Abstract
Purpose of review
Breastfeeding provides passive immunity while the neonatal immune system matures, and may also protect against chronic immune-mediated conditions long after weaning. This review summarizes current knowledge and new discoveries about human milk and mucosal immunity.
Recent findings
New data suggest that certain microbes in maternal milk may seed and shape the infant gut microbiota, which play a key role in regulating gut barrier integrity and training the developing immune system. Human milk oligosaccharides, best known for their prebiotic functions, have now been shown to directly modulate gene expression in mast and goblet cells in the gastrointestinal tract. Epidemiologic data show a reduced risk of peanut sensitization among infants breastfed by peanut-consuming mothers, suggesting a role for milk-borne food antigens in tolerance development. Cross-fostering experiments in mice suggest the soluble Toll-like receptor 2, found in human milk, may be critical in this process. Finally, interest in human milk antibodies surged during the pandemic with the identification of neutralizing severe acute respiratory syndrome coronavirus 2 antibodies in maternal milk following both natural infection and vaccination.
Summary
Human milk provides critical immune protection and stimulation to breastfed infants. Understanding the underlying mechanisms could identify new therapeutic targets and strategies for disease prevention across the lifespan.
Keywords: breastfeeding, developmental origins of health and disease, human milk, microbiota, mucosal immunity
INTRODUCTION
Mucosal membranes are composed of specialized epithelial cells covering a layer of connective tissue that lines the respiratory, digestive, and urinary tracts. The immune system, with its innate and adaptive components, is an essential element of the mucosa, which serves as the body's first line of defense against pathogens and the first point of contact for establishing tolerance toward innocuous antigens such as food proteins and commensal bacteria [1]. After full gestation, all of the basic mucosal immune elements are in place, but they exist in an immature state characterized by incomplete barrier integrity, deficiency in secretory immunoglobulin A (sIgA), reduced complement activation, and low levels of memory immune cells [2]. Therefore, infants depend on their mother's milk for passive immunity, and rely on milk and other exogenous factors to stimulate mucosal immune system development.
Besides essential nutrients, human milk is rich in cytokines, immunoglobulins, growth factors, microbiota, soluble receptors, immune cells, enzymes, lipids, and oligosaccharides [3▪,4▪,5]. These components are dynamic and change over time to match the needs of infants throughout lactation. Some factors also have a diurnal rhythm or fluctuate during a single feeding [6▪▪]. By the end of pregnancy, the mammary gland starts to produce colostrum, which is especially rich in bioactive factors that provide passive immunity to the neonate [7]. Transitional milk is secreted for about two weeks, followed by mature milk that comprises a thinner ‘fore-milk’ that becomes fattier toward the end of the feed, referred to as ‘hind-milk’ [8]. Milk composition is highly variable between women due to several fixed and modifiable factors, including genetics, diet, health status, environmental exposures, infant gestational age, and infant sex [6▪▪,8].
It is well established that breastfeeding is protective against infections and immune-mediated diseases, both during and beyond the lactation period [9,10]. For example, recent systematic reviews and meta-analyses have found that breastfed (vs. formula fed) infants have a 31% lower incidence of diarrhea and a 52% lower risk of mortality due to infectious disease in the first 2 years of life [10]. These effects are particularly critical in low resource settings where the burden of infectious disease is high and the access to clean water for preparing formula is low. In high resource settings, breastfeeding remains important for the prevention of chronic immune-mediated diseases such as asthma (9% reduced risk) and allergic rhinitis (21% reduced risk) [10]. Collectively, this evidence suggests a critical role for human milk in developing the mucosal immune system during early life, which will be the focus of this review.
Box 1.
no caption available
DEVELOPMENT OF THE MUCOSAL IMMUNE SYSTEM
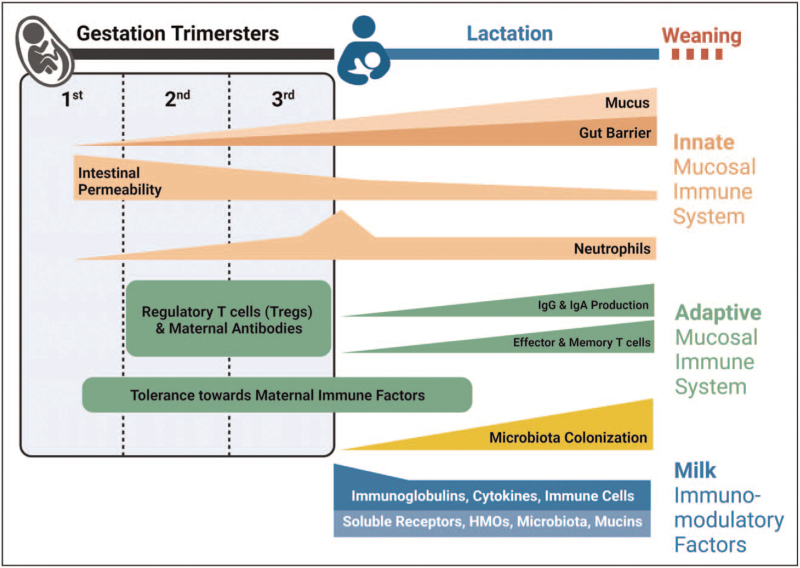
The mucosal immune system includes physical (epithelium) and chemical (mucus) barriers, signaling molecules (e.g. pattern recognition receptors), along with innate immune cells (neutrophils, macrophages, mast cells, natural killer T cells, dendritic cells [DCs]) and adaptive immune cells (B and T cells). These components overlay gastrointestinal, pulmonary, and other mucosal surfaces [11,12]. Structures of the fetal mucosal immune system are fully developed in utero by week 28; thus, neonates have a responsive but ‘immature’ immune system at birth [13]. With limited exposure to foreign antigens, the initial immune responses of neonates are essential for facilitating tolerance toward self, maternal, and bacterial antigens. However, they also have reduced functional capacity in some aspects of innate immunity. Thus, during this early developmental period, breastmilk is vital in helping to prevent infection and supporting a smooth transition from fetal to postnatal life as the mucosal immune system matures (Fig. 1).
FIGURE 1.
Timeline of innate and adaptive mucosal immune development during gestation and infancy and the bridging role of human milk. (Original). Gut barrier development beings in utero and continues until weaning, correlating with a decline in gut permeability and an increased thickness of the mucus layer. Levels of neutrophils increase toward the end of gestation and rapidly decrease to normal levels within the first hours after birth. Levels of Tregs are higher in the fetal period, which leads to immune suppression and tolerance toward maternal immune factors, such as immunoglobulins. After birth, the neonatal immune system gradually switches to an effector immune response with the increased exposure to environmental and milk immune factors, including microbiota. The innate and adaptive immune systems mature after birth, increasing the local production of mucosal antibodies. Milk components, such as microbiota, maternal cells, antibodies, cytokines, growth factors, soluble receptors, and HMOs, play an important role in promoting the normal development of the neonatal mucosal immune system (Figure 1); many are especially abundant in colostrum.
The innate mucosal immune system after birth
Innate mucosal immunity is the first line of defense against foreign antigens. It is less effective in neonates than adults, thus increasing the risk of infection in early life [14,15]. The gastrointestinal barrier and its gut-associated lymphoid tissue start to develop by 8–10 weeks of gestation [16] and continue after birth until weaning [17]. Increased intestinal epithelial permeability in the neonate allows passage of macromolecules, including maternal IgGs [18▪]. Intestinal permeability gradually declines after birth, and this process occurs more slowly in formula-fed infants [18▪,19,20], suggesting an essential role for breastmilk in such maturation. The mucosal epithelium is coated with a layer of mucus produced by goblet cells present at birth. Neonatal expression of certain mucins is low, resulting in reduced mucus layer thickness compared to adults [2].
Neutrophils are present in the fetus and increase in numbers before birth, declining to ’normal’ adult levels within few days of delivery [21]. However, their response to bacterial products is limited compared to adult neutrophils [15]. Monocytes and macrophages are also immature in neonates, with relatively low expression of innate receptors, such as Toll-like receptor 4 (TLR4) [22–24]. Moreover, the responsiveness of neonatal myeloid and plasmacytoid DCs to viral infections is limited, leading to attenuated priming of T cells and low secretion of interferons [25▪▪,26–28]. In addition, neonatal natural killer T cells, which are potent antiviral innate immune cells, produce limited interferon-γ and exhibit low cytolytic activity compared to adults [29].
The immaturity of the fetal/neonatal immune system provides opportunities for developing immune tolerance toward maternal antigens delivered in utero and via breastmilk, and for allowing optimal colonization by commensal microbes. During this period of vulnerability to infection, the infant relies on exogenous protection from maternal milk while its endogenous mucosal immune system matures.
The adaptive mucosal immune system after birth
Humoral adaptive immune responses are mediated by antibodies produced by B cells, whereas cell-mediated responses are controlled by antigen-specific T cells. These adaptive immune responses are highly specific and long-lasting, generating immunological ‘memory’ that acts quickly to eliminate subsequent insults. Sensing of antigens by the innate immune response is the critical first step to developing adaptive immunity; thus, the immature state of innate mucosal immunity at birth leads to similarly immature adaptive humoral and cellular responses.
Functional B and T cells, the key elements of adaptive immunity, can be found as early as 12 weeks after birth in the gastrointestinal tract. However, these neonatal lymphocytes differ from mature adult lymphocytes, and their primary function is biased toward immune tolerance [30]. In addition, the high expression of transforming growth factor-β (TGF-β) in fetal mucosal lymphoid tissues [31,32] favors the expansion of regulatory T cells (Tregs) [33], which actively suppress B and T cells [34], resulting in low B-cell production of sIgA among neonates [35]. IgM-producing B cells predominate for the first month after birth before switching to IgA production, which keeps increasing until 2 years of age [36,37]. Neonatal secretion of sIgA at mucosal surfaces begins between 1 and 8 weeks of age [35].
MATERNAL MILK AND MUCOSAL IMMUNITY
The World Health Organization recommends 6 months of exclusive breastfeeding, with continued breastfeeding together with appropriate complementary foods up to 2 years of age or longer [38]. A primary reason for this recommendation is that breastfeeding fills a critical gap in neonatal/infant immunity by transferring bioactive factors that provide passive immune protection and stimulate normal mucosal immune development, supporting lifelong homeostasis (Table 1 and Fig. 2).
Table 1.
Human milk components with immunomodulating activity
| Component | Immunomodulating activity | References |
| Immunoglobulins | ||
| sIgA (∼85% of total Igs in milk) | • Neutralize pathogens while supporting commensal bacteria• Regulate inflammatory responses to microbial antigens• Promote intestinal homeostasis & mucosal immune tolerance | [114,115] |
| IgGs | • Bind specific antigens (including allergenic foods) & transport them across the gut barrier through binding to intestinal FcRn, promoting tolerance | [44,46,47] |
| IgM | • Strong complement-fixing activity | [116] |
| IgE | • Binds to mast cells and basophils may benefit antiparasitic responses | [117] |
| Cytokines | ||
| TGF-β1, TGFβ-2 | • Inhibit T cell differentiation• Induce Treg expansion• Promote intestinal integrity• Stimulate B cell class switching to IgA• Promote microbial colonization• Promote mucosal tolerance | [73–79] |
| IL-4, IL-5, IL-13 | • Promote Type 2 cytokine responses and T cell differentiation | [118] |
| IL-10 | • Multiple anti-inflammatory roles and mast cell growth factor | [119] |
| TNF, IL-6, IFN-γ, IL-12 | • Drivers of inflammatory responses and Type 1 responses | [120] |
| GMCSF, GCSF, MCSF | • Granulocyte, macrophage and dendritic cell growth and differentiation factors | [121] |
| Chemokines | ||
| CCL4, CCL5, CXCL10 | • Mediate immune and stromal cell recruitment to sites of infection, damage or inflammation | [122] |
| Soluble Receptors | ||
| Innate receptors: sTLR2, sCD14 | • Bind microbial ligands, attenuate inflammatory response | [90,123,124] |
| Cytokine receptors: IL-1RA, sIL-6R, sTNF-RI, sTNF-RII | • Regulate signaling of milk-borne IL-1b, IL-6, TNF | [86,87] |
| Other | ||
| Epithelial Growth Factors: IGF-I, EGF | • Promote epithelial development and repair | [125] |
| Maternal cells (∼80% myeloid cells, 20% lymphocytes) | • Migrate into gut mucosa and/or pass into systemic circulation• Contribute to antibody responses• Regulate T cell development and responses | 56 57 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 |
| Microbiota | • Some may seed the infant gut• Compete with pathogens for ecological niche• Produce SCFAs that support epithelial proliferation & improve gut barrier• Enhance gut barrier integrity by binding innate immune receptors (e.g. TLR2) on gut epithelium | [18▪,93,98,99] |
| Oligosaccharides | • Prebiotic substrate for gut microbiota• Antiadhesive properties block host-pathogen interactions• Decoy receptor for some pathogens• Co-factor for some viral binding• Absorption into circulation: systemic activities | [104–106,126] |
| Lactoferrin | • Chelate iron• Block pro-inflammatory cytokines & free radical activity• Bacteriostatic function against pathogens requiring iron• Support selective microbiota growth, including Bifidobacteria and Lactobacilli | [4▪,109–112] |
CD, cluster of differentiation; EGF, epidermal growth factor; FcRn, neonatal Fc receptor; GCSF, granulocyte colony-stimulating factor; GMCSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon-gamma; Ig, immunoglobulin; IGF-I, insulin-like growth factor-1; IL, interleukine; MCSF, macrophage colony-stimulating factor; TGF, transforming growth factor; TLR, toll-like receptor; TNF, tumor necrosis factor.
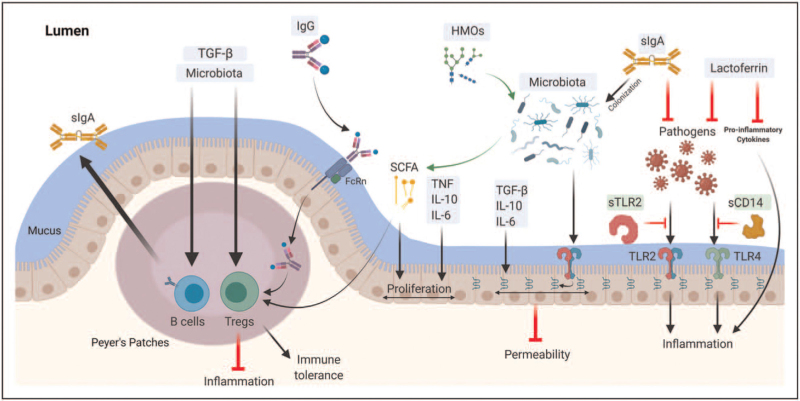
FIGURE 2.
Milk immune factors and mucosal immunity. (Original). Several aspects of the mucosal immune system are developed under the influence of human milk components in early life. Maternal sIgA is the most abundant immunoglobulin in human milk; it inhibits pathogens and facilitates colonization of commensal microbiota. Maternal IgG is present at lower levels in milk and can be found complexed with dietary antigens; these complexes can translocate across the gut epithelium through the neonatal FcRn receptor and then stimulate expansion of regulatory T cells (Tregs). Human milk cytokines, including the TGF-β family, IL-6, IL-10, and TNF, are essential in the homeostasis of mucosal immunity via enhancing epithelial integrity and proliferation, expanding Tregs and endogenous sIgA secretion from infant B cells. Lactoferrin blocks pro-inflammatory cytokines and free-radical activity, and inhibits pathogen growth by chelating iron. Human milk microbiota may colonize the infant's gut and promote the development of mucosal immunity, including intestinal integrity, Tregs expansion, and sIgA production, either directly via signaling through innate immune receptors or indirectly via metabolizing human milk oligosaccharides (HMOs) into short-chain fatty acids (SCFAs) that serve as an energy source for gut epithelial cells. Soluble receptors in milk (such as sTLR2 and sCD14) are essential in regulating the inflammatory response, acting as decoy receptors that bind pathogenic bacterial ligands.
Immunoglobulins
Human milk immunoglobulins, including IgA, IgGs, IgM, IgE, and IgD, are critical in modulating and shaping neonatal mucosal immunity [39▪,40▪▪]. Human milk is the only source of sIgA during the first month of life. sIgA comprises around 80–90% of total immunoglobulins in milk [4▪], with its highest concentration in colostrum [41]. sIgA is the first line of antigen-specific defense that can effectively neutralize pathogens while permitting commensal bacteria [4▪], suggesting a vital role in microbiota colonization and intestinal homeostasis. In addition, the majority of intestinal bacteria are coated with sIgA, which helps regulate inflammatory responses to microbial antigens [42]. Furthermore, allergic disease incidence among breastfed infants has been negatively associated with levels of sIgA in their mothers’ milk [43], indicating an essential role in mucosal immune tolerance.
IgG subtypes are also detected in human milk at much lower levels than sIgA. They can bind specific antigens, fix complement and enhance uptake of antigens. Food antigens are found complexed with IgG in maternal serum and are transferrable into breastmilk via the Fc receptor (FcRn) expressed in the mammary gland's epithelium [44]. FcRn is also found in the neonatal intestine where it mediates the translocation of the IgG-allergen complex across the gut barrier [45] where they can be taken up by FcRn-bearing antigen-presenting cells. Allergens bound to IgG have been shown to induce more Tregs and more profound immune tolerance than free allergens [46], suggesting that exposure to IgG-complexed food antigens through breastmilk may contribute to food allergy prevention [47]. This hypothesis is supported by recent data from the CHILD Cohort Study, where infants who were introduced to peanut before weaning and breastfed by mothers who regularly consumed peanuts had a reduced risk of peanut sensitization and allergy through 5 years of age [48▪].
Interest in human milk antibodies has surged during the Covid-19 pandemic, with multiple groups [49▪,50,51▪▪,52,53] showing that mothers who recover from Covid-19 have antigen-specific sIgA in their breastmilk that is presumed to protect their infants. Vaccination-induced antibodies are also present in human milk, although the profile is different (IgG-dominant) than following natural infection (IgA-dominant) [54▪▪,55].
Immune cells
Despite the challenging environment of the infant digestive system, the transfer of maternal cells continues after birth through breastmilk. As recently reviewed [56▪], these cells can migrate into the gut mucosa and may even pass into the systemic circulation where they can contribute to neonatal antibody responses and regulation of T cell responses, including their repertoire [57–60]. Myeloid cells such as monocytes, macrophages and DCs are predominant in breastmilk, making up more than 80% of total leukocytes [56▪]. Lymphocytes make up the bulk of the remaining 20%; these are predominately T cells but also include natural killer cells and subsets of B cells. The composition and function of these cells vary substantially during lactation and in response to other factors [61]. Various roles have been attributed to these maternal immune cells in milk, including microchimerism-induced immune maturation [62] and the enhancement of allergy development through IgE transport [63]. An important recent finding is the observation that, in mice, the transfer of IgA and IgA-producing cells through milk can impact the development of regulatory T cells in the offspring. These elegant cross-fostering experiments identify a novel mechanism by which immune function can be transmitted across generations independently of genetic, epigenetic or microbial factors [64▪▪].
Cytokines, growth factors, and soluble receptors
Cytokines and growth factors in milk are signaling proteins involved in cell communication, recruitment, regulation and stimulation. A wide array of these immune factors are found in breastmilk, including TGF-β1, TGF-β2, IL-10, IL-6, IL-1β, tumor necrosis factor (TNF), insulin-like growth factor (IGF)-1, IFN-γ, IL-4, IL-5, IL-12, IL-13, G-colony-stimulating factor (CSF), GM-CSF, and M-CSF [43,65–69], and the list is expanding. These immune mediators tend to be present at higher concentrations in colostrum than mature milk, indicating their potential importance in supporting immature mucosal immunity in neonates.
The most abundant cytokines in human milk, TGF-β2 and TGF-β1 [43], are scarce in infant formula [70–72]. The role of the TGF-β family in mucosal immunity is multifactorial, including inhibition of T cell differentiation into effector Th1 and Th2 cells [73,74], inducing Tregs’ expansion [75,76], promoting intestinal integrity [77], stimulating B cell class switching to IgA [78], and promoting microbial colonization [79]. According to Järvinen et al.,[80,81], higher levels of milk TGF-β1, along with IL-1β, IL-10, and IL-6, associate with a decrease in food allergy, suggesting a role of these cytokines in mucosal tolerance. Animal studies have also shown that IL-10 [82], IL-6 [83], and TNF [84] impact intestinal cell maturation and proliferation. Notably, levels of cytokines in milk and their impact on immune development are highly dependent on maternal genetics and immune system status. For example, mouse pups nursed by dams deficient in the pattern recognition receptor TLR2 have attenuated epithelial barrier function and altered responses to oral antigens [85▪].
Breastmilk also contains bioactive soluble receptors that can regulate cytokine and bacterial signaling by sequestering them away from the neonatal mucosa. For example, cytokine receptors and antagonists, including IL-1RA, sIL-6R, sTNF-RI, and sTNF-RI, are detected in human milk and are believed to regulate the signaling of milk-borne IL-1β [86–88], IL-6 [89], and TNF [86] cytokines, respectively. Soluble forms of innate immune receptors are also detected in human milk, including sTLR2 and sCD14 [90]. These soluble receptors bind to different microbial ligands leading to attenuation of the inflammatory response in infants.
Microbiota
It is now accepted that breastmilk is not sterile [91]. Our group and others have described a diverse human milk microbiome that, like other milk components, appears to be dynamic and highly variable among mothers [91,92]. Milk-borne microbiota may contribute to the developing infant gut microbiota, which promote mucosal development by competing with pathogens for the ecological niche, producing bioactive factors that improve the intestinal barrier, and educating the mucosal immune system [18▪,93,94]. Recent data from the CHILD Cohort Study identified specific microbial taxa that are commonly shared between a mother's milk and her infant's gut microbiota and showed that this 'sharing of bacteria’ is increased among exclusively breastfeeding dyads [95▪]. Certain commensal microbes, including Bifidobacteria and Lactobacilli, are enriched in the gut of breastfed (vs. formula fed) infants and have been shown to enhance intestinal barrier integrity [96,97] through binding innate immune receptors (e.g., TLR2) on the gut epithelium [98]. In addition, the digestion of human milk oligosaccharides (HMOs, not found in most infant formulas) by Bifidobacterium and Lactobacillus species in the neonatal intestine produces short-chain fatty acid (SCFA) including butyrate, acetate, and propionate [99]. These SCFAs enhance epithelial proliferation, gut barrier function, and intestinal motility [100]; they also serve as an energy source for host enterocytes, and have been associated with protection from allergic diseases [101].
Oligosaccharides
Oligosaccharides are complex glycans present in human milk in higher concentrations than any other mammalian milk, with over 150 structurally distinct HMOs identified thus far [102▪]. Each mother secretes a unique HMO profile [103]. HMOs serve a prebiotic function in human milk, acting as substrates for the neonatal gut microbiota [104]. Some HMOs can also act as decoy receptors that block microbial binding to epithelial cells by mimicking the epithelial cell receptors [105]. In addition, some HMOs can be absorbed into the circulation and interact with immune and epithelial cells, thus, modulate the neonatal immune response [106]. New in vitro data from Cheng et al. shows that HMOs may also directly enhance mucosal barrier function by modulating intestinal goblet cell gene expression [107▪]. In addition, recent experiments in human and mouse model systems have shown that the HMO disialyllacto-N-tetraose favorably modulates mast cell homeostasis, contributing to protection against necrotizing enterocolitis, an often fatal gastrointestinal disorder in preterm neonates [108▪▪].
Lactoferrin
Lactoferrin is an iron-binding glycoprotein that is highly abundant in human milk, particularly in colostrum [109]. It has a bacteriostatic function via preventing the growth of iron-dependent pathogens [110]. Lactoferrin also inhibits the binding of pathogens to host cells and regulates excess immune responses via blocking pro-inflammatory cytokines and free-radical activity [4▪,111,112]. At the same time, lactoferrin has been shown to regulate intestinal homeostasis [4▪] and support selective microbiota growth, including Bifidobacteria and Lactobacilli[113].
CONCLUSION
During infancy, the mucosal immune system is ’programmed’ through exposure to environmental, dietary, and microbial factors, with potentially life-long implications. Maternal milk is the primary source of nutrients and immune factors during this critical period of development. The beneficial impact of breastfeeding on mucosal immune development is supported by the decrease in morbidity and mortality among optimally breastfed infants. However, more research is required to understand how human milk components interact with the neonatal mucosa to support immune development. This knowledge will help inform disease prevention and treatment strategies for infants (including those who cannot be breastfed), and could also inform therapeutic approaches to immune-mediated conditions in older children and adults.
Acknowledgements
We thank Rilwan Azeez for his critical and constructive review of this article.
Financial support and sponsorship
This work was supported by a Canadian Institutes of Health Research Project Grant #156155 and a Bill & Melinda Gates Foundation Grant #INV-001734. M.B.A. is supported by Tier 2 Canada Research Chair in the Developmental Origins of Chronic Disease at the University of Manitoba and is a Fellow in the CIFAR Humans and the Microbiome Program.
Conflicts of interest
M.B.A. regularly speaks at conferences and workshops on infant nutrition. She has received speaking honoraria from Prolacta Biosciences and AstraZeneca. She has contributed without remuneration to online courses on breastmilk and the infant microbiome produced by Microbiome Courses. She serves in a volunteer capacity for the International Society for Research on Human Milk and Lactation and as a member of the National Academy of Sciences, Engineering and Medicine Committee on Scanning New Evidence on the Nutrient Content of Human Milk. She also serves on the Malaika Vx Scientific Advisory Board. B.D. and J.S.M. declare no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med 2005; 11:S45–S53. [DOI] [PubMed] [Google Scholar]
- 2.Torow N, Marsland BJ, Hornef MW, et al. Neonatal mucosal immunology. Mucosal Immunol 2017; 10:5–17. [DOI] [PubMed] [Google Scholar]
- 3▪.Pham Q, Patel P, Baban B, et al. Factors affecting the composition of expressed fresh human milk. Clin Res 2020; 15:551–558. [DOI] [PubMed] [Google Scholar]; Review summarizing the impact of maternal diet, medications, handling and storage on expressed human milk.
- 4▪.Thai JD, Gregory KE. Bioactive factors in human breast milk attenuate intestinal inflammation during early life. Nutrients 2020; 12:581–595. [DOI] [PMC free article] [PubMed] [Google Scholar]; Review describing key microbial, immunological, and metabolic components of human milk that have been implicated in intestinal inflammation and/or NEC prevention.
- 5.Ruiz L, Espinosa-Martos I, García-Carral C, et al. What's normal? Immune profiling of human milk from healthy women living in different geographical and socioeconomic settings. Front Immunol 2017; 8:696–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6▪▪.Italianer MF, Naninck EFG, Roelants JA, et al. Circadian variation in human milk composition, a systematic review. Nutrients 2020; 12:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]; Systematic review of 83 reports assessing circadian variation in the concentration of 71 human milk components.
- 7.Bryant J, Thistle J. Anatomy, Colostrum, 2020. http://www.ncbi.nlm.nih.gov/pubmed/30020628. [Google Scholar]
- 8.Ballard O, Morrow AL. Human milk composition. nutrients and bioactive factors. Pediatr Clin N Am 2013; 60:49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Infant and young child feeding: model chapter for textbooks for medical students and allied health professionals. World Health Organization, 2009. [PubMed] [Google Scholar]
- 10.Victora CG, Bahl R, Barros AJD, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 2016; 387:475–490. [DOI] [PubMed] [Google Scholar]
- 11.Bienenstock J, Befus AD. Mucosal immunology. Immunology 1980; 41:249–270. [PMC free article] [PubMed] [Google Scholar]
- 12.Brandtzaeg P. Review article: Homing of mucosal immune cells-a possible connection between intestinal and articular inflammation. Aliment Pharmacol Ther 2010; 11:24–39. [DOI] [PubMed] [Google Scholar]
- 13.Gleeson M, Cripps AW. Development of mucosal immunity in the first year of life and relationship to sudden infant death syndrome. FEMS Immunol Med Microbiol 2004; 42:21–33. [DOI] [PubMed] [Google Scholar]
- 14. Anderson RC, Dalziel JE, Gopal PK, et al. The role of intestinal barrier function in early life in the development of colitis. In: Colitis. InTech. 2012; 1–30. doi: 10.5772/25753. [Epub ahead of print] [Google Scholar]
- 15.Nussbaum C, Gloning A, Pruenster M, et al. Neutrophil and endothelial adhesive function during human fetal ontogeny. J Leukoc Biol 2013; 93:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maheshwari A, ZemlinM. Ontogeny of the intestinal immune system. Hematol Meet Rep 2006; 2:18–26. [Google Scholar]
- 17.Cummins AG, Thompson FM. Effect of breast milk and weaning on epithelial growth of the small intestine in humans. Gut 2002; 51:748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18▪.Weström B, Arévalo Sureda E, Pierzynowska K, et al. The immature gut barrier and its importance in establishing immunity in newborn mammals. Front Immunol 2020; 11:1153–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]; Review of developmental processes in the gut epithelium, how these affect macromolecular passage in different species, and implications for immune development during the perinatal period.
- 19.Catassi C, Bonucci A, Coppa GV, et al. Intestinal permeability changes during the first month: effect of natural versus artificial feeding. J Pediatr Gastroenterol Nutr 1995; 21:383–386. [DOI] [PubMed] [Google Scholar]
- 20.van Elburg RM, Uil JJ, Kokke FTM, et al. Repeatability of the sugar-absorption test, using lactulose and mannitol, for measuring intestinal permeability for sugars. J Pediatr Gastroenterol Nutr 1995; 20:184–188. [DOI] [PubMed] [Google Scholar]
- 21.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc R Soc B: Biol Sci 2015; 282:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan SR, Qing G, Byers DM, et al. Role of MyD88 in diminished tumor necrosis factor alpha production by newborn mononuclear cells in response to lipopolysaccharide. Infect Immun 2004; 72:1223–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sadeghi K, Berger A, Langgartner M, et al. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J Infect Dis 2007; 195:296–302. [DOI] [PubMed] [Google Scholar]
- 24.Al-Hertani W, Sen RY, Byers DM, et al. Human newborn polymorphonuclear neutrophils exhibit decreased levels of MyD88 and attenuated p38 phosphorylation in response to lipopolysaccharide. Clin Investig Med 2007; 30:E44–E53. [DOI] [PubMed] [Google Scholar]
- 25▪▪.Lau-Kilby AW, Turfkruyer M, Kehl M, et al. Type I IFN ineffectively activates neonatal dendritic cells limiting respiratory antiviral T-cell responses. Mucosal Immunol 2020; 13:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]; In vivo mouse study and in vitro human cell experiments show a functionally distinct response to IFN-I by neonatal dendritic cells.
- 26.Willems F, Vollstedt S, Suter M. Phenotype and function of neonatal DC. Eur J Immunol 2009; 39:26–35. [DOI] [PubMed] [Google Scholar]
- 27.De Wit D, Tonon S, Olislagers V, et al. Impaired responses to toll-like receptor 4 and toll-like receptor 3 ligands in human cord blood. J Autoimmun 2003; 21:277–281. [DOI] [PubMed] [Google Scholar]
- 28.Schüller SS, Sadeghi K, Wisgrill L, et al. Preterm neonates display altered plasmacytoid dendritic cell function and morphology. J Leukoc Biol 2013; 93:781–788. [DOI] [PubMed] [Google Scholar]
- 29.Ivarsson MA, Loh L, Marquardt N, et al. Differentiation and functional regulation of human fetal NK cells. J Clin Investig 2013; 123:3889–3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mold JE, Venkatasubrahmanyam S, Burt TD, et al. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science (80-) 2010; 330:1695–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitisin K, Saha T, Blake T, et al. Tgf-Beta signaling in development Science's STKE: signal transduction knowledge environment. 2007; 399; 1-6. doi: 10.1126/stke.3992007cm1. [DOI] [PubMed] [Google Scholar]
- 32.Mold JE, Michaëlsson J, Burt TD, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science (80-) 2008; 322:1562–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayakawa S, Ohno N, Okada S, et al. Significant augmentation of regulatory T cell numbers occurs during the early neonatal period. Clin Exp Immunol 2017; 190:268–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brugman S, Perdijk O, van Neerven RJJ, et al. Mucosal immune development in early life: setting the stage. Arch Immunol Ther Exp 2015; 63:251–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gleeson M. Development of mucosal immunity in the first year of life and relationship to sudden infant death syndrome. FEMS Immunol Med Microbiol 2004; 42:21–33. [DOI] [PubMed] [Google Scholar]
- 36.Perkkio M, Savilahti E. Time of appearance of immunoglobulin-containing cells in the mucosa of the neonatal intestine. Pediatr Res 1980; 14:953–955. [DOI] [PubMed] [Google Scholar]
- 37.Knox WF. Restricted feeding and human intestinal plasma cell development. Arch Dis Child 1986; 61:744–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feldman-Winter L, Kellams A. In-hospital formula feeding and breastfeeding duration. Pediatrics 2020; 146:1–5. [DOI] [PubMed] [Google Scholar]
- 39▪.Czosnykowska-Łukacka M, Lis-Kuberka J, Kr�lak-Olejnik B, et al. Changes in human milk immunoglobulin profile during prolonged lactation. Front Pediatr 2020; 8:428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]; A rare study analyzing human milk after the 2nd year of lactation, finding higher concentrations of protein, sIgA, and IgG.
- 40▪▪.Atyeo C, Alter G. The multifaceted roles of breast milk antibodies. Cell 2021; 184:1486–1499. [DOI] [PubMed] [Google Scholar]; Review of emerging data related to the importance of human milk antibodies in neonatal immunity and development
- 41.Castellote C, Casillas R, Ramírez-Santana C, et al. Premature delivery influences the immunological composition of colostrum and transitional and mature human milk. J Nutr 2011; 141:1181–1187. [DOI] [PubMed] [Google Scholar]
- 42.Rogier E, Frantz A, Bruno M, et al. Secretory IgA is concentrated in the outer layer of colonic mucus along with gut bacteria. Pathogens 2014; 3:390–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dawod B, Marshall JS. Cytokines and soluble receptors in breast milk as enhancers of oral tolerance development. Frontiers in Immunol 2019; 10:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cianga P, Medesan C, Richardson JA, et al. Identification and function of neonatal Fc receptor in mammary gland of lactating mice. Eur J Immunol 1999; 29:2515–2523. [DOI] [PubMed] [Google Scholar]
- 45.Yoshida M, Claypool SM, Wagner JS, et al. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity 2004; 20:769–783. [DOI] [PubMed] [Google Scholar]
- 46.Mosconi E, Rekima A, Seitz-Polski B, et al. Breast milk immune complexes are potent inducers of oral tolerance in neonates and prevent asthma development. Mucosal Immunol 2010; 3:461–474. [DOI] [PubMed] [Google Scholar]
- 47.Ohsaki A, Venturelli N, Buccigrosso TM, et al. Maternal IgG immune complexes induce food allergen- specific tolerance in offspring. J Exp Med 2018; 215:91–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48▪.Azad MB, Dharma C, Simons E, et al. Reduced peanut sensitization with maternal peanut consumption and early peanut introduction while breastfeeding. J Dev Orig Health Dis 2020; 12:811–818. [DOI] [PubMed] [Google Scholar]; Longitudinal study associating maternal peanut intake, breastfeeding and timing of peanut introduction during infancy with peanut sensitization and allergy at 5 years of age, proposing a ’triple exposure hypothesis’ about how food antigens may interact with human milk components to induce oral tolerance in the breastfed child.
- 49▪.Fox A, Marino J, Amanat F, et al. Robust and specific secretory IgA against SARS-CoV-2 detected in human milk. ISCIENCE 2020; 23:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]; Among the first publications to confirm anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) sIgA antibodies in human milk from women recovered from COVID-19.
- 50.Spatz DL, Davanzo R, Müller JA, et al. Promoting and protecting human milk and breastfeeding in a COVID-19 World. Front Pediatr 2021; 8:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51▪▪.Pace RM, Williams JE, Järvinen KM, et al. Characterization of SARS-CoV-2 RNA, antibodies, and neutralizing capacity in milk produced by women with covid-19. MBio 2021; 12:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]; Among the first publications to confirm anti-SARS-CoV-2 sIgA antibodies in human milk from women recovered from COVID-19, and demonstrate neutralizing capacity.
- 52.Lackey KA, Pace RM, Williams JE, et al. SARS-CoV-2 and human milk: what is the evidence? Maternal and child nutrition 2020; 16:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chambers C, Krogstad P, Bertrand K, et al. Evaluation for SARS-CoV-2 in breast milk from 18 infected women. JAMA 2020; 324:1347–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54▪▪.Perl SH, Uzan-Yulzari A, Klainer H, et al. SARS-CoV-2-specific antibodies in breast milk after COVID-19 vaccination of breastfeeding women. JAMA 2021; 325:2013–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]; Among the first publications to confirm anti-SARS-CoV-2 sIgA antibodies in human milk from women vaccinated against this virus.
- 55.Baird JK, Jensen SM, Urba WJ, et al. SARS-CoV-2 antibodies detected in human breast milk postvaccination. medRxiv 2021; 2021.02.23.21252328. [Preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56▪.Laouar A. Maternal leukocytes and infant immune programming during breastfeeding. Trends Immunol 2020; 41:225–239. [DOI] [PubMed] [Google Scholar]; Review of advances on how milk leukocytes impact early life immunity, focusing on development of the infant T cell repertoire and early immune responses in the periphery and gut-associated lymphoid tissue.
- 57.Cabinian A, Sinsimer D, Tang M, et al. Transfer of maternal immune cells by breastfeeding: maternal cytotoxic t lymphocytes present in breast milk localize in the peyer's patches of the nursed infant. PLoS One 2016; 11:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghosh MK, Nguyen V, Muller HK, et al. Maternal milk T cells drive development of transgenerational Th1 immunity in offspring thymus. J Immunol 2016; 197:2290–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arvola M, Gustafsson E, Svensson L, et al. Immunoglobulin-secreting cells of maternal origin can be detected in B cell-deficient mice. Biol Reprod 2000; 63:1817–1824. [DOI] [PubMed] [Google Scholar]
- 60.Ghosh MK, Muller HK, Walker AM. Lactation-based maternal educational immunity crosses MHC class I barriers and can impart Th1 immunity to Th2-biased recipients. J Immunol 2017; 199:1729–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baban B, Malik A, Bhatia J, et al. Presence and profile of innate lymphoid cells in human breast milk. JAMA Pediatr 2018; 172:594–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Molès JP, Tuaillon E, Kankasa C, et al. Breastmilk cell trafficking induces microchimerism-mediated immune system maturation in the infant. Pediatr Allergy Immunol 2018; 29:133–143. [DOI] [PubMed] [Google Scholar]
- 63.Dixon DL, Forsyth KD. Leukocytes in expressed breast milk of asthmatic mothers. Allergol Immunopathol 2017; 45:325–332. [DOI] [PubMed] [Google Scholar]
- 64▪▪.Ramanan D, Sefik E, Galván-Peña S, et al. An immunologic mode of multigenerational transmission governs a gut Treg setpoint. Cell 2020; 181:1276–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mouse studies and cross-fostering experiments uncover a lactational (nongenetic, nonepigenetic, nonmicrobial) mode of vertical transmission of the Treg homeostatic setpoint via the entero-mammary axis.
- 65.Žižka J, Kverka M, Novotná O, et al. Perinatal period cytokines related to increased risk of future allergy development. Folia Microbiol 2007; 52:549–555. [DOI] [PubMed] [Google Scholar]
- 66.Hawkes JS, Bryan DL, James MJ, et al. Cytokines (IL-1β, IL-6, TNF-α, TGF-β1, and TGF-β2) and prostaglandin E2 in human milk during the first three months postpartum. Pediatr Res 1999; 46:194–199. [DOI] [PubMed] [Google Scholar]
- 67.Calhoun DA, Lunøe M, Van D, et al. Granulocyte colony-stimulating factor is present in human milk and its receptor is present in human fetal intestine. Pediatrics 2000; 105:107–114. [DOI] [PubMed] [Google Scholar]
- 68.Ochiai S, Shimojo N, Morita Y, et al. Cytokine biomarker candidates in breast milk associated with the development of atopic dermatitis in 6-month-old infants. Int Arch Allergy Immunol 2013; 160:401–408. [DOI] [PubMed] [Google Scholar]
- 69.Hara T, Irie K, Saito S, et al. Identification of macrophage colony-stimulating factor in human milk and mammary gland epithelial cells. Pediatr Res 1995; 37:437–443. [DOI] [PubMed] [Google Scholar]
- 70.MohanKumar K, Namachivayam K, Ho TTB, et al. Cytokines and growth factors in the developing intestine and during necrotizing enterocolitis. Semin Perinatol 2017; 41:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frost BL, Jilling T, Lapin B, et al. Maternal breast milk transforming growth factor-beta and feeding intolerance in preterm infants. Pediatr Res 2014; 76:386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Böttcher MF, Jenmalm MC, Garofalo RP, et al. Cytokines in breast milk from allergic and nonallergic mothers. Pediatr Res 2000; 47:157–162. [DOI] [PubMed] [Google Scholar]
- 73.Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor β-induced inhibition of T helper type 1 differentiation. J Exp Med 2002; 195:1499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gorelik L, Fields PE, Flavell RA. Cutting edge: TGF-β inhibits Th Type 2 development through inhibition of GATA-3 expression. J Immunol 2000; 165:4773–4777. [DOI] [PubMed] [Google Scholar]
- 75.Melnik BC, John SM, Carrera-Bastos P, et al. Milk: A postnatal imprinting system stabilizing FoxP3 expression and regulatory T cell differentiation. Clin Transl Allergy 2016; 6:18.doi: 10.1186/s13601-016-0108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tran DQ. TGF-β: The sword, the wand, and the shield of FOXP3 + regulatory T cells. J Mol Cell Biol 2012; 4:29–37. [DOI] [PubMed] [Google Scholar]
- 77.Hering NA, Andres S, Fromm A, et al. Transforming growth factor-β, a whey protein component, strengthens the intestinal barrier by upregulating claudin-4 in HT-29/B6 cells. J Nutr 2011; 141:783–789. [DOI] [PubMed] [Google Scholar]
- 78.Van Vlasselaer P, Punnonen J, De Vries JE. Transforming growth factor-β directs IgA switching in human B cells. J Immunol 1992; 148:2062–2067. [PubMed] [Google Scholar]
- 79.Sitarik AR, Bobbitt KR, Havstad SL, et al. Breast milk transforming growth factor β is associated with neonatal gut microbial composition. J Pediatr Gastroenterol Nutr 2017; 65:e60–e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Järvinen KM, Suárez-Fariñas M, Savilahti E, et al. Immune factors in breast milk related to infant milk allergy are independent of maternal atopy. J Allergy Clin Immunol 2015; 135:1390–1393.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Järvinen KM, Martin H, Oyoshi MK. Immunomodulatory effects of breast milk on food allergy. Ann Allergy Asthma Immunol 2019; 123:133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Quiros M, Nishio H, Neumann PA, et al. Macrophage-derived IL-10 mediates mucosal repair by epithelial WISP-1 signaling. J Clin Investig 2017; 127:3510–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jeffery V, Goldson AJ, Dainty JR, et al. IL-6 signaling regulates small intestinal crypt homeostasis. J Immunol 2017; 199:304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bradford EM, Ryu SH, Singh AP, et al. Epithelial TNF receptor signaling promotes mucosal repair in inflammatory bowel disease. J Immunol 2017; 199:1886–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85▪.Dawod B, Haidl ID, Azad MB, et al. Toll-like receptor 2 impacts the development of oral tolerance in mouse pups via a milk-dependent mechanism. J Allergy Clin Immunol 2020; 146:631–641.e8. [DOI] [PubMed] [Google Scholar]; Cross-fostering experiments involving TLR2−/− dams demonstrate a critical role for TLR2 in regulating milk components that are essential for development of oral tolerance in pups.
- 86.Buescher ES, Malinowska I. Soluble receptors and cytokine antagonists in human milk. Pediatr Res 1996; 40:839–844. [DOI] [PubMed] [Google Scholar]
- 87.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev 2002; 13:323–340. [DOI] [PubMed] [Google Scholar]
- 88.Epstein FH, Dinarello CA, Wolff SM. The role of interleukin-1 in disease. N Engl J Med 1993; 328:106–113. [DOI] [PubMed] [Google Scholar]
- 89.Schöbitz B, Pezeshki G, Pohl T, et al. Soluble interleukin-6 (IL-6) receptor augments central effects of IL-6 in vivo. FASEB J 1995; 9:659–664. [DOI] [PubMed] [Google Scholar]
- 90.Chatterton DEW, Nguyen DN, Bering SB, et al. Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. Int J Biochem Cell Biol 2013; 45:1730–1747. [DOI] [PubMed] [Google Scholar]
- 91.Fernández L, Rodríguez JM. Human milk microbiota: origin and potential uses. Nestle Nutr Inst Workshop Ser 2020; 94:75–85. [DOI] [PubMed] [Google Scholar]
- 92.Moossavi S, Sepehri S, Robertson B, et al. Composition and variation of the human milk microbiota are influenced by maternal and early-life factors. Cell Host Microbe 2019; 25:324–335e4. [DOI] [PubMed] [Google Scholar]
- 93.Gensollen T, Iyer SS, Kasper DL, et al. How colonization by microbiota in early life shapes the immune system. Science 2016; 352:539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dzidic M, Boix-Amorós A, Selma-Royo M, et al. Gut Microbiota and mucosal immunity in the neonate. Med Sci 2018; 6:56.doi: 10.3390/medsci6030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95▪.Fehr K, Moossavi S, Sbihi H, et al. Breastmilk feeding practices are associated with the co-occurrence of bacteria in mothers’ milk and the infant gut: the CHILD Cohort Study. Cell Host Microbe 2020; 28:285–297.e4. [DOI] [PubMed] [Google Scholar]; Analysis of mother's milk and infant stool from 1249 dyads provides evidence that certain microbes are shared between mothers and infants during breastfeeding, and this sharing is reduced when breast milk is pumped and fed from a bottle.
- 96.Ohland CL, MacNaughton WK. Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol Gastrointest Liver Physiol 2010; 298:G807–G819. [DOI] [PubMed] [Google Scholar]
- 97.Lutgendorff F, Akkermans L, Soderholm J. The role of microbiota and probiotics in stress-induced gastrointestinal damage. Curr Mol Med 2008; 8:282–298. [DOI] [PubMed] [Google Scholar]
- 98.Cario E, Gerken G, Podolsky DK. Toll-Like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 2007; 132:1359–1374. [DOI] [PubMed] [Google Scholar]
- 99.Yoshioka H, Iseki K, Fujita K. Development and differences of intestinal flora in the neonatal period in breastfed and bottle-fed infants. Pediatrics 1983; 72:317–321. [PubMed] [Google Scholar]
- 100.Canani RB, Costanzo MDi, Leone L, et al. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol 2011; 17:1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cait A, Cardenas E, Dimitriu PA, et al. Reduced genetic potential for butyrate fermentation in the gut microbiome of infants who develop allergic sensitization. J Allergy Clin Immunol 2019; 144:1638–1647.e3. [DOI] [PubMed] [Google Scholar]
- 102▪.Bode L. Human milk oligosaccharides: structure and functions. Nestle Nutr Inst Workshop Ser 2020; 94:115–123. [DOI] [PubMed] [Google Scholar]; Updated review on HMOs and their physiological functions.
- 103.Azad MB, Robertson B, Atakora F, et al. Human milk oligosaccharide concentrations are associated with multiple fixed and modifiable maternal characteristics, environmental factors, and feeding practices. J Nutr 2018; 148:1733–1742. [DOI] [PubMed] [Google Scholar]
- 104.German J, Freeman S, Lebrilla C, et al. Human milk oligosaccharides: Evolution, structures and bioselectivity as substrates for intestinal bacteria. Nestle Nutr Workshop Ser Pediatr Program 2008; 62:205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bode L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology 2012; 22:1147–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Triantis V, Bode L, van Neerven JRJ. Immunological effects of human milk oligosaccharides. Front Pediatr 2018; 6:190.doi: 10.3389/fped.2018.00190. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107▪.Cheng L, Kong C, Walvoort MTC, et al. Human milk oligosaccharides differently modulate goblet cells under homeostatic, proinflammatory conditions and ER Stress. Mol Nutr Food Res 2020; 64:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cell culture experiments suggest that HMOs enhance mucus barrier function through direct modulation of intestinal goblet cell gene expression.
- 108▪▪.Zhang W, He-Yang J, Zhuang W, et al. Causative role of mast cell and mast cell-regulatory function of disialyllacto-N-tetraose in necrotizing enterocolitis. Int Immunopharmacol 2021; 96:107597–107604. [DOI] [PubMed] [Google Scholar]; In rats, the HMO DSLNT favorably modulated mast cell homeostasis and epithelium barrier function, contributing to attenuated necrotizing enerotcolitis.
- 109.Albenzio M, Santillo A, Stolfi I, et al. Lactoferrin levels in human milk after preterm and term delivery. Am J Perinatol 2016; 33:1085–1089. [DOI] [PubMed] [Google Scholar]
- 110.Buescher ES. Anti-inflammatory characteristics of human milk: how, where, why. In: Advances in Experimental Medicine and Biology 2001; 501:207–222. [DOI] [PubMed] [Google Scholar]
- 111.Palmeira P, Carneiro-Sampaio M. Immunology of breast milk. Rev Assoc Med Bras 2016; 62:584–593. [DOI] [PubMed] [Google Scholar]
- 112.Griffiths J, Jenkins P, Vargova M, et al. Enteral lactoferrin supplementation for very preterm infants: a randomised placebo-controlled trial. Lancet 2019; 393:423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vega-Bautista A, de la Garza M, Carrero JC, et al. The impact of lactoferrin on the growth of intestinal inhabitant bacteria. Int J Mol Sci 2019; 20:4707–4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mathias A, Pais B, Favre L, et al. Role of secretory IgA in the mucosal sensing of commensal bacteria. Gut Microbes 2015; 5:688–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mantis NJ, Rol N, Corthésy B. Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol 2011; 4:603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Petar P, Dubois D, Rabin BS, et al. Immunoglobulin titers and immunoglobulin subtypes. Meas Immun 2005; 158–171. [Google Scholar]
- 117.Wright AL, Sherrill D, Holberg CJ, et al. Breast-feeding, maternal IgE, and total serum IgE in childhood. J Allergy Clin Immunol 1999; 104:589–594. [DOI] [PubMed] [Google Scholar]
- 118.Paul WE, Zhu J. How are TH2-type immune responses initiated and amplified? Nat Rev Immunol 2010; 10:225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Borish L. IL-10: evolving concepts. J Allergy Clin Immunol 1998; 101:293–297. [DOI] [PubMed] [Google Scholar]
- 120.Kany S, Vollrath JT, Relja B. Cytokines in inflammatory disease. Int J Mol Sci 2019; 20:6008–6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol 2008; 8:533–544. [DOI] [PubMed] [Google Scholar]
- 122.Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity 2012; 36:705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.LeBouder E, Rey-Nores JE, Rushmere NK, et al. Soluble forms of toll-like receptor (TLR)2 capable of modulating TLR2 signaling are present in human plasma and breast milk. J Immunol 2003; 171:6680–6689. [DOI] [PubMed] [Google Scholar]
- 124.Snijders BEP, Damoiseaux JGMC, Penders J, et al. Cytokines and soluble CD14 in breast milk in relation with atopic manifestations in mother and infant (KOALA Study). Clin Exp Allergy 2006; 36:1609–1615. [DOI] [PubMed] [Google Scholar]
- 125.Donovan SM, Odle J. Growth factors in milk as mediators of infant development. Annu Rev Nutr 1994; 14:147–167. [DOI] [PubMed] [Google Scholar]
- 126.Coombes JL, Siddiqui KRR, Arancibia-Cárcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β -and retinoic acid-dependent mechanism. J Exp Med 2007; 204:1757–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]