Abstract
Purpose
Limited research has evaluated the success criteria and priorities for symptom improvement of patients with cancer to inform patient-centered care. In this study, we adapted and tested a measure of these constructs, the Patient-Centered Outcomes Questionnaire (PCOQ), for patients with advanced prostate cancer. We compared acceptable symptom severity levels following symptom treatment across 10 symptoms and identified patient subgroups based on symptom importance.
Methods
Patients with advanced prostate cancer (N = 99) participated in a one-time survey, which included a modified version of the PCOQ, standard symptom measures, and additional clinical characteristics.
Results
The modified PCOQ demonstrated construct validity through its correlations with related theoretical constructs. There was a moderate correlation between symptom severity and importance. Acceptable symptom severity levels were generally low, with sexual dysfunction having a higher acceptable severity than most other symptoms. Three patient subgroups were identified: (1) those who rated all symptoms as low in importance (n = 43); (2) those who rated all symptoms as moderately important (n = 33); and (3) those who rated all symptoms as highly important (n= 18). Subgroups were associated with functional status, fatigue, sleep problems, pain, and emotional distress.
Conclusion
The modified PCOQ demonstrated preliminary evidence of construct validity. Patients generally considered low symptom severity to be acceptable, with variations across symptoms. Results suggest that symptom severity and importance are related but distinct aspects of the symptom experience in advanced prostate cancer. Patients’ diverse priorities for symptom improvement point to the need for individualized treatment plans.
Keywords: patient-centered outcomes, patient-centered care, advanced prostate cancer, latent profile analysis, symptom importance, symptom severity
Introduction
Patient-centered care emphasizes shared decision-making and providing care that is responsive to individual needs and values [1, 2]. This approach can lead to improved patient satisfaction, health outcomes, and treatment adherence [3–5]. Within supportive cancer care, shared decision-making helps align evidence-based treatments with patients’ expectations and priorities and empowers patients to actively manage their symptoms [4, 6–8]. Researchers have rarely assessed patients’ criteria for successful symptom treatment and their priorities for symptom improvement in cancer care. A deeper understanding of patients’ views on acceptable levels of symptom severity and the importance of treating certain symptoms can strengthen patient-provider communication and facilitate the development of care plans targeting specific needs [9].
The Patient-Centered Outcomes Questionnaire (PCOQ) has been used to define treatment success and priorities for symptom improvement from the patients’ perspective [2]. Primarily used within chronic pain and Parkinson’s disease research, this measure has revealed a significant gap between patient expectations for treatment success and the actual effectiveness of existing therapies, underscoring the need for care that better aligns with patient preferences [2, 3, 5, 10–12]. Within these populations, the PCOQ has also identified patient clusters based on their ratings of the importance of improving pain, fatigue, emotional distress, and daily activities [2, 12]. This differentiation has the potential to enhance personalized care approaches.
Modified versions of the PCOQ have been used with two cancer patient groups [5, 13, 14]. First, the PCOQ was revised to focus on 10 common symptoms in metastatic breast cancer [14]. Patients indicated that fatigue, cognitive problems, and sleep problems would require the greatest reductions to consider symptom treatment successful (range = 43–49% reductions). Additionally, three distinct patient clusters based on ratings of the importance of symptom improvement were found: (1) those who rated cognitive problems, fatigue, and sleep problems as highly important, (2) those who rated pain as highly important, and (3) those who rated all symptoms as highly important. The PCOQ was also revised to focus on eight common symptoms in advanced lung cancer [13]. In general, levels of acceptable symptom severity were low and only small reductions from usual symptom severity were needed to reach acceptable levels. Four patient clusters based on ratings of the importance of symptom improvement were found: (1) those who considered all symptoms as minimally important; (2) those who rated bronchial symptoms and sleep issues as low in importance and other symptoms as moderately important; (3) those rated nausea and emotional distress as low in importance and other symptoms as moderately important; and (4) those who considered all symptoms to be highly important. Across studies, results demonstrate diversity with respect to patient success criteria and priorities for symptom treatment.
Outcomes specific to breast and lung cancers may not generalize across all advanced cancers. Patients with advanced prostate cancer often present with distinctive symptoms that significantly affect their quality of life, such as urinary problems and sexual dysfunction [15, 16]. Although studies have examined providers’ perspectives on key symptoms in prostate cancer treatment [16], limited research addresses patient perspectives on success criteria and symptom improvement priorities [17–19]. This research has primarily assessed the importance of urinary incontinence and sexual impotence in patients with early-stage prostate cancer [17–19]. These assessments were part of a decision aid for determining the appropriateness of “watchful waiting” rather than surgery or radiation.
This study adapted the PCOQ for patients with advanced prostate cancer, focusing on ten prevalent symptoms. For each symptom, we evaluated typical symptom severity, acceptable symptom severity after symptom treatment, and the importance of improving the symptom. The primary aim of this study was to evaluate the construct validity of this modified PCOQ. The Dodd Symptom Management Model suggests that patients’ symptom experiences are associated with demographics, medical factors, and outcomes such as functional status and quality of life [20]. Potential correlates of the PCOQ were selected based on this model. We hypothesized that symptom severity ratings on the PCOQ would be correlated with established symptom assessments, medical comorbidities, functional status, quality of life, and the importance of symptom improvement. As secondary aims, this study (2) compared acceptable severity levels after symptom treatment across the 10 symptoms, (3) identified patient subgroups based on symptom improvement priorities, and (4) explored demographic and clinical correlates of patient subgroups based on symptom improvement priorities.
Methods
Participants and Procedures
After Indiana University (IU) Institutional Review Board approval, participants were recruited from the IU Health University Hospital and IU Health cancer registry to participate in a one-time survey between March and August 2019. Eligibility criteria included: 1) > 3 weeks post-diagnosis of stage IV prostate cancer; 2) ≥ 18 years old; (3) English fluency; and 4) no evidence of severe cognitive impairment (≥ 3 errors on a 6-item cognitive screener) [21]. An introductory letter was sent to potential participants with information for opting out of the study. Research assistants contacted patients who did not opt out, administered the cognitive screener, and obtained consent. Online or paper surveys were sent to consenting participants based on their preference. Reminder calls or automated emails were sent as reminders to complete the survey. Participants received a $25 gift card upon survey receipt.
Measures
Demographics and Medical Factors.
Participants’ age and cancer-related information were collected from medical records. Other demographics were self-reported. Patients reported whether they were diagnosed or treated for eight common medical conditions in the past three years [22]. Using an author-constructed checklist, patients also reported their treatments in the past 3 years (i.e., over-the-counter or prescribed medication, oxygen, psychotherapy/counseling, or other treatments) for each of the ten symptoms in the modified PCOQ.
Modified PCOQ.
The original PCOQ was developed for people with chronic pain [2] and has demonstrated evidence of reliability and concurrent validity with pain, disability, and distress measures [3]. Based on a review of the literature and feedback on the PCOQ from prior cognitive interviews with patients with advanced cancer [13], we modified the PCOQ to focus on ten common symptoms in patients with prostate cancer in three sections. For each symptom, patients first rated their usual symptom severity during the past week on an 11-point scale (0 = none, 10 = worst imaginable). Participants with a usual symptom severity greater than zero were then asked: “What level of [symptom] would be acceptable to you if you were to receive treatment for [symptom]?” on an 11-point scale (0 = none, 10 = worst imaginable) and, “How important is it for you to see improvement in your level of [symptom]?” on an 11-point scale (0 = not at all important, 10 = most important).
Symptom Severity.
The 3-item Patient-Reported Outcomes Measurement Information System (PROMIS) measure was used to assess pain intensity in the past week on a 5-point scale (1 = no pain/had no pain, 5 = very severe) [23]. Four-item PROMIS measures were used to assess anxiety, depressive symptoms, fatigue, and sleep disturbance in the past week on a 5-point scale (1 = never/not at all, 5 = very much). For all PROMIS measures, T-scores anchored to the U.S. general population norms were computed (Mean = 50, SD = 10). Memorial Symptom Assessment Scale (MSAS) subscales were used to assess urination problems, diarrhea, constipation, nausea, lack of appetite, and sexual dysfunction in the past week [24]. For MSAS measures, participants rated the frequency (1 = rarely, 4 = almost constantly), severity (1 = slight, 4 = very severe), and level of distress (0 = not at all, 4 = very much) associated with each symptom.
Quality of Life.
One item from the McGill Quality of Life Questionnaire was used to assess global quality of life over the past two days on an 11-point scale (0 = very bad, 10 = excellent) [25].
Data Analysis
Descriptive statistics were computed using SPSS v.25.0. For aim 1, the construct validity of the modified PCOQ was assessed through correlations between PCOQ symptom severity ratings, PROMIS and MSAS measures of the same symptoms, and theoretically related constructs (i.e., medical comorbidities, functional status, and quality of life).
For aim 2, acceptable severity levels following symptom treatment across the ten symptoms were compared for patients who endorsed at least one symptom (i.e., usual severity rating ≥1 on a 0 to 10 scale) using linear mixed modeling in SPSS. Linear mixed modeling was chosen to allow an unbalanced design, accommodating participants who only provided acceptable severity levels for certain symptoms. In the model, acceptable symptom severity was the outcome, and symptom type (e.g., anxiety, pain) was the within-subjects predictor variable.
For aim 3, patient subgroups based on importance ratings for each of the ten symptoms were identified using latent profile analysis (LPA) in Mplus v.8 [26]. To identify the best fitting model, five models were estimated, and subgroups were added iteratively. Based on the following fit indices, acceptable model fit was determined as: 1) lower values of Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC), and sample size adjusted Bayesian Information Criterion (ssBIC), 2) statistically significant value for the bootstrap likelihood ratio test (BLRT), 3) entropy > 0.80, and 4) an interpretable model solution [27–30].
For aim 4, potential correlates of the patient subgroups were explored individually using multinomial logistic regressions with Vermunt’s 3-step approach in Mplus [31]. Examined correlates included usual severity of the ten symptoms on the modified PCOQ, demographics (i.e., age, marital status, education, employment status, and income), and medical characteristics (i.e., number of medical comorbidities, functional status, time since the advanced cancer diagnosis, cancer treatment history, symptom treatment history, and quality of life). Given the number of analyses, p-values < 0.01 were considered statistically significant.
Results
The study flow is found in Online Resource Fig. 1. Of the 197 patients who were sent recruitment mailings, 160 (81%) were reached via phone, 33 (17%) could not be reached, and 4 (2%) were deceased. Of those reached, 40 (25%) declined to participate mostly due to lack of interest or time, and 120 (75%) completed the eligibility screening. All eligible patients (n = 112) consented to participate, and most consenting patients (n = 99, 88%) completed the survey.
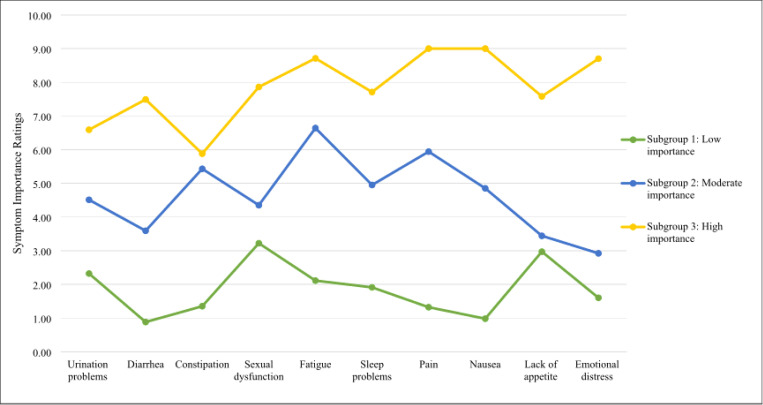
Figure 1.
Patient subgroups’ estimated marginal mean importance ratings on the Patient-Centered Outcomes Questionnaire (PCOQ). N=94.
Participants were mostly non-Hispanic White (88%), with an average age of 68.49 years (SD = 9.01) (Table 1). Participants were, on average, 3.41 years (SD = 2.83) from their advanced prostate cancer diagnosis. Most participants had a history of surgery (80%) and hormonal therapy (83%).
Table 1.
Participant demographic and medical characteristics (N = 99)
| Characteristic | Statistic |
|---|---|
| Age | |
| Mean (S.D.) | 68.49 (9.01) |
| Range | 42–87 |
| Race and Ethnicity, no. (%) | |
| Non-Hispanic White | 87 (87.88%) |
| African American/Black | 9 (9.09%) |
| Othera | 3 (3.03%) |
| Married or Living with a Partner, no. (%) | 83 (83.84%) |
| Employed, no. (%) | 40 (40.40%) |
| Level of Education, no. (%) | |
| No college | 21 (21.21%) |
| Some college | 22 (22.22%) |
| Graduated college/graduate school | 54 (54.54%) |
| Household Income,b no. (%) | |
| $0 – $30,999 | 9 (9.09%) |
| $31,000 – $50,999 | 21 (21.21%) |
| $51,000 – $99,999 | 29 (29.29%) |
| $100,000 or more | 36 (36.36%) |
| Years since Advanced or Extensive Stage Diagnosis | |
| Mean (S.D.) | 3.41 (2.83) |
| Range | 0.10–15.29 |
| Cancer Treatment History,c no. (%) | |
| Surgery | 79 (79.80%) |
| Chemotherapy | 36 (36.36%) |
| Radiation | 45 (45.45%) |
| Targeted therapy | 2 (2.02%) |
| Immunotherapy | 14 (14.14%) |
| Hormone therapy | 82 (82.83%) |
| Current Cancer Treatment,d no. (%) | |
| Chemotherapy | 12 (12.12%) |
| Radiation | 1 (1.01%) |
| Targeted therapy | 0 (0.00%) |
| Immunotherapy | 4 (4.04%) |
| Hormone therapy | 56 (56.57%) |
| Symptom Treatment History,e no. (%) | |
| Urination problems | 17 (17.17%) |
| Diarrhea | 9 (9.09%) |
| Constipation | 15 (15.15%) |
| Sexual dysfunction | 8 (8.08%) |
| Fatigue | 10 (10.10%) |
| Sleep problems | 15 (15.15%) |
| Pain | 23 (23.23%) |
| Nausea | 3 (3.03%) |
| Lack of appetite | 1 (1.01%) |
| Emotional distress | 5 (5.05%) |
| Medical Comorbidities | |
| Mean (S.D.) | 1.08 (1.04) |
| Range | 0–5 |
Multi-racial, Asian American, Native American, Hispanic, and other.
Median household income in Indiana was $57,603 according to U.S. Census 2019 data. Reference: United States Census Bureau (2020) Indiana 2019. https://data.census.gov/cedsci/profile?g=0400000US18. Accessed 2 February 2024.
Treatment any time before study completion.
Treatment ≤ 4 weeks before study completion.
Treatment in the past 3 months for a particular symptom.
PCOQ descriptive statistics are reported in Table 2. On average, patients reported experiencing 4.72 symptoms (SD = 2.37) during the past week. As evidence of the construct validity of the PCOQ (aim 1), the usual severity of all symptoms on the PCOQ were strongly correlated with standard assessments (PROMIS or MSAS) of the same symptoms, rs(97–99) = 0.56–0.89, ps< 0.01 (Table 3). Only lack of appetite was significantly correlated with medical comorbidities, r(99) = 0.26, p < 0.01, whereas other symptoms showed small, non-significant correlations with medical comorbidities. More severe constipation, fatigue, pain, nausea, lack of appetite, and emotional distress were correlated with worse functional status, rs(97–99) = 0.30–54, ps< 0.01. More severe urination problems, fatigue, sleep problems, pain, nausea, and emotional distress were correlated with worse quality of life, rs(97–99)=−0.50 – −0.30, ps< 0.01. Moderate, positive correlations were found between the severity and importance of all symptoms, rs(13–82) = 0.56–0.78, ps< 0.01, except for sexual dysfunction and lack of appetite. Although severity and importance were also positively correlated for sexual dysfunction and lack of appetite, results fell short of statistical significance, rs(22–60) = 0.27–0.48, ps< 0.05.
Table 2.
Descriptive statistics for PCOQ constructs
| Usual Severity | Acceptable Severity | Importance of Improvement | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Symptom | n | Mean | S.D. | Range | n | Mean | S.D. | Range | n | Mean | S.D. | Range |
| Urination problems | 98 | 2.36 | 2.81 | 0–10 | 59 | 2.59 | 1.78 | 0–6 | 59 | 4.00 | 3.30 | 0–10 |
| Diarrhea | 97 | 0.62 | 1.58 | 0–8 | 21 | 1.14 | 0.91 | 0–3 | 21 | 3.67 | 3.34 | 0–10 |
| Constipation | 98 | 1.06 | 2.15 | 0–10 | 26 | 2.19 | 1.83 | 0–6 | 26 | 4.23 | 3.31 | 0–10 |
| Sexual dysfunction | 97 | 4.20 | 4.17 | 0–10 | 59 | 3.66 | 2.68 | 0–10 | 60 | 4.55 | 3.70 | 0–10 |
| Fatigue | 98 | 3.62 | 2.54 | 0–10 | 81 | 2.51 | 1.74 | 0–8 | 82 | 5.05 | 3.21 | 0–10 |
| Sleep problems | 99 | 2.93 | 2.70 | 0–10 | 72 | 2.51 | 1.96 | 0–10 | 74 | 4.15 | 3.01 | 0–10 |
| Pain | 98 | 2.05 | 2.56 | 0–10 | 52 | 2.44 | 2.25 | 0–10 | 53 | 4.72 | 3.37 | 0–10 |
| Nausea | 98 | 0.40 | 1.25 | 0–7 | 14 | 1.71 | 1.59 | 0–6 | 13 | 4.46 | 3.69 | 0–10 |
| Lack of appetite | 99 | 0.85 | 1.94 | 0–10 | 22 | 3.41 | 2.32 | 0–10 | 22 | 4.82 | 3.26 | 0–10 |
| Emotional distress | 99 | 1.35 | 2.05 | 0–8 | 45 | 2.44 | 1.66 | 0–7 | 46 | 3.65 | 3.11 | 0–10 |
PCOQ Patient-Centered Outcomes Questionnaire
Table 3.
Correlations between PCOQ symptom severity and hypothesized variables
| Standardized Symptom Severity a | Medical Comorbidities b | Functional Status c | Quality of Life d | Symptom Importance e | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p-value | r | p-value | r | p-value | r | p-value | r | p-value | |
| Urination problems | 0.72 | <0.001* | 0.17 | 0.088 | 0.13 | 0.203 | −0.35 | <0.001* | 0.59 | <0.001* |
| Diarrhea | 0.88 | <0.001* | 0.08 | 0.460 | 0.16 | 0.125 | −0.11 | 0.302 | 0.57 | 0.001* |
| Constipation | 0.89 | <0.001* | 0.20 | 0.054 | 0.31 | 0.002* | −0.25 | 0.012 | 0.73 | <0.001* |
| Sexual dysfunction | 0.66 | <0.001* | 0.17 | 0.092 | 0.12 | 0.257 | −0.16 | 0.116 | 0.27 | 0.034 |
| Fatigue | 0.80 | <0.001* | 0.03 | 0.799 | 0.54 | <0.001* | −0.50 | <0.001* | 0.56 | <0.001* |
| Sleep problems | 0.71 | <0.001* | 0.09 | 0.398 | 0.26 | 0.010 | −0.36 | <0.001* | 0.60 | <0.001* |
| Pain | 0.81 | <0.001* | 0.24 | 0.018 | 0.53 | <0.001* | −0.42 | <0.001* | 0.69 | <0.001* |
| Nausea | 0.64 | <0.001* | 0.25 | 0.012 | 0.30 | 0.003* | −0.30 | 0.003* | 0.78 | 0.002* |
| Lack of appetite | 0.83 | <0.001* | 0.26 | 0.008* | 0.39 | <0.001* | −0.23 | 0.022 | 0.48 | 0.023 |
| Emotional distress | 0.57, 0.55f | <0.001* | 0.00 | 0.969 | 0.30 | 0.003* | −0.45 | <0.001* | 0.65 | <0.001* |
Pairwise correlations. PCOQ Patient-Centered Outcomes Questionnaire.
p<0.01.
Memorial Symptom Assessment Scale (MSAS) measures were used for urination problems, diarrhea, constipation, sexual dysfunction, nausea, and lack of appetite, and Patient-Reported Outcomes Measurement Information System (PROMIS) measures were used for fatigue, sleep problems, pain, and emotional distress (i.e., anxiety and depression), ns = 96–99.
ns = 97–99.
ns = 97–99.
ns = 97–99.
ns = 97–99.
Separate correlations were computed between PCOQ emotional distress and PROMIS anxiety and depression measures, rs = 0.57, 0.55, respectively.
Among participants experiencing the relevant symptom, on average, a small reduction from usual severity (1.07 to 3.32 on a 0–10 scale) was considered acceptable. Regarding aim 2, estimated marginal means obtained from the linear mixed model analysis indicated that the lowest acceptable severity was 1.29 for diarrhea and the highest acceptable severity was 3.71 for sexual dysfunction (Table 4). Comparisons of acceptable severity across symptoms indicated that sexual dysfunction had significantly higher acceptability than all other symptoms except for urination problems and lack of appetite, ps< 0.01.
Table 4.
Acceptable levels of symptom severity using linear mixed modeling (n = 97)
| Estimated Marginal Mean | S.E. | |
|---|---|---|
| Symptoms | ||
| Urination problems | 2.68 | 0.25 |
| Diarrhea | 1.29 | 0.39 |
| Constipation | 1.99 | 0.35 |
| Sexual dysfunction | 3.71 | 0.25 |
| Fatigue | 2.51 | 0.22 |
| Sleep problems | 2.51 | 0.23 |
| Pain | 2.37 | 0.26 |
| Nausea | 1.55 | 0.47 |
| Lack of appetite | 2.99 | 0.38 |
| Emotional distress | 2.42 | 0.28 |
Each symptom was rated on a 0 to 10 scale.
Regarding aim 3, patient subgroups based on symptom improvement priorities were identified through estimation of five latent profile models (Online Resource 1). The 3-subgroup model was chosen because it yielded the lowest BIC value, provided a better fit than the 4-subgroup model based on the BLRT, and was the most conceptually meaningful classification of patients. Accordingly, subgroup 1 rated all symptoms as low in importance (n = 43, 43%); subgroup 2 rated all symptoms as moderate in importance (n = 33, 33%); and subgroup 3 rated all symptoms as high in importance (n = 18, 18%) (Fig. 1, Table 5).
Table 5.
Descriptive statistics for subgroups based on symptom importance
| Subgroup 1 | Subgroup 2 | Subgroup 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Symptom Importance | Estimated Marginal Means | S.E. | Range | Estimated Marginal Means | S.E. | Range | Estimated Marginal Means | S.E. | Range |
| Urination problems | 2.32 | 0.58 | 0–10 | 4.51 | 0.66 | 0–9 | 6.59 | 0.82 | 0–10 |
| Diarrhea | 0.88 | 0.66 | 0–10 | 3.59 | 0.72 | 0–9 | 7.49 | 0.77 | 0–6 |
| Constipation | 1.35 | 0.87 | 0–5 | 5.43 | 0.97 | 0–9 | 5.88 | 0.89 | 0–10 |
| Sexual dysfunction | 3.22 | 0.67 | 0–10 | 4.35 | 0.76 | 0–10 | 7.86 | 1.02 | 0–10 |
| Fatigue | 2.11 | 0.29 | 0–10 | 6.64 | 0.34 | 0–10 | 8.71 | 0.44 | 0–10 |
| Sleep problems | 1.91 | 0.36 | 0–10 | 4.95 | 0.42 | 0–9 | 7.71 | 0.56 | 0–10 |
| Pain | 1.32 | 0.33 | 0–10 | 5.94 | 0.35 | 0–10 | 9.00 | 0.45 | 0–10 |
| Nausea | 0.98 | 1.36 | 0–3 | 4.85 | 0.92 | 0–9 | 9.00 | 1.73 | 0–10 |
| Lack of appetite | 2.97 | 0.96 | 0–8 | 3.44 | 0.90 | 0–7 | 7.58 | 0.85 | 0–10 |
| Emotional distress | 1.60 | 0.36 | 0–9 | 2.92 | 0.36 | 0–10 | 8.70 | 0.46 | 0–10 |
| Differing Variables | Mean | SD | Range | Mean | SD | Range | Mean | SD | Range |
| Functional status | 0.67 | 0.64 | 0–3 | 0.73 | 0.91 | 0–3 | 1.00 | 0.91 | 0–3 |
| Fatigue | 3.62 | 2.52 | 0–9 | 3.39 | 2.52 | 0–8 | 4.00 | 2.72 | 0–10 |
| Sleep problems | 2.86 | 2.61 | 0–9 | 2.70 | 2.62 | 0–8 | 2.72 | 2.24 | 0–7 |
| Pain | 2.02 | 2.33 | 0–8 | 1.91 | 2.59 | 0–8 | 2.17 | 2.64 | 0–7 |
| Emotional distress | 1.30 | 2.04 | 0–8 | 1.06 | 1.78 | 0–7 | 1.83 | 2.62 | 0–8 |
| n | (%) | n | (%) | n | (%) | ||||
| History of pain treatment | 9 | 20.93% | 9 | 27.27% | 5 | 27.78% | |||
Subgroup 1 Low Symptom Importance, n = 43; Subgroup 2 Moderate Symptom Importance, n = 33; Subgroup 3 High Symptom Importance, n = 18.
Regarding aim 4, differences between subgroups on demographic and clinical characteristics were explored through multinomial logistic regressions using Vermunt’s 3-step approach with subgroup 1 as the reference group. Worse functional status (OR = 6.05), higher levels of fatigue (OR = 1.61), sleep problems (OR = 1.50), pain (OR = 1.64), and emotional distress (OR = 1.64), and a history of pain treatment (OR = 13.57) were associated with a greater likelihood of being in subgroup 3 than subgroup 1 (Online Resource 2). Similarly, worse functional status (OR = 6.52), higher levels of fatigue (OR = 1.57), sleep problems (OR = 1.43), pain (OR = 1.56), and a history of pain treatment (OR = 9.85) were associated with a greater likelihood of being in subgroup 2 than subgroup 1.
Discussion
The modified PCOQ showed evidence of construct validity in patients with advanced prostate cancer. The correlations between this measure and related theoretical constructs were mostly aligned with the Dodd Symptom Management Model [20]. On average, patients experiencing symptoms considered a minor reduction in symptom severity acceptable. Acceptable symptom levels were significantly higher for sexual dysfunction than most other symptoms. Patient priorities for symptom treatment varied widely and were correlated with certain symptom levels and other clinical characteristics.
Preliminary construct validity of the PCOQ showed results that were largely consistent with our hypotheses. The severity of all symptoms on the PCOQ was strongly correlated with standard measures of the same symptoms. For the majority of symptoms, greater severity was correlated with worse functional status and quality of life. Among the symptoms, only lack of appetite, prevalent in many clinical conditions [32–34], showed a significant correlation with the number of medical comorbidities. Additionally, moderate, positive correlations were found between symptom severity and importance for all symptoms except for sexual dysfunction and lack of appetite. Findings suggest that symptom severity and importance are related yet distinct concepts. Previous research in chronic pain and cancer has found similar associations between symptom severity and patient subgroups based on symptom importance [2, 5, 11, 13, 14, 35].
Potential explanations for the moderate correlation between symptom severity and importance have rarely been assessed. In one qualitative study, patients with advanced lung cancer provided varied reasons for symptom importance ratings that extended beyond symptom severity [13]. For example, some patients reported that their ratings of symptom importance reflected their ability to tolerate the symptom or the extent to which the symptom interfered with daily activities. Others stated that their low symptom importance ratings were due to prioritizing survival over symptom management.
Patients generally preferred low symptom severity levels across all symptoms and considered minor reductions in symptom severity acceptable, although acceptability varied across symptoms. The acceptable severity levels in the current sample align with success criteria for symptom improvement in patients with chronic pain and metastatic breast and lung cancer [2, 10, 13, 14, 35]. The lowest acceptable severity ratings were found for diarrhea and nausea, emphasizing their importance as intervention targets. A previous study of patients with prostate cancer identified diarrhea as a more troubling side effect than urinary symptoms, underscoring its significance [36]. However, in this study, patients, on average, had very mild diarrhea and nausea. Conversely, sexual dysfunction, a prevalent side effect of prostate cancer therapies, had a significantly higher acceptable severity level than most symptoms. This side effect is often addressed in patient education [37], which may help facilitate adaptive coping strategies. Additionally, reduced sexual desire, a frequent side effect of treatment [38, 39], may lead to greater tolerance of sexual dysfunction. The severity and importance of sexual dysfunction were not significantly correlated in this study, consistent with its higher acceptable severity level. Fatigue severity was the most distant from its acceptable level, aligning with research that identifies fatigue as the most troublesome symptom for patients with advanced prostate cancer [40]. However, in this study, fatigue did not require substantial reduction to be considered manageable.
Three distinct subgroups of patients were identified based on the importance of seeing symptom improvement: those who rated all symptoms as low, moderate, or high in importance. This classification illustrates the variability in patient attitudes towards symptom management. Studies in advanced breast and lung cancer also identified one patient subgroup that rated all symptoms as highly important [13, 14]. Differences in the remaining patient subgroups across studies likely reflect variations in the examined symptoms and sample characteristics.
In the current study, patients who rated all symptoms as moderately or highly important were more likely to have greater physical symptoms (e.g., pain, fatigue), a history of pain treatment, and worse functional status than patients who rated all symptoms as low in importance. In addition, patients who rated all symptoms as highly important showed higher levels of emotional distress than patients who rated all symptoms as low in importance. Our findings align with the Dodd Symptom Management Model, which posits correlations between health-related characteristics and symptom experiences [20]. Other findings in advanced breast and lung cancer and chronic pain were largely inconsistent with this model; few demographic and clinical correlates of patient subgroups based on symptom importance were found [2, 10, 13, 14, 35]. Findings suggest that for patients with advanced prostate cancer, symptom importance ratings may more closely parallel other aspects of their functioning and clinical care as compared to patients with other advanced solid tumors or chronic pain.
Limitations of the current study should be noted. This study was conducted at a single academic medical center in the midwestern United States and primarily enrolled non-Hispanic white participants. The relatively small sample size may have reduced statistical power for detecting significant associations. Finally, the cross-sectional design did not allow for the evaluation of test-retest reliability and longitudinal changes.
Our findings highlight the need for personalized symptom management for patients with advanced prostate cancer. This approach would incorporate patient criteria for symptom treatment success and treatment priorities into the shared decision-making process. Specifically, patients in our study often required low symptom severity to view treatment as satisfactory, underscoring the need for tailored patient-provider discussions about expected treatment outcomes. Additionally, given the varied priorities among patients for symptom improvement, understanding individual treatment goals would inform patient-centered care. For example, providers may consider asking patients which symptom is most important to address first. Finally, it is important for providers to consider the interconnectedness of symptoms and discuss potential side effects of treatments to align with patients’ symptom management priorities. For example, previous studies indicate that treating co-occurring symptoms such as urinary problems and pain can also mitigate fatigue [41]. However, single-symptom treatments like steroids or stimulants for fatigue might worsen other symptoms, such as anxiety and sleep problems [42].
There are several important directions for future research. The psychometric properties of our modified PCOQ should be examined in larger advanced prostate cancer samples that are fully representative of the population. In addition, researchers could modify and validate the PCOQ for other advanced cancer populations. Longitudinal studies could assess changes in acceptable symptom levels and priorities for symptom improvement over the cancer trajectory. Finally, pragmatic intervention trials targeting symptom clusters could assess patient priorities for symptom improvement to guide the next steps in their care. This research will lead to patient-centered approaches to enhancing quality of life and functioning in advanced cancer.
Funding:
This work was supported by the Walther Cancer Foundation (0172.01: Mosher) and the National Cancer Institute (T32CA117865: Krueger). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Walther Cancer Foundation or National Cancer Institute.
Footnotes
Declarations
Conflicts of interest
Ekin Secinti is presently an employee and shareholder of Eli Lilly and Company, Indianapolis, IN. This study was not financially supported by and does not necessarily represent the official views of Eli Lilly and Company. The authors declare that they have no conflict of interest.
Ethics approval:
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Indiana University Institutional Review Board.
Consent to participate:
Informed consent was obtained from all individual participants included in the study.
Consent to publish:
Participants provided informed consent regarding publishing their data.
Competing Interests
Ekin Secinti is presently an employee and shareholder of Eli Lilly and Company, Indianapolis, IN. This study was not financially supported by and does not necessarily represent the official views of Eli Lilly and Company. The authors declare that they have no conflict of interest.
Additional Declarations: Competing interest reported. Ekin Secinti is presently an employee and shareholder of Eli Lilly and Company, Indianapolis, IN. This study was not financially supported by and does not necessarily represent the official views of Eli Lilly and Company.
The authors declare that they have no conflict of interest.
Contributor Information
Stella Snyder, Indiana University Indianapolis.
Ekin Secinti, Indiana University Indianapolis.
Ellen Krueger, Indiana University Indianapolis.
Nabil Adra, Indiana University School of Medicine.
Roberto Pili, Jacobs School of Medicine & Biomedical Sciences, University at Buffalo.
Nasser Hanna, Indiana University School of Medicine.
Catherine Mosher, Indiana University Indianapolis.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Barry MJ, Edgman-Levitan S (2012) Shared decision making-pinnacle of patient-centered care. N Engl J Med 366: 780–781 10.1056/NEJMp1109283 [DOI] [PubMed] [Google Scholar]
- 2.Robinson ME, Brown JL, George SZ, Edwards PS, Atchison JW, Hirsh AT, Waxenberg LB, Wittmer V, Fillingim RB (2005) Multidimensional success criteria and expectations for treatment of chronic pain: the patient perspective. Pain Med 6: 336–345 10.1111/j.1526-4637.2005.00059.x [DOI] [PubMed] [Google Scholar]
- 3.Brown JL, Edwards PS, Atchison JW, Lafayette-Lucey A, Wittmer VT, Robinson ME (2008) Defining patient-centered, multidimensional success criteria for treatment of chronic spine pain. Pain Med 9: 851–862 10.1111/j.1526-4637.2007.00357.x [DOI] [PubMed] [Google Scholar]
- 4.Etkind SN, Daveson BA, Kwok W, Witt J, Bausewein C, Higginson IJ, Murtagh FE (2015) Capture, transfer, and feedback of patient-centered outcomes data in palliative care populations: does it make a difference? A systematic review. J Pain Symptom Manage 49: 611–624 10.1016/jjpainsymman.2014.07.010 [DOI] [PubMed] [Google Scholar]
- 5.Nisenzon AN, Robinson ME, Bowers D, Banou E, Malaty I, Okun MS (2011) Measurement of patient-centered outcomes in Parkinson’s disease: what do patients really want from their treatment? Parkinsonism Relat Disord 17: 89–94 10.1016/j.parkreldis.2010.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joosten EAG, DeFuentes-Merillas L, de Weert GH, Sensky T, van der Staak CPF, de Jong CAJ (2008) Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychother Psychosom 77: 219–226 10.1159/000126073 [DOI] [PubMed] [Google Scholar]
- 7.Kehl KL, Landrum MB, Arora NK, Ganz PA, van Ryn M, Mack JW, Keating NL (2015) Association of actual and preferred decision roles with patient-reported quality of care: shared decision making in cancer care. JAMA Oncol 1: 50–58 10.1001/jamaoncol.2014.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henson LA, Maddocks M, Evans C, Davidson M, Hicks S, Higginson IJ (2020) Palliative care and the management of common distressing symptoms in advanced cancer: pain, breathlessness, nausea and vomiting, and fatigue. J Clin Oncol 38: 905–914 10.1200/jco.19.00470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell KR, Brassil KJ, Rodriguez SA, Tsai E, Fujimoto K, Krause KJ, Shay LA, Springer AE (2020) Operationalizing patient-centered cancer care: a systematic review and synthesis of the qualitative literature on cancer patients’ needs, values, and preferences. Psychooncology 29: 1723–1733 10.1002/pon.5500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Brien EM, Staud RM, Hassinger AD, McCulloch RC, Craggs JG, Atchison JW, Price DD, Robinson ME (2010) Patient-centered perspective on treatment outcomes in chronic pain. Pain Med 11: 6–15 10.1111/j.1526-4637.2009.00685.x [DOI] [PubMed] [Google Scholar]
- 11.Yi TI, Kim BK, Ha SA, Lim JY (2014) The Relationships Between Determination of Treatment Success and Emotional Factors in Patients With Chronic Musculoskeletal Pain. Ann Rehabil Med 38: 77–83 10.5535/arm.2014.38.1.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stutts LA, Robinson ME, McCulloch RC, Banou E, Waxenberg LB, Gremillion HA, Staud R (2009) Patient-centered outcome criteria for successful treatment of facial pain and fibromyalgia. J Orofac Pain 23: 47–53 [PMC free article] [PubMed] [Google Scholar]
- 13.Krueger E, Secinti E, Wu W, Hanna N, Durm G, Einhorn L, Jalal S, Mosher CE (2021) Measurement of patients’ acceptable symptom levels and priorities for symptom improvement in advanced lung cancer. Support Care Cancer 29: 5895–5904 10.1007/s00520-021-06159-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tometich DB, Mosher CE, Hirsh AT, Rand KL, Johns SA, Matthias MS, Outcalt SD, Schneider BP, Mina L, Storniolo AMV, Newton EV, Miller KD (2018) Metastatic breast cancer patients’ expectations and priorities for symptom improvement. Support Care Cancer 26: 3781–3788 10.1007/s00520-018-4244-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drudge-Coates L, Oh WK, Tombal B, Delacruz A, Tomlinson B, Ripley AV, Mastris K, O’Sullivan JM, Shore ND (2018) Recognizing symptom burden in advanced prostate cancer: a global patient and caregiver survey. Clin Genitourin Cancer 16: e411–e419 10.1016/j.clgc.2017.09.015 [DOI] [PubMed] [Google Scholar]
- 16.Yount S, Cella D, Banik D, Ashraf T, Shevrin D (2003) Brief assessment of priority symptoms in hormone refractory prostate cancer: the FACT Advanced Prostate Symptom Index (FAPSI). Health Qual Life Outcomes 1: 69 10.1186/1477-7525-1-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeliadt SB, Ramsey SD, Penson DF, Hall IJ, Ekwueme DU, Stroud L, Lee JW (2006) Why do men choose one treatment over another?: a review of patient decision making for localized prostate cancer. Cancer 106: 1865–1874 10.1002/cncr.21822 [DOI] [PubMed] [Google Scholar]
- 18.Holmboe ES, Concato J (2000) Treatment decisions for localized prostate cancer: asking men what’s important. J Gen Intern Med 15: 694–701 10.1046/j.1525-1497.2000.90842.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steginga SK, Occhipinti S, Gardiner RA, Yaxley J, Heathcote P (2002) Making decisions about treatment for localized prostate cancer. BJU Int 89: 255–260 10.1046/j.1464-4096.2001.01741.x [DOI] [PubMed] [Google Scholar]
- 20.Dodd M, Janson S, Facione N, Faucett J, Froelicher ES, Humphreys J, Lee K, Miaskowski C, Puntillo K, Rankin S, Taylor D (2001) Advancing the science of symptom management. J Adv Nurs 33: 668–676 10.1046/j.1365-2648.2001.01697.x [DOI] [PubMed] [Google Scholar]
- 21.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC (2002) Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care 40: 771–781 10.1097/01.mlr.0000024610.33213.c8 [DOI] [PubMed] [Google Scholar]
- 22.Kroenke K, Theobald D, Norton K, Sanders R, Schlundt S, McCalley S, Harvey P, Iseminger K, Morrison G, Carpenter JS (2009) The Indiana Cancer Pain and Depression (INCPAD) trial: design of a telecare management intervention for cancer-related symptoms and baseline characteristics of study participants. Gen Hosp Psychiatry 31: 240–253 10.1016/j.genhosppsych.2009.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S (2010) The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 63: 1179–1194 10.1016/jjclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Portenoy RK, Thaler HT, Kornblith AB, Lepore JM, Friedlander-Klar H, Kiyasu E, Sobel K, Coyle N, Kemeny N, Norton L (1994) The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer 30: 1326–1336 [DOI] [PubMed] [Google Scholar]
- 25.Cohen SR, Mount BM, Bruera E, Provost M, Rowe J, Tong K (1997) Validity of the McGill Quality of Life Questionnaire in the palliative care setting: a multi-centre Canadian study demonstrating the importance of the existential domain. Palliat Med 11: 3–20 10.1177/026921639701100102 [DOI] [PubMed] [Google Scholar]
- 26.Muthén LK, Muthén BO (2017) MPlus user’s guide, 8th ed. Muthén & Muthén, Los Angeles, CA [Google Scholar]
- 27.Clark SL, Muthén B (2009) Relating latent class analysis results to variables not included in the analysis. https://wwwstatmodelcom/download/relatinglcapdf
- 28.Celeux G, Soromenho G (1996) An entropy criterion for assessing the number of clusters in a mixture model. J Classif 13: 195–212 10.1007/BF01246098 [DOI] [Google Scholar]
- 29.Nylund KL, Asparouhov T, Muthén BO (2007) Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Struct Equ Modeling 14: 535–569 10.1080/10705510701575396 [DOI] [Google Scholar]
- 30.Hagenaars JA, McCutcheon AL (2002) Applied latent class analysis. Cambridge University Press, New York, NY [Google Scholar]
- 31.Vermunt JK (2010) Latent class modeling with covariates: two improved three-step approaches. Polit Anal 18: 450–469 10.1093/pan/mpq025 [DOI] [Google Scholar]
- 32.Hariyanto TI, Kurniawan A (2021) Appetite problem in cancer patients: pathophysiology, diagnosis, and treatment. Cancer Treat Res Commun 27: 100336 10.1016/j.ctarc.2021.100336 [DOI] [PubMed] [Google Scholar]
- 33.Brain K, Burrows TL, Bruggink L, Malfliet A, Hayes C, Hodson FJ, Collins CE (2021) Diet and chronic non-cancer pain: the state of the art and future directions. J Clin Med 10: 5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andreae C, Strömberg A, örestedt K (2016) Prevalence and associated factors for decreased appetite among patients with stable heart failure. J Clin Nurs 25: 1703–1712 10.1111/jocn.13220 [DOI] [PubMed] [Google Scholar]
- 35.Zeppieri G Jr, Bialosky J, George SZ (2020) Importance of outcome domain for patients with musculoskeletal pain: characterizing subgroups and their response to treatment. Phys Therapy 100: 829–845 10.1093/ptj/pzaa009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lehto U-S, Tenhola H, Taari K, Aromaa A (2017) Patients’ perceptions of the negative effects following different prostate cancer treatments and the impact on psychological well-being: a nationwide survey. Br J Cancer 116: 864–873 10.1038/bjc.2017.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albaugh JA, Sufrin N, Lapin BR, Petkewicz J, Tenfelde S (2017) Life after prostate cancer treatment: a mixed methods study of the experiences of men with sexual dysfunction and their partners. BMC Urology 17: 45 10.1186/s12894-017-0231-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canalichio K, Jaber Y, Wang R (2015) Surgery and hormonal treatment for prostate cancer and sexual function. Transl Androl Urol 4: 103–109 10.3978/j.issn.2223-4683.2015.01.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kinnaird W, Konteti VK, Mitra A, Davda R, Payne H (2021) Sexual dysfunction in men with advanced prostate cancer. Trend Urol Mens Heal 12: 7–12 10.1002/tre.800 [DOI] [Google Scholar]
- 40.Luo YH, Yang YW, Wu CF, Wang C, Li WJ, Zhang HC (2021) Fatigue prevalence in men treated for prostate cancer: a systematic review and meta-analysis. World J Clin Cases 9: 5932–5942 10.12998/wjcc.v9.i21.5932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng LR, Fuss T, Dickinson K, Ross A, Saligan LN (2019) Co-occurring symptoms contribute to persistent fatigue in prostate cancer. Oncology 96: 183–191 10.1159/000494620 [DOI] [PubMed] [Google Scholar]
- 42.Escalante CP, Manzullo EF (2009) Cancer-related fatigue: the approach and treatment. J Gen Intern Med 24: 412–416 10.1007/s11606-009-1056-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.