Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) open reading frame K8 encodes a basic region-leucine zipper protein of 237 amino acids that homodimerizes with its bZIP domain. KSHV K8 shows significant homology to the Epstein-Barr virus (EBV) immediate-early protein Zta, a key regulator in the reactivation and replication of EBV. In this study, we report that K8, like its homolog EBV Zta, interacts with cellular CREB-binding protein (CBP) in vivo and in vitro. This interaction requires the C/H3 domain of CBP and the basic region of K8. K8 represses CBP-mediated transcription by competing with limited amounts of cellular CBP, exemplified by the reduced expression from the AP-1 and human immunodeficiency virus long terminal repeat promoters.
Kaposi's sarcoma (KS)-associated herpesvirus (KSHV) is a recently discovered human gamma herpesvirus, and it is the eighth type of human herpesvirus (HHV8) (8, 36, 37). KSHV has been identified as an important pathogen in KS (8). KSHV is also associated with abnormal lymphoproliferation. In a KSHV infection, the viral DNA is found principally in B cells and in spindle cells (7). The most important step in the KSHV life cycle may be the switch from latency to lytic replication, and viral lytic replication is important in KS development (7, 31, 37). Upon chemical induction with tetradecanoyl phorbol ester acetate (TPA) or sodium butyrate, KSHV produces immediate-early viral transcripts. These transcripts encode viral transcriptional activator proteins, such as open reading frame (ORF) 50, which is necessary for inducing the lytic phase of KSHV (29, 30, 38, 43).
KSHV ORF K8 encodes an early viral protein that is activated by and expressed after KSHV ORF 50 protein (28, 39). It is a homodimerizing protein of 237 amino acids with a prototypic basic region-leucine zipper (bZIP) domain at the carboxyl terminus by in-frame splicing (28). K8 shows significant homology to the Epstein-Barr virus (EBV) immediate-early gene product Zta (also called ZEBRA, EB1, and BZLF1) (16, 28). The EBV Zta protein is a well-known bZIP family transcriptional activator, which induces the entire lytic cycle of EBV (26, 31). Zta binds to the amino-terminal region of CREB-binding protein (CBP) and interacts with CBP. This interaction enhances Zta-mediated transactivation of EBV early promoters. It also controls host cellular transcription factor activity through competition by limiting the amount of cellular CBP (1, 42).
CBP, initially known as a coactivator for the transcription factor CREB (24), functions in a variety of signaling pathways and modulates specific gene expression (14, 20). The ability of CBP to interact with multiple, signal-dependent transcription factors, including Jun, Fos, and NF-κB (5, 21, 34), suggests that this coactivator functions as a signal integrator by coordinating complex signal transduction events at the transcriptional level (4, 21). CBP also mediates transcriptional activation via intrinsic and associated histone acetyltransferase (HAT) activities and targets gene activation through association with active RNA polymerase II complex (14, 23, 25).
Here, we show that K8 interacts and colocalizes with CBP. CBP is immunoprecipitated with anti-K8 antibodies in BCBL-1 cell lines, which contain KSHV DNA. K8 binds to the C/H3 domain of CBP, which is known as the interacting domain of adenovirus E1A proteins (3, 9). The interacting domain of K8 with CBP locates within the basic region of K8. K8 represses the CBP-mediated transcription activities of AP-1 and the human immunodeficiency virus (HIV) long terminal repeat (LTR) promoters. Deletion mutants of K8 which had lost their binding activity to CBP did not repress the transcriptional activities of these promoters, and CBP was able to relieve the K8-mediated repression in a dose-dependent manner. CBP has been shown to associate with the promyelocytic leukemia (PML) protein and to be recruited to the PML oncogenic domains (PODs) (10, 11, 25). The fact that K8 interacts with CBP also coincides with the recent data indicating that K8 localizes in the PODs, in which a fraction of CBP is recruited (41).
K8 interacts with CBP in vivo.
In order to clone the full-length cDNA of K8, we performed a reverse transcription-PCR (RT-PCR) using the appropriate primers on poly(A)+ RNAs from BCBL-1 cell lines treated with TPA (20 ng/ml) for 48 h. We verified the resulting clone by sequencing and comparing it with the cDNA sequence of K8 in GenBank (accession no. AF072866) (28). 293T cells were transfected in the following ways: (i) with no DNA (Mock) and 7 μg of a hemagglutinin (HA)-tagged CBP expression vector (HA-CBP) in the presence or absence of a blank (as a negative control; pEBG; a gift from J. Jung) vector and(ii) a glutathione S-transferase (GST)-fused K8 expression plasmid (pEBG-K8; K8 cloned into the BamHI and NotI sites of pEBG) using the calcium phosphate precipitation method (15). Forty-eight hours after transfection, the cells were harvested and lysed in binding buffer (20 mM HEPES [pH 7.4], 100 mM NaCl, 1% Triton X-100, 0.5% NP-40 supplemented with protease inhibitors). The cell lysates were mixed in the buffer for 1 h at 4°C before the cell debris was removed by centrifugation. The appropriate lysates were immunoprecipitated with the addition of antibodies against HA (α-HA) or GST (α-GST) and a protein G resin (Santa Cruz Biotechnology, Santa Cruz, Calif.). The beads were washed four times, and the proteins were analyzed by 7 and 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). They were transferred to a nitrocellulose membrane, immunoblotted with anti-HA or anti-GST antibodies, and visualized with an enhanced chemiluminescence reagent according to the manufacturer's instructions (Amersham Pharmacia Biotech, Uppsala, Sweden). As shown in Fig. 1A, the anti-GST antibody immunoprecipitated GST and GST-fused K8 specifically and coimmunoprecipitated CBP from the cell extract that was cotransfected with K8, but not from the cell extracts transfected with the blank vector. The above results confirm that K8 binds to CBP in vivo.
FIG. 1.
In vivo interaction of K8 and CBP. (A) The coimmunoprecipitation of in vivo-synthesized K8 and CBP was analyzed by Western blotting. 293T cells were transfected with an HA-tagged CBP expression vector and either a blank (pEBG) vector or an expression vector carrying GST-fused K8 (pEBG-K8). The cells were harvested, lysed, and precipitated with either anti-HA antibody (α-HA) or anti-GST antibody (α-GST), and protein G resin. The proteins were analyzed by SDS-PAGE and immunoblotted with anti-HA or anti-GST antibodies. (B) Coimmunoprecipitation assay in the KSHV-positive BCBL-1 cell lines. The BJAB cell lines were used as a KSHV-negative control. BCBL-1 cell lines, with or without TPA induction (48 h), were harvested, and the appropriate lysates were precipitated and immunoblotted with either CBP-specific monoclonal antibody (α-CBP) or rabbit polyclonal anti-K8 antibody (α-K8), respectively. (C) K8 colocalizes with CBP in 293T cells. The GFP-K8 and HA-CBP expression vectors were transfected into 293T cells. Cells were fixed and immunostained 48 h after transfection. HA-CBP was detected using a rhodamine-conjugated secondary antibody against a mouse monoclonal HA antibody. (D) Colocalization of K8 and CBP in BCBL-1 cells. For the expression of lytic gene product K8, BCBL-1 cells were treated with TPA for 48 h. K8 was detected by indirect immunofluorescence using a rabbit anti-K8 antibody as a primary antibody and FITC-conjugated goat anti-rabbit IgG as a secondary antibody. CBP was detected with a mouse CBP-specific monoclonal antibody and TRITC-conjugated goat anti-mouse IgG. The nucleus of the cell was stained with DAPI.
To confirm this interaction in the physiological condition, we performed a coimmunoprecipitation assay in the KSHV-positive BCBL-1 cell line (Fig. 1B). The B-cell lymphoma BJAB cell lines were used as a KSHV-negative control. BCBL-1 cells with or without TPA induction (48 h) were harvested, and the appropriate lysates were precipitated with either mouse CBP-specific monoclonal antibody (α-CBP; Santa Cruz Biotechnology) or rabbit polyclonal anti-K8 antibody (α-K8). K8 was specifically detected in the BCBL-1 cells 48 h after TPA induction. Anti-K8 antibody coimmunoprecipitated CBP from the TPA-induced BCBL-1 cell lines, but not from BJAB or uninduced BCBL-1 cell lines. These results showed that K8 binds to CBP in KSHV-positive cell lines.
K8 colocalizes with CBP.
We next assessed whether or not K8 and CBP were colocalized in 293T cells. Green fluorescent protein (GFP)-tagged K8 (GFP-K8; K8 cloned into the EcoRI and XhoI sites of pEGFP-C1; Clontech, Palo Alto, Calif.) and HA-CBP were transfected into 293T cells. Forty-eight hours after transfection, the cells were fixed with 3.7% formaldehyde, permeabilized with 0.2% Triton X-100, and immunostained. HA-CBP was detected using a rhodamine-conjugated secondary antibody against a mouse monoclonal HA antibody (Santa Cruz Biotechnology). Imaging was performed using a confocal microscope equipped with an argon-krypton laser (LSM510; Zeiss, Oberkochen, Germany). GFP alone showed a diffuse pattern throughout the cytoplasm and nucleus (data not shown). As shown in Fig. 1C, K8 was located mainly in the nucleus (22). Cotransfection of HA-CBP with GFP-K8 yielded a yellow color indicative of colocalization in the nucleus.
To examine the colocalization of K8 and CBP in physiological conditions, we performed confocal microscopy in the TPA-induced BCBL-1 cell. Forty-eight hours after TPA induction, BCBL-1 cells were immunostained and attached to poly-l-lysine-coated glass slide. K8 was detected by indirect immunofluorescence using a rabbit anti-K8 antibody (α-K8) as primary antibody and a fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit immunoglobulin G (IgG) as the secondary antibody. CBP was detected with a mouse CBP-specific monoclonal antibody (α-CBP) and a tetramethylrhodamine isothiocyanate (TRITC)-conjugated goat anti-mouse IgG. The nucleus of the cell was stained with 4′,6-diamidino-2-phenylindole (DAPI; Sigma, St. Louis, Mo.). As shown in Fig. 1D, K8 shows a nuclear punctate pattern within nuclear background (41) and colocalizes with CBP in TPA-induced BCBL-1 cells.
K8 binds to the C/H3 domain of CBP.
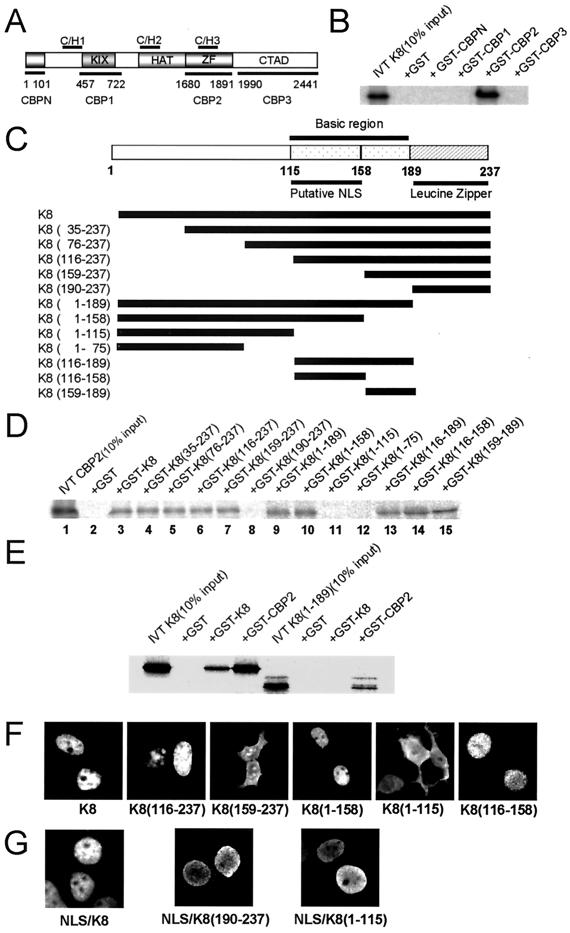
To determine the K8-binding domain of CBP, we performed GST pulldown assays with in vitro-translated K8 and deletion mutants of CBP fused to GST (Fig. 2A). CBP consists of the KIX, the HAT, the zinc finger motifs, and the carboxyl-terminal transcriptional activation domain (TAD) (17, 18, 24, 40). The CBP fragments were subcloned into pGEX4T-1 (Amersham Pharmacia Biotech). The GST-CBP fusion proteins were expressed and purified according to the manufacturer's instructions. K8 was in vitro transcribed and translated using a T7-coupled transcription-translation system (Promega, Madison, Wis.). The 35S-labeled K8 proteins were incubated with 1 μg of a GST-fused CBP fragment in binding buffer (20 mM HEPES [pH 7.4], 100 mM NaCl, 0.5% NP-40 supplemented with protease inhibitors). Glutathione-Sepharose 4B (Amersham Pharmacia Biotech) beads were then added, and the reaction mixture was incubated at 4°C overnight. The beads were then washed four times with the binding buffer. The proteins were analyzed by SDS–12% PAGE and visualized by autoradiography on a Fujix BAS-1500 (Fuji Film Co., Tokyo, Japan). In vitro-translated K8 did not bind to GST alone or to other GST-fused CBP deletion mutants except a CBP2 fragment, which contains the C/H3 conserved region (Fig. 2B). The adenovirus E1A protein also binds to the C/H3 region of CBP (3).
FIG. 2.
In vitro interaction and the interacting domains of K8 with CBP. (A) The domains of CBP and the GST-fused CBP fragments are indicated. KIX, kinase-induced domain interacting domain; ZF, zinc finger; CTAD, carboxyl-terminal transcriptional activation domain. (B) GST pulldown assays with in vitro-translated, 35S-labeled K8 and deletion mutants of CBP fused to GST. (C) Domains within K8 and the deletion mutants. The K8 fragments were introduced into pGEX4T-1. (D) GST pulldown assays were performed with in vitro-translated, 35S-labeled CBP2 and GST-fused K8 fragments. (E) GST pulldown assays of in vitro-translated K8 and its mutant K8(1–189) with GST-fused K8 and CBP2. (F) Localization of K8 deletion mutants was investigated by immunostaining 293T cells transfected with each Flag-tagged K8 deletion mutant. (G) Localization of K8 and NLS-deleted mutants K8(190–237) and K8(1–115) cloned into an NLS-containing CMV2N3T vector.
Basic domain of K8 is responsible for association with CBP2 domain.
To determine the CBP-binding domain of K8, we performed GST pulldown assays using in vitro-translated CBP2 and GST-fused K8 and deletion mutants (Fig. 2C).
Amino acids 1 to 158 of K8 are derived from exon 1 and 159 to 189 are from exon 2. The basic region of K8 is amino acids 116 to 189, which is derived from part of exon 1 (116 to 158) and exon 2 (159 to 189). This basic region of K8 is necessary for binding to CBP, because mutants which have a part of the basic region [K8(159–237) and K8(1–158)], bind to CBP2 (Fig. 2D, lanes 7 and 10), but K8(190–237) and K8(1–115) mutants, which do not contain the basic region, do not bind to CBP2 (Fig. 2D, lanes 8 and 11). It is also shown that the part of the basic region, amino acids 116 to 158 or 159 to 189, is sufficient for binding to CBP (Fig. 2D, lanes 14 and 15). The HIV Tat protein also interacts with CBP through its basic domains (19). Because many basic region-leucine zipper (bZIP) proteins dimerize by using the ZIP domain and K8 also homodimerizes with its leucine zipper domain (16, 28), we next assessed whether the leucine zipper region is required for binding to CBP when K8 is not in the form of a GST fusion protein. GST pulldown assays of in vitro-translated K8 and K8(1–189), which does not contain a Zip domain, with GST-K8 and GST-CBP2 clearly showed that a Zip domain is necessary for the homodimerization of K8, but not for binding to CBP (Fig. 2E).
NLS of K8 is within the basic region derived from exon 1.
Subcellular localization of K8 deletion mutants was tested before the mutants was compared to wild-type K8. Wild-type K8 and deletion mutants were cloned into the EcoRI and XhoI sites of pFLAG-CMV-2 (Kodak, Rochester, N.Y.) and transfected into 293T cells. Forty-eight hours after transfection, the cells were fixed, immunostained, and detected using a rhodamine-conjugated secondary antibody against a mouse monoclonal Flag antibody (Sigma). While the wild-type K8 localizes only in the nucleus (22, 35), deletion mutants without amino acids 116 to 158 [K8(159–237) and K8(1–115)] localize in the cytoplasm (Fig. 2F). Another deletion mutant that has only amino acids 116 to 158 [K8(116–158)], localizes in the nucleus, as does the wild-type K8. This finding suggests that the nuclear localization signal (NLS) of K8 is within amino acids 116 to 158. This putative NLS designation is further supported and specified by the PSORT WWW server (Kenta Nakai and Paul Horton, http://psort.ims.u-tokyo.ac.jp/). Data on this server indicate that the NLS of K8 is PTRRSKR, which is located at amino acids 123 to 129. This result is similar to the NLS of simian virus 40 large T antigen. It also corresponds to the short amino acid sequence TRRSKRRLHRKF between residues 124 and 135, which was identified by Portes-Sentis et al. (35). These data suggest that the NLS in a basic region (amino acids 116 to 158) is essential for the localization of K8 in the nucleus. Therefore, we cloned the K8 and deletion mutants into the EcoRI and XbaI sites of a CMV2N3T vector, which fuses NLS and HA tag into the amino-terminal region of a cloned protein (a gift from D. Trouche). This confirmed that all CMV2N3T clones were localized in the nucleus (Fig. 2G).
K8 represses transcription from AP-1 and HIV LTR in a CBP-dependent manner.
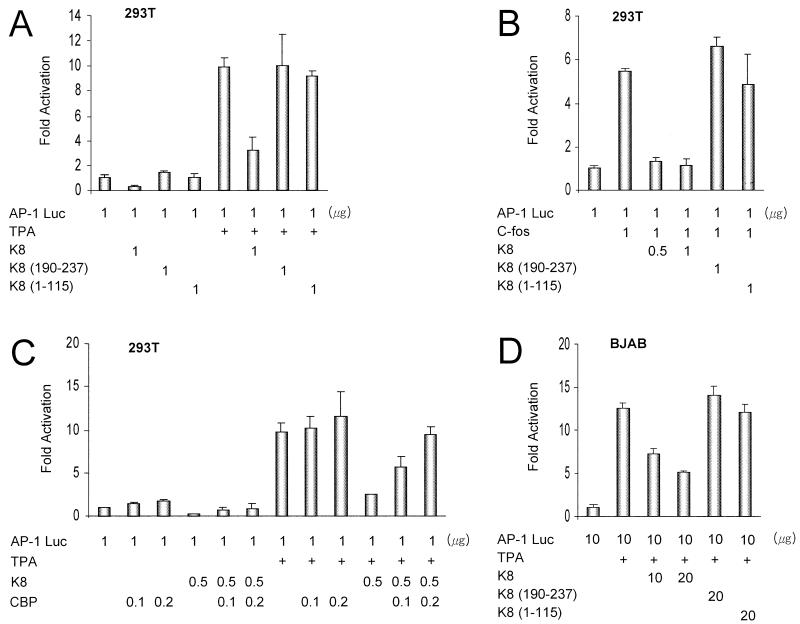
CBP is an integrator of multiple signal transduction pathways (4, 14, 20, 21). A variety of inducible genes have multiple elements for transcription factors that bind to CBP, such as AP-1 (5, 21) and HIV LTR promoters (23, 33, 34). To investigate whether the interaction of K8 and CBP has an effect on the physiological condition of cells or the viral life cycle, we tested the effect of K8 and its CBP-binding-deficient mutants on the above promoters in a transient reporter assay. We performed transient reporter assays in which 293T and BJAB cells were transiently cotransfected with a reporter and one or more of the expression vectors. BJAB cells were transfected by electroporation as described previously (30). In Fig. 3A, a reporter gene, AP-1 luciferase (Luc) (p3XAP1-tk-Luc, containing artificial AP-1-responsive elements, a gift from Y. Nagamine) and K8 expression plasmids CMV2N3T K8, K8(190–237), and K8(1–115) were transfected and assayed. The amounts of the expression plasmids are indicated in the figure. The total amounts of the expression vectors were kept constant by adding an empty cytomegalovirus (CMV) expression plasmid. K8 repressed the transcription of the AP-1 reporter with or without TPA induction, but the CBP-binding-deficient mutants did not affect the transcription of the AP-1 reporter. In Fig. 3B, pcDNA3/c-Fos (cloned into the EcoRI and XhoI sites of pcDNA3; Invitrogen, Carlsbad, Calif.) expression plasmid was used to activate the AP-1 promoter instead of TPA. K8 also represses c-Fos activity in a CBP-binding-dependent manner. To prove that the repression by K8 is due to the reduction in the limited amount of coactivator CBP available for transcriptional activation, we tested whether the repression by K8 can be overcome by addition of CBP. We found that CBP was able to relieve the K8-mediated repression of AP-1 promoter in a dose-dependent manner (Fig. 3C).
FIG. 3.
K8 represses the transcription of AP-1 in a CBP-dependent manner. (A) Transient reporter assays were performed in which 293T cells were transiently cotransfected with a reporter gene construct (AP-1 Luc) and K8 expression plasmids CMV2N3T K8, K8(190–237), and K8(1–115). The amounts of expression vectors are shown. In all assays, the fold activation was determined by luciferase activity derived from the reporter after normalizing it to β-Gal activity from a cotransfected RSV β-Gal control plasmid. All experiments were performed at least in triplicate, and the total amount of each expression vector was kept constant. The luciferase activity of AP-1 in the absence of the other expression vectors and TPA induction is normalized to a value of 1. (B) AP-1 reporter assays with induction by c-Fos expression vector instead of TPA. (C) CBP relieves the repression of AP-1 by K8 in a dose-dependent manner, but does not activate the AP-1 promoter. (D) Transient reporter assay with the same reporter and expression vectors in BJAB cell lines.
To provide further support for the CBP-dependent repression of AP-1 by K8 in physiological conditions, BJAB cells were transiently cotransfected with the same reporter plasmid and expression vectors, and similar results were observed (Fig. 3D). In all assays, the fold activation was determined by luciferase activity derived from the reporter after normalizing it to β-galactosidase (Gal) activity from a cotransfected Rous sarcoma virus (RSV) β-Gal control plasmid. All experiments were performed at least in triplicate, and the equivalent expression of each plasmid was verified by Western blotting (data not shown). The Luc activity of AP-1, in the absence of TPA or c-Fos induction and the other expression vectors, is normalized to a value of 1. Gene expression from AP-1 is regulated by a combination of the various AP-1 activators, such as c-Fos and c-Jun. Enhancement of transcription by AP-1 protein requires the presence of an additional mediator protein, CBP (5, 21). Because AP-1 requires relatively low intracellular levels of the CBP, the interaction of CBP with K8 correlates with the K8 activity to repress TPA- or c-Fos-mediated AP-1 promoter activation, similar to that of adenovirus E1A (5). The loss of CBP binding correlates with a loss of the repression activity of the K8 deletion mutants.
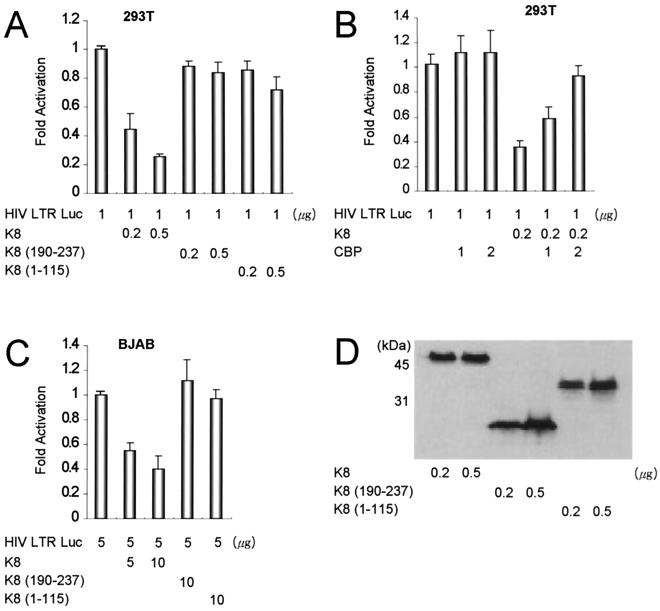
We next investigated the effect of CBP competition by K8 on another well-known viral promoter, HIV LTR. Transcriptional activation of HIV LTR is highly responsive to the transcription factor nuclear factor-κB (NF-κB) (20, 33). The binding of NF-κB to CBP is critical for NF-κB activity (13, 14, 34). CBP potentially influences the activation of HIV gene expression through its effects on NF-κB transactivation (34). 293T cells were transiently cotransfected with an HIV LTR reporter gene (pLTRluc-wt, containing a 186-bp fragment of the HIV-1 LAI strain 5′ LTR; a gift from M. Benkirane) and the indicated amounts of expression vectors. The Luc activity of pLTRluc-wt in the absence of the other expression vectors is normalized to a value of 1. Functioning similarly to the AP-1 promoter, the basal transcription from the HIV LTR was repressed in a dose-dependent manner by K8 but not by CBP-binding-deficient mutants (Fig. 4A). CBP relieved the repression of HIV LTR by K8 in a dose-dependent manner (Fig. 4B), and the same amount of CBP did not activate the HIV LTR promoter. BJAB cells were transiently cotransfected with the same reporter plasmid and expression vectors in the indicated amounts, and similar results were observed (Fig. 4C). Equivalent expression of each plasmid was verified by Western blotting with anti-HA antibody (Fig. 4D).
FIG. 4.
K8 represses the transcription of HIV LTR in a CBP-dependent manner. (A) Transient reporter assays in 293T cells with a reporter gene, HIV LTR luciferase, and the same expression vectors. The luciferase activity of HIV LTR in the absence of the other expression vectors is normalized to a value of 1. (B) CBP relieves the repression of HIV LTR by K8 in a dose-dependent manner but does not much activate the HIV LTR promoter alone. (C) Transient reporter assays with the same reporter and expression vectors in BJAB cell lines. (D) The equivalent expression of each plasmid was verified by Western blotting.
As shown in Fig. 3 and 4, K8 repressed the transcription of the AP-1 and HIV LTR promoters, but the deletion mutants K8(190–237) and K8(1–115), which do not bind to CBP, did not repress the transcription as effectively as wild-type K8. Since CBP is physiologically maintained at a limiting concentration (21), K8 then has the capacity to modulate AP-1- and NF-κB-responsive elements that contain promoters such as AP-1 and HIV LTR. K8 may inhibit transcription of other promoters through the interaction with CBP. This suggests that K8 interferes with CBP to function as a transcriptional coactivator, as does adenovirus E1A (4). Recent data showed that K8 interacts directly with p53 and represses p53-mediated transcriptional activity (32). Transcriptional activation by p53 was reported to be dependent on CBP. E1A represses p53-mediated transcription by competitively inhibiting the interaction of CBP with p53 (17, 27). Thus, it is possible that K8 represses the p53-mediated transactivation indirectly by the inhibition of p53 binding to CBP, as well as by direct interaction with p53.
Wu et al. (41) showed the association and colocalization of K8 with the KSHV pseudo-replication compartment structure and PML protein in PODs. This suggests another possibility, that K8 plays an important role in KSHV replication. Several data showed the function of PML in viral propagation, such as interferon-induced upregulation, association with viral replication, and modification of the transcriptional activities of cellular factors (2, 6, 10, 11, 25, 41). Like other herpesviruses, such as herpes simplex virus 1 (HSV-1) and human cytomegalovirus (HCMV), EBV disrupts POD upon lytic reactivation. EBV Zta can disrupt PML bodies by competing with PML for an essential small ubiquitin-related modifier-1 (SUMO-1) (2, 6). Meanwhile, KSHV did not show any loss of PML or disruption of PODs upon lytic replication (41). K8 associates with PML but does not disrupt PML. CBP has been shown to associate with the PML protein and to be recruited to the PODs (10, 11, 25). Recruitment of CBP to the PODs by PML might represent a critical regulatory step in transcriptional activation (10). It is possible that K8 can colocalize with PML in PODs and then modulate the PML activity on viral replication and transcription through the interaction with CBP.
CBP functions as a coactivator for several cellular transcription factors and is also used by viral proteins, such as adenovirus E1A and simian virus 40 T antigen, to promote viral gene transactivation (3, 9, 12). In this study, we showed that KSHV K8 protein interacts with CBP in vivo and in vitro. This interaction needs a basic region of K8 and the C/H3 region of CBP. It may alter the physiological condition of cells by competing for limited amounts of CBP, which is exemplified by the repression of gene expression from the AP-1 and HIV LTR promoter. K8 may also contribute to viral replication and transcription by association with PML that is mediated by CBP. Previously, we demonstrated that KSHV immediate-early transcript ORF 50 protein binds to CBP/p300 and modulates the transcriptional activities of the cellular transcription factors through interaction with CBP (18). K8, which is activated by and expressed after ORF 50 protein, may have a synergistic role with ORF 50 protein in changing the physiological condition of cells for propagation of KSHV.
Acknowledgments
This work was supported in part by grants from the National Research Laboratory Program of the Korea Institute of Science & Technology Evaluation and Planning (KISTEP), the Korea Science and Engineering Foundation (KOSEF) through the Protein Network Research Center at Yonsei University, and the BK21 Program of the Ministry of Education, Korea.
REFERENCES
- 1.Adamson A L, Kenney S. The Epstein-Barr virus BZLF1 protein interacts physically and functionally with the histone acetylase CREB-binding protein. J Virol. 1999;73:6551–6558. doi: 10.1128/jvi.73.8.6551-6558.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamson A L, Kenney S. Epstein-Barr virus immediate-early protein BZLF1 is SUMO-1 modified and disrupts promyelocytic leukemia bodies. J Virol. 2001;75:2388–2399. doi: 10.1128/JVI.75.5.2388-2399.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arany Z, Newsome D, Oldread E, Livingston D M, Eckner R. A family of transcriptional adaptor proteins targeted by the E1A oncoprotein. Nature. 1995;374:81–84. doi: 10.1038/374081a0. [DOI] [PubMed] [Google Scholar]
- 4.Arias J, Alberts A S, Brindle P, Claret F X, Smeal T, Karin M, Feramisco J, Montminy M. Activation of cAMP and mitogen responsive genes relies on a common nuclear factor. Nature. 1994;370:226–229. doi: 10.1038/370226a0. [DOI] [PubMed] [Google Scholar]
- 5.Bannister A J, Kouzarides T. CBP-induced stimulation of c-Fos activity is abrogated by E1A. EMBO J. 1995;14:4758–4762. doi: 10.1002/j.1460-2075.1995.tb00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell P, Lieberman P M, Maul G G. Lytic but not latent replication of Epstein-Barr virus is associated with PML and induces sequential release of nuclear domain 10 proteins. J Virol. 2000;74:11800–11810. doi: 10.1128/jvi.74.24.11800-11810.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boshoff C, Schulz T F, Kennedy M M, Graham A K, Fisher C, Thomas A, McGee J O, Weiss R A, O'Leary J J. Kaposi's sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat Med. 1995;1:1274–1278. doi: 10.1038/nm1295-1274. [DOI] [PubMed] [Google Scholar]
- 8.Boshoff C, Weiss R A. Kaposi's sarcoma-associated herpesvirus. Adv Cancer Res. 1998;75:57–86. doi: 10.1016/s0065-230x(08)60739-3. [DOI] [PubMed] [Google Scholar]
- 9.Dorsman J C, Teunisse A F, Zantema A, van der Eb A J. The adenovirus 12 E1A proteins can bind directly to proteins of the p300 transcription coactivator family, including the CREB-binding protein CBP and p300. J Gen Virol. 1997;78:423–426. doi: 10.1099/0022-1317-78-2-423. [DOI] [PubMed] [Google Scholar]
- 10.Doucas V, Tini M, Egan D A, Evans R M. Modulation of CREB binding protein function by the promyelocytic (PML) oncoprotein suggests a role for nuclear bodies in hormone signaling. Proc Natl Acad Sci USA. 1999;96:2627–2632. doi: 10.1073/pnas.96.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doucas V. The promyelocytic (PML) nuclear compartment and transcription control. Biochem Pharmacol. 2000;60:1197–1201. doi: 10.1016/s0006-2952(00)00413-5. [DOI] [PubMed] [Google Scholar]
- 12.Eckner R, Ludlow J W, Lill N L, Oldread E, Arany Z, Modjtahedi N, DeCaprio J A, Livingston D M, Morgan J A. Association of p300 and CBP with simian virus 40 large T antigen. Mol Cell Biol. 1996;16:3454–3464. doi: 10.1128/mcb.16.7.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerritsen M E, Williams A J, Neish A S, Moore S, Shi Y, Collins T. CREB-binding protein/p300 are transcriptional coactivators of p65. Proc Natl Acad Sci USA. 1997;94:2927–2932. doi: 10.1073/pnas.94.7.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodman R H, Smolik S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 2000;14:1553–1577. [PubMed] [Google Scholar]
- 15.Graham F L, van der Eb A J. Transformation of rat cells by DNA of human adenovirus 5. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90163-3. [DOI] [PubMed] [Google Scholar]
- 16.Gruffat H, Portes-Sentis S, Sergeant A, Manet E. Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) encodes a homologue of the Epstein-Barr virus bZip protein EB1. J Gen Virol. 1999;80:557–561. doi: 10.1099/0022-1317-80-3-557. [DOI] [PubMed] [Google Scholar]
- 17.Gu W, Shi X L, Roeder R G. Synergistic activation of transcription by CBP and p53. Nature. 1997;387:819–823. doi: 10.1038/42972. [DOI] [PubMed] [Google Scholar]
- 18.Gwack Y, Byun H, Hwang S, Lim C, Choe J. CREB-binding protein and histone deacetylase regulate the transcriptional activity of Kaposi's sarcoma-associated herpesvirus open reading frame 50. J Virol. 2001;75:1909–1917. doi: 10.1128/JVI.75.4.1909-1917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hottiger M O, Nabel G J. Interaction of human immunodeficiency virus type 1 Tat with the transcriptional coactivators p300 and CREB binding protein. J Virol. 1998;72:8252–8256. doi: 10.1128/jvi.72.10.8252-8256.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hottiger M O, Nabel G J. Viral replication and the coactivators p300 and CBP. Trends Microbiol. 2000;8:560–565. doi: 10.1016/s0966-842x(00)01874-6. [DOI] [PubMed] [Google Scholar]
- 21.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin S C, Heyman R A, Rose D W, Glass C K, Rosenfeld M G. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 22.Katano H, Sato Y, Kurata T, Mori S, Sata T. Expression and localization of human herpesvirus 8-encoded proteins in primary effusion lymphoma, Kaposi's sarcoma, and multicentric Castleman's disease. Virology. 2000;269:335–344. doi: 10.1006/viro.2000.0196. [DOI] [PubMed] [Google Scholar]
- 23.Kiernan R E, Vanhulle C, Schiltz L, Adam E, Xiao H, Maudoux F, Calomme C, Burny A, Nakatani Y, Jeang K T, Benkirane M, Van Lint C. HIV-1 tat transcriptional activity is regulated by acetylation. EMBO J. 1999;18:6106–6118. doi: 10.1093/emboj/18.21.6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwok R P, Lundblad J R, Chrivia J C, Richards J P, Bachinger H P, Brennan R G, Roberts S G, Green M R, Goodman R H. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature. 1994;370:223–226. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- 25.LaMorte V J, Dyck J A, Ochs R L, Evans R M. Localization of nascent RNA and CREB binding protein with the PML-containing nuclear body. Proc Natl Acad Sci USA. 1998;95:4991–4996. doi: 10.1073/pnas.95.9.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lieberman P M, Hardwick J M, Sample J, Hayward G S, Hayward S D. The zta transactivator involved in induction of lytic cycle gene expression in Epstein-Barr virus-infected lymphocytes binds to both AP-1 and ZRE sites in target promoter and enhancer regions. J Virol. 1990;64:1143–1155. doi: 10.1128/jvi.64.3.1143-1155.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lill N L, Grossman S R, Ginsberg D, DeCaprio J, Livingston D M. Binding and modulation of p53 by p300/CBP coactivators. Nature. 1997;387:823–827. doi: 10.1038/42981. [DOI] [PubMed] [Google Scholar]
- 28.Lin S F, Robinson D R, Miller G, Kung H J. Kaposi's sarcoma-associated herpesvirus encodes a bZIP protein with homology to BZLF1 of Epstein-Barr virus. J Virol. 1999;73:1909–1917. doi: 10.1128/jvi.73.3.1909-1917.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lukac D M, Renne R, Kirshner J R, Ganem D. Reactivation of Kaposi's sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology. 1998;252:304–312. doi: 10.1006/viro.1998.9486. [DOI] [PubMed] [Google Scholar]
- 30.Lukac D M, Kirshner J R, Ganem D. Transcriptional activation by the product of open reading frame 50 of Kaposi's sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J Virol. 1999;73:9348–9361. doi: 10.1128/jvi.73.11.9348-9361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller G, Heston L, Grogan E, Gradoville L, Rigsby M, Sun R, Shedd D, Kushnaryov V M, Grossberg S, Chang Y. Selective switch between latency and lytic replication of Kaposi's sarcoma herpesvirus and Epstein-Barr virus in dually infected body cavity lymphoma cells. J Virol. 1997;71:314–324. doi: 10.1128/jvi.71.1.314-324.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park J, Seo T, Hwang S, Lee D, Gwack Y, Choe J. 2000. The K-bZIP protein from Kaposi's sarcoma-associated herpesvirus interacts with p53 and represses its transcriptional activity. J Virol. 1982;74:11977–11979. doi: 10.1128/jvi.74.24.11977-11982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perkins N D, Edwards N L, Duckett C S, Agranoff A B, Schmid R M, Nabel G J. A cooperative interaction between NF-kappa B and Sp1 is required for HIV-1 enhancer activation. EMBO J. 1993;12:3551–3558. doi: 10.1002/j.1460-2075.1993.tb06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perkins N D, Felzien L K, Betts J C, Leung K, Beach D H, Nabel G J. Regulation of NF-κB by cyclin-dependent kinases associated with the p300 coactivator. Science. 1997;275:523–527. doi: 10.1126/science.275.5299.523. [DOI] [PubMed] [Google Scholar]
- 35.Portes-Sentis S, Manet E, Gourru G, Sergeant A, Gruffat H. Identification of a short amino acid sequence essential for efficient nuclear targeting of the Kaposi's sarcoma-associated herpesvirus/human herpesvirus-8 K8 protein. J Gen Virol. 2001;82:507–512. doi: 10.1099/0022-1317-82-3-507. [DOI] [PubMed] [Google Scholar]
- 36.Russo J J, Bohenzky R A, Chien M C, Chen J, Yan M, Maddalena D, Parry J P, Peruzzi D, Edelman I S, Chang Y, Moore P S. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schulz T F. Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) J Gen Virol. 1998;79:1573–1591. doi: 10.1099/0022-1317-79-7-1573. [DOI] [PubMed] [Google Scholar]
- 38.Sun R, Lin S F, Gradoville L, Yuan Y, Zhu F, Miller G. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc Natl Acad Sci USA. 1998;95:10866–10871. doi: 10.1073/pnas.95.18.10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun R, Lin S F, Staskus K, Gradoville L, Grogan E, Haase A, Miller G. Kinetics of Kaposi's sarcoma-associated herpesvirus gene expression. J Virol. 1999;73:2232–2242. doi: 10.1128/jvi.73.3.2232-2242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swope D L, Mueller C L, Chrivia J C. CREB-binding protein activates transcription through multiple domains. J Biol Chem. 1996;271:28138–28145. doi: 10.1074/jbc.271.45.28138. [DOI] [PubMed] [Google Scholar]
- 41.Wu F Y, Ahn J H, Alcendor D J, Jang W J, Xiao J, Hayward S D, Hayward G S. Origin-independent assembly of Kaposi's sarcoma-associated herpesvirus DNA replication compartments in transient cotransfection assays and association with the ORF-K8 protein and cellular PML. J Virol. 2001;75:1487–1506. doi: 10.1128/JVI.75.3.1487-1506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zerby D, Chen C J, Poon E, Lee D, Shiekhattar R, Lieberman P M. The amino-terminal C/H1 domain of CREB binding protein mediates zta transcriptional activation of latent Epstein-Barr virus. Mol Cell Biol. 1999;19:1617–1626. doi: 10.1128/mcb.19.3.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu F X, Cusano T, Yuan Y. Identification of the immediate-early transcripts of Kaposi's sarcoma-associated herpesvirus. J Virol. 1999;73:5556–5567. doi: 10.1128/jvi.73.7.5556-5567.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]