Abstract
Introduction:
There is high prevalence of non-alcoholic fatty liver disease in individuals with type 2 diabetes mellitus (T2D), and available evidence suggests higher prevalence of NASH and advanced stages of fibrosis among T2D. Data regarding prevalence of clinically significant liver fibrosis (CSLF) in individuals with T2D is scarce. We investigated the prevalence of transient elastography (TE)-proven CSLF among patients of T2D attending a diabetes clinic at a tertiary care center.
Methods:
A cross-sectional descriptive evaluation study of 603 consecutive adults with T2D was conducted to detect CSLF using TE. Steatosis was diagnosed using a controlled attenuation parameter >237 dB/m.
Results:
The prevalence of CSLF was 22.7%, and the prevalence of steatosis was 58.9% in our study. A higher body mass index (BMI) (P = 0.001), aspartate aminotransferase (AST; P = 0.0001), alanine aminotransferase (ALT; P = 0.0001), and low platelets (P = 0.0001) were independent factors associated with CSLF. Elevated ALT and AST (≥40 units/L) levels were present in only 27.7% and 37.2% of individuals with CSLF, respectively. Twenty-six (4.31%) individuals had LSM > 13.0 kPa.
Conclusion:
CSLF is highly prevalent in T2D patients attending a diabetes clinic at a tertiary care center, and the majority of such individuals have normal transaminase levels. Higher BMI, AST, and ALT values and lower platelet counts are associated with liver fibrosis.
Keywords: FibroScan, cirrhosis, clinically relevant liver fibrosis, non-alcoholic fatty liver disease, steatosis, type 2 diabetes mellitus
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is very common in people with type 2 diabetes mellitus (T2D); approximately two thirds of people with diabetes are diagnosed with NAFLD.[1] T2D itself is an aggravating factor for NAFLD and is associated with an increased risk of developing non-alcoholic steatohepatitis, liver fibrosis, cirrhosis, and hepatocellular carcinoma.[2] The European Association for the Study of the Liver (EASL), the European Association for the Study of Diabetes (EASD), and the European Association for the Study of Obesity (EASO) proposed recommendations for the diagnosis, treatment, and follow-up of NAFLD.[3] These guidelines recommended to look for liver fibrosis in people with T2D irrespective of liver enzyme levels as they are in high risk of disease progression. Biomarkers, fibrosis scores, and transient elastography (TE) are considered acceptable non-invasive tools for the identification of cases with a low risk of advanced fibrosis/cirrhosis.[3] Traditionally, liver biopsy is the gold standard for the assessment of hepatic necroinflammation and fibrosis.[4] However, a standard liver biopsy sample only represents approximately 1/50,000 of the whole liver mass, and therefore, sampling bias may occur.[4] TE measures liver stiffness in a volume that approximates a cylinder 1 cm wide and 4 cm long between 25 mm and 65 mm below the skin surface. This volume is at least 100 times bigger than a biopsy sample and is therefore far more representative of the hepatic parenchyma.[5] In a meta-analysis of nine studies, the pooled estimates of sensitivity and specificity of TE when compared to liver biopsy were 87% and 91%, respectively, for cirrhosis and 70% and 84%, respectively, for F2 (perisinusoidal and periportal/portal fibrosis) or higher disease.[6] Another meta-analysis too reported good (88%–89%) and excellent (93%–96%) accuracies of TE for diagnosing advanced fibrosis and cirrhosis, respectively.[7] Globally, TE is considered to be the non-invasive gold standard for screening of significant liver fibrosis, and therefore, we selected it as our screening modality.
The aim of our study was to estimate prevalence of TE-proven clinically significant liver fibrosis (CSLF) among patients of T2D attending a diabetes clinic at a tertiary care center.
MATERIALS AND METHODS
Study population: We conducted a cross-sectional descriptive evaluation study of 603 consecutive patients with T2D in the Department of Endocrinology between July 2022 and February 2023. We included patients aged 18 to 65 years. Men who consumed more than 30 g of alcohol per day and women who consumed more than 20 g of alcohol per day were excluded. Patients with secondary causes of hepatic steatosis (e.g., chronic use of systemic corticosteroids), positive hepatitis B surface antigen or anti-hepatitis C virus antibody, or known history of other concomitant chronic liver diseases or having thyroid dysfunction were excluded. Patients already receiving pharmacotherapy for NAFLD, like pioglitazone or saroglitazar, were also excluded.
Sample Size: Based on the anticipated prevalence of 57% and a precision of 4% at 95% confidence interval (CI), we arrived a sample size of 588. Considering the turnover of patients with long-standing diabetes mellitus, the sample size was rounded off to 600.[8]
Thus, a total of 600 participants with T2D were included in the study. The formula used to estimate the sample size was
n = Z2pq/d2
For estimation of single proportion,
Z = standard normal variant
d = precision (4%) at 95% CI
p = prevalence (57%)
q = 1-prevalence (43%)
n = (1.96)2 × 0.57 × (1 − 0.57)/(0.04)2 = 588
Clinical assessment: Comprehensive clinical assessment was performed. Anthropometric tests included body weight and body height measurements. Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. Normal weight, overweight, and obesity were defined as BMI <23 kg/m2, BMI between ≥23 and <25 kg/m2, and BMI ≥25 kg/m2, respectively, in accordance with World Health Organization (WHO) Asia Pacific guidelines.[9] Blood for complete blood count and liver function test was collected after TE and analyzed on the same day in the Central Laboratory of the institute.
Liver stiffness and CAP measurements: Liver stiffness and the controlled attenuation parameter (CAP) were measured by TE. TE was performed by a single operator with FibroScan® Mini+ 430 model by Echosens on the right lobe of the liver, through the intercostal spaces, with the participant lying flat on his or her back with the right arm laying in maximal abduction. Either M- or XL-probe was applied, according to the instructions by the manufacturer. The final liver stiffness result was expressed in kiloPascal (kPa). CAP has been designed to measure liver ultrasonic attenuation (go and return path) at 3.5MHz on the signals acquired by the FibroScan®. CAP was computed only when the associated liver stiffness measurement was valid and using the same signals as the one used to measure liver stiffness. Therefore, both stiffness and CAP were obtained simultaneously and in the same volume of liver parenchyma. Based on CAP values stipulated by the manufacturer (FibroScan by Echosens), hepatic steatosis was graded as follows: <237 dB/m: normal liver, 237–259 dB/m: grade 1 steatosis, 260–292 dB/m: grade 2 steatosis, and >292 dB/m: grade 3 steatosis.[10] Liver stiffness measurement (LSM) ≥ 8.0 kPa was taken as the cut-off for CSLF) and LSM ≥13.0 kPa for cirrhosis. This cut-off level was chosen because this is known to yield high positive predictive values (PPVs) for the presence of clinically significant fibrosis in previous studies.[11]
Statistical analysis: Statistics for categorical variables were expressed as numbers (percentages). Quantitative variables were described as means with their standard deviations or as medians with their inter-quartile range if not normally distributed. An unpaired t-test was used for comparisons of continuous variables between groups for normally distributed data, and a Mann–Whitney U-test was used for skewed data. For comparisons of categoric variables, the Chi-squared or Fisher exact test was used. A P value <0.05 was considered as statistically significant.
Ethical Aspect
The study protocol was ratified by the Institutional Ethics Committee (reg no. ECR/609/Instt/WB/2014/RR20), Nil Ratan Sircar Medical College and Hospital, Kolkata (Memo No. NRSMC/IEC/06/2023), approved on 11.01.2023, and written informed consent was obtained for participation in the study and use of the data for research and educational purposes. The study was conducted according to the current version of World Medical Association Declaration of Helsinki (2013).
RESULTS
Patient characteristics: A total of 603 participants with T2D were analyzed after application of inclusion and exclusion criteria. Fifty-two participants were excluded due to various reasons as shown in Figure 1. The mean age of our cohort was 48.21 ± 9.28 years, and 59% participants were females. The mean duration of T2D since diagnosis was 7.07 ± 5.22 years. The mean BMI of the cohort was 24.93 ± 4.00 kg/m2, 126 (20.9%) patients were overweight, 222 (36.82%) patients were found to be obese, and 169 (28.02%) patients had normal BMI. The mean LSM was 7.18 ± 5.97 kPa. The demographics, biochemical results, and co-morbidities of all study participants with T2D are summarized in Table 1. Overall, 355 (58.9%) participants had hepatic steatosis. Among them, 14.8%, 21.2%, and 22.9% individuals had CAP-based grade 1, grade 2, and grade 3 steatosis, respectively [Table 1]. 137 (22.7%) participants had LSM ≥ 8.0 kPa, suggesting CSLF. Grading of CAP-based steatosis and LSM-based fibrosis is given in Table 1.
Figure 1.
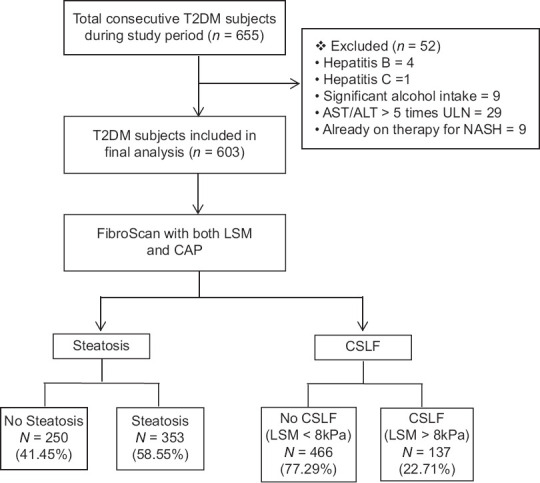
Derivation of the study cohort. LSM, liver stiffness measurement; NAFLD, non-alcoholic fatty liver disease; T2D, type 2 diabetes
Table 1.
Baseline characteristics of all participants
| Characteristics | All participants (n=603) |
|---|---|
| Age (years) | 48.21±9.28 |
| Male | 246 (40.8%) |
| Female | 357 (59.2%) |
| BMI (kg/m2) | 24.93±4.00 |
| Diabetes Duration (years) | 7.07±5.22 |
| Platelet count (*109/L) | 215.43±76.36 |
| AST (U/L) | 28.04±16.63 |
| ALT (U/L) | 27.91±18.88 |
| CAP (db/m) | 251.96±50.31 |
| Steatosis by CAP | |
| Grade 0 (<11% fat) n (%) | 248 (41.1%) |
| Grade 1 (11-33% fat) n (%) | 89 (14.8%) |
| Grade 2 (33-67% fat) n (%) | 128 (21.2%) |
| Grade 3 (>67% fat) n (%) | 138 (22.9%) |
| Transient Elastography | |
| Liver Stiffness Measurement (LSM), kPa | 7.18±5.97 |
| Clinically Significant Liver Fibrosis (CSLF), LSM >8kPa, n (%) | 137 (22.7%) |
| LSM-based cirrhosis, LSM >13.0kPa, n (%) | 26 (4.31%) |
Data are presented as mean (SD), or percentage. BMI, body mass index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; CAP, controlled attenuation parameter
Prevalence of Steatosis and associated risk factors: The prevalence of steatosis was 58.9%. Subjects with steatosis had higher BMI (26.32 vs 22.96 kg/m2, P < 0.0001), a shorter duration of diabetes (6.6 years vs 7.7 years, P < 0.0001), more elevation of AST (30.19 vs 24.97 U/L, P < 0.0001), and ALT (30.30 vs 24.50 U/L, P = 0.0002) compared to those without steatosis [Table 2]. No significant differences in age and platelet levels were observed.
Table 2.
Characteristics of T2D participants with and without steatosis
| No Steatosis | Steatosis | P | |
|---|---|---|---|
| No. of patients | 248 | 355 | - |
| Age (years) | 48.96±9.06 | 47.69±9.42 | 0.994 |
| Female | 125 (50.4%) | 232 (65.4%) | 0.0002* |
| BMI (kg/m2) | 22.96±3.59 | 26.32±3.68 | <0.0001* |
| Diabetes duration (years) | 7.70±5.30 | 6.64±5.12 | 0.0141* |
| Platelet count (*109/L) | 217.85±79.32 | 213.74±74.28 | 0.5149 |
| AST (U/L) | 24.97±13.45 | 30.19±18.24 | <0.0001* |
| ALT (U/L) | 24.50±16.92 | 30.30±19.82 | 0.0002* |
| LSM (kPa) | 6.42±4.99 | 7.71±6.52 | 0.008* |
*Statistically significant. Data are presented as mean (SD), or percentage. BMI, body mass index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LSM, liver stiffness measurement
Prevalence of CSLF and associated risk factors: CSLF was detected in 137 (22.7%) participants. Subjects with CSLF (LSM ≥ 8.0 kPa) had higher BMI (26.77 ± 4.32 vs 24.4 ± 3.75 kg/m2, P = 0.0001), more elevation of AST (40.75 vs 24.31 U/L, P = 0.0001), ALT (35.93 vs 25.56 U/L, P = 0.0001), and a lower platelet count (159.07 * 109/L vs 232 * 109/L, P = 0.0001) as compared to those without CSLF (LSM < 8.0 kPa). No significant differences in age or duration of diabetes were observed [Table 3].
Table 3.
Characteristics of T2D participants with and without CSLF
| LSM <8 | LSM >8 | P | |
|---|---|---|---|
| No. of patients | 466 | 137 | - |
| Age (years) | 47.96±9.38 | 49.08±8.94 | 0.2145 |
| Female | 279 (59.9%) | 78 (56.9%) | 0.54 |
| BMI (kg/m2) | 24.4±3.75 | 26.77±4.30 | <0.0001* |
| Diabetes duration (years) | 7.21±5.02 | 6.62±5.83 | 0.25 |
| Platelet count (*109/L) | 232±37.94 | 159±54.55 | <0.0001* |
| AST (U/L) | 24.31±10.43 | 40.75±25.33 | <0.0001* |
| ALT (U/L) | 25.56±16.02 | 35.93±24.82 | <0.0001* |
| CAP (dB/m) | 246.29±48.56 | 271.26±51.51 | <0.0001* |
*Statistically significant. Data are presented as mean (SD), or percentage. BMI, body mass index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; CAP, controlled attenuation parameter; LSM, liver stiffness measurement
Prevalence of raised transaminases in individuals with T2D and CSLF: Thirty-eight (27.7%) individuals with CSLF had elevated ALT (≥40 U/L). Fifty-one (37.2%) individuals with CSLF had elevated AST (≥40 U/L). 99 (72.3%) and 86 (62.8%) individuals with CSLF had normal ALT and AST, respectively. 80 (58%) individuals with CSLF were having both AST and ALT < 40 U/L.
Prevalence of cirrhosis and associated risk factors: Twenty-six (4.31%) participants were found to have LSM > 13.0 kPa (cirrhosis). Of these, 14 (53.8%) were males, and the mean age of those with cirrhosis was 50.26 years and the mean duration of diabetes was 6.46 ± 4.27 years.
DISCUSSION
There are several published studies on prevalence of NAFLD in T2D population from different parts of India. This however is the first cross-sectional study to report the prevalence of CSLF in T2D from the eastern part of India. The majority of epidemiological studies on NAFLD in general or in T2D population in particular are based on histological evidence of steatosis or fatty infiltration proven by ultrasonography (USG) or raised transaminase levels on biochemical testing. This study makes an effort to estimate the prevalence of CSLF among T2D patients on the basis of CAP. It is clinically relevant to establish the prevalence of CSLF in this population not only because it is a major risk factor for the progression of liver disease but also because liver fibrosis is associated with the risk of microvascular and macrovascular disease in patients with T2D.[12,13,14] Our study was unique in screening patients with TE, establishing the prevalence of both hepatic steatosis and CSLF in a single sitting. In our study, the prevalence of hepatic steatosis was 58.9%, which is in line with the regional prevalence of 57.87% in South Asia shown by Younussi et al. but lower than 84.2% described by Kuchay et al.[8,15] The prevalence of CSLF in our study was 22.7% (137/603). This result is similar to a meta-analysis of global prevalence of NAFLD and steatohepatitis in overweight and obese population where clinically significant fibrosis was present in 20.27% of overweight and 21.6% of obese patients.[16] However, the prevalence of CSLF in our study (22.7%) is somewhat less than the prevalence found in a study done by Kuchay et al., in which it was 28.2% among people with T2D.[15] Global prevalence of CSLF is heterogeneous and varies according to the method of screening, country of origin, mean age, and duration of T2D and ranges from 9.51% to 24.16%.[8] The estimated global prevalence of histologically proven NASH among patients with T2D is 37.33%.[8] The prevalence of CSLF among the Indian population is rising due to an improved socio-economic status and Western lifestyle adoption, especially in the urban population. The prevalence of steatosis in this study was numerically more in female participants (65% vs 50% in males), but prevalence of fibrosis was more in male participants (24% vs 21.8% in females). A possible explanation for this finding of lower fibrosis prevalence in females could be the lower age of females (46.8 ± 9.0 years vs 50.3 ± 9.3 years in males) and lower duration of diabetes in females (6.8 ± 4.8 years vs 7.5 ± 5.7 years in males) in our study. In menstruating women, estrogen exhibits a protective effect against the development of fibrosis by activating estrogen receptor-β on the liver, which in turn inhibits hepatic fibrosis via inhibition of hepatic stellate cells.[17] Another finding in our study was that most of the patients with CSLF were having a normal aminotransferase level. Asymptomatic patients with T2D with normal aminotransferase levels are often considered to be free of liver disease. An observational study comprising 63 participants of biopsy-proven NAFLD with normal ALT levels found that 59% of these participants already had liver fibrosis.[18] In another study among T2D patients, >50% participants had biopsy-proven NAFLD despite normal ALT levels (defined as <40 U/L).[19] Thus, a diagnosis of liver steatosis and fibrosis using elevated serum ALT levels might under-estimate the prevalence of CSLF. This observation highlights the importance of screening for liver fibrosis in T2D using appropriate measures irrespective of transaminase levels. Our study had several limitations, including its cross-sectional nature and lack of histopathology results in patients with advanced fibrosis. However, we used TE, which is a non-invasive technique to evaluate hepatic fibrosis and considered as a standard of care. Histopathological examination of a liver specimen obtained by percutaneous biopsy has traditionally been considered as the gold standard for evaluating hepatic fibrosis.[20] However, liver biopsy is an invasive and painful procedure, often with poor patient acceptance, and also carries a significant, although small, risk of life-threatening complications.[21,22] The accuracy of liver biopsy for assessing fibrosis has also been questioned due to sampling errors and intra- and inter-observer variability that may lead to over- or under-estimation of the fibrosis stage.[23,24] Even when an experienced physician performs liver biopsy and an expert pathologist interprets the results, liver biopsy has up to a 20% error rate in disease staging.[25] In addition, it is certainly not the ideal procedure for serial assessment of disease progression. TE has been endorsed as an alternative to liver biopsy by international guidelines in guiding clinical management of NAFLD and is a validated modality in a real-world setting where liver biopsy is not practical for all subjects.[3,26] However, TE has its own limitations like difficulty in measurement in obese patients (especially in patients with BMI >28 because of thick fatty thoracic belt) or in those with narrow intercostal space. Another limitation of our study was that we assessed only individuals with T2D who attended a tertiary care center and who usually have multiple metabolic co-morbidities. Therefore, our study population may represent a somewhat high-risk group, and the results may not apply to community people with T2D at large. The strength of our study is its large sample size and exclusive use of TE in all patients for fibrosis screening.
CONCLUSION
CSLF is highly prevalent in T2D patients attending a diabetes clinic at a tertiary care center with the majority of such individuals having normal transaminase levels. Higher BMI, AST, and ALT values and lower platelet counts are associated with liver fibrosis. It is therefore important to screen all T2D patients for liver fibrosis as recommended by most guidelines.
Authors’ contribution
Rajat Deb, Soumik Goswami and Nilanjan Sengupta contributed to the conception and design of the study, data analysis and manuscript writting
Arjun Baidya, Vibhu Ranjan Khare and Joydip Datta contributed in work up and participant recruitment
Kunal Jhaveri contributed in data analysis
Mousumi Das and Debes Ray contributed in laboratory work (biochemical analysis of blood samples)
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Data availability statement
Data supporting this study is not publicly available due to ethical reasons and further analysis. Data can be made available on request through personal communication to corresponding author email address.
Acknowledgment
We would like to thank all study participants for agreeing to take part in the study. We would also like to thank the scientific committee and institutional ethical committee for approval of the study.
REFERENCES
- 1.Cusi K. Nonalcoholic fatty liver disease in type 2 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2009;16:141–9. doi: 10.1097/MED.0b013e3283293015. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: Where are we?Where do we go? Hepatol Baltim Md. 2014;60:1767–75. doi: 10.1002/hep.27222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver (EASL);European Association for the Study of Diabetes (EASD);EuropeanAssociation for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454–62. doi: 10.1002/hep.23312. [DOI] [PubMed] [Google Scholar]
- 5.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835–47. doi: 10.1016/j.jhep.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound- based transient elastography for the detection of hepatic fibrosis: Systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2007;5:1214–20. doi: 10.1016/j.cgh.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Friedrich-Rust M, Poynard T, Castera L. Critical comparison of elastography methods to assess chronic liver disease. Nat Rev Gastroenterol Hepatol. 2016;13:402–11. doi: 10.1038/nrgastro.2016.86. [DOI] [PubMed] [Google Scholar]
- 8.Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol. 2019;71:793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 9.Regional Office for the Western Pacific (WPRO), World Health Organization. The Asia Pacific Perspective: Redefining Obesity and Its Treatment. St Leonards, Australia: Health Communications Australia Pty Limited; 2000. International Association for the Study of Obesity and the International Obesity Task Force; pp. 22–9. [Google Scholar]
- 10.Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Lédinghen V, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022–30. doi: 10.1016/j.jhep.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 11.Koehler EM, Plompen EP, Schouten JN, Hansen BE, Darwish Murad S, Taimr P, et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: The Rotterdam study. Hepatology. 2016;63:138–47. doi: 10.1002/hep.27981. [DOI] [PubMed] [Google Scholar]
- 12.Tolman KG, Fonseca V, Dalpiaz A, Tan MH. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care. 2007;30:734–43. doi: 10.2337/dc06-1539. [DOI] [PubMed] [Google Scholar]
- 13.Targher G, Mantovani A, Pichiri I. Nonalcoholic Fatty liver disease is independently associated with an increased incidence of chronic kidney disease in patients with type 1 diabetes. Diabetes Care. 2014;37:1729–36. doi: 10.2337/dc13-2704. [DOI] [PubMed] [Google Scholar]
- 14.Targher G, Marra F, Marchesini G. Increased risk of cardiovascular disease in non-alcoholic fatty liver disease: Causal effect or epiphenomenon? Diabetologia. 2008;51:1947–53. doi: 10.1007/s00125-008-1135-4. [DOI] [PubMed] [Google Scholar]
- 15.Kuchay MS, Choudhary NS, Mishra SK, Bano T, Gagneja S, Mathew A. Prevalence of clinically relevant liver fibrosis due to nonalcoholic fatty liver disease in Indian individuals with type 2 diabetes. JGH Open. 2021;5:915–22. doi: 10.1002/jgh3.12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quek J, Chan KE, Wong ZY, Tan C, Tan B, Lim WH. Global prevalence of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in the overweight and obese population: A systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2023;8:20–30. doi: 10.1016/S2468-1253(22)00317-X. [DOI] [PubMed] [Google Scholar]
- 17.Nagral A, Bangar M, Menezes S, Bhatia S, Butt N, Ghosh J. Gender differences in nonalcoholic fatty liver disease. Euroasian J Hepatogastroenterol. 2022;12(Suppl 1):S19–25. doi: 10.5005/jp-journals-10018-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fracanzani AL, Valenti L, Bugianesi E, Andreoletti M, Colli A, Vanni E, et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: A role for insulin resistance and diabetes. Hepatology. 2008;48:792–8. doi: 10.1002/hep.22429. [DOI] [PubMed] [Google Scholar]
- 19.Portillo-Sanchez P, Bril F, Maximos M, Lomonaco R, Biernacki D, Orsak B. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal plasma aminotransferase levels. J Clin Endocrinol Metab. 2015;100:2231–8. doi: 10.1210/jc.2015-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 21.Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: Results of a prospective nationwide survey. For the group of epidemiology of the french association for the study of the liver (AFEF) Hepatology. 2000;32:477–81. doi: 10.1053/jhep.2000.16602. [DOI] [PubMed] [Google Scholar]
- 22.Castera L, Negre I, Samii K, Buffet C. Pain experienced during percutaneous liver biopsy. Hepatology. 1999;30:1529–30. doi: 10.1002/hep.510300624. [DOI] [PubMed] [Google Scholar]
- 23.Bedossa P, Darge`re D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–57. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 24.Rousselet MC, Michalak S, Dupre F, Croue A, Bedossa P, Saint-Andre JP, et al. Sources of variability in histological scoring of chronic viral hepatitis. Hepatology. 2005;41:257–64. doi: 10.1002/hep.20535. [DOI] [PubMed] [Google Scholar]
- 25.Afdhal NH. Diagnosing fibrosis in hepatitis C: Is the pendulum swinging from biopsy to blood tests? Hepatology. 2003;37:972–4. doi: 10.1053/jhep.2003.50223. [DOI] [PubMed] [Google Scholar]
- 26.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–57. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting this study is not publicly available due to ethical reasons and further analysis. Data can be made available on request through personal communication to corresponding author email address.


