Abstract
Aim
The aim of this study was to evaluate the antimicrobial efficacy and minimum inhibitory concentration (MIC) of commercially available pediatric dentifrices containing different compositions against Streptococcus mutans and Lactobacillus activity.
Materials and methods
Four different commercially available brands of pediatric dentifrices, designated as sample I—fluoride, sample II—herbal, sample III—xylitol with nanosilver particles, and sample IV—xylitol with fluoride, along with two control groups (a positive control—ciprofloxacin and a negative control—distilled water), were tested for their antibacterial activity by measuring the zone of inhibition, followed by MIC against two dental bacterial pathogens, S. mutans strain and Lactobacillus acidophilus (LB) strain, at five different twofold dilutions of 100, 50, 25%, 12.5, and 6.25% concentrations.
Result
All four dentifrices were found to have wide variations in their effectiveness against the two tested microorganisms at 100% (pure) and 50% concentrations, with sample I having the highest activity, followed by sample IV and sample II. At 25% concentration, only sample I and sample IV showed antibacterial activity, while at 12.5 and 6.25% concentrations, none of the tested toothpastes exhibited any antibacterial activity. Sample III failed to show antibacterial activity even in pure form against the two microorganisms.
Conclusion
In our present study, the fluoride-containing pediatric dentifrice with a lower fluoride concentration (458 ppm) exhibited the highest zone of inhibition, followed by the xylitol with fluoride dentifrice and the herbal dentifrice. No zone of inhibition was observed in the nanosilver with xylitol dentifrice.
How to cite this article
Dureha R, Navit S, Khan SA, et al. Comparative Evaluation of Antimicrobial Activity and Minimum Inhibitory Concentration of Commercially Available Pediatric Dentifrices: An In Vitro Study. Int J Clin Pediatr Dent 2024;17(8):938–944.
Keywords: Antimicrobial efficacy, Fluorides, Herbal toothpaste, Minimum inhibitory concentration, Nanosilver, Toothpastes, Xylitol
Introduction
The oral cavity harbors a plethora of microorganisms with varying environmental conditions. Oral flora has an ecologically diverse microbial population, making the study of oral microbiology complex and difficult. As early as 1674, Antony van Leeuwenhoek, the father of the modern-day microscope, observed his own dental plaque and reported “little living animalcules prettily moving.”1 Numerous subsequent studies on the role of oral microflora in health and disease have followed this model. These oral microorganisms are beneficial when present in the right numbers, with a predominance of bacteria.
Carious lesions reveal a wide variety of microorganisms, of which S. mutans, L. acidophilus, various proteolytic bacteria, anaerobic organisms, etc., are the essential microorganisms involved in the initiation and progression of dental caries.2S. mutans is a gram-positive bacterium regularly found in the human oral cavity and is one of the principal microorganisms involved in the etiology of dental caries, alongside Lactobacillus spp.3 On the other hand, the further progression of carious lesions is related to Lactobacillus. These organisms are often found in large numbers in patients with rampant caries,4 particularly in association with Lactobacillus, and they play a significant role in the fermentation of carbohydrates, resulting in acid production and the demineralization of teeth.
The colonization of microorganisms on tooth surfaces is perceived as a vital etiologic factor in dental caries, gum disease, and periodontitis. Dental caries is an infectious disease in which bacteria destroy the enamel, dentin, or cementum of the teeth. Sugar present in plaque together with cariogenic bacteria can produce the disease2 by interacting in various recognized ways, including coaggregation,5 metabolic exchange, cell-cell communication,6 and exchange of genetic material.7
Thus, an antibacterial approach to reduce the risk and spread of caries is an important step forward in the modern noninvasive mode. One such technique to reduce the cariogenic bacterial load is the use of dentifrices, which has been defined by the American Dental Association as a paste used with the aid of a toothbrush to cleanse and maintain the esthetic and well-being of the oral cavity.8 One of the most common forms of oral hygiene worldwide, tooth brushing with a dentifrice is an essential step in maintaining oral health.9 Over 80% of people brush their teeth at least once or twice a day, making it the most popular method of home dental care.10
It is known that dentifrices are effective in removing cariogenic bacteria from the mouth, thereby preventing dental caries and periodontal disease. Minimum inhibitory concentration (MIC) indicates the lowest concentration of antimicrobial agent that will inhibit the visible growth of microorganism.11 Thus, dentifrices with lower MIC scores are more effective antimicrobial agents. Today, toothpaste contains a wide range of active ingredients, primarily antimicrobial ones, in order to directly inhibit plaque formation and arrest dental caries.
The rationale for performing this in vitro study was to offer information to pediatric clinicians about the microbial efficacy of commercially available pediatric dentifrices against S. mutans and L. acidophilus. This in vitro study was performed to evaluate the antimicrobial effect and MIC of commercially available pediatric dentifrices containing various active agents at different concentrations against S. mutans and L. acidophilus activity.
Materials and Methods
This in vitro study was carried out to demonstrate the antimicrobial effect and MIC of commercially available pediatric dentifrices containing fluoride, herbal, nanosilver particles with xylitol, and fluoride with xylitol formulations. After obtaining due approval from the Institutional Research and Development Committee (SDC/IRDC/2018/MDS/24), this study was carried out in the Department of Pediatric and Preventive Dentistry, in collaboration with Cytogene Research and Development Centre, Lucknow, India,
Material Used
Dentifrices
The following dentifrices were chosen for the study (Fig. 1):
Fig. 1:

Dentifrices used
Sample I: Fluoride dentifrice—sodium monoflurophosphate—0.35%, and containing fluoride—458 ppm (Cheerio Gel).
Sample II: Herbal dentifrice (Dant Kanti Junior).
Sample III: Xylitol + nanosilver particles dentifrice (Superblue).
Sample IV: Xylitol + fluoride dentifrice—sodium monofluorophosphate 0.38%, containing fluoride—500 ppm with xylitol (Kidodent).
Positive control: Ciprofloxacin 500 ppm.
Negative control: Distilled water as the active ingredient.
Tested Microorganisms
American Type Culture Collection (ATCC) culture of common oral microflora, that is, S. mutans strain (ATCC 35668) and L. acidophilus strain (ATCC 4357), was selected.
In this study, the antimicrobial activity test was done in two parts:
Zone of inhibition by disk diffusion method.
Minimum inhibitory concentration by broth dilution method.
The antimicrobial activities of pediatric dentifrices are used against S. mutans strain and L. acidophilus strain, which was cultured in this study by brain heart infusion agar and Lactobacillus MRS agar, respectively, by diffusion method.
Slurry Preparation of Dentifrices
The calculated amount of dentifrices (10.0 gm) was mixed with the measured volume of sterile distilled water (10 mL) to prepare each pediatric dentifrice sample for the slurry, to give a respective serial concentration of 100 (pure), 50, 25, 12.5, and 6.25% (toothpaste: distilled water) dilution.
Antimicrobial Assay
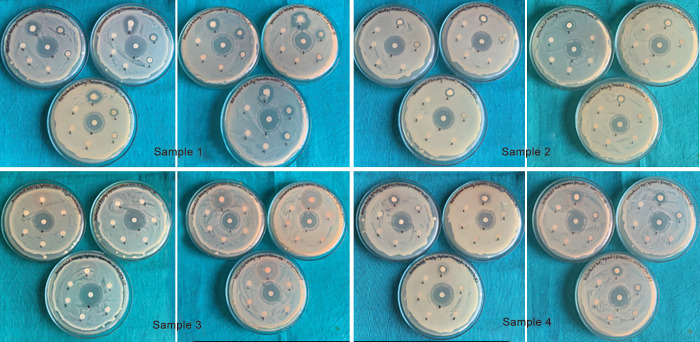
For the first part, the antimicrobial properties of prepared dentifrice slurries were investigated against S. mutans and L. acidophilus. Turbidity of 0.5 on the McFarland scale was achieved by preparing the bacterial suspension in sterile brain heart infusion broth and Lactobacillus MRS broth at 37°C for 24 hours, respectively. For each perusing, 100 μL of the bacterial suspension was spread uniformly on brain heart infusion agar and Lactobacillus MRS agar plates utilizing sterile cotton swabs. Dentifrice slurry’s antimicrobial efficacy against the test organism at various concentrations was evaluated using the diffusion method. The plates were allowed to dry. After 1 hour, 07 disks (5 mm in diameter) made of Whatman No.1 filter paper were soaked in each agar plate to test 04 different pediatric dentifrices [at concentrations 100 (pure), 50, 25, 12, and 6.25%] and two control groups at equidistance in each of the plates. The plates were then incubated for 48 hours at 37°C. Zones of microbial inhibition were measured in mm around the disc of each sample and positive control using a digital caliper. After 24 hours, the shortest distance between the outer edge of the disk and the first microbial growth was measured. The tests were performed in triplicate (coding given as A, B, C) for each set and are listed in the tables for each sample separately (Fig. 2).
Fig. 2:
Zone of inhibition by disk diffusion for different samples against S. mutans (left) and L. acidophilus (right)
Minimal Inhibitory Concentration
To determine the MIC value of the samples, the broth microdilution method was used, and the media used was brain heart infusion broth for S. mutans and MRS broth for L. acidophilus. Each well of the 96 well plates, except for the last two wells, was filled with 100 µL of the culture media. To obtain the susceptibility concentration, first, the suspension of each sample was prepared with distilled water in the ratio of 1:1, and from this suspension, 100 µL was added in the first well, then two obtain the two-fold serial dilution of 100 µL from the first well was taken and inserted into the next well. This step was repeated till the concentration reached 6.25%; a similar process was repeated for all three samples in separate plates. For the bacterial suspension preparation, the culture broth with 0.5 McFarland was diluted in the ratio 1:10 to obtain 107 CFU/mL; then, each well was loaded with 5 µL of the bacterial suspension so that the final CFU value in each well was 5 × 10 CFU/well. From the last two wells, one was considered as positive control inoculated with bacterial suspension, and no sample was added, while the second was the negative control without bacterial suspension. The plates were then covered, sealed, and incubated at 37°C. The MIC value of each sample was taken to be its lowest concentration at which no bacterial viability; that is, no growth was observed after the incubation period (Fig. 3).
Fig. 3:

Showing the results for the MIC of samples I, II, III, and IV against S. mutans and L. acidophilus
Statistical Analysis
All data were fed into Statistical Package for the Social Sciences version 22.0 software package and were analyzed using Tukey’s honest significant difference post hoc test, Chi-squared test, and analysis of variance (ANOVA). The significance level was fixed at p < 0.05 value.
Results
Dentifrices selected in this study are shown in Figure 1, where sample I contains fluoride, sample II herbal, sample III nanosilver with xylitol and sample IV fluoride with xylitol. Table 1 shows the zones of inhibition for samples I–IV at 100 (pure), 50, 25, 12.5, and 6.25% concentration. Sample I exhibited the maximum zone of inhibition, followed by sample IV and sample II, while sample III failed to show any activity even at 100% (pure) concentration against S. mutans and L. acidophilus, respectively. No inhibitory zones were seen at 12.5 and 6.25% concentrations. Figures 456 also show the zones of inhibition at 100, 50, and 25%, respectively.
Table 1:
Zone of inhibition (mm) of sample I—fluoride dentifrice (Cheeriogel), sample II—herbal dentifrice (Dant Kranti Junior), sample III—nanosilver particles + xylitol dentifrice (Superblue), and sample IV—xylitol + fluoride-containing dentifrice (Kidodent) against S. mutans and L. acidophilus. The samples were tested at 100, 50, 25, 12.5, and 6.25% concentrations, with ciprofloxacin as the positive control and distilled water as the negative control
| Part I—Zone of inhibition by disk diffusion method | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean values ± standard deviation | |||||||||||
| Concentration | 100% | 50% | 12.5% | 6.25% | |||||||
| S. no. | Sample | Zone of inhibition against S. mutans (mm) | Zone of inhibition against L. acidophilus (mm) | Zone of inhibition against S. mutans (mm) | Zone of inhibition against L. acidophilus (mm) | Zone of inhibition against S. mutans (mm) | Zone of inhibition against L. acidophilus (mm) | Zone of inhibition against S. mutans (mm) | Zone of inhibition against L. acidophilus (mm) | Zone of inhibition against S. mutans (mm) | Zone of inhibition against L. acidophilus (mm) |
| 1. | Sample I | 14.667 ± 0.577 | 16.667 ± 1.527 | 9.000 ± 0.000 | 11.667 ± 0.577 | 7.000 ± 0.000 | 7.333 ± 0.577 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 |
| 2. | Sample II | 9.667 ± 0.577 | 9.333 ± 0.577 | 6.667 ± 0.577 | 6.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 |
| 3. | Sample III | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 |
| 4. | Sample IV | 10.667 ± 0.577 | 12.000 ± 0.000 | 6.333 ± 0.577 | 8.333 ± 0.577 | 0.000 ± 0.000 | 5.333 ± 0.577 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 |
| ANOVA “f-value” | 464.333 | 221.S33 | 267.333 | 435.333 | NA | 252.667 | NA | NA | NA | NA | |
| “p-value” | 0.001 (HS) | ||||||||||
Fig. 4:

Comparative evaluation of mean zone of inhibition (mm) against S. mutans at 100% concentration (pure form) between samples I, II, III, and IV
Fig. 5:

Comparative evaluation of mean zone of inhibition (mm) against S. mutans at 50% concentration between samples I, II, III, and IV
Fig. 6:

Comparative evaluation of mean zone of inhibition (mm) against S. mutans at 25% concentration between samples I, II, III and IV
Tables 2 and 3 reveal the MIC value of the samples against the bacterial isolates. In sample III, there was no zone of inhibition at all conc. MIC value was 25% against S. mutans and L. acidophilus in sample I. MIC Value was 50.0% against S. mutans and L. acidophilus in sample II. MIC value was 50 and 25% against S. mutans and L. acidophilus in sample IV. Chi-squared test was applied to find a significant difference in MIC against S. mutans and L. acidophilus. No statistically significant difference was found in the MIC value of the samples against the bacterial isolates (p = 0.060).
Table 2:
inimum inhibitory concentration value of growth (turbidity) in sample I—fluoride dentifrice (Cheeriogel), sample II—herbal dentifrice (Dant Kranti Junior), sample III—nanosilver particles + xylitol dentifrice (Superblue), and sample IV—xylitol + fluoride-containing dentifrice (Kidodent) against S. mutans and L. acidophilus
| Part II—MIC | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| S. no. | Concentration | Sample-I | Sample-II | Sample-III | Sample-IV | ||||
| MIC value against S. mutans | MIC value against L. acidophilus | MIC value against S. mutans | MIC value against L. acidophilus | MIC value against S. mutans | MIC value against L. acidophilus | MIC value against S. mutans | MIC value against L. acidophilus | ||
| 1. | 100% (pure) | NG | NG | NG | NG | NG | NG | NG | NG |
| 2. | 50% | NG | NG | NG | NG | NG | NG | NG | NG |
| 3. | 25% | NG | NG | G | G | NG | NG | G | NG |
| 4. | 12.5% | G | G | G | G | NG | NG | G | G |
| 5. | 6.25% | G | G | G | G | NG | NG | G | G |
G, growth; NG, no growth
Table 3:
Minimum inhibitory concentration value of the samples against the bacterial isolates
| MIC value | |||
|---|---|---|---|
| Sample name | Pediatric dentifrices | S. mutans | L. acidophilus |
| Sample I | Fluoride (Cheeriogel) | 25% | 25% |
| Sample II | Herbal (Pitanjali) | 50% | 50% |
| Sample III | Nanosilver particles + xylitol (Superblue) | NG | NG |
| Sample IV | Xylitol + fluoride (Kidodent) | 50% | 25% |
| Chi-square value | 5.62 | ||
| Significance “p” value | 0.060 (NS) | ||
Discussion
Dental caries is a common chronic condition caused by interactions between diet, teeth, and oral flora. The collaboration between these three essential elements throughout a predetermined time span is fundamental for the commencement progression of caries. Microorganisms that can convert sucrose to lactic acid, such as S. mutans, L. acidophilus, and Enterococcus faecalis colonizing the oral cavity, are associated with the initiation of dental caries.12 As a result, the modern noninvasive method of managing caries has made significant progress by incorporating an antibacterial strategy.
Dental caries and periodontal illnesses are started in childhood, and their prevention needs to be done on time before they spread.13 There are many antimicrobial agents and methods for the prevention of such diseases, and toothpaste is the agent most commonly used to remove dental plaque and prevent tooth decay. An assortment of research center strategies can be utilized to assess the in vitro antimicrobial action of a concentrate or a pure compound. The most known and basic methods are the “disk diffusion method and broth or agar dilution methods.”14 The agar disk diffusion method was developed in 1904.15 For routine antimicrobial susceptibility testing, it is an official method that is utilized in many clinical microbiology laboratories. This method is used to evaluate semi-solid materials that are fluid in the presence of saliva or water, like toothpaste.16 These techniques are the most commonly used to determine the MIC of antimicrobial agents. The “gold standard” for determining an organism’s susceptibility to antimicrobials is the MIC.17
All four different commercially available pediatric dentifrices were tested for their antibacterial activity by measuring the zone of inhibition and MIC against two dental bacterial pathogens caries, that is, S. mutans and L. acidophilus, at different two-fold dilutions of 100, 50, 25, 12.5, and 6.25% concentrations. Positive control—ciprofloxacin and negative control—distilled water were used to confirm the antimicrobial around the disk. All four dentifrices were found to have wide varieties in their effectiveness against the two tested microorganisms at 100% (pure) and 50% concentrations, with sample I having the highest activity, followed by sample IV and sample II. At 25% concentration, only samples I and IV showed antibacterial activity, and at 12.5 and 6.25% concentrations, none of the tested toothpastes exhibited any antibacterial activity. Sample III failed to show antibacterial activity, even in pure form, against both the two microorganisms.
In our present study, fluoride containing pediatric dentifrices exhibited the highest zone of inhibition and lowest MIC against both microorganisms. Caries preventive effects could rise up out of both the fluoride and nonfluoride parts of the dentifrice. This was in accordance with the studies done by Malhotra et al.,18 Lodaya et al.,19 Deshpande et al.,20 and Kurian and RV,21 who all reported maximum antimicrobial activity of fluoridated toothpaste at all concentrations when compared to nonfluoridated toothpaste. Although remineralization is a major mechanism by which fluoride reduces caries and prevention of demineralization,22 fluoride can also exert antibacterial effects. In a double-blind study conducted by Winter et al.,23 no significant outcomes were seen between the two groups of 1055 and 550 ppm fluoride levels in dentifrices. Therefore, they recommended the usage of low-fluoride toothpaste for children. However, Evans et al.,24 in their in vitro study, demonstrated that S. mutans and S. sanguinis were inhibited more effectively by dentifrices with 1,450 ppm fluoride than by those with 500 ppm. The difference in results between present studies may be due to differences in active ingredients used in the dentifrice.
Pentacarbon sugars and pentitols, like xylitol, have recently been used as supplements in the manufacturing of oral hygiene products. Due to its cariostatic properties, which prevent the development of dental caries, xylitol may not only enhance the flavor of toothpaste but may also improve the environment inside the oral cavity.25 The antibacterial activity of xylitol depends on both its high frequency and concentration. A substantial difference in the zone of inhibition was observed between fluoride only and fluoride with xylitol toothpaste in our study. Fluoride-only toothpaste showed significantly better antimicrobial activity than fluoride toothpaste with xylitol toothpaste. In support of that Chi et al.26 assessed and noted that compared to fluoride toothpaste, xylitol did not provide any therapeutic benefit. The author claims that toothpaste’s surfactants may prevent xylitol from being absorbed and that sodium lauroyl sarcosinate, a surfactant in xylitol toothpaste, may also prevent fluoride from being absorbed by tooth enamel.
Scheinin et al.27 and Nivashini et al.28 inferred in an in vitro study that xylitol-containing toothpaste has less potency as an antimicrobial agent, but it can be used in children to avoid fluoride toxicity. Because clinical evidence is conflicting, the American Association of Pediatric Dentistry (AAPD) supports the utilization of xylitol as a component of an extensive system to forestall caries yet doesn’t suggest xylitol toothpaste use on the grounds that the exploration proof is uncertain.29 In the present study, both the xylitol-containing toothpaste showed less antibacterial activity than fluoride-only toothpaste.
Due to various properties like anti-inflammatory, antimicrobial, and antiseptic properties, there is a global trend among consumers to seek natural products for a healthier lifestyle.30 Corroborating the findings of our study, Sam et al.,31 in an in vivo antimicrobial study, determined that S. mutans and Lactobacillus acidophilus (LB) counts were significantly lower in the fluoride group with no reduction in the herbal group. Likewise, Deshpande et al.20 and Kurian and RV21 also found similar antimicrobial activity of herbal toothpastes, as indicated in the present study. However, Bedre et al.32 found similar antimicrobial activity in herbal and nonherbal toothpastes against S. mutans, E. coli, and Candida albicans.
In recent years, dentistry has attracted attention to nanosilver particles that possess antimicrobial properties against cariogenic bacteria.33 Surprisingly, in the present in vitro study, pediatric dentifrice containing active ingredient nanosilver particles exhibited no antimicrobial activity against both the tested microorganisms, even at full strength (100% concentration). In our study, the results contradict the study conducted by Ahmed et al.,34 in which they observed that the toothpaste with nanosilver in it was the most effective against S. mutans when compared to other conventional dentifrices. Evans et al.,24 in their in vitro study, orchestrated that the toothpaste’s primary bacterial inhibitor is sodium lauryl sulfate. Another possible reason for the lack of antibacterial property could be related to the particle size of the silver nanoparticles in the dentifrice used in the present study, though the exact particle size was not specified by the manufacturer. Studies by Noronha et al.35 and Espinosa-Cristóbal et al.36 have revealed that the size of silver nanoparticles affects their bactericidal properties—smaller diameter particles had lower inhibitory concentrations than larger diameter particles.
The present study aimed to compare and contrast the antimicrobial efficacy of four commonly used pediatric dentifrices against S. mutans and L. acidophilus bacteria. It was clear from the overall result that different toothpastes had different levels of antimicrobial activity. This is probably due to the different formulations, the concentration of the active product, and the connection it has with various components. Therefore, the present in vitro study concludes that fluoride-containing pediatric dentifrice (Cheeriogel) containing a lesser amount of fluoride concentration (458 ppm) manifested as the paramount among all the four tested pediatric dentifrice as it exhibited the highest mean zone of inhibition and least value of MIC against S. mutans and L. acidophilus. The fluoride concentration (458 ppm) of this dentifrice was in favor of our Indian scenario as fluorosis is one of the severe public health problems in India, affecting children up to 14 years of age. Thus, for children under the age of 3, the AAPD recommends using no more than a smear or rice-size amount of fluoridated toothpaste, while children between the ages of 3 and 6 should use no more than a pea-sized amount.37
According to the Bureau of Indian Standards, 1.0 mg/L is the maximum permissible limit of fluoride.38 Fluoride is a double-edged sword; when used properly and in moderation, it protects against caries to its fullest extent; however, unwise or excessive consumption may result in dental and skeletal fluorosis.39 Awareness about the sources and ill effects of fluoride must be spread in the population through oral health education. Low fluoride concentration toothpaste can be recommended for children at high-risk of dental caries. These measures can go a long way in reaping caries by preventing the benefits of fluoride while simultaneously avoiding dental fluorosis as much as possible in these areas.40
The rationale for performing this in vitro study was to offer information to pediatric clinicians about the microbial efficacy of commercially available pediatric dentifrices against S. mutans and L. acidophilus. Pediatric toothpaste must pass tests for antimicrobial activity, allowing professionals to make better clinical decisions when demonstrating these products to their patients.
However, It is essential to keep in mind that the test was conducted in vitro; consequently, it cannot be assumed that the findings regarding antimicrobial efficacy could be proportional to or transferred to the oral cavity and translated into clinical efficacy as toothpaste used in vivo. This is due to the fact that toothpaste is likely to be diluted by saliva, which is the level at which in vitro dilution buffers or loses antimicrobial properties.
Conclusion
It was clear from the overall result that different toothpastes had different levels of antimicrobial activity. This is probably because the formulations are different, the concentration of the active product, and how it interacts with other components. Therefore, the present in vitro study concludes that fluoride-containing pediatric dentifrice comprising a lesser amount of fluoride concentration (458 ppm) manifested as the paramount among all the four tested pediatric dentifrice as it exhibited the highest mean zone of inhibition and least value of MIC against S. mutans and L. acidophilus.
This in vitro study accomplishes that considering the endemic scenario of fluorosis in many parts of India (20 states, 100 districts, 60 million people) and to reduce fluoride ingestion during brushing, low fluoride concentration toothpaste can be recommended to children at high-risk to dental caries.
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Dobell C. London: Staples Press; 1932. Antony Van Leeuwenhoek and His ‘Little Animals’. [Google Scholar]
- 2.Mandal A, Singh DK, Siddiqui H, et al. New dimensions in mechanical plaque control: an overview. Indian J Dent Sci. 2017;2(9):133–139. doi: 10.4103/IJDS.IJDS_18_17. [DOI] [Google Scholar]
- 3.Marinho VT, Dos Reis AC, da Costa Valente ML. Efficacy of antimicrobial agents in dentifrices: a systematic review. Antibiotics. 2022;11(10):1413. doi: 10.3390/antibiotics11101413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thosar N, Dharmadhikari P, Baliga S, et al. Changing trends in oral hygiene and plaque control in children. J Dent Oral Care. 2016;1(2):79–73. doi: 10.15436/2379-1705.15.026. [DOI] [Google Scholar]
- 5.Kolenbrander PE, Palmer Rj JR, Rickard AH, et al. Bacterial interactions and successions during plaque development. Periodontol 2000. 2006;42(1):47–79. doi: 10.1111/j.1600-0757.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- 6.Li YH, Lau PCY, Lee JH, et al. Natural genetic transformation of Streptococcus mutans growing in biofilms. J Bacteriol. 2002;183(3):897–898. doi: 10.1128/JB.183.3.897-908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts AP, Cheah G, Ready D, et al. Transfer of TN916-like elements in microcosm dental plaques. Antimicrobial Agents Chemotherapy. 2001;45(10):2943–2946. doi: 10.1128/AAC.45.10.2943-2946.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nwankwo I, Ihesiulo S. Comparative analysis of the Antibacterial potentials of some Brands of toothpaste commonly used in Umuahia Abia State. IOSR J Pharm Biol Sci. 2014;9(3):50–54. doi: 10.9790/3008-09365054. [DOI] [Google Scholar]
- 9.Ali A, Lim XY, Wahida PF. The fundamental study of antimicrobial activity of piper betle extract in commercial toothpastes. J Herb Med. 2018;14:29–34. [Google Scholar]
- 10.Sheiham A. Dental cleanliness and chronic periodontal disease. Studies on British population. Br Dent J. 1970;129(9):413–418. doi: 10.1038/sj.bdj.4802596. [DOI] [PubMed] [Google Scholar]
- 11.Moran J, Addy M, Newcombe R. The antibacterial effect of toothpastes on the salivary flora. J Clin Periodontol. 1988;15(3):193–199. doi: 10.1111/j.1600-051x.1988.tb01569.x. [DOI] [PubMed] [Google Scholar]
- 12.Bhati N, Jaidka S, Somani R. Evaluation of antimicrobial efficacy of Aloe vera and Meswak containing dentifrices with fluoridated dentifrice: an in vivo study. J Int Soc Prev Community Dent. 2015;5(5):394–399. doi: 10.4103/2231-0762.165924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aas JA GA, Dardis SR, Lee AM, et al. Bacteria of dental caries in primary and permanent teeth in children and young adults. J Clin Microbiol. 2008;46(4):1407–1417. doi: 10.1128/JCM.01410-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharmaceut Anal. 2016;6(2):71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heatley NG. A method for the assay of penicillin. Biochem J. 1944;38(1):61–65. doi: 10.1042/bj0380061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossi A, Ferreira DC, Silva RA, et al. Antimicrobial activity of toothpastes containing natural extracts, chlorhexidine or triclosan. Braz Dent J. 2014;25(3):186–190. doi: 10.1590/0103-6440201300027. [DOI] [PubMed] [Google Scholar]
- 17.Amdrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48(suppl 1):5–6. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 18.Malhotra R, Singla S, Shashikiran ND. Comparison of antimicrobial activity of child formula dentifrices at different concentrations: an in vitro Study. Int J Clin Pediatr Dent. 2017;10(2):131–135. doi: 10.5005/jp-journals-10005-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lodaya R, Venkataraman S, Lakade L, et al. Comparative evaluation of antimicrobial efficiency of marketed children’s fluoridated toothpastes at diluted concentrations against Streptococcus mutans - an in vitro study. Int Dent Med J Adv Res. 2018;4(1):1–5. doi: 10.15713/ins.idmjar.92. [DOI] [Google Scholar]
- 20.Deshpande R, Kachare P, Sharangpani G, et al. Comparative evaluation of antimicrobial efficacy of two commercially available dentifrices (fluoridated and herbal) against salivary microflora. Int J Pharm Sci. 2014;6(6):72–74. [Google Scholar]
- 21.Kurian M, RV G. Effect of herbal and fluoride toothpaste on streptococcus mutans – a comparative study. J Pharm Sci Res. 2015;7(10):864–865. [Google Scholar]
- 22.Twetman S, Axelsson S, Dahlgren H, et al. Caries preventive effect of fluoride toothpaste: a systematic review. Acta Odontol Scand. 2003;61(6):347–345. doi: 10.1080/00016350310007590. [DOI] [PubMed] [Google Scholar]
- 23.Winter GB, Holt RD, Williams BF. Clinical trial of a low-fluoride toothpaste for young children. Int Dent J. 1989;39(4):227–225. [PubMed] [Google Scholar]
- 24.Evans A, Leishman SJ, Walsh LJ, et al. Inhibitory effects of children’s toothpastes on Streptococcus mutans, Streptococcus sanguinis and Lactobacillus acidophilus. Eur Arch Paediatr Dent. 2015;16(2):219–226. doi: 10.1007/s40368-014-0159-3. [DOI] [PubMed] [Google Scholar]
- 25.Assev S, Wåler SM, Rølla G. Are sodium lauryl sulfate-containing toothpastes suitable vehicles for xylitol. Eur J Oral Sci. 1997;105(2):178–182. doi: 10.1111/j.1600-0722.1997.tb00197.x. [DOI] [PubMed] [Google Scholar]
- 26.Chi DL, Tut OK, Milgrom P. Cluster-randomized xylitol toothpaste trial for early childhood caries prevention. J Dent Child. 2014;81(1):27–32. [PMC free article] [PubMed] [Google Scholar]
- 27.Scheinin A, Banoczy J, Szoke J, et al. Collaborative WHO xylitol field studies in Hungary. I. Three–year caries activity in institutionalized children. Acta Odontol Scand. 1985;43(6):327–337. doi: 10.3109/00016358509046517. [DOI] [PubMed] [Google Scholar]
- 28.Nivashini GSV, Muralidharan NP, Kumar V. Evaluation of anti-microbial property of toothpaste containing calcium, fluoride, xylitol and herbs against Streptococcus mutans. IJSDR. 2020;5(2):1–5. [Google Scholar]
- 29.American Academy on Pediatric Dentistry. Guideline on xylitol use in caries prevention. Pediatr Dent. 2011;3:157–160. [Google Scholar]
- 30.Khairnar MR, Dodamani AS, Karibasappa GN, et al. Efficacy of herbal toothpastes on salivary pH and salivary glucose preliminary study. J Ayurveda Integr Med. 2017;8(1):3–6. doi: 10.1016/j.jaim.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sam JE, Benin P, Beaulah RH, et al. Comparative evaluation of antibacterial efficacy of four toothpastes and mouthwashes against Streptococcus mutans and lactobacillus: an in vivo study. J Oper Dent Endod. 2016;1(2):60–65. doi: 10.5005/jp-journals-10047-0013. [DOI] [Google Scholar]
- 32.Bedre AS, Arjunkumar R, Muralidharan NP. Evaluation of concentration dependent antimicrobial efficacy of herbal and non herbal dentifrices against salivary microflora – an in vitro study. Biomed Pharmacol J. 2018;11(2):711–718. doi: 10.13005/bpj/1424. [DOI] [Google Scholar]
- 33.Besinis A, Peralta T, Handy R. Antibacterial effects of silver, titanium dioxide and silica dioxide nanoparticles compared to the dental disinfectant chlorhexidine on Streptococcus mutans using a suite of bioassays. Nanotoxicology. 2014;8(1):1–16. doi: 10.3109/17435390.2012.742935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmed F, Prashanth ST, Sindhu K, et al. Antimicrobial efficacy of nanosilver and chitosan against Streptococcus mutans, as an ingredient of toothpaste formulation: an in vitro study. J Indian Soc Pedod Prev Dent. 2019;37(1):46–54. doi: 10.4103/JISPPD.JISPPD_239_18. [DOI] [PubMed] [Google Scholar]
- 35.Noronha VT, Paula AJ, Duran G, et al. Silver nanoparticles in dentistry. Dent Mater. 2017;33(10):1110–1126. doi: 10.1016/j.dental.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Espinosa-Cristóbal LF, Martínez-Castañon GA, Martinez-Martinez RE, et al. Antibacterial effect of silver nanoparticles against Streptococcus mutans. Materials Letters. 2009;63(29):2603–2606. doi: 10.1016/j.matlet.2009.09.018. [DOI] [Google Scholar]
- 37.American Academy of Pediatric Dentistry. :262–265. Fluoride therapy. Pediatr Dent 2018;(latest revision): [Google Scholar]
- 38.Shyam R, Manjunath BC, Kumar A, et al. Prevalence of dental fluorosis and treatment needs among 11–14 years old school children in endemic fluoride areas of Haryana, India. Indian J Dent Res. 2021;32(1):110–114. doi: 10.4103/ijdr.IJDR_835_18. [DOI] [PubMed] [Google Scholar]
- 39.Sharma A, Kumar N, Sharma R. Prevalence and association of dental caries and dental fluorosis in fluoride endemic region of Mewat district, Haryana, India. Int J Oral Health Dent. 2019;5(1):27–31. doi: 10.18231/j.ijohd.2019.007. [DOI] [Google Scholar]
- 40.Rani R, Singhal R, Singhal P, et al. Prevalence of dental fluorosis and dental caries in fluoride endemic areas of Rohtak district, Haryana. J Indian Soc Pedod Prev Dent. 2022;40(2) doi: 10.4103/jisppd.jisppd_185_22. [DOI] [PubMed] [Google Scholar]