synthetic cell penetration enhancer, and 5% mPEG2kDa-DSPE to cargo dexamethasone hemisuccinate into the posterior eye segment of rat retina.[78]
Hydrogels
These are biodegradable three-dimensional polymeric particles made by conjugating one or more synthetic and nonsynthetic monomers, having a higher water content due to numerous hydrophilic functional groups, facilitating the entrapment of therapeutic agents.[79] Injectable or implantable hydrogels can be loaded with GT constructs and put in the subretinal space or vitreous humor to provide sustained release and controlled delivery of therapeutic genetic material.[80]
Nanotubes
Nanotubes have emerged as promising GT vectors due to their small size, which enables cellular penetration, and their customizable surface chemistry for targeted transportation of genetic material.[81] Carbon nanotubes (CNTs) offer unique physical properties that can aid in gene delivery.[82] Toxicity and action mechanisms of multi-walled CNTs were confirmed in human corneal epithelial cells by assessing differentially expressed genes. Furthermore, Quantitative polymerase chain reaction, colorimetric analysis, enzyme-linked immunosorbent assay, and western blotting were employed to validate the mRNA and protein expression of key genes involved in this process.[82]
Virus-Like Particles
Virus-like particles (VLPs) are self-assembling structures mimicking viral shape, lacking genetic material, and enhancing safety.[83] These offer promising prospects in GT as they do not replicate, minimizing the viral vector–associated infection risks, and can be modified for precise cell-type targeting, enhancing treatment accuracy.[84] In a previous report, co-transfection of a recombinant ABCA4 with a plasmid that expresses murine leukemia virus proteins gag and pol in HEK293T cells, formed virus-like particles (VLPs lacking viral RNA) enriched with the recombinant ABCA4.[85]
Peptide-Based Vectors
Cell-penetrating peptides facilitate cellular uptake of genetic material. These peptides can be modified to target retinal cells.[86] In one such research endeavor, Peptide for Ocular Delivery (POD)–GFP fusion protein showed successful expression in epithelial lung and kidney cells, and when injected in the subretinal region, POD–GFP delivery remained localized to the RPE and PR cells.[87] While vitreous injection targeted ganglion cells, INL, and the lens capsule, ocular surface topical application led to corneal epithelium uptake and even transduction of nonocular tissues like the skin’s epidermis.[88]
Additional delivery systems
Some other innovative techniques employed in gene delivery are electroporation, hydrodynamic delivery, and ultrasound-mediated delivery, each offering distinct advantages.[89] Electroporation applies electric pulses creating transient pores in cells’ membranes, facilitating the entry of genetic material.[90] Hydrodynamic delivery utilizes controlled large-volume solution injection to achieve efficient gene transfer through mechanical forces.[91,92] Ultrasound-mediated delivery employs focused ultrasound waves to improve cell membrane permeability, aiding in the uptake of genetic cargo.[93,94,95] These methods play a pivotal role in overcoming size constraints associated with traditional gene delivery systems, allowing efficient gene transfer. Importantly, these techniques mitigate potential insertional mutagenesis hazards, a concern linked to some viral vectors, by providing a safer and more controlled means of gene delivery.[96,97] In addition, they exhibit low immunogenicity, reducing the likelihood of an immune trigger, which is important for successful integration of therapeutic genes into target cells.
Clinical trials in retinal GT
A total of 110 retinal GT trials are listed on clinicaltrials.gov. The earliest GT clinical trial was reported in 1998 for GA. The next trial started in the year 2007. Across 2007–2013, one to four cases had been listed yearly; from 2015 to 2020, a drastic increase in cases (5–10 cases per year) was observed. The last 3 years have shown a considerable hike in the trial numbers (up to 21). While the trials listed for IRDs are shown across the years, NIRD trials appeared only in 2010 and 2011. The number of NIRD trials increased only after 2017 [Fig. 4].
Figure 4.
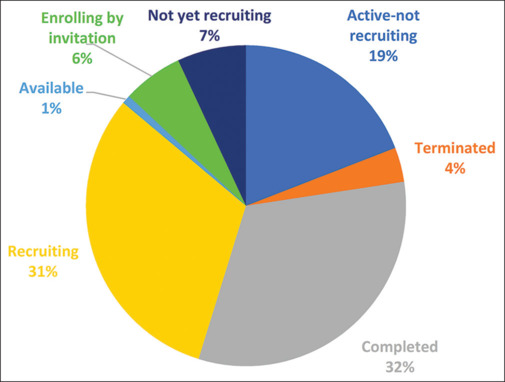
Distribution of the status of retinal gene therapy clinical trials as listed in clinicaltrials.gov until September 2023
On examining the pie chart [Fig. 4], it becomes evident that a considerable percentage of trials, amounting to 32%, have reached the completion stage. Approximately 31% of trials are actively recruiting participants, with an additional 6% enrolling participants via invitation. Furthermore, 19% of trials are currently in an active state. For a more granular insight into the trial status according to specific diseases, refer to Fig. 5.
Figure 5.
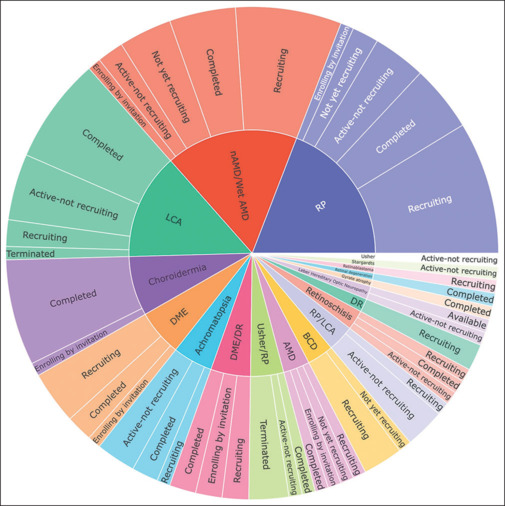
Sunburst chart representation of the disease-wise status of retinal gene therapy clinical trials as listed in clinicaltrials.gov until September 2023
When we categorize trials based on disease types, we observe that the highest number of trials focus on RP, followed by AMD and LCA. This pattern could be owing to high prevalence of RP, the significant disease burden associated with AMD, and the popularity of successful GT treatments for LCA.[98]
In contrast, diseases like CHM, DME, ACHM, BCD, dry AMD, DME with DR, US with RP, RP with LCA, retinoschisis, DR, and LHON have fewer trials listed.
The diseases with the lowest number of trials include RB, GA, retinal degeneration, US, and STGD. For a comprehensive description of genes associated with these diseases, refer to Fig. 6.
Figure 6.
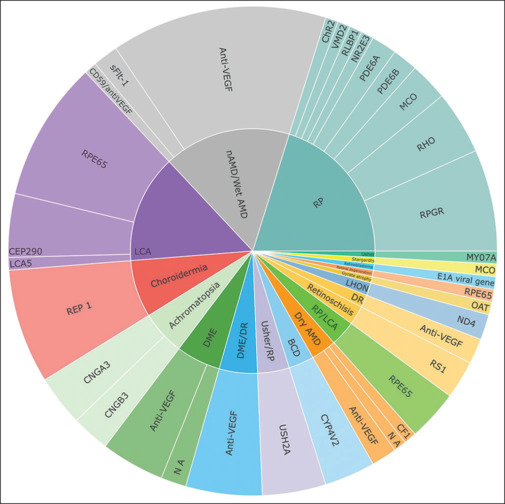
Distribution of reported clinical trials across different retinal disease groups. Inner segments of the sunburst chart represent diseases and outer segments represent the target genes reported (as listed in www.clinicaltrials.gov until September 2023). NA = not available
Limitations associated with GT
The approval and success of Luxturna in treating patients with RPE65 mutation-associated IRD have overlaid the way to develop other GT agents, with an aim to restore vision or halt PR degeneration in retinal diseases.[99] This progress holds the potential to expedite the production of therapeutics for previously untreatable conditions.[100] Beneficial effects persisted for up to 4–10 years, with ongoing follow-up extending to 15 years. Control recipients, eligible for Luxturna after 1 year, exhibited improvements during subsequent follow-ups (up to 3 years postinjection), mirroring those who received Luxturna at baseline. The therapeutic impact endured for at least 10 years in animal models, yet in the crucial Phase III trial, considerable improvements could be observed till 7.5 years.[101] Despite a safety concern with retinal detachment occurring in a patient in the fourth year, adverse reactions were asymptomatic, transient, and nonserious, resolving without sequelae.
While GT is promising in its potential, it has struggled with wide acceptance and accessibility mainly due to its high costs; Luxturna costs ~US$ 85,000 for both eyes. The high cost associated with raw materials and manufacturing is an additional bottleneck for GT vector production.[101] This has led to successful gene therapies being presented for approval only in Western world markets with higher gross domestic product and universal insurance coverage. This excludes developing nations such as India from the therapy being available, since it is not economically feasible for the biotech industry to market such therapies in our country; understandably, therapies worth multiple crores are difficult to afford for the general Indian population. Furthermore, the affected patient pool for any given gene mutated in eye diseases is relatively small; thus, developing a treatment modality, testing it in various models, regulatory processes, and trial costs, all drive the final cost of the therapy high, thereby limiting interest in its continued development. This limitation becomes evident in complexities such as RP, which involves more than 150 different gene mutations (www.sph.uth.edu/RetNet). In addition, in case of complex or multifactorial diseases, translation of GT results from animals to humans is occasionally unsuccessful owing to physiologic distinctions between animal and human eyes. Therefore, a lot of development in terms of cost-effective platform techniques and vector technologies is required to make GT acceptable and accessible to the larger patient population.
GT status in India
Currently, no treatment options are available in India for ocular genetic disorders. The incidence of RP in rural India was estimated at 1:750, which is a considerably greater ratio than in USA and other countries, primarily attributed to the higher number of consanguineous marriages in several communities.[102] GT for various diseases is being developed at several institutes around the country, such as Christian Medical College (CMC) and Vellore Institute of Technology (VIT), Vellore, Indian Institute of Technology (IIT), Mumbai, Kanpur, etc., Institute of Genomics and Integrative Biology (IGIB) New Delhi, Narayana Netralaya Foundation (NNF), Bangalore, L V Prasad Eye Institute (LVPEI), Hyderabad. Among these, scientists of the GROW research laboratory at NNF in Bengaluru are currently developing cost-effective, clinical-grade AAV GT vectors. These vectors are being designed to target a broad range of ocular disorders, including keratoconus as outlined in,[103] LCA, AMD, STGD, and others. To develop indigenous GT platforms that can be cost-effective, it is of value to establish proprietary AAV vectors for which IP rights are held in the country. Such vectors have been indigenously developed at GROW research laboratory to target several genetic diseases, including novel dual AAV vector systems,[37] representing a novel approach to accommodate larger therapeutic genes within AAV capsids. Furthermore, GROW research laboratory has taken significant steps by establishing a Good Manufacturing Practice (GMP)-grade AAV vector production facility equipped to produce entirely indigenous, high-quality cGMP-grade vectors for both ocular and systemic disorders. This progress in GT is driven not only by cutting-edge scientific developments, but also by the support of various government agencies and regulatory bodies.
Future perspectives: To ensure sustained growth in this field, it is imperative that the government plays a pivotal role by increasing financial support and ensuring stability in funding. Furthermore, involving patients and caregiver groups at specific regulatory checkpoints is crucial. This engagement can raise awareness about disorders of genetic origin and underscore the significance of early diagnosis. In addition, there is room for improvement in streamlining regulatory processes to promote both scientists and industry players to launch more trials while maintaining scientific rigor. Simultaneously, the Indian GT community must prioritize innovation in GT products, economical raw material production, and comprehensive clinical trial design development. Overall, by fostering greater awareness and collaboration among patient communities, clinicians, scientists, and government-sponsored organizations, we can bridge the information gap and expedite the deployment of GT and cell therapy, ultimately benefiting a multitude of patients with genetic disorders.
Author’s contribution
AK, GPM, and AG drafted the manuscript, and PB, TMB, RS, and AG edited the final manuscript.
Financial support and sponsorship:
Narayana Nethralaya Foundation, Bengaluru, Karnataka, India.
Conflicts of interest:
There are no conflicts of interest.
Clinical trials categorized based on specific retinal diseases as reported in clinicaltrials.gov
Acknowledgements
The authors express their gratitude to Ravi Kiran M, Junior Research Fellow at the GROW Research Laboratory, for assisting with data visualization. They also extend their appreciation to the Narayana Netralaya Foundation for their generous financial support.
References
- 1.Berger W, Kloeckener-Gruissem B, Neidhardt J. The molecular basis of human retinal and vitreoretinal diseases. Prog Retin Eye Res. 2010;29:335–75. doi: 10.1016/j.preteyeres.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen KH PB, Tadi P. StatPearls Publishing; Treasure Island (FL): 2023. Anatomy, Head and Neck: Eye Retina. [PubMed] [Google Scholar]
- 3.Ginn SL, Amaya AK, Alexander IE, Edelstein M, Abedi MR. Gene therapy clinical trials worldwide to 2017: An update. J Gene Med. 2018;20:e3015. doi: 10.1002/jgm.3015. doi: 10.1002/jgm. 3015. [DOI] [PubMed] [Google Scholar]
- 4.Gonçalves GAR, Paiva RMA. Gene therapy: Advances, challenges and perspectives. Einstein. 2017;15:369–75. doi: 10.1590/S1679-45082017RB4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navale S, Bhosale K, Mohite M, Navale S. Hemgenix as first gene therapy for treatment of Haemophilia B. Int Adv Res Sci Commun Technol. 2022;2:89–94. [Google Scholar]
- 6.Prado DA, Acosta-Acero M, Maldonado RS. Gene therapy beyond Luxturna: A new horizon of the treatment for inherited retinal disease. Curr Opin Ophthalmol. 2020;31:147–54. doi: 10.1097/ICU.0000000000000660. [DOI] [PubMed] [Google Scholar]
- 7.Davidsohn N, Pezone M, Vernet A, Graveline A, Oliver D, Slomovic S, et al. A single combination gene therapy treats multiple age-related diseases. Proc Natl Acad Sci U S A. 2019;116:23505–11. doi: 10.1073/pnas.1910073116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roosing S, Thiadens AA, Hoyng CB, Klaver CC, den Hollander AI, Cremers FP. Causes and consequences of inherited cone disorders. Prog Retin Eye Res. 2014;42:1–26. doi: 10.1016/j.preteyeres.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Diakatou M, Manes G, Bocquet B, Meunier I, Kalatzis V. Genome editing as a treatment for the most prevalent causative genes of autosomal dominant retinitis pigmentosa. Int J Mol Sci. 2019;20:2542. doi: 10.3390/ijms20102542. doi: 10.3390/ijms20102542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heath Jeffery RC, Mukhtar SA, McAllister IL, Morgan WH, Mackey DA, Chen FK. Inherited retinal diseases are the most common cause of blindness in the working-age population in Australia. Ophthalmic Genet. 2021;42:431–9. doi: 10.1080/13816810.2021.1913610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez-Fernandez De La Camara C, Nanda A, Salvetti AP, Fischer MD, MacLaren RE. Gene therapy for the treatment of X-linked retinitis pigmentosa. Expert Opin Orphan Drugs. 2018;6:167–77. doi: 10.1080/21678707.2018.1444476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358:2231–9. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 13.Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: Short-term results of a phase I trial. Hum Gene Ther. 2008;19:979–90. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han Z, Conley SM, Naash MI. Gene therapy for stargardt disease associated with ABCA4 gene. In: Ash JD, Grimm C, Hollyfield JG, Anderson RE, LaVail MM, Bowes Rickman C, editors. Retinal Degenerative Diseases. New York, NY: Springer; 2014. pp. 719–24. [DOI] [PubMed] [Google Scholar]
- 15.McClements ME, Barnard AR, Singh MS, Charbel Issa P, Jiang Z, Radu RA, et al. An AAV dual vector strategy ameliorates the stargardt phenotype in adult ABCA4−/− mice. Hum. Gene Ther. 2018;30:590–600. doi: 10.1089/hum.2018.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michaelides M, Hirji N, Wong SC, Besirli CG, Zaman S, Kumaran N, et al. First-in-human gene therapy trial of AAV8-hCARp.hCNGB3 in adults and children with CNGB3-associated Achromatopsia. Am J Ophthalmol. 2023;253:243–51. doi: 10.1016/j.ajo.2023.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Montioli R, Bellezza I, Desbats MA, Voltattorni CB, Salviati L, Cellini B. Deficit of human ornithine aminotransferase in gyrate atrophy: Molecular, cellular, and clinical aspects. Biochim Biophys Acta Proteins Proteom. 2021;1869:140555. doi: 10.1016/j.bbapap.2020.140555. doi: 10.1016/j.bbapap. 2020.140555. [DOI] [PubMed] [Google Scholar]
- 18.Ng DSC, Lai TYY, Ng TK, Pang CP. Genetics of bietti crystalline dystrophy. Asia-Pacific J Ophthalmol. 2016;5:245–52. doi: 10.1097/APO.0000000000000209. [DOI] [PubMed] [Google Scholar]
- 19.Lam BL, Davis JL, Gregori NZ. Choroideremia gene therapy. Int Ophthalmol Clin. 2021;61:185–93. doi: 10.1097/IIO.0000000000000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacLaren RE, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, Seymour L, et al. Retinal gene therapy in patients with choroideremia: Initial findings from a phase 1/2 clinical trial. Lancet. 2014;383:1129–37. doi: 10.1016/S0140-6736(13)62117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahel JA, Newman NJ, Yu-Wai-Man P, Vignal-Clermont C, Carelli V, Biousse V, et al. Gene therapies for the treatment of leber hereditary optic neuropathy. Int Ophthalmol Clin. 2021;61:195–208. doi: 10.1097/IIO.0000000000000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cukras C, Wiley HE, Jeffrey BG, Sen HN, Turriff A, Zeng Y, et al. Retinal AAV8-RS1 gene therapy for X-linked retinoschisis: Initial findings from a phase I/IIa trial by intravitreal delivery. Mol Ther. 2018;26:2282–94. doi: 10.1016/j.ymthe.2018.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dinculescu A, Link BA, Saperstein DA. Retinal gene therapy for Usher syndrome: Current developments, challenges, and perspectives. Int Ophthalmol Clin. 2021;61:109–24. doi: 10.1097/IIO.0000000000000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melluso A, Secondulfo F, Capolongo G, Capasso G, Zacchia M. Bardet-Biedl syndrome: Current perspectives and clinical outlook. Ther Clin Risk Manag. 2023;19:115–32. doi: 10.2147/TCRM.S338653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.globenewswire.com ALSA ventures launches novel gene therapy portfolio company Axovia therapeutics to treat ciliopathies. 2023 Available from: https://www.globenewswire.com/news-release/2023/09/19 / 2745463/0/en/ALSA-Ventrues-la%20unches-novel-Gene-Therapy-portfolio-company-Axovia-Therapeutics-to-treat-Ciliopathies.html . [Last accessed on 2023 Oct 25] [Google Scholar]
- 26.Lin F-L, Wang P-Y, Chuang Y-F, Wang J-H, Wong VHY, Bui BV, et al. Gene therapy intervention in neovascular eye disease: A recent update. Mol Ther. 2020;28:2120–38. doi: 10.1016/j.ymthe.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2007-An update. J Gene Med. 2007;9:833–42. doi: 10.1002/jgm.1100. [DOI] [PubMed] [Google Scholar]
- 28.Gelfman CM, Grishanin R, Bender KO, Nguyen A, Greengard J, Sharma P, et al. Comprehensive preclinical assessment of ADVM-022, an intravitreal anti-VEGF gene therapy for the treatment of neovascular AMD and diabetic macular edema. J Ocul Pharmacol Ther. 2021;37:181–90. doi: 10.1089/jop.2021.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reid CA, Nettesheim ER, Connor TB, Lipinski DM. Development of an inducible anti-VEGF rAAV gene therapy strategy for the treatment of wet AMD. Sci Rep. 2018;8:11763. doi: 10.1038/s41598-018-29726-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Millington-Ward S, Chadderton N, Finnegan LK, Post IJM, Carrigan M, Nixon R, et al. RPE-directed gene therapy improves mitochondrial function in murine dry amd models. Int J Mol Sci. 2023;24:3847. doi: 10.3390/ijms24043847. doi: 10.3390/ijms24043847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barot M, Gokulgandhi MR, Patel S, Mitra AK. Microvascular complications and diabetic retinopathy: Recent advances and future implications. Future Med Chem. 2013;5:301–14. doi: 10.4155/fmc.12.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J-H, Roberts GE, Liu G-S. Updates on gene therapy for diabetic retinopathy. Curr Diabetes Rev. 2020;20:22. doi: 10.1007/s11892-020-01308-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Musat O, Cernat C, Labib M, Gheorghe A, Toma O, Zamfir M, et al. Diabetic macular edema. Rom J Ophthalmol. 2015;59:133–6. [PMC free article] [PubMed] [Google Scholar]
- 34.Cruz-Gálvez CC, Ordaz-Favila JC, Villar-Calvo VM, Cancino-Marentes ME, Bosch-Canto V. Retinoblastoma: Review and new insights. Front Oncol. 2022;12:963780. doi: 10.3389/fonc.2022.963780. doi: 10.3389/fonc. 2022.963780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pascual-Pasto G, Bazan-Peregrino M, Olaciregui NG, Restrepo-Perdomo CA, Mato-Berciano A, Ottaviani D, et al. Therapeutic targeting of the RB1 pathway in retinoblastoma with the oncolytic adenovirus VCN-01. Sci Transl Med. 2019;11:eaat9321. doi: 10.1126/scitranslmed.aat9321. doi: 10.1126/scitranslmed.aat9321. [DOI] [PubMed] [Google Scholar]
- 36.Cheng S-Y, Punzo C. Update on viral gene therapy clinical trials for retinal diseases. Hum Gene Ther. 2022;33:865–78. doi: 10.1089/hum.2022.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panikker P, Roy S, Ghosh A, Poornachandra B, Ghosh A. Advancing precision medicines for ocular disorders: Diagnostic genomics to tailored therapies. Front Med. 2022;9:906482. doi: 10.3389/fmed.2022.906482. doi: 10.3389/fmed. 2022.906482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarkar S, Panikker P, D’Souza S, Shetty R, Mohan RR, Ghosh A. Corneal regeneration using gene therapy approaches. Cells. 2023;12:1280. doi: 10.3390/cells12091280. doi: 10.3390/cells12091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li C, Samulski RJ. Engineering adeno-associated virus vectors for gene therapy. Nat Rev Genet. 2020;21:255–72. doi: 10.1038/s41576-019-0205-4. [DOI] [PubMed] [Google Scholar]
- 40.Arsenijevic Y, Berger A, Udry F, Kostic C. Lentiviral vectors for ocular gene therapy. Pharmaceutics. 2022;14:1605. doi: 10.3390/pharmaceutics14081605. doi: 10.3390/pharmaceutics14081605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drag S, Dotiwala F, Upadhyay AK. Gene therapy for retinal degenerative diseases: Progress, challenges, and future directions. Invest Ophthalmol Vis Sci. 2023;64:39–56. doi: 10.1167/iovs.64.7.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sahu B, Chug I, Khanna H. The ocular gene delivery landscape. Biomolecules. 2021;11:1135. doi: 10.3390/biom11081135. doi: 10.3390/biom11081135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vollrath D, Feng W, Duncan JL, Yasumura D, D’Cruz PM, Chappelow A, et al. Correction of the retinal dystrophy phenotype of the RCS rat by viral gene transfer of Mertk. Proc Natl Acad Sci U S A. 2001;98:12584–9. doi: 10.1073/pnas.221364198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu W, Liu S, Li P, Yao K. Retinitis pigmentosa: Progress in molecular pathology and biotherapeutical strategies. Int J Mol Sci. 2022;23:4883. doi: 10.3390/ijms23094883. doi: 10.3390/ijms23094883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao Z, Anselmo AC, Mitragotri S. Viral vector-based gene therapies in the clinic. Bioeng Transl Med. 2022;7:e10258. doi: 10.1002/btm2.10258. doi: 10.1002/btm2.10258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maier P, Kalle Cv, Laufs S. Retroviral vectors for gene therapy. Future Microbiol. 2010;5:1507–23. doi: 10.2217/fmb.10.100. [DOI] [PubMed] [Google Scholar]
- 47.Calame M, Cachafeiro M, Philippe S, Schouwey K, Tekaya M, Wanner D, et al. Retinal degeneration progression changes lentiviral vector cell targeting in the retina. PLoS One. 2011;6:e23782. doi: 10.1371/journal.pone.0023782. doi: 10.1371/journal.pone. 0023782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naso MF, Tomkowicz B, Perry WL, 3rd, Strohl WR. Adeno-associated virus (AAV) as a vector for gene therapy. Biodrugs. 2017;31:317–34. doi: 10.1007/s40259-017-0234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang D, Tai PW, Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov. 2019;18:358–78. doi: 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balakrishnan B, R Jayandharan G. Basic biology of adeno-associated virus (AAV) vectors used in gene therapy. Curr Gene Ther. 2014;14:86–100. doi: 10.2174/1566523214666140302193709. [DOI] [PubMed] [Google Scholar]
- 51.Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, et al. Clades of adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78:6381–8. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee EJ, Guenther CM, Suh J. Adeno-associated virus (AAV) vectors: Rational design strategies for capsid engineering. Curr Opin Biomed Eng. 2018;7:58–63. doi: 10.1016/j.cobme.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katada Y, Kobayashi K, Tsubota K, Kurihara T. Evaluation of AAV-DJ vector for retinal gene therapy. Peer J. 2019;7:e6317. doi: 10.7717/peerj.6317. doi: 10.7717/peerj. 6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gehrke M, Diedrichs-Möhring M, Bogedein J, Büning H, Michalakis S, Wildner G. Immunogenicity of novel AAV capsids for retinal gene therapy. Cells. 2022;11:1881. doi: 10.3390/cells11121881. doi: 10.3390/cells11121881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu Z, Yang H, Colosi P. Effect of genome size on AAV vector packaging. Mol Ther. 2010;18:80–6. doi: 10.1038/mt.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patel A, Zhao J, Duan D, Lai Y. New York: Springer; 2019. Design of AAV Vectors for Delivery of Large or Multiple Transgenes. [DOI] [PubMed] [Google Scholar]
- 57.Nelson CE, Gersbach CA. Engineering delivery vehicles for genome editing. Annu Rev Chem Biomol Eng. 2016;7:637–62. doi: 10.1146/annurev-chembioeng-080615-034711. [DOI] [PubMed] [Google Scholar]
- 58.Shtykalova S, Deviatkin D, Freund S, Egorova A, Kiselev A. Non-viral carriers for nucleic acids delivery: Fundamentals and current applications. Life. 2023;13:903. doi: 10.3390/life13040903. doi: 10.3390/life13040903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trapani I, Auricchio A. Has retinal gene therapy come of age? From bench to bedside and back to bench. Hum Mol Genet. 2019;28:R108–18. doi: 10.1093/hmg/ddz130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aukunuru JV, Ayalasomayajula SP, Kompella UB. Nanoparticle formulation enhances the delivery and activity of a vascular endothelial growth factor antisense oligonucleotide in human retinal pigment epithelial cells. J Pharm Pharmacol. 2003;55:1199–206. doi: 10.1211/0022357021701. [DOI] [PubMed] [Google Scholar]
- 61.Ramamoorth M, Narvekar A. Non viral vectors in gene therapy-An overview. J Clin Diagn Res. 2015;9:GE01–6. doi: 10.7860/JCDR/2015/10443.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davis HL, Demeneix BA, Quantin B, Coulombe J, Whalen RG. Plasmid DNA is superior to viral vectors for direct gene transfer into adult mouse skeletal muscle. Hum. Gene Ther. 1993;4:733–40. doi: 10.1089/hum.1993.4.6-733. [DOI] [PubMed] [Google Scholar]
- 63.Hassan S, Keshavarz-Moore E, Ward J. A cell engineering strategy to enhance supercoiled plasmid DNA production for gene therapy. Biotechnol Bioeng. 2016;113:2064–71. doi: 10.1002/bit.25971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gemayel MC, Bhatwadekar AD, Ciulla T. RNA therapeutics for retinal diseases. Expert Opin Biol Ther. 2021;21:603–13. doi: 10.1080/14712598.2021.1856365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kuijper EC, Bergsma AJ, Pijnappel WP, Aartsma‐Rus A. Opportunities and challenges for antisense oligonucleotide therapies. J Inherit Metab Dis. 2021;44:72–87. doi: 10.1002/jimd.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li T, Yang Y, Qi H, Cui W, Zhang L, Fu X, et al. CRISPR/Cas9 therapeutics: Progress and prospects. Signal Transduct Target Ther. 2023;8:36. doi: 10.1038/s41392-023-01309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li F, Wing K, Wang JH, Luu CD, Bender JA, Chen J, et al. Comparison of CRISPR/Cas endonucleases for in vivo retinal gene editing. Front Cell Neurosci. 2020;14:570917. doi: 10.3389/fncel.2020.570917. doi: 10.3389/fncel. 2020.570917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jain A, Zode G, Kasetti RB, Ran FA, Yan W, Sharma TP, et al. CRISPR-Cas9 based treatment of myocilin-associated glaucoma. Proc Natl Acad Sci U S A. 2017;114:11199–204. doi: 10.1073/pnas.1706193114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Palpant NJ, Dudzinski D. Zinc finger nucleases: Looking toward translation. Gene Ther. 2013;20:121–7. doi: 10.1038/gt.2012.2. [DOI] [PubMed] [Google Scholar]
- 70.Moehle EA, Rock JM, Lee Y-L, Jouvenot Y, DeKelver RC, Gregory PD, et al. Targeted gene addition into a specified location in the human genome using designed zinc finger nucleases. Proc Natl Acad Sci U S A. 2007;104:3055–60. doi: 10.1073/pnas.0611478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yanik M, Müller B, Song F, Gall J, Wagner F, Wende W, et al. In vivo genome editing as a potential treatment strategy for inherited retinal dystrophies. Prog Retin Eye Res. 2017;56:1–18. doi: 10.1016/j.preteyeres.2016.09.001. doi: 10.1016/j.preteyeres.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 72.Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm Sin B. 2016;6:287–96. doi: 10.1016/j.apsb.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wassmer SJ, Carvalho LS, György B, Vandenberghe LH, Maguire CA. Exosome-associated AAV2 vector mediates robust gene delivery into the murine retina upon intravitreal injection. Sci Rep. 2017;7:45329. doi: 10.1038/srep45329. doi: 10.1038/srep45329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yavuz B, Pehlivan SB, Vural İ, Ünlü N. In vitro/in vivo evaluation of dexamethasone-PAMAM dendrimer complexes for retinal drug delivery. J Pharm Sci. 2015;104:3814–23. doi: 10.1002/jps.24588. [DOI] [PubMed] [Google Scholar]
- 75.Kambhampati SP, Bhutto IA, Wu T, Ho K, McLeod DS, Lutty GA, et al. Systemic dendrimer nanotherapies for targeted suppression of choroidal inflammation and neovascularization in age-related macular degeneration. J Control Release. 2021;335:527–40. doi: 10.1016/j.jconrel.2021.05.035. [DOI] [PubMed] [Google Scholar]
- 76.Kambhampati SP, Clunies-Ross AJ, Bhutto I, Mishra MK, Edwards M, McLeod DS, et al. Systemic and intravitreal delivery of dendrimers to activated microglia/macrophage in ischemia/reperfusion mouse retina. Invest Ophthalmol Vis Sci. 2015;56:4413–24. doi: 10.1167/iovs.14-16250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.healio.com First patient enrolled in phase 1/2 study of hydroxyl dendrimer imaging agent. 2023 Available from: https://www.healio.com/news/neurology/20230220/first-patient-enrolled-in-phase-12-study-of-hydroxyl-dendrimer-imaging-agent . [Last accessed on 2023 Oct 25] [Google Scholar]
- 78.Tawfik M, Chen F, Goldberg JL, Sabel BA. Nanomedicine and drug delivery to the retina: Current status and implications for gene therapy. Naunyn Schmiedebergs. Arch Pharmacol. 2022;395:1477–507. doi: 10.1007/s00210-022-02287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Al-Amin MD, Mastrotto F, Subrizi A, Sen M, Turunen T, Arango-Gonzalez B, et al. Tailoring surface properties of liposomes for dexamethasone intraocular administration. J Control Release. 2023;354:323–36. doi: 10.1016/j.jconrel.2023.01.027. [DOI] [PubMed] [Google Scholar]
- 80.Rafael D, Guerrero M, Marican A, Arango D, Sarmento B, Ferrer R, et al. Delivery systems in ocular retinopathies: The promising future of intravitreal hydrogels as sustained-release scaffolds. Pharmaceutics. 2023;15:1484. doi: 10.3390/pharmaceutics15051484. doi: 10.3390/pharmaceutics15051484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seah I, Zhao X, Lin Q, Liu Z, Su SZZ, Yuen YS, et al. Use of biomaterials for sustained delivery of anti-VEGF to treat retinal diseases. Eye. 2020;34:1341–56. doi: 10.1038/s41433-020-0770-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mahor A, Singh PP, Bharadwaj P, Sharma N, Yadav S, Rosenholm JM, et al. Carbon-based nanomaterials for delivery of biologicals and therapeutics: A cutting-edge technology. C. 2021;7:19. [Google Scholar]
- 83.Luo X, Xie D, Su J, Hu J. Inflammatory genes associated with pristine multi-walled carbon nanotubes-induced toxicity in ocular cells. Int J Nanomed. 2023;18:2465–84. doi: 10.2147/IJN.S394694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ren D, Fisson S, Dalkara D, Ail D. Immune responses to gene editing by viral and non-viral delivery vectors used in retinal gene therapy. Pharmaceutics. 2022;14:1973. doi: 10.3390/pharmaceutics14091973. doi: 10.3390/pharmaceutics14091973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chao C-N, Yang Y-H, Wu M-S, Chou M-C, Fang C-Y, Lin M-C, et al. Gene therapy for human glioblastoma using neurotropic JC virus-like particles as a gene delivery vector. Sci Rep. 2018;8:2213. doi: 10.1038/s41598-018-19825-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Biswas-Fiss EE, Affet S, Biswas SB. Expression of the human retina specific ABC transporter, ABCA4, in virus-like particles. Investig Ophthalmol Vis Sci. 2012;53:1610. [Google Scholar]
- 87.Lehto T, Kurrikoff K, Langel Ü. Cell-penetrating peptides for the delivery of nucleic acids. Expert Opin Drug Deliv. 2012;9:823–36. doi: 10.1517/17425247.2012.689285. [DOI] [PubMed] [Google Scholar]
- 88.Johnson LN, Cashman SM, Read SP, Kumar-Singh R. Cell penetrating peptide POD mediates delivery of recombinant proteins to retina, cornea and skin. Vis Res. 2010;50:686–97. doi: 10.1016/j.visres.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cashman SM, Sadowski SL, Morris DJ, Frederick J, Kumar-Singh R. Intercellular trafficking of adenovirus-delivered hsv vp22 from the retinal pigment epithelium to the photoreceptors-implications for gene therapy. Mol Ther. 2002;6:813–23. doi: 10.1006/mthe.2002.0806. [DOI] [PubMed] [Google Scholar]
- 90.Yarmush ML, Golberg A, Serša G, Kotnik T, Miklavčič D. Electroporation-based technologies for medicine: Principles, applications, and challenges. Annu Rev Biomed. 2014;16:295–320. doi: 10.1146/annurev-bioeng-071813-104622. [DOI] [PubMed] [Google Scholar]
- 91.Lambricht L, Lopes A, Kos S, Sersa G, Préat V, Vandermeulen G. Clinical potential of electroporation for gene therapy and DNA vaccine delivery. Expert Opin Drug Deliv. 2016;13:295–310. doi: 10.1517/17425247.2016.1121990. [DOI] [PubMed] [Google Scholar]
- 92.Vandermeulen G, Vanvarenberg K, De Beuckelaer A, De Koker S, Lambricht L, Uyttenhove C, et al. The site of administration influences both the type and the magnitude of the immune response induced by DNA vaccine electroporation. Vaccine. 2015;33:3179–85. doi: 10.1016/j.vaccine.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 93.Sayed N, Allawadhi P, Khurana A, Singh V, Navik U, Pasumarthi SK, et al. Gene therapy: Comprehensive overview and therapeutic applications. Life Sci. 2022;294:120375. doi: 10.1016/j.lfs.2022.120375. doi: 10.1016/j.lfs. 2022.120375. [DOI] [PubMed] [Google Scholar]
- 94.Suda T, Yokoo T, Kanefuji T, Kamimura K, Zhang G, Liu D. Hydrodynamic delivery: Characteristics, applications, and technological advances. Pharmaceutics. 2023;15:1111. doi: 10.3390/pharmaceutics15041111. doi: 10.3390/pharmaceutics15041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nakamura S, Ando N, Watanabe S, Akasaka E, Ishihara M, Sato M. Hydrodynamics-based transplacental delivery as a useful noninvasive tool for manipulating fetal genome. Cells. 2020;9:1744. doi: 10.3390/cells9071744. doi: 10.3390/cells9071744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Walsh APG, Gordon HN, Peter K, Wang X. Ultrasonic particles: An approach for targeted gene delivery. Adv Drug Deliv Rev. 2021;179:113998. doi: 10.1016/j.addr.2021.113998. doi: 10.1016/j.addr. 2021.113998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Song S, Lyle MJ, Noble-Vranish ML, Min-Tran DM, Harrang J, Xiao W, et al. Ultrasound-mediated gene delivery of factor VIII plasmids for hemophilia A gene therapy in mice. Mol Ther Nucleic Acids. 2022;27:916–26. doi: 10.1016/j.omtn.2022.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bolt MW, Brady JT, Whiteley LO, Khan KN. Development challenges associated with rAAV-based gene therapies. J Toxicol Sci. 2021;46:57–68. doi: 10.2131/jts.46.57. [DOI] [PubMed] [Google Scholar]
- 99.Patel U, Boucher M, de Léséleuc L, Visintini S. CADTH Issues in Emerging Health Technologies. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2016. Voretigene Neparvovec: An Emerging Gene Therapy for the Treatment of Inherited Blindness. [PubMed] [Google Scholar]
- 100.Kang C, Scott LJ. Voretigene neparvovec: A review in RPE65 mutation-associated inherited retinal dystrophy. Mol Diagn Ther. 2020;24:487–95. doi: 10.1007/s40291-020-00475-6. [DOI] [PubMed] [Google Scholar]
- 101.Bennett J, Maguire AM. Lessons learned from the development of the first FDA-approved gene therapy drug, voretigene neparvovec-rzyl. Cold Spring Harb Perspect Med. 2023;13:a041307. doi: 10.1101/cshperspect.a041307. doi: 10.1101/cshperspect.a041307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.De Luca M, Cossu G. Cost and availability of novel cell and gene therapies: Can we avoid a catastrophic second valley of death?: Can we avoid a catastrophic second valley of death? EMBO Rep. 2023;24:e56661. doi: 10.15252/embr.202256661. doi: 10.15252/embr. 202256661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gopinath C. Gene therapy for retinal diseases. In: Singh AD, editor. Advances in Vision Research. Essentials in Ophthalmology. Singapore: Springer; 2021. [Google Scholar]
- 104.Ghosh A, Yue Y, Duan D. Efficient transgene reconstitution with hybrid dual AAV vectors carrying the minimized bridging sequences. Hum Gene Ther. 2011;22:77–83. doi: 10.1089/hum.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ling S, Yang S, Hu X, Yin D, Dai Y, Qian X, et al. Lentiviral delivery of co-packaged Cas9 mRNA and a Vegfa-targeting guide RNA prevents wet age-related macular degeneration in mice. Nat Biomed Eng. 2021;5:144–56. doi: 10.1038/s41551-020-00656-y. [DOI] [PubMed] [Google Scholar]
- 106.Stradiotto E, Allegrini D, Fossati G, Raimondi R, Sorrentino T, Tripepi D, et al. Genetic aspects of age-related macular degeneration and their therapeutic potential. Int J Mol Sci. 2022;23:13280. doi: 10.3390/ijms232113280. doi: 10.3390/ijms232113280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Palmowski-Wolfe A, Stingl K, Habibi I, Schorderet D, Tran HV. Novel PDE6B mutation presenting with retinitis pigmentosa-A case series of three patients. Klin Monbl Augenheilkd. 2019;236:562–7. doi: 10.1055/a-0811-5480. [DOI] [PubMed] [Google Scholar]
- 108.Petit L, Lhériteau E, Weber M, Le Meur G, Deschamps JY, Provost N, et al. Restoration of vision in the PDE6β-deficient dog, a large animal model of rod-cone dystrophy. Mol Ther. 2012;20:2019–30. doi: 10.1038/mt.2012.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rao GN, Cotlier E. Ornithine delta-aminotransferase activity in retina and other tissues. Neurochem Res. 1984;9:555–62. doi: 10.1007/BF00964382. [DOI] [PubMed] [Google Scholar]
- 110.Brody LC, Mitchell GA, Obie C, Michaud J, Steel G, Fontaine G, et al. Ornithine delta-aminotransferase mutations in gyrate atrophy. Allelic heterogeneity and functional consequences. J Biol Chem. 1992;267:3302–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical trials categorized based on specific retinal diseases as reported in clinicaltrials.gov


