Abstract
Aims and background
Midazolam is commonly used as a preanesthetic medication for behavior management of children. The current study is conducted to find out the effect of midazolam through nasal and oral routes as a premedicament in pediatric patients treated under general anesthesia. The main aims of the study were: to compare the effect of oral syrup and intranasal spray as preanesthetic medication; to record the undesirable side effects of midazolam by both routes.
Materials and methods
The patients aged 2–6 years of either sex were randomly divided into two equal groups of 30 each—group I: oral; group II: intranasal.
Results
The oral and intranasal routes of midazolam were found to be equally effective and provided adequate sedation for easy separation from the parents and cooperation from children during the induction of anesthesia with minimal side effects.
Conclusion
Based on the study results, we can conclude that both oral and intranasal midazolam can be used as preanesthetic medication for pediatric dental patients treated under general anesthesia.
Clinical significance
In pediatric patients, the oral route should be preferred for midazolam premedication in comparison to the intranasal route.
How to cite this article
Swati, Shah RK, Tandon S, et al. Comparative Evaluation of Oral and Intranasal Administration of Midazolam as Preanesthetic Medication in Pediatric Dental Patients Treated under General Anesthesia. Int J Clin Pediatr Dent 2024;17(8):881–886.
Keywords: Behavior management, General anesthesia, Intranasal midazolam sedation and dental treatment, Midazolam sedation, Oral midazolam, Pharmacological management, Premedication
Introduction
Addressing the dental needs of uncooperative children who require extensive treatment remains a significant and persistent challenge for a pediatric dentist. Since most of the treatment has to be performed under local anesthesia, the cooperation of pediatric patients becomes imperative. Obstacles to treatment can include young age, dental anxiety, or difficulties with behavior management. Behavior management problems are defined as disruptive behavior that results in the delay of treatment or makes treatment impossible.1 To perform dental treatment in these children effectively and efficiently, some nonpharmacological and pharmacological behavior management techniques can be used. The majority of children can be adequately treated with behavior modification techniques such as tell, show, and do (TSD) and voice control; however, many children continue to struggle with tolerating dental treatment. In such cases, pharmacological behavior management techniques, such as sedation or general anesthesia, can be considered as methods for reducing anxiety to facilitate dental treatment.2
In the present-day scenario, parents often find it difficult to accept traditional behavior management techniques. These techniques may create traumatic memories in children, potentially resulting in lifelong dental phobia.3 In today’s scenario, the use of pharmacological behavior management techniques has increased. However, many children experience severe anxiety and apprehension when separated from their parents or family for anesthesia.4 The unfamiliar faces and environment inside the operatory may heighten the child’s sense of insecurity.5 This preoperative anxiety can significantly impact the child’s psychological and emotional state in the long term6 and is linked to a higher incidence of nightmares, separation anxiety, eating disorders, and an increased fear of physicians.7 Several behavioral modification techniques are available to allay preoperative anxiety in children. Nonpharmacological approaches, such as friendly visits to build rapport with the child and briefing them about the procedure, along with parental presence in the operating room, can help reduce the child’s anxiety but may not be entirely effective. Pharmacological methods like sedative premedication may be more effective.8 The ideal sedative should have a rapid onset, predictable duration, and quick recovery. In clinical practice, the most commonly used drugs for premedication are ketamine, clonidine, fentanyl, buprenorphine, and midazolam.
Midazolam is an ideal and potent imidazobenzodiazepine that possesses hypnotic, sedative, and anxiolytic properties. It has a rapid onset of action with an elimination half-life of approximately 2 hours. It is highly lipophilic at physiological pH and water-soluble in an acidic medium.9
Midazolam is used as a sedative premedication via various routes like intramuscular,10,11 rectal,12 oral,13 intranasal,14,15 and sublingual,16,17 with each route having its own pros and cons. Midazolam, when administered intramuscularly, is not only painful but also carries the risk of developing needle phobia, especially in young children. With rectal administration, the absorption of the drug is usually very unpredictable and uncomfortable for the children. In this respect, the sublingual route appears to be more advantageous,18 but the child needs to keep the drug sublingually for at least 30 seconds to be effective. The oral route is also common in children; however, owing to high first-pass metabolism, it has low bioavailability.19 The intranasal route of administration has advantages owing to the high vascularity of the nasal mucosa and bypassing the hepatic first-pass metabolism. This allows rapid and nearly complete systemic absorption of the drug within 1–2 hours.20
Materials and Methods
The in vivo study was conducted in the Department of Pediatric and Preventive Dentistry, Government Dental College and Hospital, Jaipur, and ethical clearance was obtained from the ethical committee of the institute.
Sample Selection
A total of 60 healthy children aged 2–6 years of either gender, were included in the study. The children were randomly divided into two groups, with 30 children in each group, A and B, respectively.
Group A: Midazolam 0.5 mg/kg was given orally.
Group B: Midazolam 0.2 mg/kg was administered intranasally.
Prior to the commencement of the study, parents were thoroughly informed about the associated risks and benefits. They were also required to sign a written consent form in their local dialect.
Inclusion Criteria
Healthy children aged 2–6 years of either gender.
Children classified under American Society of Anesthesiologists (ASA) I and II.
Children requiring dental procedures with a duration of 20 minutes to 2 hours.
Children with Frankl’s behavior rating I and II.
Exclusion Criteria
Children with anemia (hemoglobin <9 gm/dL).
Children with any coagulation disorders.
Children with nasal infection or nasal pathology.
Children taking any other sedative drugs.
Children with any cardiac or renal diseases.
Children hypersensitive to any drugs used in the study.
Preanesthetic clearance was obtained, and peroral instructions were duly explained to the parents prior to the treatment under general anesthesia. Baseline vitals were recorded, and then in group A, commercially available midazolam syrup (MEZOLAM 0.5 mg/kg) was administered with the help of a premeasuring cup. In group B, midazolam was administered using an atomizer spray (INSED 0.2 mg/kg), with the dose divided equally between each nostril. Prior to the procedure under anesthesia and following drug administration, the vitals, sedation score, and separation score were recorded at 5-minute intervals for a period of 30 minutes.
The following scales were used to record the sedation and separation scores.
Sedation Scale
Score 1: Patient is agitated and clinging to parents and/or crying.21
Score 2: Patient is alert and aware but not clinging to parent, may whimper, not crying.
Score 3: Patient is calm, sitting or lying comfortably with spontaneous eye opening.
Score 4: Patient is drowsy, sitting or lying comfortably with eyes closed but responding to minor stimulation.
Score 5: Patient’s eyes closed, arousable but not responding to minor stimulus.
Separation and Induction Scale
Score 1: Excellent—patient unafraid, cooperative, asleep.21
Score 2: Good—slight fear or crying, quiet with reassurance.
Score 3: Fair—moderate fear, crying not quiet with reassurance.
Score 4: Poor—crying, need for restraint.
Postoperative recovery was assessed at 10-minute intervals for a period of 30 minutes on a 10-point Post Anesthesia Care Unit (PACU) Assessment and Recovery Score scale on the following parameters: respiration, activity, consciousness, circulation, preoperative blood pressure (BP), and temperature.
Post Anesthesia Care Unit Assessment and Recovery Score
Respiration
Apneic = 022
Dyspnea or limiting breathing = 1
Able to breathe deeply and cough freely = 2
Activity
Able to move no extremities voluntarily or on command = 0
Able to move two extremities voluntarily or on command = 1
Able to move four extremities voluntarily or on command = 2
Consciousness
Nonresponsive = 0
Responding to stimuli = 1
Awake = 2
Circulation, Preoperative Blood Pressure
BP >120% of preanesthetic level = 0
BP 111–120% of preanesthetic level = 1
BP <110% of preanesthetic level = 2
Temperature
Axillary temperature <35.0 or >35.5°C = 0
Axillary temperature between 35.0 and 35.5°C = 1
Axillary temperature 35.6–37.5°C = 2
Children were shifted to the pediatric ward when they were fully awake, able to move all extremities, breathe adequately, cough effectively, have SpO2 greater than 98%, and the PACU score was 10.
Side effects such as nausea and vomiting, bradycardia, drop in O2 saturation, hypertension, and respiratory depression were observed.
Results
Data obtained from a total of 60 children who met all the inclusion criteria were subjected to statistical analysis and randomly categorized into two groups:
Group A: 30 patients received midazolam orally.
Group B: 30 patients received midazolam intranasally.
It is evident from Table 1 and Figure 1 that when comparing the oral and nasal routes with respect to sedation scores at various time intervals, no statistical difference (p > 0.05) was observed between the two routes.
Table 1:
Comparison of different routes with respect to sedation score
| Group | N | Median | Mean rank | Sum of ranks | p-value* | |
|---|---|---|---|---|---|---|
| Baseline | Oral | 30 | 1 | 32.83 | 985.00 | 0.227 |
| Nasal | 30 | 1 | 28.17 | 845.00 | ||
| 5 minutes | Oral | 30 | 2 | 28.10 | 843.00 | 0.251 |
| Nasal | 30 | 2 | 32.90 | 987.00 | ||
| 10 minutes | Oral | 30 | 2.5 | 27.67 | 830.00 | 0.256 |
| Nasal | 30 | 3 | 33.33 | 1000.00 | ||
| 15 minutes | Oral | 30 | 3 | 31.32 | 939.50 | 0.671 |
| Nasal | 30 | 3 | 29.68 | 890.50 | ||
| 20 minutes | Oral | 30 | 3 | 29.20 | 876.00 | 0.496 |
| Nasal | 30 | 3 | 31.80 | 954.00 | ||
| 25 minutes | Oral | 30 | 3 | 28.80 | 864.00 | 0.387 |
| Nasal | 30 | 3 | 32.20 | 966.00 | ||
| 30 minutes | Oral | 30 | 4 | 28.88 | 866.50 | 0.375 |
| Nasal | 30 | 4 | 32.12 | 963.50 |
*Mann–Whitney test
Fig. 1:

Comparison of different routes with respect to sedation score
It is evident from Table 2 and Figure 2 that there were no statistically significant differences (p > 0.05) with respect to separation and induction scores between the oral and nasal groups throughout the study period.
Table 2:
Comparison of different routes with respect to separation score and induction score
| Group | N | Median | Mean rank | Sum of ranks | p-value* | |
|---|---|---|---|---|---|---|
| Baseline | Oral | 30 | 3 | 28.00 | 780.00 | 0.169 |
| Nasal | 30 | 4 | 33.00 | 1050.00 | ||
| 5 minutes | Oral | 30 | 3 | 28.98 | 869.50 | 0.419 |
| Nasal | 30 | 3 | 32.02 | 960.50 | ||
| 10 minutes | Oral | 30 | 3 | 27.50 | 825.00 | 0.111 |
| Nasal | 30 | 3 | 33.50 | 1005.00 | ||
| 15 minutes | Oral | 30 | 2 | 28.87 | 866.00 | 0.383 |
| Nasal | 30 | 2 | 32.13 | 964.00 | ||
| 20 minutes | Oral | 30 | 2 | 30.98 | 929.50 | 0.730 |
| Nasal | 30 | 2 | 30.02 | 900.50 | ||
| 25 minutes | Oral | 30 | 2 | 31.42 | 942.50 | 0.531 |
| Nasal | 30 | 2 | 29.58 | 887.50 | ||
| 30 minutes | Oral | 30 | 2 | 28.60 | 858.00 | 0.192 |
| Nasal | 30 | 2 | 32.40 | 972.00 |
*Mann–Whitney test
Fig. 2:

Comparison of different routes with respect to separation score and induction score
Table 3 and Figure 3 show the comparison of recovery scores in the oral and nasal groups at various time intervals. There was no statistically significant difference between the two groups (p > 0.05) with respect to recovery scores at these time intervals.
Table 3:
Comparison of different routes with respect to recovery score
| Group | N | Median | Mean rank | Sum of ranks | p-value* | |
|---|---|---|---|---|---|---|
| 10 minutes | Oral | 30 | 8 | 28.38 | 851.50 | 0.327 |
| Nasal | 30 | 8 | 32.62 | 978.50 | ||
| 20 minutes | Oral | 30 | 9 | 27.23 | 817.00 | 0.128 |
| Nasal | 30 | 9.5 | 33.77 | 1013.00 | ||
| 30 minutes | Oral | 30 | 10 | 30.32 | 909.50 | 0.918 |
| Nasal | 30 | 10 | 30.68 | 920.50 |
*Mann–Whitney test
Fig. 3:
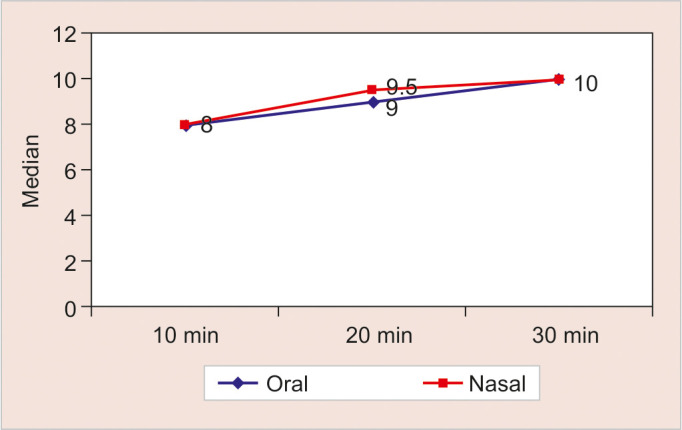
Comparison of different routes with respect to recovery score
It is evident from Table 4 that only 1 (33.3%) patient in the oral group showed nausea and vomiting, and only 1 (33.3%) patient in the nasal group showed a drop in O2 saturation. No patients showed bradycardia, hypertension, or respiratory depression.
Table 4:
Comparison of different routes with respect to side effects
| Oral n = 30 (100%) | Nasal n = 30 (100%) | |
|---|---|---|
| Nausea and vomiting | 1 (3.33%) | 0 |
| Bradycardia | 0 | 0 |
| Drop in O2 saturation | 0 | 1 (3.33%) |
| Hypertension | 0 | 0 |
| Respiratory depression | 0 | 0 |
Table 5 shows that 4 (13.33%) out of 30 patients in the nasal group experienced watering of the eyes, 10 (33.33%) patients exhibited sneezing, 10 (33.33%) patients cried during drug administration, and only 1 (3.33%) patient spat after drug administration. In the oral group, no patients showed watering of the eyes or nose, and no patients exhibited sneezing. However, 6 (20%) patients cried, and 2 (6.67%) patients spat the drug. This indicates that the orally administered drug is more acceptable than the nasal administration.
Table 5:
Acceptance of the route
| Oral n = 30 (100%) | Nasal n = 30 (100%) | |
|---|---|---|
| Watering of eyes | 0 | 4 (13.33%) |
| Sneezing | 0 | 10 (33.3%) |
| Crying | 6 (20%) | 10 (33.3%) |
| Spitting | 2 (6.67%) | 1 (3.33%) |
Discussion
In the present study, demographic parameters such as age, gender, and weight of the children were comparable, and no statistical differences were noted between the two groups. Children between 2 and 6 years of age were selected for the study, as this age-group is more susceptible to separation anxiety due to their limited understanding.23 One group received 0.5 mg/kg midazolam orally, and the other group received 0.3 mg/kg midazolam intranasally, as no added advantage of higher doses for sedation and anxiolysis properties, as well as lower chances of side effects, were reported in previous studies.24,25 The patient’s acceptance of nasal midazolam was improved by spray administration compared to drop instillation; therefore, an atomizer nasal spray was used instead of nasal drops.26
When comparing the oral and nasal routes with respect to sedation scores at various time intervals, no statistically significant differences were noted between the two routes. In the intranasal group, the onset of sedation was faster due to the quick and almost complete absorption of the drug, facilitated by the rich blood supply of the nasal mucosa. The systemic bioavailability of the drug is greater with the intranasal route compared to the oral route, as it avoids first-pass hepatic metabolism.20 A previous study reported that in the intranasal group, a faster sedation score of 3 or more was achieved at 15 and 30 minutes compared to the oral group, with results being statistically significant, which was not observed in this study. This difference may be due to the previous study using 0.5 mg/kg midazolam intranasally, while the present study used only 0.2 mg/kg intranasally.27 Another similar study compared 0.3 mg/kg intranasal midazolam and 0.5 mg/kg oral midazolam in preschool children and found that cooperation scores at the time of separation from parents and at the time of mask application were similar in both groups, which was statistically insignificant.28
All vitals, including heart rate, respiratory rate, BP, and O2 saturation, were noted at baseline and at 5-minute intervals for a period of 30 minutes in both the oral and nasal groups, with no statistical differences observed. Postoperative recovery scores were assessed at 10-minute intervals for a period of 30 minutes. Children were shifted to the pediatric ward from the PACU when a score of 10 was achieved, indicating regular and deep breathing, effective coughing, movement of all extremities, and SpO2 greater than 98%. At 10 minutes, the median score was 8 in both the oral and intranasal groups, with the difference being statistically insignificant. At 20 minutes, the median score was 9 in the oral group and 9.5 in the intranasal group, indicating faster recovery in children who received midazolam via the intranasal route, though these differences were statistically insignificant. At 30 minutes, all children in both groups had a score of 10 and were fit for transfer to the ward. A previous study observed that the time in the PACU was similar for children who received midazolam either nasally, rectally, or orally.29
Side effects such as nausea and vomiting, bradycardia, drop in O2 saturation, hypertension, and respiratory depression were observed. Nausea and vomiting were observed in only 1 (3.33%) patient in the oral group. A drop in O2 saturation was observed in only 1 (3.33%) patient in the nasal group. No other side effects such as hypertension, bradycardia, or respiratory depression were observed in any patients in either group. Acceptability of the route was assessed by observing watering of eyes and nose, sneezing, and crying. In the nasal group, 4 (13.33%) patients experienced watering of the eyes and nose, and sneezing was seen in 10 (33.33%) patients, which may be due to irritation of the nasal mucosa from the acidic preparation of midazolam (pH 3.34). Crying was observed in 6 (20%) patients who received midazolam through the nasal route, compared to 10 (33.33%) patients who received midazolam orally. In the nasal group, only 1 (3.33%) patient spat the drug, while in the oral group, 2 (6.67%) patients spat the drug. The median sedation score at 30 minutes was equal for both the oral and nasal groups, but subjects with intranasal administration showed more movement and less sleep than those with oral administration. Based on these observations, the oral route is more acceptable for midazolam premedication in pediatric patients compared to the nasal route; however, the nasal route offers a quicker onset of action. A previous study also observed similar side effects, such as nasopharyngeal irritation, bad mouth taste, watering of the eyes, dizziness, and nasal congestion, with the intranasal route.30
Conclusion
We can conclude that both the oral and intranasal routes for midazolam premedication are equally effective and safe for pediatric dental patients treated under general anesthesia; however, the intranasal route has a quicker and more rapid onset of action.
Clinical Significance
Based on the entire observations, we recommend the oral route of midazolam premedication over the intranasal route because it is more acceptable to pediatric patients. Intranasal midazolam can cause irritation and burning of the nasal mucosa, making the child more reluctant.
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Klingberg G, Berggren U, Carlsson SG, et al. Child dental fear: cause-related factors and clinical effects. Eur J Oral Sci. 1995;103(6):405–412. doi: 10.1111/j.1600-0722.1995.tb01865.x. [DOI] [PubMed] [Google Scholar]
- 2.Enger DJ, Mourino AP. A survey of 200 pediatric dental general anaesthesia cases. J Dent Child. 1985;52:36–41. [PubMed] [Google Scholar]
- 3.Damle SG, Gandhi M, Laheri V. Comparison of oral ketamine and oral midazolam as sedative agents in pediatric dentistry. J Indian Soc Pedod Prev Dent. 2008;26:97–101. [PubMed] [Google Scholar]
- 4.Beeby DG, Huges JOM. Behaviour of unsedated children in the anaesthetic room. Br J Anaesth. 1980;52:279–281. doi: 10.1093/bja/52.3.279. [DOI] [PubMed] [Google Scholar]
- 5.Korsch BM. The child and operating room. Anesthesiology. 1975;431:251–257. doi: 10.1097/00000542-197508000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Kain ZN. Perioperative Psychological Trauma in Children, 1st edition. Philadelphia: WB Saunders; 1990. [Google Scholar]
- 7.Kain ZN, Mayes LC, O’Connor TZ, et al. Preoperative anxiety in children: predictors and outcomes. Arch Pediatr Adolesc Med. 1996;150:1238–1245. doi: 10.1001/archpedi.1996.02170370016002. [DOI] [PubMed] [Google Scholar]
- 8.Rita L, Cox JM, Seleny FL, et al. Ketamine hydrochloride for pediatric premedication: comparison to pentazocine. Anesth Analg. 1974;53:375. [PubMed] [Google Scholar]
- 9.Clerk RSJ. Intravenous anaesthetic agents: induction and maintenance. In: Healy TEJ, Cohen PJ, editors. Wylie and Churchill-Davidson’s A Practice of Anaesthesia, 6th edition. London: Edward Arnold; 1995. p. 98. (Eds). p. [Google Scholar]
- 10.Rita L, Seleny FL, Mazurek A, et al. Intramuscular midazolam for pediatric preanethestic sedation: a double blind controlled study with morphine. Anesthesiology. 1985;63:528–531. doi: 10.1097/00000542-198511000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Taylor MB, Vine PR, Hatch DJ. Intramuscular midazolam premedication in small children. A comparison with papaveretum and hyoscine. Anaesthesia. 1986;41:21–26. doi: 10.1111/j.1365-2044.1986.tb12698.x. [DOI] [PubMed] [Google Scholar]
- 12.Saint-Maurice C, Meistleman C, Rey E, et al. The pharmacokinetics of rectal midazolam for premedication in children. Anesthesiology. 1986;65:536–538. doi: 10.1097/00000542-198611000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Raybould D, Bradshaw EG. Premedication for day case surgery. Anaesthesia. 1987;42:591–595. doi: 10.1111/j.1365-2044.1987.tb03081.x. [DOI] [PubMed] [Google Scholar]
- 14.Abrams R, Morrison JE, Villasenor A, et al. Safety and effectiveness of intranasal administration of sedative medications (ketamine, midazolam or sufentanil) for urgent brief pediatric dental procedures. Anesth Prog. 1993;40:63–66. [PMC free article] [PubMed] [Google Scholar]
- 15.Wilton NCT, Leigh J, Rosen DR, et al. Preanesthetic sedation of preschool children using intranasal midazolam. Anesthesiology. 1988;69:972–975. doi: 10.1097/00000542-198812000-00032. [DOI] [PubMed] [Google Scholar]
- 16.Karl HW, Rosenburger JL, Lrach MG, et al. Transmucosal administration of midazolam for premedication of pediatric patients. Comparison of nasal and sublingual routes. Anesthesiology. 1993;78:885–891. doi: 10.1097/00000542-199305000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Khalil S, Philbrook L, Rabb M, et al. Sublingual midazolam premedication in children: a dose response study. Pediatr Anesth. 1998;8:461–465. doi: 10.1046/j.1460-9592.1998.00286.x. [DOI] [PubMed] [Google Scholar]
- 18.Pandit UA, Collier PJ, Malviya S, et al. Oral tranmucosal midazolam premedication for preschool children. Can J Anesth. 2001;48(2):191–195. doi: 10.1007/BF03019734. [DOI] [PubMed] [Google Scholar]
- 19.Cox RG, Nemish U, Ewen A, et al. Evidence-based clinical update: does premedication with oral midazolam lead to improved behavioural outcomes in children? Can J Anesth. 2006;53(12):1213–1219. doi: 10.1007/BF03021583. [DOI] [PubMed] [Google Scholar]
- 20.Bojrkman S, Rigemar G, Idvall J. Pharmacokinetics of midazolam given as intranasal spray to adult surgical patients. Br J Anaesth. 1997;79:575–580. doi: 10.1093/bja/79.5.575. [DOI] [PubMed] [Google Scholar]
- 21.Karl HW, Keifer AT, Rosenberger JL, et al. Comparison of the safety and efficacy of intranasal midazolam or sufentanil for premedication of anaesthesia of paediatric patients. Anaesthesiology. 1992;76:209–215. doi: 10.1097/00000542-199202000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Davis PJ, Tome JA, McGowan FX, Jr,, et al. Preanaesthetic mediation with intranasal midazolam for brief paediatric surgical procedures. Effect on recovery and hospital discharge times. Anaesthesiology. 1995;82:2–5. doi: 10.1097/00000542-199501000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Weksler N, Ovadia L, Muati G, et al. Nasal ketamine for pediatric premedication. Can J Anesth. 1993;40:119–121. doi: 10.1007/BF03011307. [DOI] [PubMed] [Google Scholar]
- 24.McMillan CO, Spahr-Schopfer IA, Sikich N, et al. Premedication of children with oral midazolam. Can J Anaesth. 1992;39(6):545–550. doi: 10.1007/BF03008315. [DOI] [PubMed] [Google Scholar]
- 25.Bhakta P, Ghosh BR, Mukherjee G, et al. Evaluation of intranasal midazolam for preanasthetic sedation in paediatric patients. Ind J Anaesth. 2007;51(2):111–116. [Google Scholar]
- 26.Primosch RE, Guelmann M. Comparison of drops versus spray administration of intranasal midazolam in two- and three-year-old children for dental sedation. Pediatr Dent. 2005;27(5):401–408. [PubMed] [Google Scholar]
- 27.Koppal R, Adarsh ES, Ambi U, et al. Comparison of the midazolam transnasal atomizer and oral midazolam for sedative premedication in paediatric Cases. JCDR. 2011;5(5):932–934. [Google Scholar]
- 28.Alex S, Coelho B, Ambareesha M. Comparison of intranasal and oral midazolam as premedicant drug in preschool children. J Anaesth Clin Pharmacol. 2008;24(3):333–336. [Google Scholar]
- 29.Chhibber AK, Fickling K, Lustik SJ. Pre-anesthetic midazolam: a randomized trial with three different routes of administration. J Anesth Clinic Res. 2011;2:1. doi: 10.4172/2155-6148.1000118. [DOI] [Google Scholar]
- 30.Wermeling DP, Record KA, Kelly TH, et al. Pharmacokinetics and pharmacodynamics of a new intranasal midazolam and formulation in healthy volunteers. Anesth Analg. 2006;103:344–349. doi: 10.1213/01.ane.0000226150.90317.16. [DOI] [PubMed] [Google Scholar]


