Abstract
We established a porcine lung epithelial cell line designated St. Jude porcine lung cells (SJPL) and demonstrated that all tested influenza A and B viruses replicated in this cell line. The infectivity titers of most viruses in SJPL cells were comparable to or better than those in MDCK cells. The propagation of influenza viruses from clinical samples in SJPL cells did not lead to antigenic changes in the hemagglutinin molecule. The numbers of both Sia2-3Gal and Sia2-6Gal receptors on SJPL cells were greater than those on MDCK cells. Influenza virus infection of SJPL cells did not lead to apoptosis, as did infection of MDCK cells. No porcine endogenous retrovirus was detected in SJPL cells, and in contrast to MDCK cells, SJPL cells did not cause tumors in nude mice.
The isolation and propagation of influenza viruses from clinical samples are important in surveillance, studies of host range and pathogenesis, and vaccine production. However, the number of primary cell types and continuous cell lines that support influenza virus replication is limited (8, 10, 20, 31). The primary epithelial cells of human adenoid and primary rhesus monkey kidney tissue support replication of influenza viruses (6, 21). Cell lines such as Vero, MRC-5, Madin-Darby canine kidney (MDCK), and baby hamster kidney (BHK) also support the growth of influenza viruses, but of these cell lines, MDCK is the best for isolation and propagation of influenza viruses (8, 10, 11, 31).
One important issue regarding the pathogenesis of influenza viruses is how influenza viruses that infect one species acquire the ability to infect another. Pigs are regarded as possible “mixing vessels” in which human-avian influenza A virus reassortants are generated (36). Therefore, a porcine lung epithelial cell line would be very useful in studying factors that influence interspecies transmission.
A porcine lung epithelial cell line could also be useful in producing more effective influenza virus vaccines. Currently, available vaccine is produced in embryonated eggs using egg-adapted variants of the influenza virus. Adaptation of human influenza A and B viruses in eggs can result in the selection of variants that contain hemagglutinin (HA) molecules that are antigenically and structurally different from those of the original influenza viruses in the clinical samples (16, 33, 35). Because of the antigenic changes in the HA molecule of the vaccine virus, the vaccine may not offer complete protection against infection by the circulating influenza virus. HA molecules of influenza virus isolated and grown in mammalian cells have been shown to be identical to those of the original influenza viruses in clinical samples (17, 32). Currently, no mammalian cell line is licensed for use in the production of human influenza vaccine. Therefore, an alternative mammalian cell line that supports the replication of all subtypes of influenza viruses is needed for vaccine production. A porcine lung epithelial cell line could fulfill this need.
There are two major morphologically and biochemically distinct modes of cell death: apoptosis (programmed cell death) and necrosis (5, 42). Apoptotic cells retain membrane integrity even after disintegration into apoptotic bodies (42). Apoptotic bodies and cells are phagocytosed by macrophages, which prevent an inflammatory reaction (30), while cells dying by necrosis lose membrane integrity, which results in inflammation (42). It was reported that infection of epithelial cells such as MDCK and HeLa cells by influenza viruses leads to apoptosis (38). However, it is not clear whether infection of epithelial cells of respiratory tracts by influenza viruses results in apoptosis or necrosis.
An epithelial cell line (St. Jude porcine lung [SJPL] cells) was spontaneously established from the normal lungs of a normal 4-week-old female Yorkshire pig as previously described (37) at St. Jude Children's Research Hospital. This cell line was continuously cultured in Dulbecco's modified Eagle's medium (DMEM; Life Technologies, Gaithersburg, Md.) supplemented with 10% fetal bovine serum, 1% sodium pyruvate, 1% l-glutamine, 1.4% MEM nonessential amino acids, and 1% antibiotic-antimycotic solution (Sigma Chemical Co., St. Louis, Mo.). SJPL cells were epithelial cell-like, and the morphology was not changed at passage 60. SJPL cells were positive for an epithelial cell marker, cytokeratin (data not shown).
Expression of influenza virus proteins and influenza virus-induced CPE in SJPL cells.
To determine the cytopathic effect (CPE) of influenza virus in SJPL cells, confluent monolayers of SJPL cells in a six-well plate (Becton Dickinson, Franklin Lakes, N.J.) were infected with A/Sydney/5/97 (H3N2) (multiplicity of infection [MOI], 2), and DMEM containing 0.3% bovine serum albumin (Life Technologies) and 1 μg of TPCK (tosylsulfonyl phenylalanyl chloromethyl ketone)-treated trypsin per ml were added to each well. CPE was determined 48 h after infection.
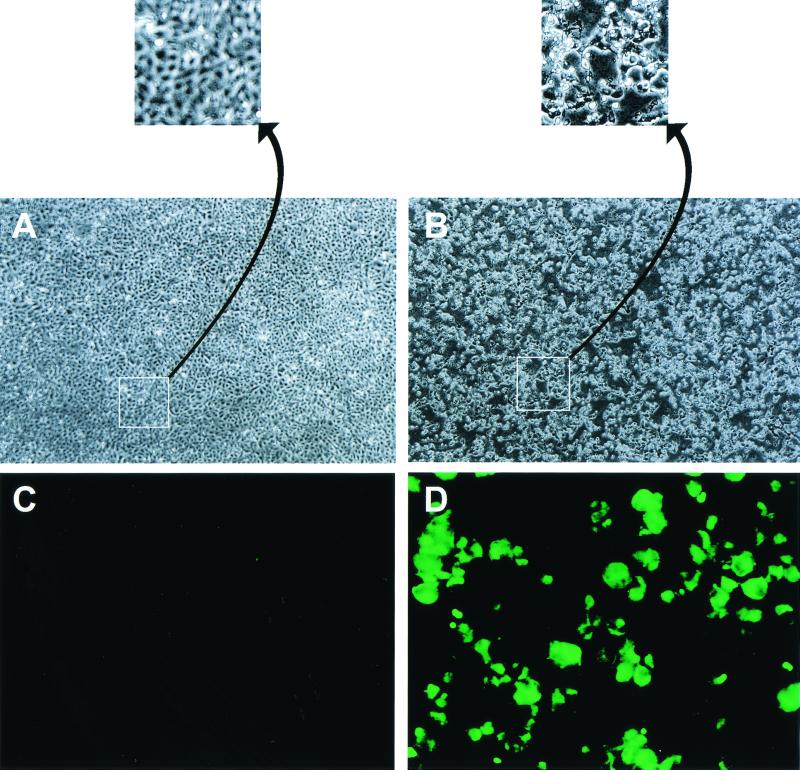
MDCK cells infected with influenza viruses show marked cytopathogenicity. SJPL cells infected with A/Sydney/5/97 (H3N2) (MOI, 2) showed a strong CPE (Fig. 1B), but uninfected cells showed no sign of cell damage (Fig. 1A). The indication of CPE in MDCK cells and SJPL cells infected with influenza viruses was different. Approximately 16 h after infection, pores on the monolayer of MDCK cells started to appear, and the size of pores increased until the entire monolayer was destroyed and detached from the surface of the flask. In contrast, the apical surfaces of SJPL cells started to be destroyed at 18 h after infection, and the area of the apical surface that was destroyed continued to increase, but some cells (20%) remained attached to the flask surface even after 72 h.
FIG. 1.
CPE and nucleoprotein expression of SJPL cells infected with A/Sydney/5/97 (H3N2). (A and B) Confluent monolayers of SJPL cells in six-well plates were infected with A/Sydney/5/97 (H3N2) (MOI, 2), and 48 h later CPE was evaluated (B). Uninfected cells were used as a control (A). Magnification, ×5 (A and B) and 232% further (insets). (C and D) Confluent monolayers of SJPL cells in two-well Lab-Tek chamber slides were infected with A/Sydney/5/97 (H3N2) (MOI, 5), and 16 h later the cells were incubated with a monoclonal antibody to nucleoprotein and a secondary FITC-labeled goat anti-mouse immunoglobulin antibody (D). Uninfected cells were used as a control (C). Magnification, ×20.
To determine whether the newly established SJPL cell line could express structural proteins of influenza virus, we used a monoclonal antibody to nucleoprotein in an indirect immunofluorescent assay to detect the viral protein in SJPL cells that had been infected with A/Sydney/5/97 (H3N2). SJPL cells infected with an H3N2 (A/Sydney/5/97) influenza virus (MOI, 5) for 1 h were incubated overnight at 37°C before they were fixed with 80% cold acetone in water. The fixed cells were incubated on ice with a mouse monoclonal antibody to nucleoprotein of influenza A virus for 30 min and then incubated on ice with fluorescein isothiocyanate (FITC)-labeled goat anti-mouse immunoglobulin G for 30 min before cells were evaluated by using a fluorescence microscope. The results showed that the cytoplasms and nuclei of infected cells contained nucleoprotein (Fig. 1D) but similar regions of uninfected cells did not (Fig. 1C).
Replication efficiencies of mammalian, avian, and the highly pathogenic H5N1 influenza viruses in SJPL cells.
We compared the replication efficiencies of human, swine, equine, and highly pathogenic H5N1 influenza viruses in SJPL cells with those in MDCK cells. Confluent monolayers of SJPL and MDCK cells were infected with 10 50% egg-infective doses (EID50) of each virus. Viral titration was performed by using SJPL cells for viruses that had been grown in SPJL cells and MDCK cells for viruses that had been grown in MDCK cells. SJPL and MDCK cells (5 × 105 per well) were seeded in six-well tissue culture plates and allowed to grow to confluence. Cells were washed with phosphate-buffered saline (PBS, pH 7.2) twice and infected with 10 EID50 (i.e., 10 times the virus dose that will infect 50% of the eggs in a population) for 1 h at 37°C in a humidified incubator containing 5% CO2. Supernatants containing viruses were collected 50 h after infection for virus titration. Supernatants from virus-infected cells were serially diluted 10-fold in medium (DMEM for SJPL-grown virus and MEM for MDCK-grown virus) containing 0.3% bovine serum albumin and 1 μg of TPCK-trypsin per ml, and 0.1 ml of the dilutions was added to four replicate wells in a 96-well plate. Seventy-two hours after infection, the presence of virus was determined by HA with 0.5% chicken red blood cells. The virus titers were expressed as the log10 50% tissue culture infective dose (TCID50) per milliliter.
Most of the human viruses grew to higher titers in SJPL cells than in MDCK cells (Table 1). The titers of human viruses grown in SJPL cells ranged from 2.00 to 6.50 log10 TCID50/ml, whereas the titers of the same viruses grown in MDCK cells ranged from <0.1 to 4.25 log10 TCID50/ml. Although A/Bel/42 (H1N1) did not grow to detectable levels in MDCK cells, it replicated in this cell line when higher doses of virus were used for infection. Replication efficiency of swine influenza viruses was also greater in SJPL cells than in MDCK cells (Table 1). The virus titers in SJPL cells ranged from 3.75 to 6.50 log10 TCID50/ml, whereas the titers for the same viruses in MDCK cells ranged from 1.75 to 5.50 log10 TCID50/ml. The replication efficiencies of equine influenza viruses were lower than those of the swine viruses in SJPL cells and in MDCK cells. The range of titers was 1.00 to 3.50 log10 TCID50/ml. We assessed the difference in replication efficiencies of the mammalian viruses in the two cell lines by performing analysis of variance for the two-way layout with replicates. Sufficient evidence was found at the 0.05 level of significance to conclude that the replication efficiencies of mammalian influenza viruses are significantly higher in SJPL cells than in MDCK cells (P < 0.0001).
TABLE 1.
Comparison of replication efficiencies of mammalian influenza A and B viruses in SJPL cells with those in MDCK cells
| Virus strain | Virus titer (log10 TCID50/ml)a
|
|
|---|---|---|
| SJPL | MDCK | |
| A/PR/8/34 (H1N1) | 4.5, 4.0 | 3.0, 2.75 |
| A/Bel/42 (H1N1) | 2.5, 2.0 | — |
| A/Japan/305/57 (H2N2) | 5.0, 4.75 | 3.5, 3.0 |
| A/Korea/68 (H2N2) | 5.5, 5.0 | 4.0, 3.75 |
| A/HK/1/68 (H3N2) | 4.5, 4.0 | 3.75, 3.5 |
| A/Port Chalmers/1/73 (H3N2) | 6.25, 6.5 | 4.0, 4.25 |
| B/Lee/40 | 5.5, 5.0 | 3.75, 3.5 |
| A/Swine/Ned/3/80 (H1N1) | 6.0, 6.25 | 4.25, 4.0 |
| A/Swine/Guelph/41848/97 (H3N2) | 6.5, 6.0 | 3.5, 3.25 |
| A/Swine/TX/4199-2/98 (H3N2) | 6.0, 6.25 | 5.5, 5.0 |
| A/Swine/NC/35922/98 (H3N2) | 3.75, 3.75 | 2.0, 1.75 |
| A/Equine/KY/1/81 (H3N8) | 2.0, 2.0 | 2.0, 1.75 |
| A/Equine/Prague/1/56 (H7N7) | 1.0, 1.0 | 1.0, 1.0 |
| A/Equine/Alaska/29759/91 (H3N8) | 3.25, 3.50 | 3.0, 3.0 |
| A/Equine/London/1416173 (H7N7) | 3.5, 3.25 | 3.0, 3.25 |
Values of two independent experiments are given — the levels of virus were below the limit of detection (<0.1).
Avian species harbor all subtypes of influenza A viruses (12). Because avian influenza viruses are potential pandemic influenza viruses in humans, we determined the replication efficiencies of avian viruses in SJPL cells and in MDCK cells. All representative avian influenza viruses replicated in SJPL cells; the range of titers was 1.50 to 5.25 log10 TCID50/ml. In MDCK cells, most representatives of the avian virus subtypes replicated, but the avian influenza viruses A/Mallard/Alberta/119/98 (H1N1), A/RuddyTurnstone/DE/259/98 (H9N9), A/Mallard/Alberta/223/98 (H10N8), and A/Shorebird/DE/224/97 (H13N6) did not grow in MDCK cells when the cells were inoculated with a dose of 10 EID50. Higher inoculating doses of these viruses resulted in their replication in MDCK cells. The virus titers in MDCK cells ranged from <0.1 to 5.25 log10 TCID50/ml (Table 2). The replication efficiencies of avian influenza viruses in SJPL cells were higher than in MDCK cells (P < 0.0001).
TABLE 2.
Comparison of replication efficiencies of avian influenza viruses in SJPL cells with those in MDCK cells
| Virus strain | Virus titer (log10 TCID50/ml)a
|
|
|---|---|---|
| SJPL | MDCK | |
| A/Mallard/Alberta/119/98 (H1N1) | 3.5, 3.0 | — |
| A/Mallard/Alberta/205/98 (H2N9) | 4.5, 4.75 | 5.0, 5.25 |
| A/Shorebird/DE/207/98 (H3N8) | 3.0, 3.0 | 2.5, 2.5 |
| A/Mallard/Alberta/47/98 (H4N1) | 5.0, 5.25 | 2.25, 2.0 |
| A/Duck/Singapore/3/97 (H5N3) | 4.5, 4.0 | 3.0, 3.0 |
| A/Ruddy Turnstone/DE/69/98 (H6N8) | 3.75, 3.5 | 3.0, 2.75 |
| A/Chicken/NY/13307-3/95 (H7N2) | 4.25, 4.5 | 2.0, 2.0 |
| A/Red Knot/DE/254/94 (H8N4) | 4.5, 4.0 | 1.0, 1.0 |
| A/Ruddy Turnstone/DE/259/98 (H9N2) | 3.0, 2.75 | — |
| A/Mallard/Alberta/223/98 (H10N8) | 3.0, 3.0 | — |
| A/Shorebird/DE/11/95 (H11N9) | 5.0, 4.75 | 4.5, 4.0 |
| A/Mallard/Alberta/52/97 (H12N5) | 2.0, 2.25 | 1.0, 1.0 |
| A/Shorebird/DE/224/97 (H13N6) | 1.5, 1.75 | — |
| A/Mallard/Astrakhan/263/82 (H14N5) | 5.0, 5.25 | 4.5, 4.25 |
| A/Wedgetailed Shearwater/Western Australia/2576/79 (H15N9) | 5.25, 5.0 | 5.0, 4.75 |
Values of two independent experiments are given. —, the levels of virus were below the limit of detection (<0.1).
We compared the replication efficiencies of the highly pathogenic H5N1 influenza viruses in SJPL cells with those of the same viruses in MDCK cells. A/HK/156/97 (H5N1), which was originally isolated from a 3-year-old boy, replicated well in MDCK cells and in SJPL cells; the titers ranged from 5.0 to 5.25 log10 TCID50/ml (Table 3). The addition of 1 μg of TPCK-treated trypsin per ml to the infection media did not make a difference in viral yield in either cell line. The replication efficiencies of the highly pathogenic H5N1 viruses were significantly greater in SJPL cells than in MDCK cells (P = 0.0021).
TABLE 3.
Comparison of replication efficiencies of H5N1 influenza viruses in SJPL cells with those in MDCK cells
| Virus strain | Virus titer (log10 TCID50/ml)a
|
|
|---|---|---|
| SJPL | MDCK | |
| A/Chicken/HK/258/97 | 6.5, 6.5 (6.5) | 5.0, 4.75 (4.5) |
| A/HK/156/97 | 5.0, 5.0 (5.5) | 5.0, 5.25 (5.0) |
| A/Goose/HK/W374/97 | 5.5, 5.0 (5.5) | 5.0, 5.25 (5.0) |
| A/Goose/HK/437-4/99 | 3.0, 3.25 (3.5) | 2.0, 1.75 (3.0) |
| A/Chicken/HK/728/97 | 1.5, 1.0 (2.5) | 1.75, 2.0 (1.5) |
Values of two independent experiments are given. The values in parentheses are the virus titers after 1 μg of TPCK-treated trypsin per ml was added to the infection media.
Kinetics of influenza virus replication in SJPL cells.
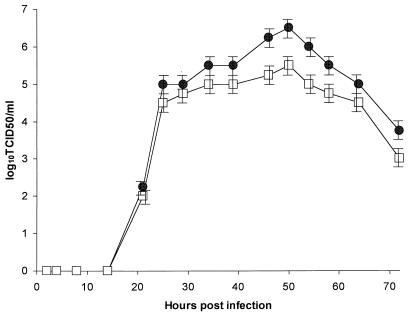
To determine the kinetics of virus replication in SJPL cells, we compared the growth curve of representative virus in SJPL cells with that of the same representative virus in MDCK cells. An influenza virus, B/Memphis/1/84, was used. To minimize the effect that the species difference in cell lines might have on virus replication, we used SJPL cells to determine the titer of viruses that were initially grown in SJPL cells and MDCK cells to determine the titer of viruses that were initially grown in MDCK cells. We titrated B/Memphis/1/84 in eggs by using serial 10-fold dilutions. Monolayers of SJPL and MDCK cells in a six-well plate were infected with 10 EID50 of virus and were incubated at 37°C in 5% CO2. At different times after infection, 50 μl of supernatant was removed. The presence of viruses in supernatant was determined by TCID50. The growth pattern of B/Memphis/1/84 in SJPL cells was similar to that in MDCK cells (Fig. 2). The time of peak viral growth in both SJPL and MDCK cells was 50 h after infection, and after that time, the viral titers started to decline. At 72 h after infection, the infectious viral titers were approximately 102- to 103-fold less than those at 50 h after infection.
FIG. 2.
Influenza virus growth in SJPL and MDCK cells. Monolayers of SJPL (●) and MDCK (□) cells in a six-well plate were infected with 10 EID50 of B/Memphis/1/84, and aliquots were harvested on different days after infection. The results are from three independent experiments.
Primary influenza virus isolation from human clinical samples and antigenic stability of cultured viruses.
Because some strains of human influenza viruses from clinical samples do not grow in eggs, MDCK cells are often used for the growth and isolation of influenza viruses from human clinical samples. Recently isolated human influenza viruses require adaptation to eggs before their variants can grow in eggs. We determined whether SJPL cells could be used to grow and isolate influenza viruses from clinical gargle samples. MDCK cells were used for comparison. Confluent monolayers of SJPL and MDCK cells in a 24-well tissue culture plate were infected with 0.1 ml of human clinical sample collected in sterile PBS. Fourteen influenza viruses in 20 human clinical samples were grown and isolated in SJPL cells and in MDCK cells. Results of hemagglutination inhibition assays indicated that all the isolates were influenza A viruses (H3N2). The viral titers in both cell lines ranged from 2.0 to 4.5 log10 TCID50/ml, with means of 3.196 (MDCK cells) and 3.300 (SJPL cells) log10 TCID50/ml.
The hallmark of influenza virus propagation in mammalian cells is that the growth of human influenza viruses in this type of cell usually does not lead to antigenic change; this result is in contrast to the antigenic changes that occur in influenza viruses cultured in eggs (34). To determine whether the growth of influenza A virus from clinical samples can lead to changes in amino acids of HA1, SJPL and MDCK cells were inoculated with clinical gargle samples from which A/Memphis/4/99 (H3N2) had been isolated. As a control, the virus in the clinical samples was adapted to eggs. After three passages, the viral RNA was isolated with the RNeasy mini kit (Qiagen, Santa Clara, Calif.), subjected to reverse transcription-PCR (RT-PCR; SuperScript preamplification system; Life Technologies), and the gene segment encoding the HA1 region was sequenced. Critical amino acids (Lys-154, Gln-156, Lys-173, Ser-186, Leu-194, Ser-199, Arg-220, Asn-246, and Thr-248) of the HA1 in the virus from the original clinical sample were identical to those of the HA1 in the viruses passaged three times in MDCK cells and in SJPL cells (Table 4). In addition, the rest of the predicted amino acid sequences within HA1 were identical in the original virus and those grown in SJPL cells and in MDCK cells. Unlike the viruses grown in MDCK cells and in SJPL cells, influenza virus passaged in eggs contained two amino acid substitutions in the HA (194L→I and 220R→S).
TABLE 4.
Comparison of critical amino acids in the HA1 region of viruses grown in MDCK cells, in SJPL cells, and in eggsa
| Source of virus | Amino acid at the indicated position in HA1
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 154 | 156 | 173 | 186 | 194 | 199 | 220 | 246 | 248 | |
| Original saliva sample | K | Q | K | S | L | S | R | N | T |
| MDCK | K | Q | K | S | L | S | R | N | T |
| SJPL | K | Q | K | S | L | S | R | N | T |
| Eggs | K | Q | K | S | I | S | S | N | T |
Human H3N2 (A/Memphis/4/99) influenza virus was passaged three times in MDCK cells, in SJPL cells, and in eggs before the nucleotide sequences were determined. Boldface indicates variation from the original sequence.
Influenza virus receptors on SJPL cells.
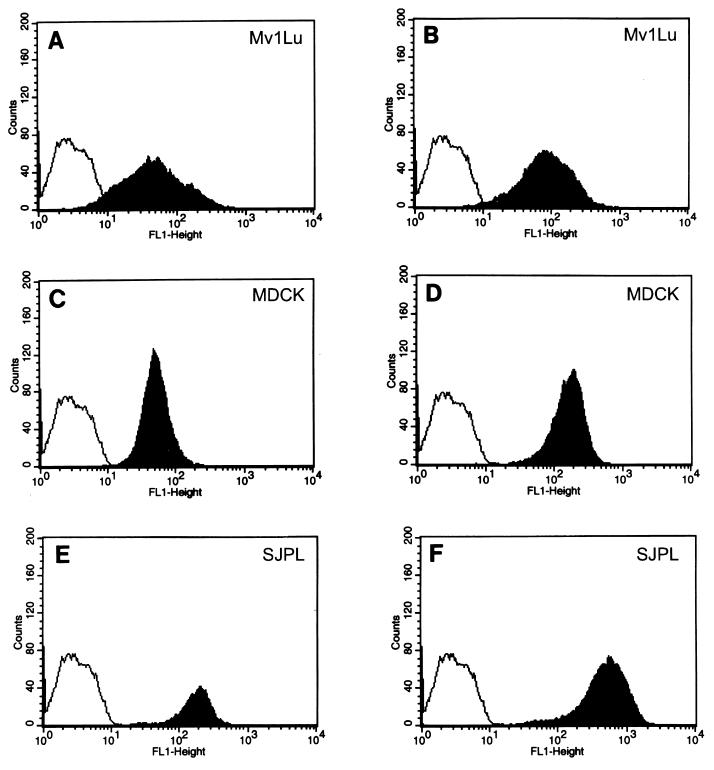
Influenza viruses enter cells by binding to sialylglycoconjugates on the surface (3, 4, 9, 26, 29). We determined whether influenza virus receptors were present on the newly established SJPL cell line. Receptor specificity was evaluated by incubating the cells with digoxigenin (DIG)-labeled lectins and FITC-labeled anti-DIG antibody (DIG glycan differentiation kit; Roche Molecular Biochemicals, Indianapolis, Ind.) and then performing flow-cytometric analysis (Fig. 3). MDCK cells and Mv1Lu cells were included as controls. SJPL, MDCK, and Mv1Lu cells expressed Sia2-3Gal- and Sia2-6Gal-containing sialylglycoconjugates on their surfaces, but the numbers of receptors on the cell surface differed among the cell lines (Fig. 3). The peak log intensity of Sia2-3Gal-containing receptors on the surfaces of Mv1Lu cells was 1.4, and that of Sia2-6Gal-containing receptors was 1.7; the peak log intensity of Sia2-3Gal-containing receptors on the surfaces of MDCK cells was 1.65, and that of Sia2-6Gal-containing receptors was 2.3; and the peak log intensity of Sia2-3Gal-containing receptors on the surfaces of SJPL cells was 2.3, and that of Sia2-6Gal-containing receptors was 2.75. Therefore, SJPL cells have more Sia2-3Gal- and Sia2-6Gal-containing receptors than the other two cell lines (Fig. 3E and F).
FIG. 3.
Influenza virus receptors on SJPL and MDCK cells. SJPL cells, MDCK cells, and Mv1Lu cells (3 × 106 of each) were incubated with the DIG-labeled lectins Maackia amurensis agglutinin (MAA), which binds specifically to Sia2-3Gal, and Sambucus nigra agglutinin (SNA), which binds specifically to Sia2-6Gal. The cells were incubated with a FITC-labeled anti-DIG antibody and then subjected to flow-cytometric analysis. (A) Mv1Lu cells after MAA binding; (B) Mv1Lu cells after SNA binding; (C) MDCK cells after MAA binding; (D) MDCK cells after SNA binding; (E) SJPL cells after MAA binding; (F) SJPL cells after SNA binding. The controls for SJPL, MvILu, and MDCK were populations of cells that were stained only with FITC-labeled anti-DIG antibody (unshaded profiles).
Influenza virus-induced damage in SJPL cells and in MDCK cells.
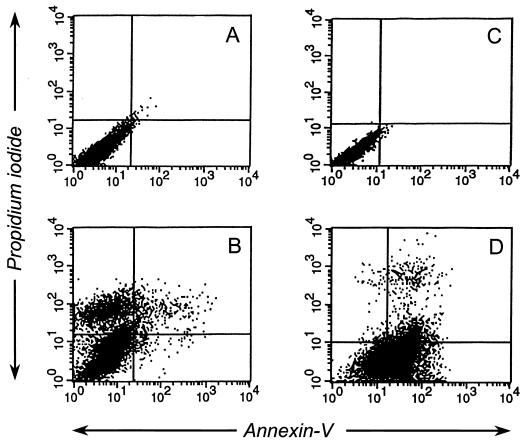
It has been reported that influenza infection of epithelial cells leads to apoptosis (13, 38). Using an annexin V binding assay, a propidium iodide staining assay (Annexin-V-Fluos staining kit; Roche Molecular Biochemicals), and a DNA fragmentation assay, we determined whether influenza virus infection of the newly established porcine lung epithelial cell line also causes apoptosis. Annexin V is a Ca2+-dependent phospholipid-binding protein with a high affinity for phosphatidylserine that translocates from the inner side of the plasma membrane to the outer side during an early stage of apoptosis but not during necrosis (7, 25). Propidium iodide binds to DNA of necrotic cells, which have damaged membranes, but does not bind to DNA of apoptotic cells with intact membranes. Ten hours after infection, 43% of SJPL cells infected with A/Sydney/5/97 (H3N2) influenza virus were stained with propidium iodide, but less than 1% of the population was detected with the Fluos-conjugated annexin V (Fig. 4B). Approximately 60% of MDCK cells infected with A/Sydney/5/97 (H3N2) bound to annexin V, and less than 1% were weakly stained with propidium iodide (Fig. 4D). Uninfected SJPL cells and MDCK cells did not either bind to annexin V or stain with propidium iodide (Fig. 4A and C).
FIG. 4.
Annexin V-Fluos assays and propidium iodide staining of SJPL and MDCK cells infected with A/Sydney/5/97 (H3N2). Ten hours after the cells were infected with A/Sydney/5/97 (H3N2) (MOI, 5), they were incubated with annexin V-Fluos and propidium iodide. (A) Uninfected SJPL cells; (B) infected SJPL cells; (C) uninfected MDCK cells; (D) infected MDCK cells.
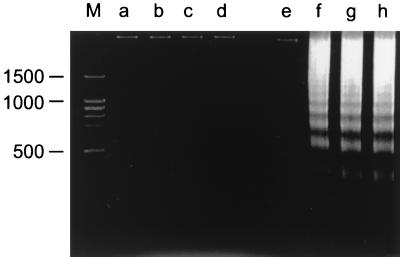
We also determined DNA fragmentation of SJPL cells infected with influenza viruses. The fragmentation of cellular DNA was determined as described previously (13, 14) but with slight modifications. Approximately 5 × 106 MDCK cells and 5 × 106 SJPL cells were infected with influenza viruses (MOI, 5), and cells were harvested 18 h after infection. The harvested cells were washed in PBS and resuspended in 500 μl of ice-cold lysis buffer (10 mM Tris [pH 7.5], 0.5% Triton X-100 [Sigma]) before they were incubated on ice for 30 min. The lysates were centrifuged for 10 min at 12,000 × g at room temperature to remove cellular debris and high-molecular-weight DNA, and the supernatants were extracted once with buffered phenol and once with buffered phenol-chloroform. DNA in the supernatants was collected by ethanol precipitation with 3 M sodium acetate (pH 5.2). DNA was dissolved in 20 μl of sterile water, treated with RNase A (Sigma), and subjected to electrophoresis through 2% agarose (GTG SeaKem agarose; FMC BioProducts, Rockland, Maine). SJPL cells infected with A/Sydney/5/97 (H3N2), A/Chicken/NY/13307-3/95 (H7N2), and A/Swine/IA/17672/88 (H1N1) did not show signs of DNA fragmentation (Fig. 5), whereas MDCK cells infected with A/Sydney/5/97 (H3N2), A/Chicken/NY/13307-3/95 (H7N2), and A/Swine/IA/17672/88 (H1N1) exhibited DNA fragmentation. Uninfected SJPL cells and MDCK cells did not show signs of DNA fragmentation.
FIG. 5.
DNA fragmentation in SJPL and MDCK cells. SJPL cells and MDCK cells were infected with A/Sydney/5/97 (H3N2), A/Chicken/NY/13307-3/95 (H7N2), or A/Swine/IA/17672/88 (H1N1) (MOI, 5), and 18 h later DNA was collected for analysis. The collected DNA was subjected to electrophoresis through a 2% agarose gel. The lanes contained DNA from the following sources: M, molecular marker; a, uninfected SJPL cells; b, SJPL cells infected with A/Sydney/5/97 (H3N2); c, SJPL cells infected with A/Chicken/NY/13307-3/95 (H7N2); d, SJPL cells infected with A/Swine/IA/17672/88 (H1N1); e, uninfected MDCK cells; f, MDCK cells infected with A/Sydney/5/97 (H3N2); g, MDCK cells infected with A/Chicken/NY/13307-3/95 (H7N2); h, MDCK cells infected with A/Swine/IA/17672/88 (H1N1).
Reverse transcriptase assay, RT-PCR analysis to detect PERV, and tumorigenicity.
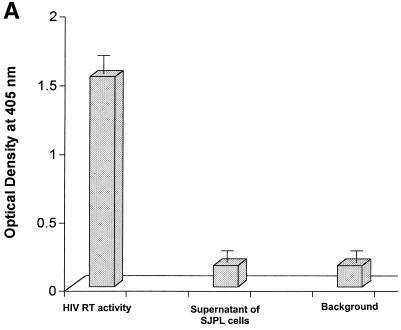
Recent progress in the field of xenotransplantation has raised concerns about the possible transmission of porcine endogenous retrovirus (PERV) to humans. Results of previous studies showed that PERV from a porcine kidney cell line (PK15) can infect many types of human cell lines (kidney, lung, muscle, and lymphoid) in vitro (22, 28, 41). If PERV from the SJPL cell line is present in the growth medium, then the SJPL cell line would not be a suitable candidate for use in influenza virus vaccine production. Because of this possibility of PERV contamination, we assayed the level of reverse transcriptase activity in tissue culture supernatant of SJPL cells using a nonradioactive reverse transcriptase assay kit (Roche Molecular Biochemicals); with this assay, we could detect any type of retrovirus that was present. Reverse transcriptase activity was not detected in supernatants of SJPL cells, i.e., the levels of activity were similar to background levels (optical density at 450 nm of 0.05). In contrast, high levels of reverse transcriptase activity (optical density at 450 nm of 1.5) were detected in medium containing the positive control HIV-1 reverse transcriptase (Fig. 6A).
FIG. 6.
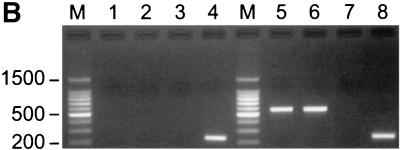
Reverse transcriptase assay and RT-PCR analysis. (A) Reverse transcriptase activity in the supernatant of SJPL cells was evaluated by using a nonradioactive reverse transcriptase assay. HIV-1 reverse transcriptase (0.125 ng/well in a 96-well plate) was used as a positive control, and ABTS [2′,2-azino-di(3-ethyl-benzthiazoline-6-sulfonic acid)] solution alone was used as a negative control. The sample was tested by the assay four times. (B) Tissue culture supernatants and cellular RNAs were collected from SJPL cells and PK-15 cells. RT-PCR was performed as described in the text. Lanes: M, 100-bp DNA marker; 1, tissue culture supernatant of SJPL cells; 2, cellular RNAs of SJPL cells; 3, cellular RNAs of SJPL cells without reverse transcriptase; 4, cellular RNAs of SJPL cells with β-actin primers; 5, tissue culture supernatant of PK-15 cells; 6, cellular RNAs of PK-15 cells; 7, cellular RNAs of PK-15 cells without reverse transcriptase; 8, cellular RNAs of PK-15 cells with β-actin primers.
We also tested the presence of PERV in SJPL cells using PERV-specific polymerase primers (41). As a positive control, the porcine kidney cell line PK-15 was used. RNA was extracted from cell culture supernatant using the RNeasy mini kit, and total cellular RNA was collected from cells using TRIzol reagent (Life Technologies). RNA was reverse transcribed with a SuperScript first-strand synthesis system for RT-PCR (Life Technologies) using random hexamers (50 ng). The cDNA was amplified with the PERV-specific primers PB905 (5′CCGCAGGGATGGGTTTGGCAAAGCA3′) and PB906 (5′ACGTACTGGAGGAGGGTCACCTGA′) for 30 cycles at 94°C for 30 s, 60°C for 30 s, and 72°C for 1 min (41). The PCR products were electrophoresed on a 1.5% agarose gel. A DNA ladder (100 bp) was used as a marker. PERV was detected in both tissue culture supernatants and cellular RNAs of PK-15 cells (Fig. 6B, lanes 5 and 6) but was not detected in either tissue culture supernatants or cellular RNAs of SJPL cells (Fig. 6B, lanes 1 and 2). PCR products were sequenced to identify PERV-specific sequences (data not shown). To confirm that PERV was from cellular RNAs rather than genomic DNAs, reverse transcriptase was omitted during cDNA synthesis (Fig. 6B, lanes 3 and 7). As an internal control, β-actin primers were used (Fig. 6B, lanes 4 and 8). PERV-specific protease primers were also used as described previously (28). We could amplify PERV in tissue culture supernatants and cellular RNAs of PK-15 cells but not in SJPL cells (data not shown).
It is known that MDCK cells cause tumors in nude mice (10). We decided to test whether SJPL cells could cause tumors in nude mice. SJPL or MDCK cells (3 × 106/mouse) were inoculated into the dorsal necks of six nude mice, and tumor development was monitored daily. The nude mice inoculated with MDCK cells developed tumors around the neck and shoulders 3 weeks postinfection, and all six nude mice developed tumors by 5 weeks postinfection. In contrast, the six nude mice inoculated with SJPL cells did not develop tumors (data not shown).
Efforts have been made to find cell lines that support the productive replication of influenza virus and allow the virus to maintain its original antigenicity. African green monkey kidney (Vero) cells fully support replication of influenza A and B viruses (10), but influenza viruses have to be extensively adapted before they can be grown in this cell line. Repeated addition of trypsin to the culture medium is also needed for multicycle growth of the influenza viruses (18). BHK cells also support replication of influenza viruses, but growth in BHK cells, like that in eggs, results in the selection of receptor-binding variants of human influenza viruses (11). MDCK cells are considered to be the best cell line for supporting the growth of influenza viruses, but this cell line causes cancer in nude mice (10; this study); MDCK cells have not been licensed for use in the production of vaccine. Cold-adapted influenza virus vaccine can be produced in specific-pathogen-free eggs, but in a pandemic, the supply of this type of egg would not be sufficient to meet demand.
An important advantage of SJPL cells for use in influenza vaccine production is that propagation of human influenza A viruses in these cells did not lead to antigenic changes in the HA molecule. Current vaccines prepared from human influenza viruses adapted for growth in eggs contain variants whose HA molecules differ from that of the original human virus by at least one or two amino acids (16, 33). This variation may result in the immune escape of influenza viruses in immunized humans. In a mouse model of influenza virus infection, a single amino acid substitution in the HA molecule rendered a candidate vaccine for an influenza A virus (H3) ineffective (19). In that study, the virus with a substitution at Lys-156 in the HA molecule was poorly immunogenic and did not prevent infection by viruses that had been grown in MDCK cells. SJPL cells may be a good candidate for use in producing a better human vaccine that maintains the antigenicity of the original virus.
SJPL cells express Sia2-3Gal- and Sia2-6Gal-containing sialylglycoconjugates, which serve as receptors for influenza virus. Ito et al. reported that receptors for human and avian influenza viruses are present on the surfaces of pig tracheal cells and suggested that this finding may explain how pigs serve as mixing vessels in which influenza viruses that can cause pandemics are created (15). However, Mv1Lu and MDCK cells also express both receptors on their cell surface. It is possible that unknown cellular factors in addition to receptors on the cell surface are responsible for creating an environment within the pig for the generation of human-avian virus reassortants.
Replication of influenza viruses, even those of the same subtype, appeared to differ in SJPL and MDCK cells. Most subtypes of avian H5N1 influenza viruses isolated during an outbreak in the Hong Kong poultry markets in 1997 grew well in SJPL cells and in MDCK cells, but the titers of A/Chicken/HK/728/97 in SJPL cells and in MDCK cells and those of the A/Goose/HK/437-4/97 in MDCK cells were 10- to 106.5-fold less than those of other viruses (Table 3). Matrosovich et al. (27) reported that the receptor binding properties of H5N1 viruses isolated from humans and birds in the Hong Kong markets in 1997 are typical of avian but not human viruses. These H5N1 viruses predominantly bind to Sia2-3Gal-containing sialylglycoconjugates. H5N1 viruses with a carbohydrate at position 158 of HA bind more weakly to ovomucoid containing Sia2-3Gal determinants than do H5N1 viruses without a carbohydrate at the same residue. Because A/Chicken/HK/728/97 (H5N1) does not contain a carbohydrate at position 158 of its HA molecule, the low titer of this virus in the SJPL and MDCK cells is probably not due to weak binding to the surface receptors. In both cell lines, the titers of A/Chicken/HK/258/97 (H5N1) with a carbohydrate at position 158 of HA were higher than those of other H5N1 viruses. In addition to receptor binding, other factors, including the set of genes within the virus, also appear to be important in supporting replication in cell culture and, possibly, in vivo.
Our findings indicate that influenza virus-induced damage of SJPL cells is due to necrosis rather than apoptosis; however, previous studies showed that influenza virus infection of MDCK and HeLa cells results in apoptosis (13, 38). The possible mechanisms of apoptosis in HeLa and MDCK cells were reported. Influenza virus infection of HeLa cells triggered the expression of Fas and Fas ligand on the cell surface. Fas (CD95) is a cell surface receptor that transduces apoptotic signal (2, 40). Apoptosis occurred in infected MDCK cells with low levels of Bcl-2, but apoptosis was inhibited when MDCK cells were stably transfected with a Bcl-2 expression plasmid (13). Bcl-2 is known to regulate the mitochondrial permeability transition pore (23, 24, 43). The difference in influenza virus-induced damage among cell lines may be due to the origins of the cells. However, at this time, we do not know why SJPL cells do not undergo apoptosis like MDCK or HeLa cells.
Our study indicates that SJPL cells do not express PERVs. It was reported that the established porcine cell lines express PERVs. (1, 39). The reason why SJPL cells do not express PERVs may be differences in the source of the original tissues.
Acknowledgments
This work was supported by Public Health Service grants AI29680 and A795357 and Cancer Center Support (CORE) grant CA-21765 from the National Institutes of Health and by the American Lebanese Syrian Associated Charities (ALSAC).
We thank Scott Krauss and David Walker for excellent technical support, Nadine Finley and Alice Herren for manuscript preparation, and Julia Cay Jones for editorial assistance.
REFERENCES
- 1.Armstrong J A, Porterfield J S, Madrid A T D. C-type virus particles in pig kidney cell lines. J Gen Virol. 1971;10:195–198. doi: 10.1099/0022-1317-10-2-195. [DOI] [PubMed] [Google Scholar]
- 2.Ashkenazi A, Dixit V M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 3.Carroll S M, Paulson J C. Differential infection of receptor-modified host cells by receptor-specific influenza viruses. Virus Res. 1985;3:165–179. doi: 10.1016/0168-1702(85)90006-1. [DOI] [PubMed] [Google Scholar]
- 4.Connor R J, Kawaoka Y, Webster R G, Paulson J C. Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology. 1994;205:17–23. doi: 10.1006/viro.1994.1615. [DOI] [PubMed] [Google Scholar]
- 5.Duvall E, Wyllie A H. Death and the cell. Immunol Today. 1986;7:115–119. doi: 10.1016/0167-5699(86)90152-0. [DOI] [PubMed] [Google Scholar]
- 6.Endo Y, Carroll K N, Ikizler M R, Wright P F. Growth of influenza A virus in primary, differentiated epithelial cells derived from adenoids. J Virol. 1996;70:2055–2058. doi: 10.1128/jvi.70.3.2055-2058.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fadok V A, Voelker D R, Campbell P A, Cohen J J, Bratton G L, Henson P M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 8.Frank A L, Couch R B, Griffis C A, Baxter B D. Comparison of different tissue cultures for isolation and quantitation of influenza and parainfluenza viruses. J Clin Microbiol. 1979;10:32–36. doi: 10.1128/jcm.10.1.32-36.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gambryan A S, Tuzikov A B, Piskarev V E, Yamnikova S S, Lvov D K, Robertson J S, Bovin N V, Matrosovich M N. Specification of receptor-binding phenotypes of influenza virus isolates from different hosts using synthetic sialylglycopolymers: non-egg-adapted human H1 and H3 influenza A and influenza B viruses share a common high binding affinity for 6′-sialyl(N-acetyllactosamine) Virology. 1997;232:345–350. doi: 10.1006/viro.1997.8572. [DOI] [PubMed] [Google Scholar]
- 10.Govorkova E A, Murti G, Meignier B, de Taisne D E C, Webster R G. African green monkey kidney (Vero) cells provide an alternative host cell system for influenza A and B viruses. J Virol. 1996;70:5519–5524. doi: 10.1128/jvi.70.8.5519-5524.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Govorkova E A, Matrosovich M N, Tuzikov A B, Bovin N V, Gerdil C, Fanget B, Webster R G. Selection of receptor-binding variants of human influenza A and B viruses in baby hamster kidney cells. Virology. 1999;262:31–38. doi: 10.1006/viro.1999.9892. [DOI] [PubMed] [Google Scholar]
- 12.Hinshaw V S, Webster R G. The natural history of influenza A viruses. In: Beard A S, editor. Basic and applied influenza research. Boca Raton, Fla: CRC Press; 1982. pp. 79–104. [Google Scholar]
- 13.Hinshaw V S, Olsen C W, Dybdahl-Sissoko N, Evans D. Apoptosis: a mechanism of cell killing by influenza A and B viruses. J Virol. 1994;68:3667–3673. doi: 10.1128/jvi.68.6.3667-3673.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito T, Couceiro J N, Kelm S, Baum L G, Krauss S, Castrucci M R, Donatelli L, Kida H, Paulson J C, Webster R G, Kawaoka Y. Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J Virol. 1998;72:7367–7373. doi: 10.1128/jvi.72.9.7367-7373.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz J M, Naeve C W, Webster R G. Host cell-mediated variation in H3N2 influenza viruses. Virology. 1987;156:386–395. doi: 10.1016/0042-6822(87)90418-1. [DOI] [PubMed] [Google Scholar]
- 17.Katz J M, Wang M, Webster R G. Direct sequencing of the HA gene of influenza (H3N2) virus in original clinical samples reveals sequence identity with mammalian cell-grown virus. J Virol. 1990;64:1808–1811. doi: 10.1128/jvi.64.4.1808-1811.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaverin N V, Webster R G. Impairment of multicycle influenza virus growth in Vero (WHO) cells by loss of trypsin activity. J Virol. 1995;69:2700–2703. doi: 10.1128/jvi.69.4.2700-2703.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kodihalli S, Justewicz D M, Gubareva L V, Webster R C. Selection of a single amino acid substitution in the hemagglutinin molecule by chicken eggs can render influenza A virus (H3) candidate vaccine ineffective. J Virol. 1995;69:4888–4897. doi: 10.1128/jvi.69.8.4888-4897.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lau S C, Scholtissek C. Abortive infection of Vero cells by an influenza A virus (FPV) Virology. 1995;212:225–231. doi: 10.1006/viro.1995.1473. [DOI] [PubMed] [Google Scholar]
- 21.Lennette E H, Schmidt N J, editors. Diagnostic procedures for viral and rickettsial infections. 4th ed. New York, N.Y: American Public Health Association, Inc.; 1969. [Google Scholar]
- 22.Le Tissier P, Stoye J P, Takeuchi Y, Patience C, Weiss R A. Two sets of human-tropic pig retrovirus. Nature. 1997;389:681–682. doi: 10.1038/39489. [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Kim C N, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 24.Mancini M, Nicholson D W, Roy S, Thornberry N A, Peterson E P, Casciola-Rosen L A, Rosen A. The caspase-3 precursor has a cytosolic and mitochondrial distribution: implications for apoptotic signaling. J Cell Biol. 1998;140:1485–1495. doi: 10.1083/jcb.140.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin S J, Reutelingsperger C P, McGahon A J, Rader J A, van Schie R C, LaFace D M, Green D R. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matrosovich M N, Gambaryan A S, Teneberg S, Piskarev V E, Yamnikova S S, Lvov D K, Robertson J S, Karlsson K A. Avian influenza A viruses differ from human viruses by recognition of sialyloligosaccharides and gangliosides and by a higher conservation of the HA receptor-binding site. Virology. 1997;233:224–234. doi: 10.1006/viro.1997.8580. [DOI] [PubMed] [Google Scholar]
- 27.Matrosovich M, Zhou N, Kawaoka Y, Webster R. The surface glycoproteins of H5 influenza viruses isolated from humans, chickens, and wild aquatic birds have distinguishable properties. J Virol. 1999;73:1146–1155. doi: 10.1128/jvi.73.2.1146-1155.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patience C, Takeuchi Y, Weiss R A. Infection of human cells by an endogenous retrovirus of pigs. Nat Med. 1997;3:282–286. doi: 10.1038/nm0397-282. [DOI] [PubMed] [Google Scholar]
- 29.Paulson J C. Interactions of animal viruses with cell surface receptors. In: Conn M, editor. The receptors. Vol. 2. Orlando, Fla: Academic Press; 1985. pp. 131–219. [Google Scholar]
- 30.Platt N, da Silva R P, Gordon S. Recognizing death: the phagocytosis of apoptotic cells. Trends Cell Biol. 1998;8:365–372. doi: 10.1016/s0962-8924(98)01329-4. [DOI] [PubMed] [Google Scholar]
- 31.Reina J, Fernadez-Baca V, Blanco I, Munar M. Comparison of Madin-Darby canine kidney cells (MDCK) with a green monkey continuous cell line (Vero) and human lung embryonated cells (MRC-5) in the isolation of influenza A virus from nasopharyngeal aspirates by shell vial culture. J Clin Microbiol. 1997;35:1900–1901. doi: 10.1128/jcm.35.7.1900-1901.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robertson J S, Bootman J S, Nicolson C, Major D, Robertson E W, Wood J M. The hemagglutinin of influenza B virus present in clinical material is a single species identical to that of mammalian cell-grown virus. Virology. 1990;179:35–40. doi: 10.1016/0042-6822(90)90270-2. [DOI] [PubMed] [Google Scholar]
- 33.Robertson J S, Naeve C W, Webster R G, Bootman J S, Newman R, Schild G C. Alterations in the hemagglutinin associated with adaptation of influenza B virus to growth in eggs. Virology. 1985;143:166–174. doi: 10.1016/0042-6822(85)90105-9. [DOI] [PubMed] [Google Scholar]
- 34.Robertson J S, Cook P, Attwell A M, Williams S P. Replicative advantage in tissue culture of egg-adapted influenza virus over tissue-culture derived virus: implications for vaccine manufacture. Vaccine. 1995;13:1583–1588. doi: 10.1016/0264-410x(95)00085-f. [DOI] [PubMed] [Google Scholar]
- 35.Schild G C, Oxford J S, de Jong J C, Webster R G. Evidence for host-cell selection of influenza virus antigenic variants. Nature. 1983;303:706–709. doi: 10.1038/303706a0. [DOI] [PubMed] [Google Scholar]
- 36.Scholtissek C, Burger H, Kistner O, Shortridge K F. The nucleoprotein as a possible major factor in determining host specificity of influenza H3N2 viruses. Virology. 1985;147:287–294. doi: 10.1016/0042-6822(85)90131-x. [DOI] [PubMed] [Google Scholar]
- 37.Seo S H, Collisson E W. Specific cytotoxic T lymphocytes are involved in in vivo clearance of infectious bronchitis virus. J Virol. 1997;71:5173–5177. doi: 10.1128/jvi.71.7.5173-5177.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takizawa T, Matsukawa S, Higuchi Y, Nakamura S, Nakanishi Y, Fukuda R. Induction of programmed cell death (apoptosis) by influenza virus infection in tissue culture cells. J Gen Virol. 1993;74:2347–2355. doi: 10.1099/0022-1317-74-11-2347. [DOI] [PubMed] [Google Scholar]
- 39.Tumilowicz J J, Hung C-L, Kramarsky B. Concurrent replication of a papovavirus and a C-type virus in the CCL 33 porcine cell line. In Vitro. 1979;15:922–928. doi: 10.1007/BF02618050. [DOI] [PubMed] [Google Scholar]
- 40.Wallach D, Varfolomeev E E, Malinin N L, Goltsev Y V, Kovalenko A V, Boldin M P. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- 41.Wilson C A, Wong S, Muller J, Davidson C E, Rose T M, Burd P. Type C retrovirus released from porcine primary peripheral blood mononuclear cells infects human cells. J Virol. 1998;72:3082–3087. doi: 10.1128/jvi.72.4.3082-3087.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wyllie A H, Kerr J F R, Currie A R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 43.Zamzami N, Susin S A, Marchetti P, Hirsch T, Gomez-Monterrey I, Castedo M, Kroemer G. Mitochondrial control of nuclear apoptosis. J Exp Med. 1996;183:1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]