Abstract
Aim
To evaluate and compare the clinical and radiographic success of NeoPUTTY® and Biodentine™ as indirect pulp treatment (IPT) materials in primary molars.
Materials and methods
This clinical trial was conducted on children aged 5–9 years. Class I carious lesions in primary molars indicated for IPT were divided into two groups—group I, NeoPUTTY®, and group II, Biodentine™. IPC was performed as per the standard protocols. The treated teeth were evaluated for clinical and radiographic success, along with the presence of a dentinal bridge at 6 and 12 months, by three blind examiners independently. All the data were tabulated, and statistical analysis was performed using the Mann–Whitney U test.
Results
Interexaminer reliability was analyzed using Fleiss κ statistics, and it showed “good” agreement. Clinical success was 100% in both groups at 6- and 12-month follow-up, while radiographic success was also 100% at 6-month follow-up for both groups. However, at 12-month follow-up, it was 93.33% for group I and 100% for group II. The difference was statistically nonsignificant. The presence of a dentinal bridge at 12-month follow-up was seen in 86.66% of cases in group I and 100% of cases in group II, but there was no statistical difference observed between them with a p-value of 0.555.
Conclusion
Within the limitations of the study, we conclude that NeoPUTTY® and Biodentine™ are equally effective as IPT agents in primary teeth.
How to cite this article
Kothari P, Mathur A, Tirupathi S, et al. Comparative Clinical and Radiographic Success Rate of Bioceramic Premix vs Biosilicate-based Medicament as Indirect Pulp Treatment Materials in Primary Molars: A Double-blind Randomized Trial with a Follow-up of 12 Months. Int J Clin Pediatr Dent 2024;17(7):748–753.
Keywords: Biodentine™, Children, NeoPUTTY®, Pulp therapy, Success
Introduction
Indirect pulp treatment (IPT) is a minimally invasive technique that is found to be an effective treatment option used for deep carious lesions in carefully selected cases and presents a success rate of 70–96.85% in primary teeth.1-9 It preserves the inner layer of dentin near the pulp, which contains intact collagen possessing the potential to remineralize. IPT limits the chances of progression of caries and lessens the possibility of pulpal exposure, which in turn prevents the further deterioration of the pulp–dentin complex and promotes pulpal healing. It allows the inception of an environment where the cariogenic pathway is interrupted, leading to the formation of a tertiary dentin bridge.10 Over the years, calcium hydroxide has served as a gold standard material in IPT cases since its introduction in 1939 by Zander. However, a few recent studies reported that the survival of the pulp on longer follow-up declined when calcium hydroxide was used for pulp capping. Reasons for this could be attributed to its nonadhesive nature, tunnel defects in the dentin, and its dissolution over time, which further lead to microleakage and failure due to the reentry of microorganisms into the pulp–dentin complex.11 With the advent of new biomimetic materials like Biodentine™ and mineral trioxide aggregate, the demerits of calcium hydroxide as pulp capping agents were suppressed, and more predictable outcomes were achieved.12 Garrocho-Rangel et al. and Boddeda et al. used Biodentine™ as an IPT agent in primary teeth and found it to be successful at 12-month follow-up.13,14 Because of its superior interactions with dentin and pulp, low solubility, no adverse effect on tooth color, tertiary dentin formation, and bioactivity, Biodentine™ is nowadays preferred over calcium hydroxide as an indirect pulp capping material.15,16 A new bioactive and bioceramic material, NeoPUTTY®, was launched in 2020 by Nusmile. It is a noncytotoxic premixed MTA with superior handling properties, promotes hydroxyapatite formation to trigger the healing process, and has the advantage of a shorter setting time.17 It provides dimensional stability, which ensures a gap-free seal.18 Gullen et al. stated that NeoPUTTY® has the property of calcium release and can be used in vital pulp therapies.19
There are many in vitro studies on NeoPUTTY® so far, but there is very little evidence of its use in clinical practice and its efficacy as an IPT agent. The present study is a pioneering in vivo study evaluating the effectiveness of NeoPUTTY® as an IPT agent. The study was undertaken with the aim of evaluating the clinical and radiographic success rate of NeoPUTTY® and comparing it with the previously proven IPT agent Biodentine™.
Materials and Methods
Study Design and Population
This in vivo clinical trial was undertaken in the outpatient department of Pediatric and Preventive Dentistry, Dr. D. Y. Patil Dental College and Hospital, Dr. D. Y. Patil Vidyapeeth (Deemed to be University), Pune, Maharashtra, India. Prior to the start of the study, ethical clearance was obtained from the Institutional Ethics Committee (DPDYCH/EC/648/36/2021). Children aged 5–9 years with no underlying medical conditions and with Frankl’s behavior rating of 3 and 4 were considered for the study. The inclusion criteria were children with at least one primary molar with an occlusal, cavitated, and active carious lesion extending deep into the dentin and showing clinical signs and symptoms of reversible pulpitis. On radiographic evaluation, primary molars with caries involving two-thirds of the dentin thickness approximating the pulp, without any periapical or furcal pathology, were included.20 Children showing disruptive behavior, presence of internal resorption, more than one-third of physiological root resorption, and pulpal exposure during the operative procedure were excluded from the study. Participants’ parents were informed about the purpose of the study, and those who agreed to participate were finally included in the study after providing written informed consent. Considering 80% power, a confidence interval of 1.96, [standard deviation (SD) 0.6; standard error (SE) 0.5], the calculated sample size was 30. Children (n = 30) were randomly divided into two different groups depending on the type of IPT agent—group I, NeoPUTTY® (Nusmile), and group II, Biodentine™ (Septodont). The study included three independent examiners apart from the operator. Because of the visual appearance of the two materials, blinding of the operator was not possible in the present study. However, the three examiners who independently evaluated the clinical and radiographic success were completely blinded to the group allocation. All clinical procedures were carried out by a single trained operator. Local anesthesia was administered before the rubber dam application using 2% lidocaine with epinephrine to minimize discomfort. The carious lesion was removed using a round bur mounted on a slow-speed handpiece. Only infected dentin, which was soft and easily scrapable, was removed using a sterile spoon excavator, while hard and leathery affected dentin was kept intact on the floor of the cavity.13,14,20,21 Infected and affected dentin was clinically distinguished depending on hardness, tactile sensation, and visual representation.13,22 Once the affected dentin was reached, caries removal was stopped. In case of pulp exposure while excavating the caries, a pulpotomy was performed, and the patient was excluded from the study. After caries excavation, the IPT material was mixed and dispensed according to the manufacturer’s instructions. In group I, NeoPUTTY® was used as the IPT material. NeoPUTTY® is available as a premixed syringe that can be directly placed in the cavity or dispensed onto a glass slab and carried to the cavity using a carrier. The thickness of NeoPUTTY® as an IPT material was kept at a minimum of 1.5 mm, and excess material was removed using a cotton dampened with saline (Fig. 1). In group II, Biodentine™ was placed on the pulpal floor. It is available as a capsule and liquid. One drop of the liquid and one-fifth part of the powder were mixed in the dispenser provided by the manufacturer for manual mixing. Once mixed, the material was carried to the cavity using an instrument and condensed on the pulpal floor to ensure good adaptation (Fig. 2). In the present study, we only included patients with occlusal caries with intact marginal ridges to avoid the need for placing stainless steel crowns, so the dentinal bridge could be visualized on radiographs during the follow-up period. Final restoration was done using type IX GIC in both groups. Baseline postoperative radiographs were taken using RVG (Figs 3 and 4).
Fig. 1:

Image showing placement of NeoPUTTY® in the cavity
Fig. 2:
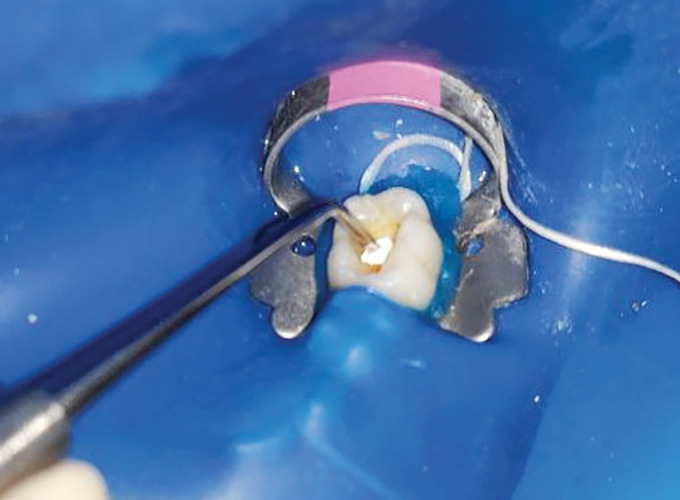
Image showing placement of Biodentine™ in the cavity
Fig. 3:

Preoperative baseline before the placement of NeoPUTTY® for IPT
Fig. 4:

Preoperative baseline before the placement of Biodentine™ for IPT
Success Criteria
Patients were recalled after 6 and 12 months and evaluated for clinical and radiographic success by three blind examiners who had been trained for this purpose. All the patients were evaluated for clinical success, radiographic success, and the presence of a dentinal bridge (Figs 5 and 6).2,13,14,21 The criteria used for clinical success were—the absence of pain, tenderness on percussion, discoloration of teeth, sinuses, fistulas, swelling, and pathological mobility. The criteria used for radiographic success were the absence of furcation or periapical radiolucency, periodontal ligament (PDL) space widening, and internal and external root resorption. Apart from the radiographic evaluation for the absence of any pathology, the presence of a dentinal bridge as evident on radiographs, was also recorded. Any tooth which presented symptoms of irreversible pulpitis at clinical evaluation or/and any pathology on radiographic evaluation was recorded as a treatment failure and either pulpectomies or extracted.
Fig. 5:

Around 12-month follow-up after the placement of NeoPUTTY® for IPT
Fig. 6:

Around 12-month follow-up after the placement of Biodentine™ for IPT
Statistical Analysis
All the data was tabulated, and statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) software. Interexaminer reliability was assessed using Fleiss–Kappa statistics. Intergroup comparison was done using the Mann–Whitney U test, and intragroup comparison was evaluated using the Wilcoxon rank test; a p-value < 0.05% was considered statistically significant.
Results
The total sample size was 30 (15 in each group), with the age range of the children included in the study being 5–9 years and a mean age of 6.5 years. Out of the 30 children, there were 10 boys and 20 girls. At the 12-month follow-up, two patients did not report, so the sample size was considered as 30 at 6-month follow-up and 28 at 12-month follow-up. A total of three examiners evaluated the clinical and radiographic success independently. Interexaminer reliability was analyzed using Fleiss κ statistics and was graded as “good” for both groups. In group I, the agreement score was 1.00 for clinical and radiographic success at the 6-month follow-up, while it was 0.812 for clinical and radiographic success at the 12-month follow-up. In group II, the agreement score was 1.00 for clinical success at both 6- and 12-month follow-up, while it was 0.812 for radiographic success at both 6- and 12-month follow-up. In group I, at the 6-month follow-up, clinical and radiographic success was found to be 100%. At the 12-month follow-up, all cases showed 100% clinical success with no signs or symptoms, while radiographic success was observed in 14 out of 15 teeth (93.33%), with one tooth showing the presence of radiolucency on the pulpal floor. In group II, clinical success and radiographic success on 6-month follow-up was 100%; that is, all 15 cases treated with Biodentine™ were successful. On 12 months of follow-up, clinical success and radiographic success were 100%, that is, 13 out of 13 (Figs 3 to 6 and Table 1). On intergroup comparison between groups I and II, for clinical success and radiographic success at 6 months, the mean rank was 15.50 for both the groups with a Mann–Whitney U score of 112.50 and p-value of 1.00. The difference between the clinical and radiographic success at 6 months of follow-up among the two groups was statistically insignificant. On 12 months of follow-up, the mean rank was 14.50 for both groups, with a Mann–Whitney score of 97.50 and a p-value of 1.00. The difference was not significant when comparing the clinical success. For radiographic success at 12-month follow-up, the mean rank was 14.93 and 14.00 for groups I and II, respectively, with a Mann–Whitney score of 91.00 and p-value of 0.786 (Table 2).
Table 1:
Clinical and radiographic success of group I (NeoPUTTY®) and group II (Biodentine™) at 6- and 12-month follow-up
| Success | 6 months | 12 months |
|---|---|---|
| The clinical success of group I | 15/15 100% |
15/15 100% |
| Radiographic success of group I | 15/15 100% |
14/15 93.33% |
| The clinical success of group II | 15/15 100% |
13/13 100% |
| The radiographic success of group II | 15/15 100% |
13/13 100% |
Table 2:
Intergroup comparison of clinical and radiographic success between groups I and II
| Group | N | Mean rank | Mann–Whitney U | p-value | |
|---|---|---|---|---|---|
| Clinical success at 6 months | 1 | 15 | 15.50 | 112.500 | 1.00 |
| 2 | 15 | 15.50 | |||
| Total | 30 | ||||
| Radiographic success at 6 months | 1 | 15 | 15.50 | 112.500 | 1.00 |
| 2 | 15 | 15.50 | |||
| Total | 30 | ||||
| Clinical success at 12 months | 1 | 15 | 14.50 | 97.500 | 1.00 |
| 2 | 13 | 14.50 | |||
| Total | 28 | ||||
| Radiographic success at 12 months | 1 | 15 | 14.93 | 91.00 | 0.786 |
| 2 | 13 | 14.00 | |||
| Total | 28 |
Dentine Bridge
When the presence of a dentinal bridge was evaluated in the NeoPUTTY® group, it was found that at the 6-month follow-up, 80% of the cases (12 out of 15) showed a dentinal bridge, and at the 12-month follow-up, 86.66% of cases (13 out of 15) showed a dentinal bridge (Table 3). When the presence of dentinal bridge was evaluated in group II, it was found that on 6-month follow-up, 93.33% of the cases, that is, 14 out of 15 showed the dentinal bridge, and on 12-month follow-up, it was seen 100% cases, that is, in all reported cases. Interexaminer reliability was good with an agreement of 0.821 on 6-month follow-up and 1.00 on 12-month follow-up when dentinal bridge was compared in group I, while in group II, the agreement score was 0.801 on 6-month follow-up and 0.812 on 12-month follow-up when the dentinal bridge was compared when the presence of dentinal bridge was evaluated and compared between groups I and II on 6-month follow-up, the difference was statistically insignificant with mean rank of 16.50 and 14.50 for groups I and II respectively, Mann–Whitney score of 97.500 and p-value of 0.539. On 12-month follow-up, the mean rank for groups I and II was 15.37 and 3.50, the Mann–Whitney score was 84.500, the p-value was 0.555, and the difference was insignificant (Table 4).
Table 3:
Presence of dentinal bridge in groups I and II on 3- and 6-month follow-up
| Parameter | 6 months | 12 months |
|---|---|---|
| Presence of a dentinal bridge in group I | 12/15 80% |
13/15 86.66% |
| Presence of dentinal bridge in group II | 14/15 93.33% |
13/13 100% |
Table 4:
Intergroup comparison of dentinal bridge between groups I and II on 6- and 12-month follow-up
| Group | N | Mean rank | Mann–Whitney U | p-value | |
|---|---|---|---|---|---|
| Presence of dentinal bridge at 6 months | 1 | 15 | 16.50 | 97.500 | 0.539 |
| 2 | 15 | 14.50 | |||
| Total | 30 | ||||
| Presence of dentinal bridge at 12 months | 1 | 15 | 15.37 | 84.500 | 0.555 |
| 2 | 13 | 13.50 | |||
| Total | 28 |
Discussion
The success of IPT hinges on the formation of reactionary dentin, which is a biological process involving the proliferation, migration, and activation of progenitor cells at the site of injury.23,26 IPT causes a mild injury, resulting in the upregulation of odontoblasts and other pulp cells to secrete a reactionary type of tertiary dentin matrix.8 This sequence of biological events provides a greater window of opportunity for success when a bioactive material is used for pulp capping, as it provides a source of calcium, which is responsible for the formation of hydroxyapatite crystals. In the present study, we found good radiographic and clinical success in the Biodentine™ group at the 12-month follow-up. This is consistent with a study conducted by Garrocho-Rangel et al., where they found a success rate of 98.3% at 12-month follow-up when Biodentine™ was used as an IPC agent in primary teeth.13 The success of Biodentine™ as an indirect pulp capping agent in primary teeth was also reported by Boddeda et al. and Sahin et al., with a mean success rate of 100% at 12-month follow-up and 100% at 24-month follow-up, respectively.14,27 Similarly, when8 assessed as an IPT agent by Chauhan et al., they found clinical success of 100% on 6-month follow-up.21 Biodentine™ is not only a biocompatible and bioactive material but also provides a greater zone of inhibition against Streptococcus mutans and Enterococcus faecalis.28 In a study done by Arora et al., they found that the ability of Biodentine™ to seal is very similar to the hydroxyapatite crystals.29 Furthermore, the presence of silicon ions in Biodentine™ enhances cell proliferation and differentiation, making it a more promising pulp therapy agent.30
The reason for the success of Biodentine™ could be attributed to its sealing ability, resulting in minimal chances of microleakage, its potential to interact with odontoblasts to form a calcific barrier, and its antimicrobial nature.31 In vital pulp therapies, a common cause of failure is microleakage, which leads to the reinfection of the pulp. When Biodentine™ is used, its ability to form hydroxyapatite ions at the margins leads to better marginal integrity, which in turn minimizes the chances of reentry of pathogens, providing better outcomes.32 Good clinical and radiographic success was also observed when NeoPUTTY® was used as an IPT material. It is composed of a water-free organic liquid, so it sets only in vivo or in the presence of moisture. When used as an IPT material, it absorbs moisture from dentinal tubules and sets within 4 hours. Although its setting time is longer, it has significant washout resistance, allowing the dentist to rinse slightly and finish the restoration immediately after placing the material. Although direct comparisons with other in vivo studies could not be made, as this study is a pioneer in evaluating NeoPUTTY® as an IPT material, indirect conclusions can be drawn from the in vitro studies conducted on NeoPUTTY® since its introduction into the market. The success of NeoPUTTY® as an IPT agent could be attributed to its calcium release, which, as proven in the study by Lozano-Guillén, aids in the formation of hydroxyapatite crystals.19 Its mineralization potential and ability to induce osteogenic differentiation from human dental pulp stem cells (HDPSCs) further explain its role in the formation of a dentinal bridge.17,33 In both the study groups, we found the presence of dentinal bridges in a maximum number of cases. The present study confirmed the presence of dentinal bridge in maximum cases when treated with Biodentine™ on 6-month follow-up. Previous studies also suggest that dentin is formed faster during the first few months, followed by a declination of rate with time.34 This result is in accordance with the study done by Chauhan et al. and Kim et al., where they stated that Biodentine™ can aid in faster deposition of tertiary dentin in a shorter period of time.21,35 Usually, it takes a minimum of 3–8 weeks to form a thick dentinal bridge after using Biodentine™ in vital pulp therapies.36 NeoPUTTY® forms hydroxyapatite crystals in two stages. The first stage is hydration—when the material is placed into the cavity, it becomes hydrated upon contact with water from surrounding tissues. This process results in the formation of calcium and hydroxide ions, which react with phosphate to form hydroxyapatite crystals. These crystals help in the formation of tertiary dentin. This mechanism explains the presence of a dentinal bridge in 13 cases in the NeoPUTTY® group.
According to the results of this study, NeoPUTTY® and Biodentine™ were equally effective as IPT agents in primary molars. However, NeoPUTTY® offers additional advantages—it is a premixed cement, which makes it less time-consuming and eliminates the chances of errors in manual mixing. Its nontacky consistency and immediate washout resistance aid in the easy placement of the material, even in uncooperative children, making it more helpful in pediatric dentistry. Additionally, NeoPUTTY® has a longer working time compared to Biodentine™ and sets only after placement into the cavity. In the present study, we did not use a caries detector dye and relied on the clinical judgment of the trained clinician to evaluate the complete removal of infected dentin. In previous research, it was found that these dyes do not stain the bacteria; instead, they stain the less mineralized organic structure, which is present in infected and affected dentin as well.37 Hence, to avoid excessive removal of dentin, dyes were not used. One of the major strengths of this study is that the subjects were randomized into the study groups. The evaluation of clinical success, radiographic success, and dentinal bridge formation was performed independently by three blinded examiners. The success of each individual case was determined based on the highest number of similar responses recorded by these examiners.
Conclusion
Within the limitations of the study, both NeoPUTTY® and Biodentine™ are effective IPT materials, showing good clinical and radiographic success. No statistically significant difference was observed between NeoPUTTY® and Biodentine™ at the 6- and 12-month follow-ups. However, more clinical trials with larger sample sizes and longer follow-ups should be conducted to further establish NeoPUTTY® as an IPT agent.
Orcid
Aditi Mathur https://orcid.org/0000-0002-6098-0253
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Coll JA, Seale NS, Vargas K, et al. Primary tooth vital pulp therapy: a systematic review and meta-analysis. Pediatr Dent. 2017;39(1):16–123. [PubMed] [Google Scholar]
- 2.Casagrande L, Bento LW, Dalpian DM, et al. Indirect pulp treatment in primary teeth: 4-year results. Am J Dent. 2010;23(1):34–38. [PubMed] [Google Scholar]
- 3.Al-Zayer MA, Straffon LH, Feigal RJ, et al. Indirect pulp treatment of primary posterior teeth: a retrospective study. Pediatr Dent. 2003;25(1):29–36. [PubMed] [Google Scholar]
- 4.Mathur VP, Dhillon JK, Logani A, et al. Evaluation of indirect pulp capping using three different materials: a randomized control trial using cone-beam computed tomography. Indian J Dent Res. 2016;27(6):623–629. doi: 10.4103/0970-9290.199588. [DOI] [PubMed] [Google Scholar]
- 5.Farooq NS, Coll JA, Kuwabara A, et al. Success rates of formocresol pulpotomy and indirect pulp therapy in the treatment of deep dentinal caries in primary teeth. Pediatr Dent. 2000;22(4):278–286. [PubMed] [Google Scholar]
- 6.Falster CA, Araujo FB, Straffon LH, et al. Indirect pulp treatment: in vivo outcomes of an adhesive resin system vs calcium hydroxide for protection of the dentin-pulp complex. Pediatr Dent. 2002;24(3):241–248. [PubMed] [Google Scholar]
- 7.Marchi JJ, Froner AM, Alves HL, et al. Analysis of primary tooth dentin after indirect pulp capping. J Dent Child. 2008;75(3):295–300. [PubMed] [Google Scholar]
- 8.Franzon R, Casagrande L, Pinto AS, et al. Clinical and radiographic evaluation of indirect pulp treatment in primary molars: 36 months follow-up. Am J Dent. 2007;20(3):189–192. [PubMed] [Google Scholar]
- 9.Pinto AS, de Araújo FB, Franzon R, et al. Clinical and microbiological effect of calcium hydroxide protection in indirect pulp capping in primary teeth. Am J Dent. 2006;19(6):382–386. [PubMed] [Google Scholar]
- 10.Fuks AB. Current concepts in vital primary pulp therapy. Eur J Paediatr Dent. 2002;3(3):115–120. [PubMed] [Google Scholar]
- 11.Sangwan P, Sangwan A, Duhan J, et al. Tertiary dentinogenesis with calcium hydroxide: a review of proposed mechanisms. Int Endod J. 2013;46(1):3–19. doi: 10.1111/j.1365-2591.2012.02101.x. [DOI] [PubMed] [Google Scholar]
- 12.Song M, Yu B, Kim S, et al. Clinical and molecular perspectives of reparative dentin formation: lessons learned from pulp-capping materials and the emerging roles of calcium. Dent Clin North Am. 2017;61(1):93–110. doi: 10.1016/j.cden.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrocho-Rangel A, Quintana-Guevara K, Vázquez-Viera R, et al. Bioactive tricalcium silicate-based dentin substitute as an indirect pulp capping material for primary teeth: a 12-month follow-up. Pediatr Dent. 2017;39(5):377–382. [PubMed] [Google Scholar]
- 14.Boddeda KR, Rani CR, V Vanga NR, et al. Comparative evaluation of Biodentine™, 2% chlorhexidine with RMGIC and calcium hydroxide as indirect pulp capping materials in primary molars: an in vivo study. J Indian Soc Pedod Prev Dent. 2019;37(1):60–66. doi: 10.4103/JISPPD.JISPPD_213_17. [DOI] [PubMed] [Google Scholar]
- 15.Kunert M, Lukomska-Szymanska M. Bio-inductive materials in direct and indirect pulp capping—a review article. Materials. 2020;13(5):1204. doi: 10.3390/ma13051204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajasekharan S, Martens LC, Cauwels RGEC, et al. Biodentine™ material characteristics and clinical applications: a 3 year literature review and update. Eur Arch Paediatr Dent. 2018;19(1):1–22. doi: 10.1007/s40368-018-0328-x. [DOI] [PubMed] [Google Scholar]
- 17.Hondares TC. Birmingham: The University of Alabama at Birmingham; 2022. An evaluation of the in vitro antibacterial, biocompatibility, and mineralization properties of six calcium silicate-based pulp capping materials [dissertation]. [Google Scholar]
- 18.Yavuz Y, Kotanli S, Doğan MS, et al. Comparisons of microleakage and scanning electron microscope SEM analyses of the use of different pulp coverage materials. Makara J Health Res. 2022;26(2):141–145. doi: 10.7454/msk.v26i2.1396. [DOI] [Google Scholar]
- 19.Lozano-Guillén A, López-García S, Rodríguez-Lozano FJ, et al. Comparative cytocompatibility of the new calcium silicate-based cement NeoPUTTY® versus NeoMTA Plus and MTA on human dental pulp cells: an in vitro study. Clin Oral Investig. 2022;26(12):7219–7228. doi: 10.1007/s00784-022-04682-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.George V, Janardhanan SK, Varma B, et al. Clinical and radiographic evaluation of indirect pulp treatment with MTA and calcium hydroxide in primary teeth (in-vivo study). J Indian Soc Pedo Prev Dent. 2015;33(2):104–110. doi: 10.4103/0970-4388.155118. [DOI] [PubMed] [Google Scholar]
- 21.Chauhan A, Dua P, Saini S, et al. In vivo outcomes of indirect pulp treatment in primary posterior teeth: 6 months’ follow-up. Contemp Clinic Dent. 2018;9(Suppl 1):S69–S73. doi: 10.4103/ccd.ccd_48_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pulp therapy for primary and immature permanent teeth. Pediatr Dent. 2018;40(6):343–351. [PubMed] [Google Scholar]
- 23.Gronthos S, Mankani M, Brahim J, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97(25):13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gronthos S, Brahim J, Li W, et al. Stem cell properties of human dental pulp stem pulp stem cell. J Dent Res. 2002;81(8):531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 25.Miura M, Gronthos S, Mingrui Z, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci. 2003;100(10):5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray PE, García-Godoy F. Stem cell responses in tooth regeneration. Stem Cells Dev. 2004;13:255–262. doi: 10.1089/154732804323099181. [DOI] [PubMed] [Google Scholar]
- 27.Sahin N, Saygili S, Akcay M. Clinical, radiographic, and histological evaluation of three different pulp-capping materials in indirect pulp treatment of primary teeth: a randomized clinical trial. Clin Oral Investig. 2021;25(6):3945–3955. doi: 10.1007/s00784-020-03724-4. [DOI] [PubMed] [Google Scholar]
- 28.Jain AS, Gupta AS, Agarwal R. Comparative evaluation of the antibacterial activity of two biocompatible materials is Biodentine and MTA when used as a direct pulp capping agent against streptococcus mutans and Enterococcus faecalis-an in vitro study.™. Endodontology. 2018;30(1):66–68. doi: 10.4103/endo.endo_66_17. [DOI] [Google Scholar]
- 29.Arora V, Nikhil V, Sharma N, et al. Bioactive dentin replacement. J Dent Med Sci. 2013;12:51–57. doi: 10.9790/0853-1245157. [DOI] [Google Scholar]
- 30.Peng W, Liu W, Zhai W, et al. Effect of tricalcium silicate on the proliferation and odontogenic differentiation of human dental pulp cells. J Endod. 2011;37(9):1240–1246. doi: 10.1016/j.joen.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 31.Kaul S, Kumar A, Jasrotia A, et al. Comparative analysis of Biodentine™, calcium hydroxide, and 2% chlorhexidine with resin-modified glass ionomer cement as indirect pulp capping materials in young permanent molars. J Contemp Dent Pract. 2021;22(5):511–516. [PubMed] [Google Scholar]
- 32.Soliman AF, Abu-Hamila NA, El-Ebiary MA. Assessment of Biodentine as an indirect pulp capping material in young permanent molars™. Tanta Dent J. 2019;16:1–5. doi: 10.4103/tdj.tdj_16_18. [DOI] [Google Scholar]
- 33.Sun Q, Gustin JW, Tian FC, et al. Effects of pre-mixed hydraulic calcium silicate putties on osteogenic differentiation of human dental pulp stem cells in vitro. J Dent. 2021;108:103653. doi: 10.1016/j.jdent.2021.103653. [DOI] [PubMed] [Google Scholar]
- 34.Cohen S, Burns RC. Pathways of Pulp, 5th edition. St Louis: Mosby; 1994. pp. 179–218. [Google Scholar]
- 35.Kim J, Song YS, Min KS, et al. Evaluation of reparative dentin formation of ProRoot MTA, Biodentine and BioAggregate using micro-CT and immunohistochemistry. Restor Dent Endod. 2016;41(1):29–36. doi: 10.5395/rde.2016.41.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tziafa C, Koliniotou-Koumpia E, Papadimitriou S, et al. Dentinogenic responses after direct pulp capping of miniature swine teeth with biodentine. J Endod. 2014;40(12):1967–1971. doi: 10.1016/j.joen.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 37.McComb D. Caries-detector dyes–how accurate and useful are they? J Can Dent Assoc. 2000;66(4):195–198. [PubMed] [Google Scholar]


