Abstract
Advances in nanotechnology have been changing the face of dentistry with their diverse range of dental applications. Silver nanoparticles (AgNPs) are a relatively new breakthrough in dentistry.
Aim
The main objective of this paper is to discuss the current progress in the field of dentistry and highlight the aspects regarding silver nanoparticle incorporation, emphasizing the properties, applications, and advantages of nanosilver fluoride (NSF) that it brings to dentistry.
Materials and methods
An extensive electronic scientific search was conducted on published articles in various databases, such as Medline (PubMed), CENTRAL (Cochrane), Scopus, and Web of Science, using the search terms AgNPs, nano dentistry, caries prevention, and oral health. Further brief communications, randomized controlled trials (RCT), in vitro research, and animal studies written in English were also considered. Case reports, editorial reviews, and opinion letters were excluded from the first phase of our research.
Results
Pertaining to various kinds of literature reviews in journals, around 345 articles were retrieved. After screening, about 28 articles met all the selection criteria, focusing on NSF for the contemporary management of dental caries, emphasizing microinvasive therapeutic methods that can successfully halt the progression of caries at the initial level and minimize the loss of sound tooth structure.
Conclusion
Due to its exceptional properties and wide range of clinical applications, AgNPs incorporated in fluoride may be employed as an effective, affordable, and improved anticaries agent that brings about superior enhancements in the fields of orthodontics, restorative dentistry, and pediatric and preventive dentistry.
How to cite this article
Thimmaiah C, Thomas NA, Baskaradoss JK, et al. Mapping the Dental Applications of Nanosilver Fluoride: A Narrative Review. Int J Clin Pediatr Dent 2024;17(7):833–837.
Keywords: Caries prevention, Nano dentistry, Nanosilver fluoride, Oral health
Introduction
Dental caries, a biofilm-sugar-influenced disease, affects the hardness of dental tissues.1 Even now, it is a serious health concern despite improvements in dental care. Managing and arresting these lesions at an initial level is gaining momentum against conventional restorative approaches. Various materials are available in the market; among them, silver is gaining popularity because of its anticaries, antimicrobial, and antirheumatic properties in the field of dentistry.2
Silver has improved dramatically from being used only as dental amalgam since the 18th century. Various alternative preparations providing more advantages were studied, and silver diamine fluoride (SDF) has emerged remarkably as a silver bullet in dentistry. SDF is routinely used for managing dental caries in deciduous and permanent teeth as a noninvasive procedure.3 By hindering cariogenic bacteria, it increases the synergistic antibacterial effects of fluoride and silver. In addition, it benefits fluoride as a remineralizing agent for the demineralized tooth surface and converts hydroxyapatite crystals into fluorapatite, decreasing the susceptibility of the surface to demineralization.4 Nevertheless, the application of SDF creates reversible black lesions on the oral tissues due to the oxidation of silver ions, and the unfavorable metallic taste can be a major pitfall of its use.3-6
Modern dentistry has evolved significantly over time. Contemporary advances in emerging nanotechnology have resulted in a rapidly growing field with numerous biomedical science applications for the eradication of various dental problems that have been a foremost issue for dentists. The evolution of second-generation nano-scaled silver particles (NSP) due to their exceptional characteristics, has attracted attention. Silver nanoparticles (AgNPs), with a high specific surface area and a high fraction of surface atoms, exhibit these characteristics. As particle size decreases, the surface area-to-volume ratio of NSPs increases dramatically, leading to significant changes in their physical, chemical, and biological properties. With a variety of dental applications, these formulations are undergoing rapid growth and are perhaps the most modern breakthrough in cariology over traditional silver products.1,4 AgNPs are made up of 20–15,000 smaller diameter (<100 nm) silver atoms. Because of its large surface-to-volume ratio, AgNPs have antimicrobial properties against cariogenic bacteria, including Streptococcus mutans and Lactobacillus dynamic biofilm. They facilitate caries arrest in enamel and dentin without staining the teeth, are nonirritating, cost-effective, and remain stable for up to 3 years.6,7 Furthermore, it is effective against residual bacteria in prepared cavities and secondary caries at the margins of restoration because of the synergistic effect of fluoride and AgNPs.8 This review offers insight into the current aspects regarding the incorporation of AgNPs in modern dental biomaterials.
PICO analysis—P = adult population; I = use of silver nano fluoride; C = conventional products/no comparison; and O = evolution of oral health.
Materials and Methods
Search Strategy
A comprehensive electronic literature search was conducted on published review articles, original research, and systematic reviews using databases such as Medline (PubMed), CENTRAL (Cochrane), Scopus, and Web of Science using the search terms Nanosilver fluoride (NSF), nano dentistry, caries prevention, oral health. Only full-text articles written in English were selected. All duplicates, case reports, editorial reviews, and articles irrelevant to the topic were excluded from the selection process.
Results
After a reliable, vast electronic literature search through various databases, search in PubMed, Scopus, and Web of Sciences databases using the free-text terms “((nanosilver fluoride) OR (nano dentistry) AND (caries prevention) (oral health))” showed 570 reviews and systematic reviews articles published until 2022. After the duplicates were removed, around 345 articles were selected. The selection process for the articles followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines, as shown in Figure 1. After the screening, only 28 full-text articles, which met the inclusion criteria and highlighted the scope and future aspects of NSF in dentistry, were selected, and the remaining articles that were not relevant were excluded from the study.
Fig. 1:
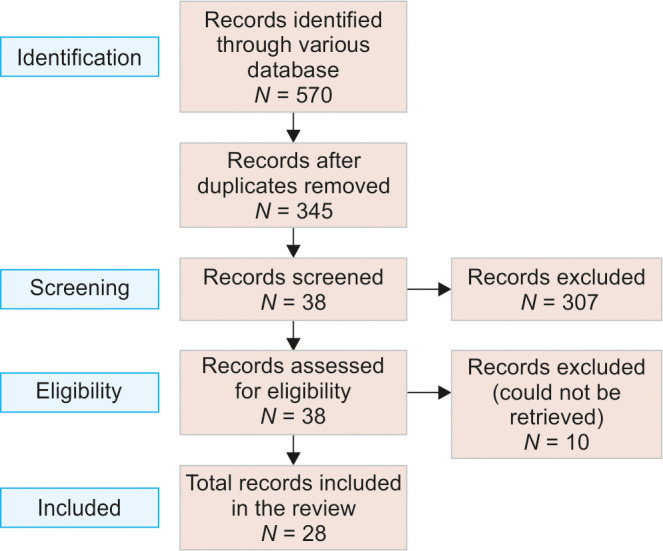
Flowchart of the review process and search strategy according to the PRISMA statement
Discussion
Physical Properties and Preparation
Nanosilver-based preparations of 1–10 nm size demonstrate significant antibacterial and anticariogenic properties as they shield the tooth against S. mutans, the pathogen responsible for dental caries.9 It is composed of chitosan (28,585 μg/mL), silver (376.5 μg/mL), and sodium fluoride (NaF) (5,028.3 μg/mL).10 Chitosan is a polysaccharide substance comprising N-acetylglucosamine and glucosamine copolymers, which functions as a carrier to stabilize AgNPs.3,8 According to an in vitro study, chitosan prevents the release of minerals from hard tissues, thereby preventing demineralization.6 NSF application is a simple, noninvasive, cost-effective procedure that can be executed in a community setup. Additionally, it reduces the risk of cross-infections.6,10 Compared to SDF, 600 and 1500 ppm of NSF are associated with reduced dentin staining (vs 30 and 38%, respectively).11 Similarly, compared to 38% SDF, 5% NSF is eight times more economical.12 Hence, this simple procedure is not only beneficial as an anticariogenic agent in patients who are uncooperative and children with special healthcare needs (CHSCN) but it can also be considered a global asset in caries prevention programs at community levels.13
Antibacterial Action of Nanosilver Fluoride
Silver nanoparticles have both bacteriostatic and bactericidal properties. The smaller (1–10 nm) particles have greater antibacterial efficacy, and their spherical shape increases the surface area of bacterial surfaces and facilitates particle penetration.5 Silver ions can have direct interactions with bacterial cells, changing their morphology and internal structure and ultimately causing cell death. AgNPs’ antibacterial action depends on their capacity to pierce bacterial cell walls, which causes direct and indirect lipid peroxidation that degrades the cell membrane, obstructs DNA replication and repair, suppresses the respiratory protein, and other effects.3,9,14 The antibacterial effect is dependent on the concentration and rate of ionic silver release. Based on this, SNPs with <50 nm size are commonly used in various research studies.
Anticariogenic Action of Nanosilver Fluoride
The antibacterial and anti-demineralizing characteristics of chitosan make it a stabilizing agent. The fluoride added to NSF minimizes the loss of enamel minerals and has shown a substantial capacity to reduce the growth of bacterial biofilms due to its anti-adherence and anticariogenic effects. To achieve antibacterial effects, silver is commonly used in the form of nitrates.6,15 However, when NSFs are used, the surface area accessible for exposure to the microbial community is significantly increased. The anticariogenic action of NSF is brought about by the cumulative effect of chitosan, AgNPs, and sodium fluoride. The NSF colloidal formulation has antibacterial properties, prevents the growth of cariogenic biofilms, and promotes tooth remineralization.14-16
In the early phases of a carious lesion, minimally invasive treatments have become increasingly important for preventing and arresting its progression. Four fundamental criteria should be followed for minimal intervention dentistry to be practiced, which were implemented according to the new policy paper created for the World Dental Federation.
Early recognition and evaluation of potential caries risk factors.
Control the disease through the reduction of cariogenic flora.
Regeneration and remineralization of early lesions.
Based on the improved understanding of the caries process and the advent of advanced adhesive restorative materials have led to the development of the idea of minimal intervention dentistry. The future of dentistry involves the creation of materials that can remineralize dentinal lesions as well as intelligent biomimetic materials that can replace the missing tooth structure. Among these various materials, a novel material called NSF was introduced by Targino et al.19 and Haghgoo et al.,20 who first conducted various experiments incorporating nanosilver particles and fluoride, which demonstrated antimicrobial activity and lower cytotoxicity than SDF.
Applications of Nanosilver Fluoride in Dental Caries Management
Silver nanoparticles incorporated in fluoride may enhance the mechanical features and antibacterial properties of dental materials and have become useful tools for various dental applications in the field of preventive dentistry, endodontics, periodontics, restorative dentistry, and orthodontics.
Nanosilver Fluoride as a Remineralizing Agent
Nozari et al.2 determined the remineralization ability in the enamel of primary anterior teeth using three different materials—sodium fluoride varnish, nanohydroxyapatite serum, and NSF. It was observed that the NSF group showed the greatest remineralization compared to the other groups. Teixeira et al. formulated a dentifrice with NSF that potentially stopped the pH from decreasing and demonstrated the ability to control demineralization. Additionally, this novel dentifrice showed better antibacterial activity, preventing bacterial adhesion and exhibiting superior potential in the prevention of dental caries. The NSF dentifrice has bacteriostatic properties but not bactericidal properties, which helps preserve the normal microbiome of the oral cavity.8 These solutions not only arrest demineralization but also prevent future progression. The bactericidal potential of silver, along with the remineralization potential of fluorides, will preserve tooth enamel against acid attacks.
Currently, various formulations of NSF with different concentrations are available, showing promising results in arresting existing carious lesions, enhancing enamel remineralization of both deciduous and permanent teeth, and preventing future carious lesions. Moreover, these do not stain the tooth surfaces.21 According to Zhi et al., while silver and fluoride ions cause remineralization, silver ions, upon penetrating demineralized enamel, precipitate and cause enamel hardening. Moreover, the spherical shape of NSF and 3.2–1.2 nm size enhances the contact surface available for reaction.7 Silva et al. reported superior efficacy of NSF in terms of decreasing pH and preventing cell-surface adhesion of S. mutans and tooth surface when compared to sodium fluoride. It was concluded that NSF is simple to use and much more effective than conventional fluorides for the treatment of incipient carious lesions.22
Nanosilver Incorporated Sodium Fluoride Varnish
Tirupathi et al. evaluated the efficacy of 5% nanosilver incorporated sodium fluoride (NSSF) varnish vs 38% SDF applied on a yearly basis. They concluded that NSSF was not inferior to SDF in preventing caries progression on dentin in deciduous molars.12 Nozari et al. reported a higher remineralization capacity of NSF varnish compared to nanohydroxyapatite serum NaF varnishes.2 While the antibacterial properties of NSF and polyvinylpyrrolidone fluoride varnishes against S. mutans are similar to SDF varnish, NSF has shown antibacterial effects against E. faecalis which has higher resistant to antibacterial agents than S. mutans. The study showed a 3% increased efficacy of NSF compared to SDF in inhibiting E. faecalis biofilm formation.23,24
Nanosilver Particles Incorporated in a Sealant
Ghasempour et al. evaluated the efficacy of sealants with nanosilver particles, with and without fluoride. It was concluded that the addition of fluoride to AgNPs results in significant inhibition of S. mutans growth.25 However, another study showed that the in vitro remineralization potential of NSF was less than that of SDF and sodium fluoride. The NSF varnish had more inconsistencies, more mineral deposits, and a lower surface microhardness when compared to SDF and NaF varnish.26
Nanosilver Fluoride for Arresting Dental Caries
After applying two drops of NSF (10 mg) with a microbrush at regular intervals for 1 month, a study by Nagireddy et al. showed greater caries arrest after 7 days, 5, and 12 months. While the single-surface carious lesions demonstrated caries arrest, the amount of caries arrest at multiple surfaces was less significant.3 Additionally, Dos Santos et al. confirmed that upon treatment with NSF, the dentine becomes hard and has a crumbly consistency.6
Nanosilver Fluoride as a Pretreatment in Sealants
Deep fissures pretreated with SDF or NSF before sealant placement improved the protection against microleakage and decreased the risk of caries under the sealant when used therapeutically, according to a study by El habashy et al.4 The goal of pretreating fissures with SDF or NSF before applying sealant is to combine the physical sealing property of fissures with the antimicrobial and remineralizing actions of these agents. This approach is extremely beneficial in caries prevention, especially in high-risk patients. According to Nanda and Naik, pretreatment with NSF prevents secondary caries formation in restored teeth, thereby decreasing the incidence of treatment failure.13
Nanosilver Fluoride Incorporated into Glass Ionomer Cement
Nanosilver particles were added to a traditional GIC due to the growing interest in the usage of NSF. It can be recommended for use in restorations of greater stress-bearing sites, as this modification showed improved mechanical and bond strength properties.27
Nanosilver Fluoride Incorporated in Orthodontic Brackets
Fixed orthodontic therapy is often associated with mild enamel demineralization and the development of white spot lesions.28 Nanosilver coated orthodontic bracket is effective in preventing S. mutans induced demineralization and development of white spot lesions in patients undergoing fixed orthodontic therapy. The primary concern is that the physical and chemical properties should not be adversely altered, even if AgNPs have been added to standard orthodontic adhesives and appliances, resulting in excellent clinical performance.29
Disinfection and Sanitization of Deep Dentinal Lesions
The antimicrobial property and high penetration ability of SNP is beneficial during disinfection and cleaning of deep dentinal cavity preparation and caries removal. They eliminate the residual bacteria present in the tooth cavity preparation.1
Due to their unique physical and biochemical characteristics in contrast to their macro- and microcomplements, AgNPs have attracted attention among the many current nanomaterials. Silver nanoparticles demonstrate significant antibacterial and anti-adherence activity against S. mutans and can be used as effective growth inhibitors. AgNPs have risen as one of the most potent antibacterial agents due to their large surface area-to-volume ratios.12,20
Despite the antibacterial and remineralizing properties, silver nanomaterials are prone to oxidation and can become aggregative. The free silver ions that enter mammalian cells and combine with oxygen to create reactive oxygen species contribute to the toxicity. This process increases the oxidative stress, resulting in deleterious effects. However, in the oral cavity, the cytotoxic effects of AgNPs are comparatively less.13 The severity of cytotoxicity of NSF can be associated with the size, shape, surface area, and charge of the AgNPs as well as the amount of nonspecific oxidative damage.1 Secondly, the use of perilous chemical agents such as acetic acid and sodium borohydride as reducing agents in the production of NSF are considered hazardous, highlighting the risk of this product. However, Targino et al. reported no toxic effects on erythrocytes with 1–5% nanosilver solutions, suggesting NSF is more biocompatible than SDF.19
Future Scope
The approach to treating dental caries has drastically changed from invasive treatments to minimally invasive techniques. Studies evaluating the long-term stability, cost-effectiveness of the material, and its safety in the oral cavity are limited.21 Therefore, long-term community-based studies evaluating the efficacy, safety, and tolerability of NSF are warranted to recommend its use in community setup.30 Another shortcoming is that there are limited research studies regarding the effectiveness of NSF on secondary caries, dentinal hypersensitivity, and root surface caries. Carrying out studies on evaluating the efficacy of NSF on these aspects as well as further conducting clinical studies to know the effectiveness of NSF hold scope for future research since most of the studies are laboratory in vitro studies.
Conclusion
The studies that we reviewed and screened are largely highlighting the benefits and enhancements that the nanostructured materials provide to the area of dentistry. Because of the high surface-volume ratio, the use of AgNPs is quickly progressing due to their potent antimicrobial action in dentistry. Although the benefits of NSF in the management of insidious and enamel surface caries as demonstrated favorable results, the clinical efficacy and overall advantages on patients’ oral health need to be monitored. Hence, further research is warranted to evaluate its efficacy on dentinal hypersensitivity and root surface caries. Nonetheless, the use of nanoparticles in dental practice is gaining popularity and will be part of mainstream dental practice in the near future.
Author Contributions
All the authors have contributed equally to gathering information for the review article.
Orcid
Charisma Thimmaiah https://orcid.org/0000-0002-4920-4624
Nithya Annie Thomas https://orcid.org/0000-0002-9314-4171
Jagan K Baskaradoss https://orcid.org/0000-0002-0032-0464
Swetha R K https://orcid.org/0009-0002-4928-043X
Anagha Chonat https://orcid.org/0000-0003-1739-2285
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Fakhruddin KS, Egusa H, Ngo HC, et al. Clinical efficacy and the antimicrobial potential of silver formulations in arresting dental caries: a systematic review. BMC Oral Health. 2020;20(1):160. doi: 10.1186/s12903-020-01133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nozari A, Ajami S, Rafiei A, et al. Impact of nano hydroxyapatite, nano silver fluoride and sodium fluoride varnish on primary teeth enamel remineralization: an in vitro study. J Clin Diagn Res. 2017;11(9):ZC97–ZC100. doi: 10.7860/JCDR/2017/30108.10694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagireddy VR, Reddy D, Kondamadugu S, et al. Nanosilver fluoride—a paradigm shift for arrest in dental caries in primary teeth of schoolchildren: a randomized controlled clinical trial. Int J Clin Pediatr Dent. 2019;12(6):484–490. doi: 10.5005/jp-journals-10005-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El habashy L, El tekeya M. The effect of enamel pre-treatment with silver diamine fluoride versus nano silver fluoride on the microleakage of fissure sealant: in vitro study. Egypt Dent J. 2020;66(4):1931–1938. doi: 10.21608/EDJ.2020.34925.1167. [DOI] [Google Scholar]
- 5.Al-Nerabieah Z, Arrag EA, Comisi JC, et al. Effectiveness of a novel nano-silver fluoride with green tea extract compared with silver diamine fluoride: a randomized, controlled, non-inferiority trial. Int J Dent Oral Sci. 2020;26;:753–761. doi: 10.19070/2377-8075-20000148. [DOI] [Google Scholar]
- 6.Dos Santos VE, Jr,, Vasconcelos Filho AA, Targino AGR, et al. A new “Silver-Bullet” to treat caries in children - nano silver fluoride: a randomized clinical trial. J Dent. 2014;42(8):945–951. doi: 10.1016/j.jdent.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Zhi QH, Lo EC, Kwok AC. An in vitro study of silver and fluoride ions on remineralization of demineralized enamel and dentine. Aust Dent J. 2013;58(1):50–56. doi: 10.1111/adj.12033. [DOI] [PubMed] [Google Scholar]
- 8.Teixeira JA, Silva AVCE, Dos Santos Júnior VE, et al. Effects of a new nano-silver fluoride-containing dentifrice on demineralization of enamel and Streptococcus mutans adhesion and acidogenicity. Int J Dent. 2018;2018:1351925. doi: 10.1155/2018/1351925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez CC, Sokolonski AR, Fonseca MS, et al. Applications of silver nanoparticles in dentistry: advances and technological innovation. Int J Mol Sci. 2021;22(5):2485. doi: 10.3390/ijms22052485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vijayakumar M, Sabari Lavanya SJ, Ponnudurai Arangannal JJ, et al. Nano silver fluoride-overview. Eur J Mol Clin Med. 2020;7:6573–6580. [Google Scholar]
- 11.Espíndola-Castro LF, Rosenblatt A, Galembeck A, et al. Dentin staining caused by nano-silver fluoride: a comparative study. Operat Denti. 2020;45(4):435–441. doi: 10.2341/19-109-L. [DOI] [PubMed] [Google Scholar]
- 12.Tirupathi S, Svsg N, Rajasekhar S, et al. Comparative cariostatic efficacy of a novel nanosilver fluoride varnish with 38% silver diamine fluoride varnish a double-blind randomized clinical trial. J Clin Exp Dent. 2019;11(2):e105–e112. doi: 10.4317/jced.54995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin IX, Zhao IS, Mei ML, et al. Use of silver nanomaterials for caries prevention: a concise review. Int J Nanomed. 2020;15:3181–3191. doi: 10.2147/IJN.S253833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nanda KJ, Naik S. An in-vitro comparative evaluation of pre-treatment with nanosilver fluoride on inhibiting secondary caries at tooth restoration interface. Cureus. 2020;12(5):e7934. doi: 10.7759/cureus.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JS, Kuk E, Yu KN, et al. Antimicrobial effects of silver nanoparticles. Nanomed. 2007;3(1):95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Pushpalatha C, Bharkhavy KV, Shakir A, et al. The anticariogenic efficacy of nanosilver fluoride. Front Bioeng Biotechnol. 2022;10:931327. doi: 10.3389/fbioe.2022.931327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Showkat N, Singh G, Singla K, et al. Minimal invasive dentistry: literature review. J Curr Med Res Opin. 2020;3(9):631–636. doi: 10.15520/jcmro.v3i09.340. [DOI] [Google Scholar]
- 18.Walsh LJ, Brostek AM. Minimum intervention dentistry principles and objectives. Aust Dent J. 2013;58(Suppl 3):16. doi: 10.1111/adj.12045. [DOI] [PubMed] [Google Scholar]
- 19.Targino AG, Flores MA, dos Santos Junior VE, et al. An innovative approach to treating dental decay in children. A new anti-caries agent. J Mater Sci Mater Med. 2014;25(8):2041–2047. doi: 10.1007/s10856-014-5221-5. [DOI] [PubMed] [Google Scholar]
- 20.Haghgoo R, Saderi H, Eskandari M, et al. Evaluation of the antimicrobial effect of conventional and nanosilver-containing varnishes on oral streptococci. J Dent (Shiraz) 2014;15(2):57–62. [PMC free article] [PubMed] [Google Scholar]
- 21.Butrón Téllez Girón C, Hernández Sierra JF, DeAlba-Montero I, et al. Therapeutic use of silver nanoparticles in the prevention and arrest of dental caries. Bioinorg Chem Appl. 2020;2020:8882930. doi: 10.1155/2020/8882930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva A, Teixeira JA, Mota CCBO, et al. In vitro morphological, optical and microbiological evaluation of nanosilver fluoride in the remineralization of deciduous teeth enamel. Nanotechnol Rev. 2018;7(6):509–520. doi: 10.1515/ntrev-2018-0083. [DOI] [Google Scholar]
- 23.Burdușel AC, Gherasim O, Grumezescu AM, et al. Biomedical applications of silver nanoparticles: an up-to-date overview. Nanomaterials (Basel) 2018;8(9):681. doi: 10.3390/nano8090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soekanto SA, Marpaung LJ, Djais AA, et al. Efficacy of propolis fluoride and nano silver fluoride for inhibition of streptococcus mutans and enterococcus faecalis biofilm formation. Int J App Pharm. 2017;9(2):51–54. doi: 10.22159/ijap.2017.v9s2.13. [DOI] [Google Scholar]
- 25.Ghasempour M, Molana Z, Alaghemand H, et al. Anti streptococcus mutans non fluoride and fluoride containing sealants after adding nano-silver particles. J Dent Med. 2014;27(1):16–23. [Google Scholar]
- 26.Akyildiz M, Sönmez IS. Comparison of remineralizing potential of nano silver fluoride, silver diamine fluoride and sodium fluoride varnish on artificial caries: an in vitro study. Oral Health Prev Dent. 2019;17(5):469–477. doi: 10.3290/j.ohpd.a42739. [DOI] [PubMed] [Google Scholar]
- 27.Jowkar Z, Jowkar M, Shafiei F. Mechanical and dentin bond strength properties of the nanosilver enriched glass ionomer cement. J Clin Exp Dent. 2019;11(3):e275–e281. doi: 10.4317/jced.55522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ali A, Ismail H, Amin K. Effect of nanosilver mouthwash on prevention of white spot lesions in patients undergoing fixed orthodontic treatment-a randomized double-blind clinical trial. J Dent Sci. 2022;17(1):249–255. doi: 10.1016/j.jds.2021.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jasso-Ruiz I, Velazquez-Enriquez U, Scougall-Vilchis RJ, et al. Silver nanoparticles in orthodontics, a new alternative in bacterial inhibition: in vitro study. Prog Orthod. 2020;21(1):24. doi: 10.1186/s40510-020-00324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang XF, Liu ZG, Shen W, et al. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci. 2016;17(9):1534. doi: 10.3390/ijms17091534. [DOI] [PMC free article] [PubMed] [Google Scholar]


