Abstract
Purpose of review
The purpose of this review is to summarize available data on fertility, fertility preservation, pregnancy and parenthood following lung transplantation for people with cystic fibrosis (pwCF).
Recent findings
In the era of cystic fibrosis transmembrane conductance regulator (CFTR) modulator use, oral therapies that positively impact fundamental CFTR protein abnormalities, the number of pregnancies has increased dramatically with a concomitant decrease in lung transplantation. Nonetheless, some pwCF still require lung transplantation as a life-saving measure, and a fraction of those individuals desires parenthood. Cystic fibrosis (CF) providers infrequently discuss fertility preservation with pwCF, and pwCF feel uneducated about their fertility options posttransplant. However, because the immunosuppression required to successfully maintain lung allografts may impact future fertility, pwCF should receive genetic and reproductive counseling prior to lung transplantation. While pregnancies posttransplantation are high-risk, selected females with CF may be able to pursue this path to parenthood.
Summary
Although there is a paucity of data specific to pwCF who have undergone lung transplantation, recently developed general guidelines should inform discussions regarding fertility, pregnancy and parenthood in pwCF who desire parenthood following lung transplantation for optimal shared decision-making.
Keywords: cystic fibrosis, fertility, lung transplantation, parenthood, pregnancy
INTRODUCTION
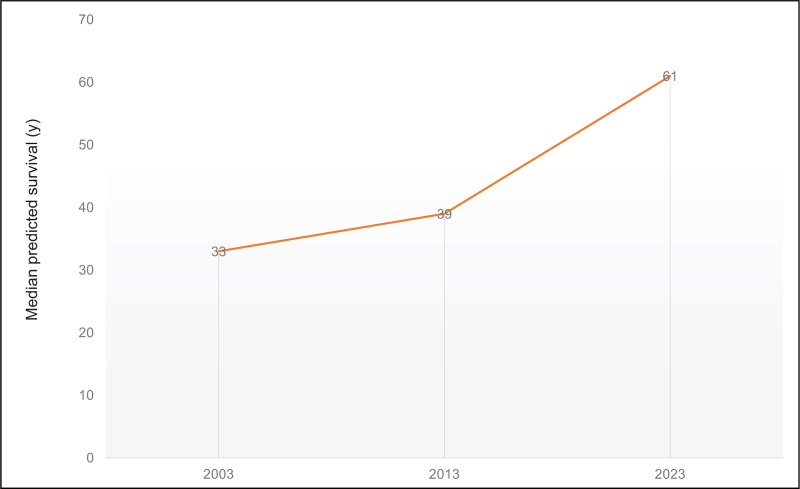
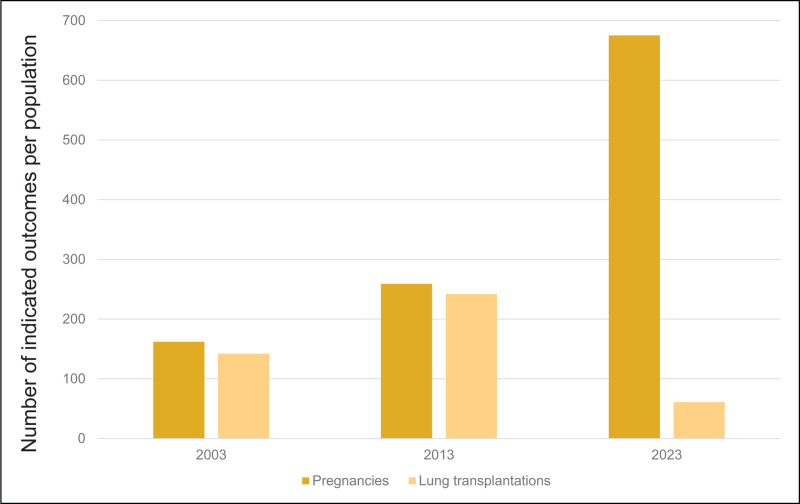
When cystic fibrosis (CF) was described as a clinical entity in the 1930 s, the diagnosis was associated with death in infancy or early childhood [1]. With the advances we have made in CF care and treatment, the predicted survival for a child born today in the United States (US) is 61 years of age [2] (Fig. 1). However, that predicted survival includes the majority of people with CF in the US and other high-income countries who have access to oral CF transmembrane conductance regulator (CFTR) modulator therapy; therapy that improves lung function, nutritional status, quality of life and pulmonary exacerbations [3–5]. The health impacts conferred by CFTR modulators have been so substantial that there are now more than ten times as many pregnancies in people with CF as lung transplants in the US [2] (Fig. 2). However, for those pwCF with variants not amenable to CFTR modulators and those who had very advanced disease at the time CFTR modulators were approved, lung transplantation may still be the only life-saving treatment option.
FIGURE 1.
Median predicted survival of individuals with cystic fibrosis the last 20 years the US CF Foundation Patient Registry [2].
FIGURE 2.
Rates of pregnancy and lung transplantation over the last 20 years the US CF Foundation Patient Registry [2,39].
Box 1.
no caption available
Those pwCF whose hope for a future has been bolstered by CFTR modulators have expressed a desire to become parents [6,7]. However, some pwCF who have undergone organ transplantation also wish to consider parenthood. Historically, pursuit of pregnancy and/or parenthood following transplant was highly discouraged, but more recently, guidelines have emerged to facilitate this choice for select posttransplant individuals [8▪▪]. In this review, we will discuss the available data on fertility, fertility preservation, pregnancy and partner pregnancy and parenthood in people with CF who have undergone lung transplantation.
Case presentation
A 20-year-old female with CF (fwCF) with moderate CF lung disease who is heterozygous for F508del and class I variants transitions from the pediatric CF program. Following transition, she is very adherent to recommended therapies, but contracts a viral illness and experiences a severe pulmonary exacerbation with a 50% decrease in her lung function. In spite of hospitalization, her lung function does not recover and she now requires oxygen with minimal exercise. You discuss pulmonary rehabilitation and lung transplant referral. She agrees to both referrals, but states that she has heard that it is difficult to have a baby after lung transplant and asks if there is anything she can do to ensure that she can still have a child someday?
FERTILITY
In 1968, Kaplan et al. [9] at the CF center in Boston sought to determine why many of the fwCF in their clinic were able to conceive, but the males with CF (mwCF) were not. Through physical examination, semen analysis and pathological specimen analysis, they demonstrated that the majority of mwCF were infertile based on absence of the vas deferens and parts of the epididymis. Subsequently, case series of two fetuses with genotypes F508del/F508del and F508del/G542 at 12 and 18 weeks, respectively, reported intact vas deferens in both male fetuses suggesting that as occurs in the pancreas, the pathological process caused by absent CFTR function results in in-utero loss of the vas deferens [10]. However, while most mwCF have CBAVD, they do produce sperm. Therefore, for those with the financial means to pursue assisted reproduction, retrieval of sperm from the testes or epididymis coupled with in-vitro fertilization and embryo implantation for the male's partner can successfully lead to pregnancy [11].
In contrast to mwCF, fwCF have reproductive anatomy that is similar to that of women without CF, thus the majority of fwCF can become pregnant without assisted reproduction. Factors that historically contributed to fwCF having lower fertility than those in the general population included malnutrition, delayed puberty, reduced ovarian reserve, and thick, acidic cervical mucus (caused by CFTR dysfunction in the epithelium of the uterus) [12,13]. More recently, Shteinberg et al. [14] reported a subfertility rate of 35% in fwCF; pancreatic insufficiency and older age at first pregnancy attempt were risk factors. Critically, the data gathered in the Shteinberg study was in the pre-CFTR modulator era. Roe et al. [15] showed that fwCF treated with the most effective approved CFTR modulator, elexacaftor-tezacaftor-ivacaftor (ETI) had cervical mucus quality comparable to that of females without CF. This improved cervical mucus quality has undoubtedly contributed to the increased pregnancy rate in fwCF in the USA (Fig. 2).
Following lung transplantation, fertility may be impaired for both mwCF and fwCF based on the immunosuppressive regimen required to maintain their transplants. (See Table 1). For example, for both sexes, glucocorticoid dosing in the first 2 years postlung transplantation may inhibit the hypothalamic-pituitary-gonadal axis [16]. In one study of more than 1000 organ transplant recipients, more than 20% of females were unable to achieve pregnancy [17▪▪]. In males, use of sirolimus may lead to decreased sperm counts and dysmotility resulting in reduction in the likelihood of partner pregnancy [16]. Because of the potential impairment in fertility resulting from required posttransplant immunosuppression, discussions regarding future fertility should occur early in the process of transplant referral [18].
Table 1.
Immunosuppressive agents in pregnancy
| Drug class | Safety for use in pregnancy | Risk of teratogenicity | Special instruction | Safety for use while breastfeeding |
| Corticosteroids | Relatively well tolerated at contemporary clinical dosing | Cleft palate may occur with high doses used in the first trimester (over, 10–15 mg prednisolone daily) | Continue treatment with corticosteroids when indicated | Yes |
| Calcineurin inhibitors Cyclosporine Tacrolimus |
Relatively well tolerated at contemporary clinical dosing | No teratogenic potential in human registries | Frequent monitoring of levels (every 2–4 weeks) | Yes |
| mTOR inhibitors Everolimus Sirolimus |
Insufficient data to affirm safety | Limited data in humans; potential risk from animal studies | Discontinue 6–12 weeks before planned conception; evaluate risk vs. benefit on case-by-case basis in heart transplant recipients with CAV | Limited evidence |
| Mycophenolate products Mycophenolate mofetil Mycophenolate sodium |
No | Teratogen: risk of spontaneous abortion and congenital malformations | Discontinue 6–12 weeks before planned conception or immediately if unplanned | Not recommended |
| Azathioprine | No evidence of teratogenic effect in human studies | May use in place of mycophenolate depending on patient's risk of rejection | Relatively well tolerated at contemporary clinical dosing | No evidence of teratogenic effect in human studies |
| Belatacept | Insufficient data to affirm safety | Limited data to establish risk | Pregnancy while on belatacept is strongly discouraged | Not recommended |
CAV, cardiac allograft vasculopathy; mTOR, mammalian target of rapamycin.
Reproduced with permission from [8▪▪].
Discussions regarding saving or protecting eggs, sperm or reproductive tissue for use in creating future biological children, ‘fertility preservation’ is a standard part of oncology visits in people of child-bearing age requiring cancer treatment [19]; CF providers have cited content knowledge barriers to engaging individuals with CF under their care in similar conversations [20▪]. To understand the perspective of pwCF, Ladores et al. [21] conducted a mixed methods study in fwCF to describe their knowledge, experiences and fertility preservation concerns. Although the majority (78%) reported that they wanted to have a child in the future most (74%) stated that their providers had not initiated discussions regarding fertility preservation. Similarly, mwCF were recruited for a two-phase study that was assessing fertility preservation knowledge and also testing a telehealth intervention to improve it. More than 80% stated that they want to have children, but only approximately half (44%) had received information from their CF team regarding infertility [22▪▪]. These studies suggest that provider education is paramount to improving the experience of care regarding fertility preservation prior to lung transplantation.
PREGNANCY
As noted above, pregnancy in fwCF has become much more common in the era of CFTR modulators (Fig. 2), but still remains rare in fwCF after a lung transplantation. As a result, large studies on the impact of pregnancy on maternal and fetal health in the setting of postlung transplant recipients with CF are still lacking. Preconception counseling is essential to assess risks, optimize health and discuss family planning options.
Based on currently available data (largely collected prior to widespread use of CFTR modulators), pregnancy is feasible, but maternal and fetal complications after a lung transplant are unfortunately higher than in the general population. In an analysis published in 2012 using data from the National Transplantation Pregnancy Registry, investigators found that in 30 pregnancies (12 with CF), more than half of women had live births, with few associated life-threatening complications and no reports of permanent disability in the infants [23]. However, the incidence of preterm birth was 60% and there were 11 infant complications and two neonatal deaths. Relative to other transplant recipients, mean gestational age was similar but mean birth weight was lower for babies born to fwCF (1980 g in CF vs. 2349 g in others). There was a lower rate of spontaneous abortion in fwCF (25% in CF vs. 33% in others), but there was also a higher rate of organ rejection reported during pregnancy in fwCF (25% in CF vs. 11% in others). Given reports of increased potential for graft loss, some lung transplant centers recommend that recipients avoid pregnancy all together, while others use published guidelines to consider carefully select individuals who may have safe and successful pregnancies [8▪▪]. Data from the 2022 Transplant Pregnancy Registry International show that only 58% of 50 lung transplant recipients had adequate graft function at last follow-up [17▪▪]. Basic therapies such as calcineurin inhibitors (CNIs), cyclosporine and tacrolimus will require frequent monitoring and potential dose adjustments in association with maternal weight changes or gastrointestinal issues (e.g. gastroesophageal reflux, impaired motility) that complicate the absorption of immunosuppressants and therefore increase the risk of both acute and chronic rejection (CLAD). Certain immunosuppressive agents should be avoided such as mycophenolate mofetil, which is known to be teratogenic [8▪▪].
Medications such as prednisone, azathioprine and calcineurin inhibitors have limited information but are thought to be relatively well tolerated (See Table 1 for further medication information during pregnancy and lactation) [8▪▪]. It is therefore important to consider timing of pregnancy after lung transplant to ideally achieve the lowest necessary doses of immunosuppressive medications during pregnancy and lactation. The higher rates of allograft rejection and more aggressive immunosuppression utilized following lung transplantation support the need for an at least 2 years posttransplant period of stability before pregnancy [8▪▪].
As with safety of medication use in pregnancy, recent reviews and recommendations of medications frequently used in lactating fwCF are published [24,25]. Two key concerns of fwCF when considering lactation are use of medications and nutritional expenditure. Lactation does require significant caloric expenditure (often an additional 500 kcal/day) and restriction of energy/calorie intake is discouraged [26]. Weight loss postpartum can be rapid in fwCF with some returning to their prepregnancy weight within the first 6 weeks postpartum and others not returning to their prepregnancy weight even after 2 years postpartum without aggressive perinatal care [27]. CFTR modulators have improved nutritional status in many eligible pwCF, but the impact of these therapies on weight in lung transplant recipients peri-partum is unknown. Regardless of medication regimen and nutritional status, some clinicians advise against pregnancy if there is evidence of any chronic lung allograft dysfunction in the past year or if there is ongoing maternal infection that may impact the fetus such as cytomegalovirus (CMV) infection, one of the leading causes of congenital neurologic impairment [8▪▪,28]. Other potential contraindications to pregnancy include uncontrolled comorbidities such as diabetes or hypertension, chronic kidney disease (eGFR < 30 ml/min/1.73 m2) or the presence of donor-specific antibodies that might increase the risk of rejection [8▪▪]. Diabetes is a particularly unique challenge for fwCF. CF-related diabetes (CFRD) is common in adults with CF with an estimated prevalence of 30% (CFFPR) and even more common during a pregnancy where 14–20% of women will receive a diagnosis of gestational diabetes (GDM) while pregnant [29,30]. The likelihood of either CFRD or GDM is even higher for patients with severe pancreatic insufficiency and for a lung transplant recipient on chronic prednisone [31]. The management of diabetes shares similar goals as for females without CF and is based on risk mitigation of hyperglycemia-related adverse pregnancy outcomes. As with the general population, insulin is a first-line therapy to treat diabetes during a pregnancy, but fwCF may have higher insulin requirements due to their competing need for adequate nutritional intake. Relative to other lung transplant recipients, other unique challenges in fwCF include malabsorption of fat-soluble vitamins secondary to pancreatic insufficiency, gastroesophageal reflux and gastroparesis, which are all likely to be worsened during pregnancy and may impact on CNI plasma levels [32].
Pregnancies are being followed in the Transplant Pregnancy Registry International long-term [17▪▪]. As of the 2022 annual report, out of 50 pregnant lung transplant recipients, 53% experienced hypertension, 14% experience preeclampsia and 10% had biopsy-proven rejection. Of the 68 pregnancies in lung transplant recipients, 64% resulted in live births, 26% were born at less than 32 weeks’ gestation, 50% delivered by Cesarean section and 7% experienced neonatal death less than 30 days after birth. Maternal deaths after lung transplant were high at 36%. Importantly, more than half of these pregnancies were unplanned. These data are consistent with larger meta-analysis data reported in thoracic transplant recipients that include up to 385 pregnancies in heart or lung transplant recipients [33]. Despite the high risk, successful pregnancies are possible in fwCF after lung transplantation, but this should be discussed in detail between providers and patients to consider timing with medications, optimize comorbidities and include needed expertise such as genetic counselors, high-risk obstetricians, CF and transplant physicians, and endocrinologists as needed. There is ongoing research into the outcomes of pregnancies in pwCF post lung transplant, focusing on maternal health, fetal development and long-term implications, but little is known in the era of CFTR modulators. Currently, a longitudinal prospective observation study called ‘Maternal and Fetal Outcomes in the Era of Modulators’ (MAYFLOWERS) sponsored by the CF Foundation and supported by the CF Therapeutics Development Network, is taking place in 41 CF centers across the USA. Over 280 FwCF pre or postlung transplant and on or not on CFTR modulators will be enrolled and followed during pregnancy, delivery and up to 2 years postpregnancy to assess maternal and fetal outcomes and may provide additional information on pregnancy in fwCF with or without a lung transplant in the modern era of CF care [34]. An open discussion between care teams and transplant recipient is needed to discuss the risks and benefits surrounding pregnancy, and ideally will include more longitudinal data in the future.
PARENTHOOD
Although the TPRI describes the outcomes of pregnancies in women who have undergone lung transplant as noted above, there are very few men who have undergone lung transplantation who have fathered children, thus outcomes specifically for lung transplant recipients have not been reported in previous or current registry reports [17▪▪,35]. A specific evaluation of all male solid-organ transplant recipients who had received pharmaceutical agents containing mycophenolic acid reported that the outcomes for partner pregnancies were similar to those in the general population [35].
There is minimal current literature describing the outcomes of parenthood in people with CF, and none specifically focused on those who have undergone lung transplantation. Kazmerski et al. [36] utilized the United Kingdom (UK) CF Registry to evaluate the short-term health outcomes of people with CF (fwCF who became pregnant and mwCF whose partner became pregnant) in the year following parenthood. Lung function and BMI declined and pulmonary exacerbations increased in the year following parenthood. The impacts of parenthood on lung function decline were somewhat mitigated by single or dual-combination modulator use. No parents who had a history of lung transplant were included in the study, but the results are likely still somewhat applicable to people with CF postlung transplant who continue to experience extra-pulmonary comorbidity from CF. Similarly, the on-going Health Outcomes of Parents with CF (HOPeCF) study (NCT05829694) is excluding those with lung transplantation, but will provide additional insights into outcomes for people with CF who become parents; these results may also have relevance for those parents with CF who have required lung transplantation.
RETURNING TO THE CASE PRESENTATION
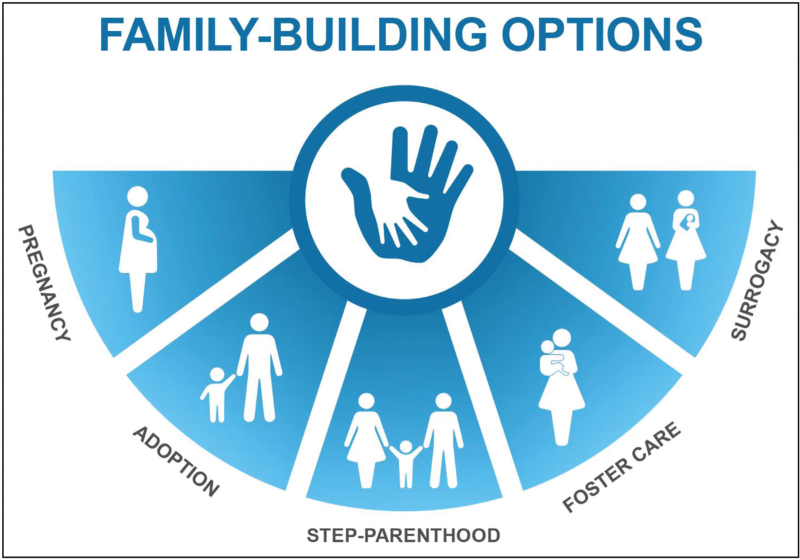
Based on her question regarding pregnancy postlung transplantation and your own knowledge of her options, you explain that you can refer her to a genetic counselor and that she could consider oocyte cryopreservation under the guidance of a fertility specialist [37]. You advise her to discuss the specific guidelines regarding pregnancy postlung transplant with her transplant team who will strongly advise her to use contraception for at least 2 years posttransplantation before attempting to become pregnant [8▪▪]. Finally, you discuss alternative paths to parenthood [38] (Fig. 3).
FIGURE 3.
Options for paths to parenthood. Original figure created and National Jewish Health and previously published in [38].
CONCLUSION
Although the number of lung transplantations has decreased dramatically in the era of CFTR modulators for most people with CF in HIC, lung transplantation continues to be the only life-saving treatment option for those with end-stage disease. For fwCF and mwCF, pretransplantation discussions regarding fertility preservation counseling are essential because transplant-necessitated immunosuppression may limit or eliminate options for unassisted conception posttransplant. Historically, pregnancy, postlung transplantation was discouraged. Today, for highly selected individuals, pregnancy after lung transplantation is a well tolerated and viable option if these requirements are met: adequate and stable graft function, no episodes of allograft rejection in the prior year, no maternal infections that may impact upon the fetus, and stable dosing of maintenance nonteratogenic immunosuppression. Because although maternal and fetal outcomes posttransplant are still relatively precarious compared to those without transplant, transparent counseling is essential for the shared decisions-making process. Additional data regarding long-term morbidity and mortality for people with CF who choose to become parents posttransplantation are needed.
Acknowledgements
The authors appreciate the individual with CF who was willing to include her de-identified story for this manuscript.
Financial support and sponsorship
J.T.C. and R.J. receive grant support from the Cystic Fibrosis Foundation (JAIN21Y3, TAYLOR19Y3) to support the CF Foundation TDN Sexual Health and Reproduction and Gender research working group. R.J. and J.T.C. receive grant support from the Cystic Fibrosis Foundation for the Maternal and Fetal Outcomes in the Era of Modulators (MAYFLOWERS) study (MAYFLOWER-TAYLOR21A0, JAIN21A0).
Conflicts of interest
In the last 36 months, J.T.C. has received grants to her institution from the Cystic Fibrosis Foundation, the National Institutes of Health, Vertex Pharmaceuticals Incorporated, Eloxx, and 4DMT; has received fees from Vertex Pharmaceuticals Incorporated related to consultation on clinical research design; and has served on advisory boards and/or provided clinical trial design consultation for 4DMT. J.T.C. served on a DMC for AbbVie. J.T.C. serves as the adult patient care representative to the CFF Board of Trustees, and on the CF Foundation's Clinical Research Executive Committee, Clinical Research Advisory Board, as immediate past chair of the CF TDN's Sexual Health, Reproduction and Gender Research Working Group, and as Co-Chair of the Heath Equity Team Science Awards study section. She also serves on the scientific advisory board for Emily's Entourage, and on the ATS Respiratory Health Awards Working Group and as Chair-Elect of the International Conference Committee. She is an Associate Editor for the Journal of Cystic Fibrosis and a member of the International Advisory Board for the Lancet Respiratory Medicine Journal. JTC serves on the Clinical Trials Review (CTLR) Study section for the National Institutes of Health/National Heart, Blood, Lung Institute.
In the last 36 months, R.J. has received grants to her University from the CF Foundation, Vertex Pharmaceutical, 4DMT, Insmed, Sound Pharma, and Armata for clinical trial participation. R.J. has also received consulting fees from Boerhinger Ingelheim, Insmed and Recode for serving on their scientific advisory committee, from Syneos for serving on a DSMB, and from Vertex for serving on their Innovation Awards committee.
In the last 36 months, A.S. has received grants to her from Spanish Scientific Society, and Carlos III Institute, National Health Institute, government of Spain. A.S. has also received consulting fees from Vertex Pharmaceutical, Chiesi, Astellas and Gilead; Chiesi for serving on their scientific advisory committee, and from Vertex for serving on their Circle of Care committee.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Andersen DH. Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathologic study. Am J Dis Children 1938; 56:344–399. [Google Scholar]
- 2.Foundation CF. 2023 Cystic Fibrosis Foundation Patient Registry Highlights Handout. 2024; Baltimore, Maryland: https://www.cff.org/medical-professionals/patient-registry [Google Scholar]
- 3.Middleton PG, Mall MA, Drevinek P, et al. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med 2019; 381:1809–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heijerman HGM, McKone EF, Downey DG, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet 2019; 394:1940–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barry PJ, Mall MA, Alvarez A, et al. Triple therapy for cystic fibrosis Phe508del-gating and -residual function genotypes. N Engl J Med 2021; 385:815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kazmerski TM, Sawicki GS, Miller E, et al. Sexual and reproductive healthcare utilization and preferences reported by young women with cystic fibrosis. J Cyst Fibros 2018; 17:64–70. [DOI] [PubMed] [Google Scholar]
- 7.Odie S, Karpel L. [Parenthood challenged by cystic fibrosis. Experience of affected parents]. Rev Mal Respir 2022; 39:420–426. [DOI] [PubMed] [Google Scholar]
- 8▪▪.Kittleson MM, DeFilippis EM, Bhagra CJ, et al. Reproductive health after thoracic transplantation: an ISHLT expert consensus statement. J Heart Lung Transplant 2023; 42:e1–e42. [DOI] [PubMed] [Google Scholar]; This multidisciplinary consensus statement provides critical selection criteria and recommended timing for choosing women likely to have the best pregnancy outcomes following lung transplantation.
- 9.Kaplan E, Shwachman H, Perlmutter AD, et al. Reproductive failure in males with cystic fibrosis. N Engl J Med 1968; 279:65–69. [DOI] [PubMed] [Google Scholar]
- 10.Gaillard DA, Carre-Pigeon F, Lallemand A. Normal vas deferens in fetuses with cystic fibrosis. J Urol 1997; 158:1549–1552. [PubMed] [Google Scholar]
- 11.McBride JA, Kohn TP, Mazur DJ, et al. Sperm retrieval and intracytoplasmic sperm injection outcomes in men with cystic fibrosis disease versus congenital bilateral absence of the vas deferens. Asian J Androl 2021; 23:140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kopito LE, Kosasky HJ, Shwachman H. Water and electrolytes in cervical mucus from patients with cystic fibrosis. Fertil Steril 1973; 24:512–516. [PubMed] [Google Scholar]
- 13.Ahmad A, Ahmed A, Patrizio P. Cystic fibrosis and fertility. Curr Opin Obstet Gynecol 2013; 25:167–172. [DOI] [PubMed] [Google Scholar]
- 14.Shteinberg M, Lulu AB, Downey DG, et al. Failure to conceive in women with CF is associated with pancreatic insufficiency and advancing age. J Cyst Fibros 2019; 18:525–529. [DOI] [PubMed] [Google Scholar]
- 15.Roe AH, Koelper N, McAllister A, et al. Cervical mucus quality in females with and without cystic fibrosis. J Cyst Fibros 2023; 22:804–805. [DOI] [PubMed] [Google Scholar]
- 16.Leroy C, Rigot JM, Leroy M, et al. Immunosuppressive drugs and fertility. Orphanet J Rare Dis 2015; 10:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17▪▪.Moritz M, Constantinescu S, Coscia L. Annual report. Transplant Pregnancy Registry International (TPRI). 2023; Philadelphia, Pennsylvania: https://www.transplantpregnancyregistry.org/ [Google Scholar]; This international registry report contains the most up-to-date information regarding maternal pregnancy and paternal partner pregnancy following thoracic transplanation.
- 18.Ladores S, Bray LA, Brown J. If we would have known’: a couple's regret over a missed opportunity to have a biological child after lung transplantation. J Patient Exp 2018; 5:320–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol 2018; 36:1994–2001. [DOI] [PubMed] [Google Scholar]
- 20▪.Corcoran J, Campbell C, Ladores S. Provider perspectives on fertility and fertility preservation discussions among women with cystic fibrosis. Inquiry 2023; 60:469580231159488. [DOI] [PMC free article] [PubMed] [Google Scholar]; This manuscript reports the only qualitative study regarding fertility preservation discussions by providers who care for people with CF and highlights the lack of provider knowledge on this topic.
- 21.Ladores S, Campbell CM, Bray LA, et al. Fertility preservation in women with cystic fibrosis prelung transplantation: A mixed methods study. J Adv Nurs 2022; 78:532–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22▪▪.Woods BM, Bray LA, Campbell SB, et al. Implementation and evaluation of a fertility preservation telehealth counseling intervention for males with cystic fibrosis. J Cyst Fibros 2024; S1569-1993:00780-X. [DOI] [PubMed] [Google Scholar]; This manuscript describes a two-part study that demonstrates that the lack of baseline knowledge of many men with CF regarding their fertility and fertility preservation options, and that use of a telehealth coaching session was acceptable and effective for improving such knowledge.
- 23.Shaner J, Coscia LA, Constantinescu S, et al. Pregnancy after lung transplant. Prog Transplant 2012; 22:134–140. [DOI] [PubMed] [Google Scholar]
- 24.Middleton PG, Gade EJ, Aguilera C, et al. ERS/TSANZ Task Force Statement on the management of reproduction and pregnancy in women with airways diseases. Eur Respir J 2020; 55:1901208. [DOI] [PubMed] [Google Scholar]
- 25.Kroon M, Akkerman-Nijland AM, Rottier BL, et al. Drugs during pregnancy and breast feeding in women diagnosed with cystic fibrosis - an update. J Cyst Fibros 2018; 17:17–25. [DOI] [PubMed] [Google Scholar]
- 26.Edenborough FP, Borgo G, Knoop C, et al. Guidelines for the management of pregnancy in women with cystic fibrosis. J Cyst Fibros 2008; 7: (Suppl 1): S2–S32. [DOI] [PubMed] [Google Scholar]
- 27.Cheng EY, Goss CH, McKone EF, et al. Aggressive prenatal care results in successful fetal outcomes in CF women. J Cyst Fibros 2006; 5:85–91. [DOI] [PubMed] [Google Scholar]
- 28.Zammarchi L, Lazzarotto T, Andreoni M, et al. Management of cytomegalovirus infection in pregnancy: is it time for valacyclovir? Clin Microbiol Infect 2020; 26:1151–1154. [DOI] [PubMed] [Google Scholar]
- 29.Renton M, Priestley L, Bennett L, et al. Pregnancy outcomes in cystic fibrosis: a 10-year experience from a UK centre. Obstet Med 2015; 8:99–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jain R, Kazmerski TM, Zuckerwise LC, et al. Pregnancy in cystic fibrosis: review of the literature and expert recommendations. J Cyst Fibros 2021; 21:387–395. [DOI] [PubMed] [Google Scholar]
- 31.Giacobbe LE, Nguyen RH, Aguilera MN, et al. Effect of maternal cystic fibrosis genotype on diabetes in pregnancy. Obstet Gynecol 2012; 120:1394–1399. [DOI] [PubMed] [Google Scholar]
- 32.Chambers DC, Cherikh WS, Harhay MO, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult lung and heart-lung transplantation Report-2019; Focus theme: donor and recipient size match. J Heart Lung Transplant 2019; 38:1042–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Acuna S, Zaffar N, Dong S, et al. Pregnancy outcomes in women with cardiothoracic transplants: a systematic review and meta-analysis. J Heart Lung Transplant 2020; 39:93–102. [DOI] [PubMed] [Google Scholar]
- 34.Jain R, Magaret A, Vu PT, et al. Prospectively evaluating maternal and fetal outcomes in the era of CFTR modulators: the MAYFLOWERS observational clinical trial study design. BMJ Open Respir Res 2022; 9:e001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones A, Clary MJ, McDermott E, et al. Outcomes of pregnancies fathered by solid-organ transplant recipients exposed to mycophenolic acid products. Prog Transplant 2013; 23:153–157. [DOI] [PubMed] [Google Scholar]
- 36.Kazmerski TM, Jain R, Lee M, Taylor-Cousar JL. Parenthood impacts short-term health outcomes in people with cystic fibrosis. J Cyst Fibros 2022; 21:662–668. [DOI] [PubMed] [Google Scholar]
- 37.Hagege E, Sokteang S, Ayoubi JM, de Ziegler D. Fertility preservation counseling: old indications, novel perspectives. Fertil Steril 2024; 121:553–554. [DOI] [PubMed] [Google Scholar]
- 38.Kazmerski TM, West NE, Jain R, et al. Family-building and parenting considerations for people with cystic fibrosis. Pediatr Pulmonol 2021; 57: (Suppl 1): S75–S88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Foundation CF. 2022 Annual Data Report. Baltimore, Maryland: Cystic Fibrosis Foundation; 2023. https://www.cff.org/medical-professionals/patient-registry. [Google Scholar]