Abstract
Purpose of review
Obstructive sleep apnea (OSA) is a common chronic condition that affects over a billion people worldwide and is associated with adverse cardio- and cerebrovascular consequences. Currently, the go-to clinical measure that determines the presence and severity of OSA is the apnea-hypopnea index (AHI). The AHI captures the frequency of respiratory events due to changes in ventilation that are associated with either oxygen desaturations or arousal from sleep. The AHI is poorly correlated to adverse outcomes in OSA with poor prognostic ability. To overcome the limitations of AHI and perhaps driven by the ease of acquisition, several studies have suggested characterizing nocturnal hypoxia in OSA, termed as “hypoxic burden”. The purpose of this review is to focus on the hypoxic burden in OSA, its various definitions, and its utility in moving OSA diagnosis beyond the AHI.
Recent findings
Several measures and definitions of hypoxic burden have been proposed and studied that show promise in overcoming limitations of AHI and also have a greater prognostic ability than AHI. More recently, area-based measures that attempt to characterize the depth and duration of oxygen desaturations, i.e., nocturnal hypoxia in OSA, have been shown to better relate to incident cardiovascular disease than AHI. In this review, we delve into the evidence for these novel area-based metrics and also delve into the pathophysiological concepts underlying nocturnal hypoxia while cautioning the reader on interpretation of the recent findings relating hypoxic burden to adverse outcomes in OSA.
Summary
In this review on hypoxic burden, we focus on the need that has driven the sudden influx of studies assessing hypoxic burden for various outcomes of OSA, its underlying pathophysiology, the various definitions, and clinical relevance. We hope that the reader can appreciate the nuances underlying hypoxic burden in OSA and suggest the need for a cohesive framework for moving beyond the AHI with hypoxic burden.
Keywords: apnea-hypopnea index, cardiovascular disease, hypoxic burden, oxygen desaturation, sleep apnea
INTRODUCTION
Obstructive sleep apnea (OSA) is a common chronic disorder that is estimated to affect over a billion people worldwide [1] and when left untreated, OSA is associated with adverse consequences such as daytime sleepiness, cardiovascular disease (CVD) and neurocognitive impairment [2]. OSA is characterized by repetitive events of either complete (apneas) or partial upper airway collapse (hypopneas). Immediate consequences of these repetitive events include oxygen desaturation and arousal from sleep. Regardless of the underlying mechanisms, be it anatomic or nonanatomic, that cause upper airway collapse, the immediate consequences of oxygen desaturation and arousal from sleep are commonly observed in almost all OSA patients. It is thus not surprising that the apnea-hypopnea index (AHI) which considers the frequency of respiratory events associated with either oxygen desaturation or arousals from sleep has become the prevailing measure of both the presence and the severity of OSA as a disease. However, AHI is inconsistently related to adverse consequences of OSA [3,4▪▪,5–13]. While epidemiological studies suggest that OSA, determined by AHI, is associated with CVD mortality and morbidity [14–18], baseline AHI or changes in AHI with treatment of OSA fail to predict adverse outcomes [19]. Further, pretreatment AHI poorly predicts the degree of clinical improvement that is obtained with treatment of OSA. As such, it is not unexpected that randomized control trials that have hypothesized that treatment of OSA would lead to lower incidence of CVD, have not yet been successful [20,21].
One possible reason that AHI, as a universally used metric for presence of OSA as well as its severity, is inconsistently associated with adverse outcomes, is that it fails to capture the depth and breadth of OSA as a disease. It has been shown that not only individuals have differing degrees of ventilatory deficit, but the responses to those ventilatory deficit differ as well [22–24]. AHI disregards this heterogeneity and groups individuals into similar groups, and while one way to capture this heterogeneity might be to design a completely new measure, or perhaps two, one for the presence and another for severity of OSA, a simpler alternative might be to re-examine AHI and its usage. AHI can be considered a fixed combination of three possibly independent domains: ventilatory, hypoxic, and arousal. Several studies have suggested that measures that characterize OSA along these domains have better prognostic ability than AHI. Butler et al. studied event duration of respiratory events and found that it was associated with CVD more so than AHI [3]. Along the respiratory domain, work from our group also suggests that the breath-by-breath amplitudes, derived in an automated fashion, is a strong predictor of incident CVD than AHI [4▪▪,25▪▪]. Azarbarzin et al. studied the area under candidate oxygen desaturation events and similarly found that their measure of nocturnal hypoxia was better related to CVD than AHI [26]. And along the arousal domain, measures of arousal intensity were likewise shown to be stronger predictors of CVD than AHI [22]. Furthermore, work from our group also suggests that a data-driven combination of measures along these three domains may better predict immediate and long-term consequences of OSA [25▪▪].
In our continually evolving understanding of OSA as a disease, evidence from several studies suggests that changes in ventilation, whether due to single or multiple factors underlying pathogenesis of OSA, precipitates changes in blood gas which leads to increased nocturnal hypoxia that may or may not culminate in arousal from sleep [27,28]. It is thought that hypoxia dominant OSA [29], subtype of OSA in which ventilatory changes during the night result in oxygen desaturation, but not necessarily arousal from sleep, can increase vascular inflammation, sympathetic nervous system activity, and as a result may lead to an increased risk for CVD. As such, it is thought that when considering cardiovascular disease, assessing severity of OSA should perhaps be made synonymous with assessing severity of nocturnal hypoxia.
Box 1.
no caption available
PATHOPHYSIOLOGICAL CONCEPTS UNDERLYING HYPOXIC BURDEN
Arguably the crucial behavior that governs deleterious effects of hypoxia in OSA is its intermittent nature. It was reported that 2–4 weeks of intermittent hypoxia leads to increased daytime blood pressure, sympathetic nerve activity, mean pulmonary artery pressure, in healthy individuals, possibly through renin-angiotensin mechanisms [30–34]. Alternative pathways include blood platelets through which intermittent hypoxia may lead to CVD [35]. These effects are different from those observed in sustained hypoxic conditions [36▪▪]. As a result, it is hypothesized that characterizing the intermittent nature of hypoxia in OSA is key.
Several experiments suggest that reduced preevent SpO2 is a robust indicator of the rate of postevent SpO2 decline [37–39]. More recently, data from Azarbarzin et al. as well as ours, suggest that the tendency to desaturate is dependent on the preevent ventilatory deficit (or burden) [40,41▪▪,42]. As a result, baseline SpO2 as well as baseline ventilatory deficit may be crucial parameters that determine the tendency to desaturate in OSA patients. Further, reduced baseline SpO2 also contributes to faster desaturations due to the sigmoidal nature of the oxyhemoglobin dissociation curve at lower partial pressures of oxygen. It is argued that OSA patients with shorter events may have the most rapid oxygen desaturations perhaps due to underlying metabolic syndromes and/or increases in abdominal visceral adipose tissue that leads to decreased lung volumes and thus leading to faster oxygen desaturations [38]. While ventilatory changes may be the largest contributor of nocturnal hypoxia, other aspects such as metabolic syndrome should be considered when assessing the relationship between hypoxic burden and adverse outcomes. Although it may be parsimonious to link nocturnal hypoxia to adverse outcomes in OSA such as cardiovascular disease, the causal mechanisms underlying this relationship are not fully known.
EVALUATING HYPOXIC BURDEN IN OBSTRUCTIVE SLEEP APNEA
All currently studied metrics of hypoxic burden rely on the use of the pulse oximetry signal obtained during a routine sleep study or nocturnal polysomnography (NPSG). It is worth noting that hypoxic burden as a concept is different from oxygen desaturation metrics that are termed “hypoxic burden”. In OSA, hypoxic burden as a concept is defined to be measure the load of nocturnal hypoxia and it is assumed that any measure of OSA-related hypoxic burden would also account for the intermittent nature of oxygen desaturations. On the other hand, as will be discussed in depth below, published metrics that characterize oxygen desaturations overnight that are termed as “hypoxic burden” refer to a particular method of defining the underlying nocturnal hypoxia in OSA. As such, conceptually, there is only one hypoxic burden in OSA, but the ways to derive it could be several.
Pulse oximetry measured oxygen saturation (SpO2) forms the basis for all hypoxic burden measures. Pulse oximetry is routinely acquired during NPSG and although its administration is relatively standardized across different sleep labs, key parameters of each pulse oximeter must be considered to ensure fair comparison and reproducibility of developed methods for hypoxic burden. Most notably, depending on the manufacturer, the averaging time for each pulse oximeter is different and several hypoxic burden measures may be sensitive to it. Further, although pulse oximetry administration as part of a routine NPSG is also standardized across sleep labs, sampling rates should be standardized to 1 Hz to avoid any nonphysiological artifacts. The preprocessing of SpO2 signals (e.g., removal of invalid signal periods, disconnects etc.) for evaluating hypoxic burden is another consideration that must be noted when comparing metrics or determining their utility.
Broadly, the definitions can be categorized into three groups: index-based, Time-based, and area-based measures. All measures aim to characterize intermittency of nocturnal hypoxia and their severity in OSA.
Index-based hypoxic burden measures
The oxygen desaturation index (ODI), like the AHI, measures rate of desaturation events without regard for ventilatory disturbances that may have precipitated the changes in oxygen desaturation. Most standard ODI measures are ODI3 and ODI4 which include oxygen desaturation events that are either more than 3% or 4% from a predefined “baseline”. In addition to the level of desaturation (3 or 4 or >4), several other parameters are embedded within a given definition of ODI: search window surrounding candidate respiratory events, and baseline [43,44]. Despite these considerations, ODI is associated with incident cardiovascular events across several studies, albeit with a poor correlation [28]. In addition to ODI3, ODI4, some studies have also considered ODI2 and ODI5. As with AHI and its varied definitions, ODI measures whether 2,3,4,or 5% are inconsistently related to adverse outcomes in OSA [45]. While simple, the various forms of ODI, their inconsistent relationship to [43] outcomes, and lack of standardized rules [45], have further mystified the use of ODI in OSA.
Time-based hypoxic burden measures
Some of the most commonly used measures of nocturnal hypoxia that are time-based include time below 90% of oxygen saturation (T90) or its variants that consider 85% (T85), 80% (T80) etc. as thresholds. A crucial aspect of these time-based measures as in the case of the index-based measures is the “time” variable. While standard NPSG and other EEG-equipped home sleep test devices can measure true sleep time, and thus the T90 (likewise T85, T80) measure can be either recording time below 90%, or time in sleep below 90% of oxygen saturation. Some studies have even suggested using a percentage-based measure, i.e., % of sleep below 90% of oxygen saturation [27,46]. All forms of T90, be it with differing threshold levels, or with sleep time vs. recording time, have shown to be inconsistently associated with hypertension [47], major cardiovascular events (MACE) [27], CVD [48], right ventricular dysfunction [49▪▪], and type 2 diabetes [50]. Although simple to calculate, T90 ignores the heterogeneity in the depth of desaturations between OSA patients. Further, it is unclear how the OSA severity could be categorized using T90.
Area-based hypoxic burden measures
Given the disadvantages of the index- and time-based measures of nocturnal hypoxia, several groups have attempted to utilize area-based measures. Fundamentally, area-based measures characterize the depth and duration of the individual oxygen desaturation events. These area-based measures use the SpO2 trace and calculate the area bounded below by the SpO2 trace and above by either a predefined baseline, a nominal baseline value of 100%, or more sophisticated methods that do not require an upper bound. Table 1 lists the different Area-based hypoxic burden measures along with their definitions and the outcomes against which the metric was tested.
Table 1.
Area based oxygen desaturation metrics that can be candidates for “hypoxic burden” in OSA
| Authors | Metric name | Definition | Automated? | Outcomes |
| Azarbarzin et al.[26,56▪] | Hypoxic burden [HB] | Sum of areas bounded below by SpO2 Nadir and above by a predefined baseline for each event based on search of candidate desaturations | Yes; however requires search window parameters | • CV risk • CPAP treatment to reduce CV events |
| de Chazal et al.[52] | Respiratory event desaturation transient area [REDTA] | Sum of the area between the SpO2 trace and the 100% baseline for all manually scored respiratory events | Yes; however requires manually scored respiratory events and search window parameters | • CV risk |
| Karhu et al.[53▪▪] | Desaturation severity [DesSev] | Sum of desaturation areas bounded below by the SpO2 Nadir and above by a “desaturation baseline” that is based on the starting point of the desaturation | Yes; however requires manually scored respiratory events | • CV risk • Daytime Sleepiness |
| Linz et al.[55] | Hypoxia load [HL] | Total area bounded below by the raw SpO2 curve and above by the 100% value | Yes; no parameters required | • CV risk • CPAP treatment to reduce CV events |
| Parekh et al.[4▪▪,57] | Hypoxic burden [HB] | Sum of areas bounded below by SpO2 Nadir and above by the left- and right-peak of the desaturation event | Yes; no parameters required | • CV risk • Daytime sleepiness • Hypertension • CPAP treatment success for vigilance |
CPAP, continuous positive airway pressure; CV, cardiovascular.
Broadly categorized, area-based measures either rely on manually marked respiratory events or are fully automated. Azarbarzin et al. utilized manually marked respiratory events as the precursor for a search into candidate desaturation events that were then analyzed using an area-based calculation [26]. They termed this measure as hypoxic burden and its units were %minutes per hour of sleep. An automated version of their algorithm, that requires no manual marking of events was recently published [51▪]. It is worth noting that this method still requires manual intervention regarding the search window for candidate events. Automated or not, the hypoxic burden by Azarbarzin et al. showed an association with cardiovascular events across two large cohorts (sleep heart health study) and the osteoporotic fractures in men study (MrOS). de Chazal et al. proposed a novel parameter called the respiratory event desaturation transient area (REDTA) as an alternative for the hypoxic burden with units of % hours [52]. Instead of using a predefined baseline that varies based on the respiratory event preceding the candidate desaturation event, REDTA assumes the baseline to be 100%, which is used as the upper bound, and calculates the area above the SpO2 trace. REDTA is available to other researchers using a freely available software named ABOSA [53▪▪]. In studies, REDTA was shown to be better than both ODI3 and T90 in predicting CVD mortality, and was associated with impaired next-day vigilance in OSA [43]. It is worth noting that the search window for both the method by de Chazal et al. and by Azarbarzin et al. is population or dataset specific and further research is needed into appropriate search windows that can be utilized with these measures.
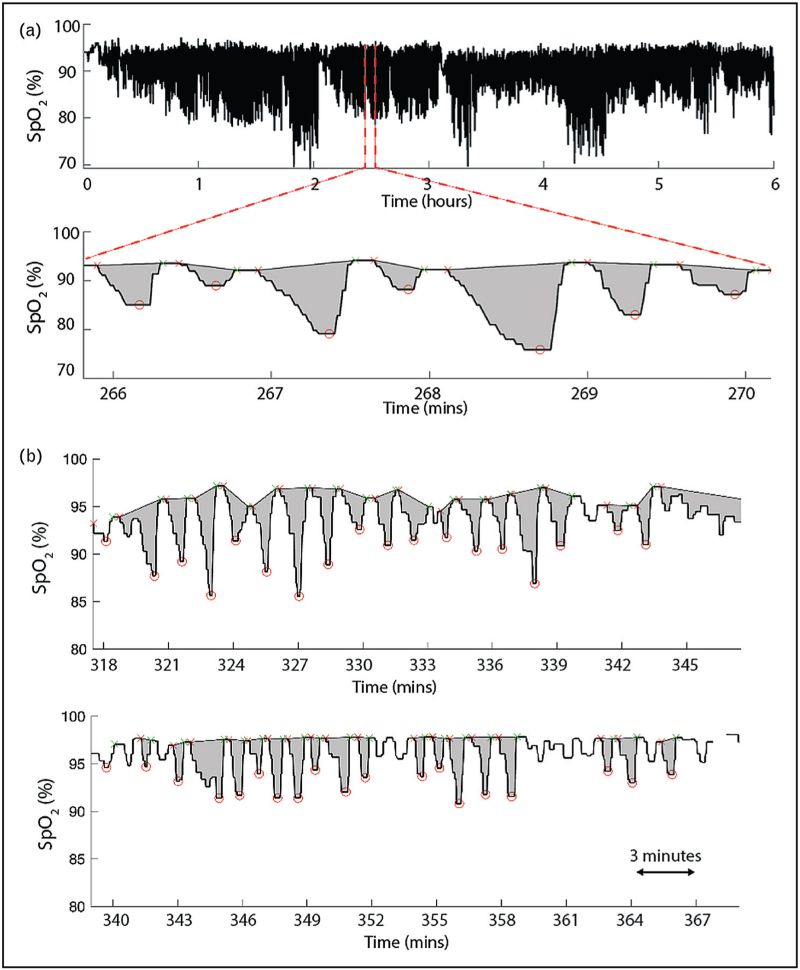
Fully automated area-based measures include the desaturation severity (DesSev) [54], hypoxic load (HL), as well as the novel hypoxic burden by our group [25▪▪,42]. DesSev uses the left peak associated with a candidate desaturation event as the baseline, where the desaturation event is defined by the left and right peaks corresponding a nadir. The area for DesSev (%) is then calculated bounded above by the baseline and bounded below by the SpO2 nadir. It was shown that daytime sleepiness was associated with DesSev, with a stronger relation than AHI and ODI, and DesSev was a strong predictor of CVD events. The hypoxic load measure by Linz et al. considers the total area between the 100% baseline value of SpO2 and the raw SpO2 trace. The hypoxic load is a fully automated measure and was shown to be associated with epicardial fat volume in patients with myocardial infarcts [55]. The hypoxic burden measure proposed by our group, considers both the left and right peaks associated with a candidate desaturation event (See Fig. 1a) and bounded below by the SpO2 nadir of the candidate event in calculation of the area. This hypoxic burden measure is fully automated, including the handling of disconnects or noisy signals, and was shown to be a stronger predictor of CVD mortality than AHI. Among the measures described above, data on night-to-night variability (either in-lab or at-home) were only available for our area-based measure. Recent evidence also suggest that in patients with different nocturnal hypoxia profiles, area-based measures such as the hypoxic burden, may be better able to distinguish them than ODI, T90 or other index- or time-based measures (e.g., see Fig. 1b). It is worth noting that although manually marked respiratory events (or any information about respiratory events) is not needed for this particular variation of hypoxic burden, it should be utilized with caution in a population of patients with comorbidities other than OSA.
FIGURE 1.
(a) Area-based measurement of “hypoxic burden” that does not require any manually marked respiratory events. (b) Nocturnal hypoxia profiles of two patients with similar AHI, ODI, and T90, but different area-based hypoxic burden.
CLINICAL RELEVANCE OF HYPOXIC BURDEN
Ultimately, utility of novel measures that characterize hypoxic burden in OSA is judged by their clinical relevance. Overnight pulse oximetry is a relatively low-cost endeavor that can be used to monitor patients either in clinic or remotely. Thus, the measures of hypoxic burden are significantly relevant to the clinic. Although it is rare to base a diagnosis for a patient solely on a single measure, that is, without regard to patient history, based on the availability of pulse oximetry, its ease of use, perhaps hypoxic burden can serve to be used as the go-to measure for OSA. Further research is needed into what constitutes normal vs. abnormal levels of hypoxic burden. Consider this that most of the studies that have analyzed the relationship between hypoxic burden and cardiovascular disease, have used one group as a reference group, and as such a logical question is whether that reference group constitutes “normal/no OSA” group. Further, it is not yet clear how a given value of hypoxic burden would guide treatment preference in OSA, given that on continuous positive airway pressure (CPAP), hypoxic burden theoretically should be zero or at the very least a relatively lower value which remains to be defined. Recent study by Azarbarzin et al. and data from our group provides evidence using a secondary analysis of the APPLES study that baseline hypoxic burden may be able to predict treatment response in OSA as it relates to vigilance and daytime sleepiness [56▪,57]. However, it must be noted that the predictive power was still not sufficient for use on an individual level, which is crucial for it to be embedded in a clinic as a diagnostic aid. As such, data are currently lacking on whether hypoxic burden can outperform AHI in predicting immediate response to treatment in the clinic. A technological hurdle that must be crossed when implementing hypoxic burden in the clinic is the fact that current CPAP devices do not have any measure of nocturnal hypoxia and doing so would require putting additional burden on the patient.
CONCLUSION
The history of AHI, the evolution of its variations (e.g., with/without arousal etc.), similarly ODI and its variations, as well as T90, is a cautionary tale as we look forward to utilizing “hypoxic burden” as a measure beyond the AHI in characterizing OSA. Already, a number of area-based measures have been published in the field, all termed hypoxic burden, that may possibly lead to confusion for researchers and clinicians interested in fully characterizing nocturnal hypoxia in OSA patients. Although a clear path forward is not evident currently, perhaps a simple change and consensus around nomenclature may suffice. Whether area-based measures that are the holy grail of a single measure that is capable of capturing the underlying pathophysiology of nocturnal hypoxia in OSA, and one that shows promise in predicting adverse consequences of OSA, remains to be tested.
Acknowledgements
None.
Financial support and sponsorship
A.P. was supported by the NIH grants R01HL171813, R21HL165320, R21HL173733, K25HL151912.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med 2019; 7:687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet 2014; 383:736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler MP, Emch JT, Rueschman M, et al. Apnea-hypopnea event duration predicts mortality in men and women in the sleep heart health study. Am J Respir Crit Care Med 2019; 199:903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4▪▪.Parekh A, Kam K, Wickramaratne S, et al. Ventilatory burden as a measure of obstructive sleep apnea severity is predictive of cardiovascular and all-cause mortality. Am J Respir Crit Care Med 2023; 208:1216–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]; First study that assessed the contribution of hypoxic burden to ventilatory burden in assessing severity of OSA as well as its relationship to adverse outcomes in OSA.
- 5.Anitua E, Duran-Cantolla J, Almeida GZ, et al. Predicting the night-to-night variability in the severity of obstructive sleep apnea: the case of the standard error of measurement. Sleep Sci 2019; 12:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antic NA, Catcheside P, Buchan C, et al. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep 2011; 34:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold WC, Guilleminault C. Upper airway resistance syndrome 2018: nonhypoxic sleep-disordered breathing. Expert Rev Respir Med 2019; 13:317–326. [DOI] [PubMed] [Google Scholar]
- 8.Azarbarzin A, Sands SA, Taranto-Montemurro L, et al. The sleep apnea-specific hypoxic burden predicts incident heart failure. Chest 2020; 158:739–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batool-Anwar S, Kales SN, Patel SR, et al. Obstructive sleep apnea and psychomotor vigilance task performance. Nat Sci Sleep 2014; 6:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhat S, Gupta D, Akel O, et al. The relationships between improvements in daytime sleepiness, fatigue and depression and psychomotor vigilance task testing with CPAP use in patients with obstructive sleep apnea. Sleep Med 2018; 49:81–89. [DOI] [PubMed] [Google Scholar]
- 11.Kainulainen S, Duce B, Korkalainen H, et al. Severe desaturations increase psychomotor vigilance task-based median reaction time and number of lapses in obstructive sleep apnoea patients. Eur Respir J 2020; 55:1901849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malhotra A, Ayappa I, Ayas N, et al. Metrics of sleep apnea severity: beyond the AHI. Sleep 2021; 44:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohammadieh AM, Sutherland K, Cistulli PA. Moving beyond the AHI. J Clin Sleep Med 2021; 17:2335–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation 2010; 122:352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009; 6:e1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottlieb DJ, Yao Q, Redline S, et al. Does snoring predict sleepiness independently of apnea and hypopnea frequency? Am J Respir Crit Care Med 2000; 162 (Pt 1):1512–1517. [DOI] [PubMed] [Google Scholar]
- 17.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Association of sleep characteristics and cognition in older community-dwelling men: the MrOS sleep study. Sleep 2011; 34:1347–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X, Wang R, Zee P, et al. Racial/Ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015; 38:877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel SR, White DP, Malhotra A, et al. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med 2003; 163:565–571. [DOI] [PubMed] [Google Scholar]
- 20.Peker Y, Glantz H, Eulenburg C, et al. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. the RICCADSA Randomized Controlled Trial. Am J Respir Crit Care Med 2016; 194:613–620. [DOI] [PubMed] [Google Scholar]
- 21.McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375:919–931. [DOI] [PubMed] [Google Scholar]
- 22.Amatoury J, Azarbarzin A, Younes M, et al. Arousal intensity is a distinct pathophysiological trait in obstructive sleep apnea. Sleep 2016; 39:2091–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sowho M, Amatoury J, Kirkness JP, et al. Sleep and respiratory physiology in adults. Clin Chest Med 2014; 35:469–481. [DOI] [PubMed] [Google Scholar]
- 24.Lv R, Liu X, Zhang Y, et al. Pathophysiological mechanisms and therapeutic approaches in obstructive sleep apnea syndrome. Signal Transduct Target Ther 2023; 8:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25▪▪.Wickramaratne S, Kam K, Tolbert T, et al. Combination of ventilatory, hypoxic, and arousal burden predicts short- and long-term consequences of OSA better than the apnea-hypopnea index. Am J Respir Crit Care Med 2023; 207:A5968–A5969. [Google Scholar]; First study that utilized combination of ventilatory, hypoxic, and arousal burden in OSA using an AI/ML framework and assessed its relationship to mortality.
- 26.Azarbarzin A, Sands SA, Stone KL, et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J 2019; 40:1149–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumert M, Immanuel SA, Stone KL, et al. Composition of nocturnal hypoxaemic burden and its prognostic value for cardiovascular mortality in older community-dwelling men. Eur Heart J 2020; 41:533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Punjabi NM, Newman AB, Young TB, et al. Sleep-disordered breathing and cardiovascular disease: an outcome-based definition of hypopneas. Am J Respir Crit Care Med 2008; 177:1150–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas RJ, Terzano MG, Parrino L, et al. Obstructive sleep-disordered breathing with a dominant cyclic alternating pattern - -a recognizable polysomnographic variant with practical clinical implications. Sleep 2004; 27:229–234. [DOI] [PubMed] [Google Scholar]
- 30.Norman D, Loredo JS, Nelesen RA, et al. Effects of continuous positive airway pressure versus supplemental oxygen on 24-h ambulatory blood pressure. Hypertension 2006; 47:840–845. [DOI] [PubMed] [Google Scholar]
- 31.Beaudin AE, Hanly PJ, Raneri JK, et al. Impact of intermittent hypoxia on human vascular responses during sleep. Exp Neurol 2022; 347:113897. [DOI] [PubMed] [Google Scholar]
- 32.Beaudin AE, Waltz X, Hanly PJ, et al. Impact of obstructive sleep apnoea and intermittent hypoxia on cardiovascular and cerebrovascular regulation. Exp Physiol 2017; 102:743–763. [DOI] [PubMed] [Google Scholar]
- 33.Foster GE, Hanly PJ, Ahmed SB, et al. Intermittent hypoxia increases arterial blood pressure in humans through a renin-angiotensin system-dependent mechanism. Hypertension 2010; 56:369–377. [DOI] [PubMed] [Google Scholar]
- 34.Hanly PJ. Mechanisms and management of central sleep apnea. Lung 1992; 170:1–17. [DOI] [PubMed] [Google Scholar]
- 35.Gabryelska A, Lukasik ZM, Makowska JS, et al. Obstructive sleep apnea: from intermittent hypoxia to cardiovascular complications via blood platelets. Front Neurol 2018; 9:635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36▪▪.Minoves M, Hazane-Puch F, Moriondo G, et al. Differential impact of intermittent vs. sustained hypoxia on HIF-1, VEGF and proliferation of HepG2 cells. Int J Mol Sci 2023; 24:6875. [DOI] [PMC free article] [PubMed] [Google Scholar]; Experimental study assessing the impact of sustained vs. intermitten hypoxia on tumor cells that sheds light on why the intermittent nature of hypoxia in OSA is deleterious.
- 37.Abler LL, O’Driscoll CA, Colopy SA, et al. The influence of intermittent hypoxia, obesity and diabetes on male genitourinary anatomy and voiding physiology. Am J Physiol Renal Physiol 2021; 321:F82–F92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jun JC. Dying with OSA, or from it: a cautionary note about novel hypoxia metrics. Am J Respir Crit Care Med 2022; 206:1563–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strohl KP, Altose MD. Oxygen saturation during breath-holding and during apneas in sleep. Chest 1984; 85:181–186. [DOI] [PubMed] [Google Scholar]
- 40.Azarbarzin A, Labarca G, Kwon Y, et al. Physiological consequences of upper airway obstruction in sleep apnea. Chest 2024; 1–10. [DOI] [PubMed] [Google Scholar]
- 41▪▪.Labarca G, Vena D, Hu WH, et al. Sleep apnea physiological burdens and cardiovascular morbidity and mortality. Am J Respir Crit Care Med 2023; 208:802–813. [DOI] [PMC free article] [PubMed] [Google Scholar]; First study to assess the contribution of arousal burden and hypoxic burden in OSA to cardiovascular disease using retrospective data.
- 42.Parekh A, Kam K, Wickramaratne S, et al. Ventilatory burden as a measure of obstructive sleep apnea severity is predictive of cardiovascular and all-cause mortality. Am J Respir Crit Care Med 2023; 208:1216–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He S, Cistulli PA, de Chazal P. A review of novel oximetry parameters for the prediction of cardiovascular disease in obstructive sleep apnoea. Diagnostics (Basel) 2023; 13: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gyulay S, Olson LG, Hensley MJ, et al. A comparison of clinical assessment and home oximetry in the diagnosis of obstructive sleep apnea. Am Rev Respir Dis 1993; 147:50–53. [DOI] [PubMed] [Google Scholar]
- 45.Ng Y, Joosten SA, Edwards BA, et al. Oxygen desaturation index differs significantly between types of sleep software. J Clin Sleep Med 2017; 13:599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang L, Ou Q, Shan G, et al. Independent association between oxygen desaturation index and cardiovascular disease in non-sleepy sleep-disordered breathing subtype: a chinese community-based study. Nat Sci Sleep 2022; 14:1397–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L, Wei DH, Zhang J, et al. Time under 90% oxygen saturation and systemic hypertension in patients with obstructive sleep apnea syndrome. Nat Sci Sleep 2022; 14:2123–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sutherland K, Sadr N, Bin YS, et al. Comparative associations of oximetry patterns in obstructive sleep apnea with incident cardiovascular disease. Sleep 2022; 45:zsac179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49▪▪.Huang Z, Duan A, Hu M, et al. Implication of prolonged nocturnal hypoxemia and obstructive sleep apnea for pulmonary hemodynamics in patients being evaluated for pulmonary hypertension: a retrospective study. J Clin Sleep Med 2023; 19:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]; Large retrospective study of hypoxic burden measures (T90) and its relationship to pulmonary hypertension.
- 50.Chang JL, Goldberg AN, Alt JA, et al. International consensus statement on obstructive sleep apnea. Int Forum Allergy Rhinol 2023; 13:1061–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51▪.Esmaeili N, Labarca G, Hu WH, et al. Hypoxic burden based on automatically identified desaturations is associated with adverse health outcomes. Ann Am Thorac Soc 2023; 20:1633–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]; First semi-automated method to assess hypoxic burden in OSA.
- 52.de Chazal P, Sadr N, Dissanayake H, et al. Predicting cardiovascular outcomes using the respiratory event desaturation transient area derived from overnight sleep studies. Annu Int Conf IEEE Eng Med Biol Soc 2021; 2021:5496–5499. [DOI] [PubMed] [Google Scholar]
- 53▪▪.Karhu T, Leppanen T, Toyras J, et al. ABOSA – freely available automatic blood oxygen saturation signal analysis software: Structure and validation. Comput Methods Programs Biomed 2022; 226:107120. [DOI] [PubMed] [Google Scholar]; A freely available software to analyze a variant of hypoxic burden and related metrics.
- 54.Kulkas A, Tiihonen P, Eskola K, et al. Novel parameters for evaluating severity of sleep disordered breathing and for supporting diagnosis of sleep apnea-hypopnea syndrome. J Med Eng Technol 2013; 37:135–143. [DOI] [PubMed] [Google Scholar]
- 55.Linz D, Colling S, Nussstein W, et al. Nocturnal hypoxemic burden is associated with epicardial fat volume in patients with acute myocardial infarction. Sleep Breath 2018; 22:703–711. [DOI] [PubMed] [Google Scholar]
- 56▪.Pinilla L, Esmaeili N, Labarca G, et al. Hypoxic burden to guide CPAP treatment allocation in patients with obstructive sleep apnoea: a post hoc study of the ISAACC trial. Eur Respir J 2023; 62:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]; Study assessing the utility of hypoxic burden to predict improvement in sleep apnea using continuous positive airway pressure treatment using retrospective data
- 57. Gaynor-Sodeifi K, Greenberg D, Varga A, et al. 0485 Can hypoxic burden predict improvement in sleepiness and cognition in response to CPAP treatment in OSA patients? Sleep. 2024;47(Suppl 1):A209. [Google Scholar]