Abstract
Purpose of review
To provide the most recent literature on our understanding behind the pathogenesis and the treatment of calcinosis in dermatomyositis.
Recent findings
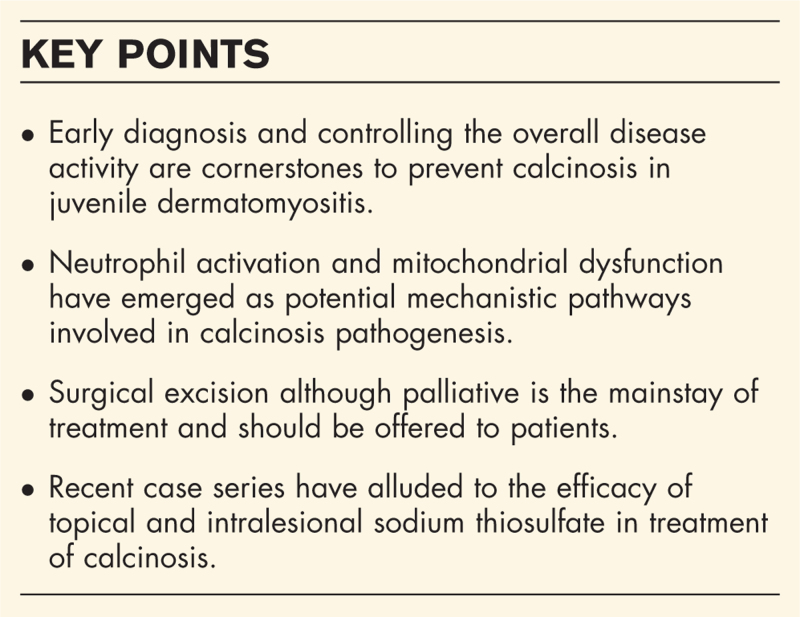
Early diagnosis and controlling the overall disease activity are cornerstones to prevent calcinosis in juvenile dermatomyositis. Observational cohort studies showed that prolonged state of inflammation and features of vascular dysfunction like digital ulcers and abnormal nailfold capillaries are associated with calcinosis. Neutrophil activation and mitochondrial dysfunction have recently emerged as potential mechanistic pathways involved in calcinosis pathogenesis. Few recent case series have alluded to the efficacy of topical and intralesional sodium thiosulfate, while JAK inhibitors appear to be newer promising therapy in juvenile dermatomyositis.
Summary
Calcinosis in dermatomyositis consists of deposition of insoluble calcium compounds in the skin and other tissues. It is prevalent in up to 75% of patients with juvenile dermatomyositis and up to 20% in adult dermatomyositis. While it leads to significant patient morbidity, we do not yet understand the pathogenesis in its entirety. Surgical excision although palliative is the mainstay of treatment and should be offered to patients. All available treatment options are only based on very low level of evidence.
Keywords: calcinosis, dermatomyositis, JAK inhibitors, mitochondria, neutrophil, sodium thiosulfate, therapeutics
INTRODUCTION
Dermatomyositis is an autoimmune connective tissue disease with a wide phenotypic heterogeneity. While its hallmark features include distinctive skin manifestations and myopathy, it also exhibits other manifestations like inflammatory arthritis, calcinosis, interstitial lung disease, malignancy and occasional gastrointestinal involvement, often correlated with specific autoantibodies [1–6]. In dermatomyositis, calcinosis is dystrophic, with insoluble calcium compounds (hydroxyapatite or carbonate apatite) deposited in the skin and other tissues, in the setting of normal serum calcium and phosphorus levels [7]. Its prevalence in juvenile dermatomyositis (JDM) ranges from 20 to 75%, depending on the racial demographics and composition of the study population and up to 20% in adult dermatomyositis [1,4,8–14].
Clinically, calcinosis in dermatomyositis can present as superficial or deep dermal nodules (sponge-like appearance on plain radiographs), tumoral form (mass-like appearance on plain radiographs) and extensive sheet-like where it is termed as calcinosis universalis [15]. A more recent study employing whole-body computed tomography (CT) identified five distinct patterns of calcinosis in dermatomyositis: clustered, disjoint, interfascial, confluent and fluid-filled [16]. Extremities, trunk and especially pressure points are commonly affected areas. Calcinotic lesions often cause significant amount of pain, reduced range of motion when near joints, ulceration and secondary infections, all contributing to the morbidity in dermatomyositis. A recent analysis of patient-reported surveys showed a significant physical and psychosocial burden from dermatomyositis in almost 50% of the respondents also leading to unemployment [17]. Moreover, a cost-analysis study determined that dermatomyositis patients incurred a five-fold higher total annual costs compared to matched controls [18].
Box 1.
no caption available
CLINICAL ASSOCIATIONS
Although calcinosis is much more frequent in JDM compared to adult dermatomyositis patients [10], it is frequently associated with prolonged state of persistent disease and/or severe disease activity in both [12,19]. Delay in diagnosis further adds to this risk especially in JDM patients [12,20]. While the presence of digital ulcers is associated with the presence of calcinosis in adult dermatomyositis [21], nailfold capillary abnormalities at baseline were predictive of development of calcinosis in JDM patients [22].
The role of race and ethnicity in development of calcinosis has been studied in several recent investigations, with inconsistent results ranging from lower to higher prevalence of calcinosis in individuals from racial and ethnic minorities as compared to White patients [23–25]. However, none of the studies have adjusted for disease severity which could be a potential confounding factor.
The presence of MDA-5 (melanoma differentiation associated protein 5) antibody and/or NXP-2 (nuclear matrix protein-2) antibody is highly associated with calcinosis in dermatomyositis amongst most racial groups [1,2,4,6,26,27]. Moreover, the presence of calcinosis is associated with poor disease outcomes in JDM patients with NXP-2 antibodies [28▪▪].
PATHOGENESIS OF CALCINOSIS
Although the exact mechanism(s) of calcinosis pathogenesis are not fully understood, the following pathways, particularly neutrophil activation [29] and mitochondrial dysfunction [30▪▪], seem to have recently emerged as novel potential processes participating in the pathogenesis of calcinosis.
Calcinosis and neutrophil activation
Despite the emergence of new advanced technologies, including spatial transcriptomics and single cell RNA sequencing, the presence and role of infiltrating neutrophils in the skin and muscle of JDM has been challenging to assess, not only due to the short life span of neutrophils but also the lack of reliable animal models of JDM calcinosis. We recently made the novel observation of neutrophils infiltrating calcified muscle tissue, engulfing mineral particles [29]. These are, to our knowledge, the first visual representations of neutrophils engaging with the local calcification, participating in its removal. However, uptake of calcium crystals promoted neutrophil-mediated inflammation and extrusion of neutrophil extracellular traps (NETs) [29].
Although we have been able to determine some of the main mechanisms contributing to neutrophil-mediated inflammation and damage in tissue, mechanisms promoting neutrophil infiltration into calcified tissue are not known. Levels of potent chemoattractant molecules, including IL-8 and complement components, have been associated with neutrophil activation in JDM [29]. Further, calcified tissue also contains mitochondrial-derived N-formyl methionine peptides [30▪▪], a potent chemoattractant signaling through formyl peptide receptor 1 (FPR1). Although we have established N-formyl methionine as an important chemoattractant molecule in several autoimmune conditions [31,32], its role in JDM pathogenesis, and in particular in calcinosis development, is still to be determined.
Neutrophil activation is also seen in peripheral blood with levels of NETs elevated in patients with calcinosis [29,33,34]. The elevated levels of NETs in peripheral blood could be due to increased frequency of low-density granulocytes spontaneously releasing NETs [34], presence of circulating immune complexes [29,33], or reduced degradation of the formed NETs [29,33], likely due to presence of NET-protective antibodies similar to what has been described in other autoimmune conditions [35]. Of note, though neutrophils are activated, both in peripheral blood and tissue, a causal link to calcinosis is not clear. The data indicate a response to calcinosis, and/or circulating danger-associated molecular patterns. However, it is also possible that neutrophil-mediated inflammation could initiate and/or amplify the pathogenesis of calcinosis. Future studies are warranted to explore the role of neutrophils in vivo in the development of calcinosis and/or local tissue inflammation. Should their role in the pathogenesis be validated, several therapeutic approaches could be considered in limiting their recruitment and activation, chief of which would be reducing calcium crystal deposits in tissue as discussed below and in more detail in prior work [36▪]. There are currently no treatment specifically targeting neutrophils. Several companies are developing inhibitors of NET formation, primarily focusing on PAD. However, given NET formation in JDM seem to be PAD-independent [29], other avenues will have to be considered. Potential targets would be NADPH oxidase inhibitors, enzymes involved in NET formation (such as neutrophil elastase), and/or targeting NET degradation itself by DNases [37]. Finally, main chemotactic signals, for example, IL-8, N-formyl methionine, and C5a, are all present in JDM, and should be explored for their role in recruiting inflammatory myeloid cells into tissue [29,38,39].
Calcinosis and mitochondria
Mitochondria play an essential role in calcium homeostasis within the cell, linking scavenging of calcium through the mitochondrial calcium uniporter (MCU) with uptake of phosphate for the assembly of amorphous hydroxyapatite [40]. In assessing muscle biopsies from JDM patients with calcinosis, we made the novel finding of profound intramitochondrial calcification in skeletal muscle cells [30▪▪]. In recapitulating these findings in vitro, mitochondrial calcification was amplified by type I interferons, commonly seen in patients with JDM [30▪▪]. Mechanistically, type I interferons caused mitochondrial stress, oxidizing MCU resulting in unregulated uptake of calcium and phosphate to the mitochondria, subsequently precipitating as hydroxyapatite [30▪▪]. The calcified mitochondria failed to perform oxidative phosphorylation, and released its nucleic acids into the cytosolic compartment, triggering interferon production through the cGAS/STING pathway establishing a vicious cycle of inflammation-mediated mitochondrial calcification and cell death.
Mitochondria were not only retained within the skeletal muscle cell, but also released into tissue, likely as a result of cell death. These observations translated to elevated levels of extracellular mitochondria, including mitochondrial DNA, in peripheral blood of patients with JDM, primarily associated with calcinosis. Of note, mitochondria, due to their prokaryotic origin, are immunogenic. Other than inflammation, such as mediated by oxidized mitochondrial DNA [41▪], extracellular mitochondria can also trigger development of antimitochondrial antibodies. Antimitochondrial antibodies are frequently seen in rheumatic diseases, including anticardiolipin antibodies, targeting mitochondrial-derived phospholipids [42]. Using an in-house flow cytometry assay, measuring antibodies targeting outer mitochondrial membrane antigens, 40% of patients with JDM had antimitochondrial antibodies. Further, presence of antimitochondrial antibodies was enriched in patients with calcinosis (close to 70%). In patients without diagnosis of calcinosis, presence of antimitochondrial antibodies predicted development of future calcinosis [30▪▪]. If validated, these findings indicate that mitochondrial dysfunction, extrusion and formation of antimitochondrial antibodies are early events in the calcinosis pathogenesis. This could provide for an important therapeutic window prior to clinical development of calcinosis where interventions could have substantial effect in limiting, or even preventing, calcinosis. In vitro, targeting mitochondrial ROS completely blunted mitochondrial calcification and subsequent inflammation. Prior work in animal models of other rheumatic diseases, including lupus [43] suggest scavenging mitochondrial ROS may be a potential target to consider in JDM, in particular in preventing excessive mitochondrial damage and calcification. Other therapeutic options will be discussed in more detail below, including JAKi that potently may interfere with inflammation/interferon-mediated amplification of the calcinosis pathway.
Vascular dysfunction
A single-center retrospective study on JDM patients (N = 172) found that the presence of nailfold capillary abnormalities at baseline was highly predictive of future calcinosis, when adjusted for age, biological sex, and disease duration (hazard ratio = 4.98) [28▪▪]. Apolipoprotein A-1 (apoA-1), a component of HDL molecule, is a major inhibitor of vessel wall inflammation. A cross-sectional study of 27 dermatomyositis patients, showed significantly lower circulating apoA-1 levels in the calcinosis group, when adjusted for age, disease duration, and severity [odds ratio (OR) = 11.49] [44]. These studies indicate a potential role for vascular dysfunction in calcinosis development in dermatomyositis.
Genetic associations
Whole genome sequencing of systemic sclerosis (SSc) and dermatomyositis patients (N = 50) with a severe calcinosis phenotype showed the presence of rare singleton variants in ABCC6 and ENPP1 genes. These genes regulate generation of inorganic pyrophosphate, an inhibitor of mineralization [36▪]. The role of the identified gene variants in regulation of inorganic pyrophosphate is currently unknown.
IMAGING MODALITIES
Plain radiographs have been an age-old modality to detect calcifications, but their two-dimensional perspective often falls short of accurate assessment. Low-dose whole-body CT seems promising in capturing the physical burden of calcinosis [16]. While its cost-effectiveness must be studied further, ultrasound has emerged as an easy point-of-care diagnostic modality in rheumatology clinics [45] and its ability to detect calcinosis is currently being studied in SSc.
Employing durometry to identify calcinosis by measuring its firmness also fared well in a recent study, especially in sites such as upper neck/clavicle as well as upper and lower extremities [46].
THERAPEUTICS
Calcinosis in dermatomyositis continues to be an unmet need, not only because of its unclear pathogenesis, but also due to the lack of large clinical trials and validated outcome measures. Most of the treatments that are currently employed are based on low research evidence. While a comprehensive list of pharmacological therapies has been outlined in a recent review article [36▪], we have only included the latest published data on pharmacological and nonpharmacological approaches herein.
Nonpharmacological approach
Surgical excision
Despite being a palliative option, surgical excision seems to be the most effective way to treat bulky calcinotic lesions or calcinosis presenting around joints restricting range of motion [47].
Minimally invasive procedures
Few case series reported improvement of dystrophic calcinosis when treated with intralesional sodium thiosulfate (STS) given as monthly injections of 1–1.2 ml of 250 mg/ml STS solution per lesion [48–50]. However, a small double-blind controlled study (N = 4) where each participant contributed one lesion each to the treatment and control group, only one lesion responded in the treatment group, and overall, there was no difference between the treatment and control groups at the end of 3 months [49].
Shiari et al.[51] conducted an open label clinical trial of intralesional infliximab with local anesthesia to five patients with JDM and calcinotic lesions less than 5 cm2, given every week over a period of 16 weeks. Dosing at 20 mg infliximab for lesions 1.5 cm2 or less and 40 mg for lesions 1.5–5 cm2 showed significant reduction in the size of the lesions [51].
Microneedling with inkless tattoo showed nearly 80% reduction of calcinotic nodules in one dermatomyositis patient, probably by stimulating the intrinsic wound healing cascade [52▪].
Further studies are needed to validate these initial, and yet unproven, findings.
Pharmacological approach
Sodium thiosulfate
STS is a calcium chelating agent and may increase the solubility of calcium salts. A study on topical STS (25% STS compounded) in patients with ectopic calcifications (N = 28), including 14 dermatomyositis patients (six adults, eight children), showed higher number of responders (radiographically determined) in children compared to adults (54.5 vs. 17.6%). While most patients did not experience any pain or adverse effects related to the local application, one dermatomyositis patient reported transient increase in local inflammation and pain, which later resolved spontaneously, allowing the patient to resume STS treatment [53▪▪]. A case report of a 44-year-old patient with calcinosis in dermatomyositis, showed an analgesic response to topical 20% STS compounded 1 : 1 with vaseline, when applied daily for 1 month [54].
A single-center retrospective study of seven patients with dystrophic calcinosis who received either intravenous (i.v.) or oral STS, administered over a mean of 4.5 months for i.v. and 29.1 months for oral formulations, respectively, showed a partial response in four patients. Pain, ulceration and inflammation associated with calcinosis improved with this treatment.
Bisphosphonates
Bisphosphonates can reduce bone turnover and impede macrophage activity leading to reduced inflammation. Bisphosphonates are frequently used in JDM-associated calcinosis. A retrospective study on 42 JDM patients with calcinosis showed that drugs affecting calcium/phosphorus metabolism, including bone metabolism, were significantly associated with improvement of calcinosis [26]. A previous case series reported improvement of calcinosis in four of six JDM patients treated with i.v. pamidronate or oral alendronate 70 mg per week over a period of 3 months [55]. Also, a more recent case report on JDM-associated calcinosis showed significant reduction in the calcinotic burden radiographically when treated with IV pamidronate for 2 years [56].
JAKi (Janus kinase inhibitors)
The JAK-STAT signal transduction system mediates inflammation via several inflammatory mediators, including type I interferons. A retrospective study on 88 JDM patients treated with at least 3 months of tofacitinib showed reduction in calcinotic burden in 12 of 17 patients with calcinosis [57]. Another retrospective study of 75 JDM patients showed that tofacitinib relieved calcinosis in three of six patients who had refractory JDM and calcinosis [58].
While a case series of three patients with adult dermatomyositis (2 had anti-NXP2 positivity) showed radiographic improvement within 3 months [59], an independent case report showed significant regression of calcinosis along with lowering the interferon score in an anti-NXP2 positive dermatomyositis patient when treated for 9 months with tofacitinib [60].
Another JAKi, baricitinib, also halted the progression of calcinosis in a recalcitrant anti-NXP-2 positive JDM patient with severe calcinosis [61].
Tumor necrosis factor inhibitors
High levels of TNF-α (tumor necrosis factor-α), released by activated macrophages and T cells, are seen in patients with active JDM and calcinosis. A retrospective study in JDM patients, receiving either infliximab or adalimumab for at least 3 months, showed improvement in 54% of those with calcinosis (N = 28) over a median treatment duration of 2.7 years [62].
Intravenous immunoglobulin
Intravenous immunoglobulin (IVIG) has been shown to reduce disease activity in dermatomyositis by reducing the deposition of membrane attack complex in the affected tissues as well as decreasing T-cell activation. Among the various immunomodulatory and calcium-modifying therapies, IVIG seemed to show the greatest benefit (hazard ratio 1.95) for calcinosis based on a recent retrospective study of the CARRA registry (Childhood Arthritis and Rheumatology Research Alliance) (N = 63) [63]. A few case reports have shown that IVIG improves calcinosis at doses of 2 g/kg/month after five to six courses in both juvenile as well as adult dermatomyositis [64,65].
CONCLUSION
Calcinosis contributes to significant morbidity in dermatomyositis patients yet remains a greatly unmet need in terms of available treatments. Neutrophil activation, mitochondrial dysfunction, and vascular dysregulation seem to play a role in the development of calcinosis. Prompt diagnosis and controlling the overall disease activity can help prevent calcinosis in JDM. While bisphosphonates have been an age-old treatment for calcinosis in JDM, JAKi are emerging as a promising avenue for anti-NXP-2 positive dermatomyositis patients with high interferon scores. Microneedling and intralesional STS treatment of calcinotic nodules are novel approaches, but need larger studies to validate their effectiveness. Surgical resection still provides the most effective and immediate relief and should be considered for accessible lesions.
Acknowledgements
The authors are grateful for generous funding provided by CureJM in support of this study (C.L).
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Horn S, Minden K, Speth F, et al. Myositis-specific autoantibodies and their associated phenotypes in juvenile dermatomyositis: data from a German cohort. Clin Exp Rheumatol 2022; 40:433–442. [DOI] [PubMed] [Google Scholar]
- 2.Manwatkar A, Padiyar S, Nair A, et al. Clinical profile of anti-NXP-2 antibody-positive inflammatory myositis and outcome in an Indian population. Clin Rheumatol 2023; 42:3289–3297. [DOI] [PubMed] [Google Scholar]
- 3.Sener S, Batu ED, Cuceoglu MK, et al. Features of myositis-specific and myositis-associated antibodies and antinuclear antibody patterns in patients with juvenile dermatomyositis. Pediatr Rheumatol 2022; 20: (Suppl 2): [Google Scholar]
- 4.Sitthi C, Khaosut P. Characteristics and outcomes of juvenile dermatomyositis (JDM) in Thai children: experience from a tertiary referral center. J Med Assoc Thailand 2023; 106:867–874. [Google Scholar]
- 5.Fu Y, Gu L, Chen J, et al. Severe gastrointestinal involvements in patients with adult dermatomyositis with anti-NXP2 antibody. RMD Open 2024; 10:e003901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuki I, Miwako S, Sae I, et al. Antinuclear matrix protein 2 antibody-positive idiopathic inflammatory myopathies represent extensive myositis without dermatomyositis-specific rash. Rheumatol (Oxford) 2022; 61:1222–1227. [DOI] [PubMed] [Google Scholar]
- 7.Mormile I, Mosella F, Turco P, et al. Calcinosis cutis and calciphylaxis in autoimmune connective tissue diseases. Vaccines (Basel) 2023; 11: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Garf K, El-Garf A, Salah S, et al. A juvenile dermatomyositis: demographics, characteristics and disease outcome in an Egyptian cohort. Clin Exp Rheumatol 2022; 40:450–456. [DOI] [PubMed] [Google Scholar]
- 9.Felix A, Delion F, Louis-Sidney F, et al. Juvenile dermatomyositis in Afro-Caribbean children: a cohort study in the French West Indies. Pediatr Rheumatol Online J 2023; 21:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loarce-Martos J, Larena C, Blazquez MA, et al. Clinical characteristics of juvenile idiopathic inflammatory myopathy and comparison with adult patients analysis from a multicentric cohort in Spain. J Clin Rheumatol 2022; 28:E195–E202. [DOI] [PubMed] [Google Scholar]
- 11.Tennelli A, Patro D. Juvenile dermatomyositis: clinical characteristics, myositis specific antibody profile and disease course in a tertiary care centre in south India. Indian J Rheumatol 2023; 18:S226. [Google Scholar]
- 12.Cakan M, Ozdel S, Karadag SG, et al. Initial manifestations and risk factors for calcinosis in juvenile dermatomyositis: a retrospective multicenter study. North Clin Istanb 2023; 10:298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baya Chatti A, Naceur I, Achour TB, et al. Clinical and serological features of dermatomyositis in Tunisia. Ann Rheum Dis 2023; 82:1675–1676. [Google Scholar]
- 14.Valenzuela A, Chung L. Subcutaneous calcinosis: is it different between systemic sclerosis and dermatomyositis? J Scleroderma Relat Disord 2022; 7:7–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Yao HH. Diffuse subcutaneous calcinosis in dermatomyositis. QJM 2024; hcae045. [DOI] [PubMed] [Google Scholar]
- 16.Cervantes BA, Gowda P, Rider LG, et al. Development of a computed tomography calcium scoring technique for assessing calcinosis distribution, pattern and burden in dermatomyositis. Rheumatology (Oxford) 2024; 63:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christopher Stine L, Feldman J, Smith T, et al. Dermatomyositis burden of disease manifestations and QOL impact: patient reported survey results. Ann Rheum Dis 2023; 82:2095–2096. [Google Scholar]
- 18.Bensimon A, Chen K, Normal A, et al. Healthcare resource utilization and cost burden of dermatomyositis. Ann Rheum Dis 2022; 81:716–717. [Google Scholar]
- 19.Mehta P, Lawrence A, Gupta L, et al. Long-standing and poorly controlled disease in juvenile dermatomyositis is associated with calcinosis: a real-world experience from a low-middle income country. Rheumatol Int 2023. [DOI] [PubMed] [Google Scholar]
- 20.Tyagi R, Pandiarajan V, Basu S. The association of delay in diagnosis of juvenile dermatomyositis with various and clinical and laboratory parameters: experience of a centre in north india. Clin Exp Rheumatol 2023; 41:484–486. [Google Scholar]
- 21.Valenzuela A, Baron M, Rodriguez-Reyna TS, et al. Calcinosis is associated with ischemic manifestations and increased disability in patients with systemic sclerosis. Semin Arthritis Rheum 2020; 50:891–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nozawa T, Bell-Peter A, Marcuz JA, et al. Early abnormal nailfold capillary changes are predictive of calcinosis development in juvenile dermatomyositis. J Rheumatol 2022; 49:1250–1255. [DOI] [PubMed] [Google Scholar]
- 23.Kam O, Osborne S, Wescott R, et al. Prevalence of calcinosis cutis in the United States using the All of Us research database. J Am Acad Dermatol 2024; 90:405–406. [DOI] [PubMed] [Google Scholar]
- 24.Yi BY, Marrs J, Acharya P, et al. Risk factors for developing calcinosis in juvenile dermatomyositis: subcutaneous and myofascial edema in initial magnetic resonance imaging. Rheumatol Int 2023. [DOI] [PubMed] [Google Scholar]
- 25.Lee M, Brown LS, Sontheimer R, Chong BF. 1316 Increased muscle involvement and younger age are distinctive features in dermatomyositis patients with skin of color. J Invest Dermatol 2023; 143:S225. [Google Scholar]
- 26.Aggarwal R, Sil A, Dod A, et al. Catching up with calcinosis: analysis of clinical characteristics, myositis specific antibodies and response to therapy in juvenile dermatomyositis in a cohort from a tertiary care centre in north India. Indian J Rheumatol 2023; 18:S220. [Google Scholar]
- 27.Padiyar S, Nair A, Yadav B, et al. Clinical characteristics, therapeutics, and treatment outcomes of adult patients with antimelanoma differentiation-associated gene 5 dermatomyositis: a single-center experience from South India. Indian J Rheumatol 2023; 18:205–211. [Google Scholar]
- 28▪▪.Toplak N, Pimpale Chavan P, Rosina S, et al. Is anti-NXP2 autoantibody a risk factor for calcinosis and poor outcome in juvenile dermatomyositis patients? Case series. Front Pediatr 2022; 9:810785. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article highlights that the presence of calcinosis is associated with disease severity and poor disease outcomes in JDM.
- 29.Duvvuri B, Pachman LM, Morgan G, et al. Neutrophil extracellular traps in tissue and periphery in juvenile dermatomyositis. Arthritis Rheumatol 2020; 72:348–358. [DOI] [PubMed] [Google Scholar]
- 30▪▪.Duvvuri B, Pachman LM, Hermanson P, et al. Role of mitochondria in the myopathy of juvenile dermatomyositis and implications for skeletal muscle calcinosis. J Autoimmun 2023; 138:103061. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article highlights how mitochondrial dysfunction plays into the pathogenesis of calcinosis.
- 31.Duvvuri B, Baddour AA, Deane KD, et al. Mitochondrial N-formyl methionine peptides associate with disease activity as well as contribute to neutrophil activation in patients with rheumatoid arthritis. J Autoimmun 2021; 119:102630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuley R, Duvvuri B, Wallin JJ, et al. Mitochondrial N-formyl methionine peptides contribute to exaggerated neutrophil activation in patients with COVID-19. Virulence 2023; 14:2218077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duvvuri B, Pachman L, Moore R, et al. Mitochondrial ROS as a regulator of calcinosis in juvenile dermatomyositis. American College of Rheumatology Convergence 2020. [Google Scholar]
- 34.Torres-Ruiz J, Carrillo-Vázquez DA, Leal-Alanis A, et al. Low-density granulocytes and neutrophil extracellular traps as biomarkers of disease activity in adult inflammatory myopathies. J Clin Rheumatol 2022; 28:e480–e487. [DOI] [PubMed] [Google Scholar]
- 35.Michailidou D, Kuley R, Wang T, et al. Neutrophil extracellular trap formation in antineutrophil cytoplasmic antibody-associated and large-vessel vasculitis. Clin Immunol 2023; 249:109274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36▪.Davuluri S, Duvvuri B, Lood C, et al. Calcinosis in dermatomyositis: origins and possible therapeutic avenues. Best Pract Res Clin Rheumatol 2022; 36:101768. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides an extensive overview of therapeutic options for calcinosis.
- 37.Bonilha CS, Veras FP, de Queiroz Cunha F. NET-targeted therapy: effects, limitations, and potential strategies to enhance treatment efficacy. Trends Pharmacol Sci 2023; 44:622–634. [DOI] [PubMed] [Google Scholar]
- 38.Duvvuri B, Gonzalez-Chapa JA, Pachman LM, et al. The emerging role of Growth Differentiation Factor 15 as a potential disease biomarker in juvenile dermatomyositis. Rheumatology (Oxford) 2023. [DOI] [PubMed] [Google Scholar]
- 39.Honda M, Shimizu F, Sato R, Nakamori M. Contribution of complement, microangiopathy and inflammation in idiopathic inflammatory myopathies. J Neuromuscul Dis 2024; 11:5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duvvuri B, Lood C. Mitochondrial calcification. Immunometabolism 2021; 3:e210008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41▪.Wilkinson MGL, Moulding D, McDonnell TCR, et al. Role of CD14+ monocyte-derived oxidised mitochondrial DNA in the inflammatory interferon type 1 signature in juvenile dermatomyositis. Ann Rheum Dis 2023; 82:658–669. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study highlights the important role of mitochondria in JDM pathogenesis.
- 42.Becker YLC, et al. The role of mitochondria in rheumatic diseases. Nat Rev Rheumatol 2022; 18:621–640. [DOI] [PubMed] [Google Scholar]
- 43.Lood C, Blanco LP, Purmalek MM, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med 2016; 22:146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bae S, Shahbazian A, Wang J, Charles-Schoeman C. Lower HDL-associated apolipoprotein A-I levels associate with presence of calcinosis in adult dermatomyositis. Arthritis Rheumatol 2021; 73:1438–1440. [Google Scholar]
- 45.Fairchild RM, Deluna MD, Golovko V, et al. Evolution and impact of a dedicated ultrasound clinic on clinical rheumatology practice at an academic medical center. Semin Arthritis Rheum 2023; 63:152276. [DOI] [PubMed] [Google Scholar]
- 46.Nelson M, Schiffenbauer A, Kim H, et al. Intra-rater validation of calcinosis durometer measurements in juvenile and adult dermatomyositis. Arthritis Rheumatol 2022; 74:325–327. [Google Scholar]
- 47.Akinfenwa SA, Mary JW, Memon A, et al. I’m well, but i’m not: a case report of recurrent calcinosis cutis in a patient with well controlled juvenile dermatomyositis. J Gen Intern Med 2023; 38:S615–S1615. [Google Scholar]
- 48.López-Sundh AE, Quintana-Sancho A, Durán-Vian C, et al. Clinical and ultrasound response to intralesional sodium thiosulfate for the treatment of calcinosis cutis in the setting of systemic sclerosis. A case-based review. Clin Rheumatol 2021; 40:2985–2989. [DOI] [PubMed] [Google Scholar]
- 49.Winter AR, Klager S, Truong R, et al. Efficacy of intralesional sodium thiosulfate for the treatment of dystrophic calcinosis cutis: a double-blind, placebo-controlled pilot study. JAAD Int 2020; 1:114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tubau C, Cubiró X, Amat-Samaranch V, et al. Clinical and ultrasonography follow-up of five cases of calcinosis cutis successfully treated with intralesional sodium thiosulfate. J Ultrasound 2022; 25:995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shiari R, Khalili M, Zeinali V, et al. Local injection of infliximab into calcinosis lesions in patients with juvenile dermatomyositis (JDM): a clinical trial. Pediatr Rheumatol Online J 2024; 22:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52▪.Motlaghzadeh Y, Tabatabai LS, Longo E, Sellmeyer DE. Regression of calcinosis cutis after inkless tattoo in a patient with dermatomyositis: therapeutic potential of microneedling. Osteoporos Int 2022; 33:2449–2452. [DOI] [PubMed] [Google Scholar]; This article is the first to use microneedling in calcinosis in dermatomyositis.
- 53▪▪.Gauffenic A, Ratsimbazafy V, Ostertag A, et al. Effectiveness of topical sodium thiosulfate for ectopic calcifications and ossifications. Results of the CATSS-O study. Semin Arthritis Rheum 2023; 63:152306. [DOI] [PubMed] [Google Scholar]; This article highlights the efficacy of topical sodium thiosulfate in calcinosis associated with juveinle dermatomyositis.
- 54.Haddadin R, Ahadiat O. Calcinosis cutis: case report of topical sodium thiosulfate (STS) treatment in the context of dermatomyositis. Skin 2022; 6:318–320. [Google Scholar]
- 55.Tayfur AC, Topaloglu R, Gulhan B, Bilginer Y. Bisphosphonates in juvenile dermatomyositis with dystrophic calcinosis. Mod Rheumatol 2015; 25:615–620. [DOI] [PubMed] [Google Scholar]
- 56.Janarthanan M, Mohan M, Murali A. Bisphosphonate therapy for juvenile dermatomyositis-associated calcinosis and metaphyseal zebra lines. BMJ Case Rep 2022; 15:e252814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang J, Sun L, Shi X, et al. Janus kinase inhibitor, tofacitinib, in refractory juvenile dermatomyositis: a retrospective multicentral study in China. Arthritis Res Ther 2023; 25:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou Q, et al. Analysis of the clinical characteristics and therapeutic effect of refractory juvenile dermatomyositis to tofacitinib. Chin J Pediatr 2023; 61:538–542. [DOI] [PubMed] [Google Scholar]
- 59.Shneyderman M, Ahlawat S, Christopher-Stine L, Paik JJ. Calcinosis in refractory dermatomyositis improves with tofacitinib monotherapy: a case series. Rheumatology (Oxford) 2021; 60:e387–e388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robert M, Gallay L, Garnier L, et al. Contribution of the Interferon score in the management of an anti-NXP2 dermatomyositis patient with calcinosis successfully treated with tofacitinib. Joint Bone Spine 2023; 90:105532. [DOI] [PubMed] [Google Scholar]
- 61.Mastrolia MV, Orsini SI, Marrani E, et al. Efficacy of Janus kinase inhibitor baricitinib in the treatment of refractory juvenile dermatomyositis complicated by calcinosis. Clin Exp Rheumatol 2023; 41:402–403. [DOI] [PubMed] [Google Scholar]
- 62.Campanilho-Marques R, Deakin CT, Simou S, et al. Retrospective analysis of infliximab and adalimumab treatment in a large cohort of juvenile dermatomyositis patients. Arthritis Res Ther 2020; 22:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yi B, Wahezi D, Covert L, et al. Treatment response and outcomes of 63 cases of juvenile dermatomyositis-associated calcinosis. Arthritis Rheumatol 2023; 75:18–20. [Google Scholar]
- 64.Shahani L. Refractory calcinosis in a patient with dermatomyositis: response to intravenous immune globulin. BMJ Case Rep 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Touimy M, Janani S, Rachidi W, et al. Calcinosis universalis complicating juvenile dermatomyositis: improvement after intravenous immunoglobulin therapy. Joint Bone Spine 2013; 80:108–109. [DOI] [PubMed] [Google Scholar]