Summary
Fluorescence single molecule imaging comprises a variety of techniques that involve detecting individual fluorescent molecules. Many of these techniques involve localizing individual fluorescent molecules with precisions below the diffraction limit, which limits the spatial resolution of (visible) light-based microscopes. These methodologies are widely used to image biological structures at the nanometer scale by fluorescently tagging the structures of interest, elucidating details of the biological behavior observed.
Two common techniques are single-molecule localization microscopy (SMLM), (Betzig et al., 2006; Fazel & Wester, 2022; Hell, 2007; Lidke et al., 2005; Rust et al., 2006; van de Linde et al., 2011) which is used to produce 2D or 3D super-resolution images of static or nearly static structures, and single-particle tracking (SPT) (Shen et al., 2017), which follows the time course of one or a very small number of moving tagged molecules. SMLM often involves distributions of particles at medium to high density, while SPT works in a very low density domain. These procedures all require intensive numerical computation, and the methods are tightly interwoven.
Statement of need
SMITE is a MATLAB-based toolbox that provides analysis tools for fluorescence single molecule imaging with an emphasis on single molecule localization microscopy (SMLM) and single-particle tracking (SPT). The SMITE toolbox consists of a MATLAB infrastructure with some C and CUDA code embedded to provide CPU/GPU speed-ups for particularly expensive computations. The source code for SMITE has been archived to GitHub: https://github.com/LidkeLab/smite
SMITE is designed around the concept that a parameter structure, the Single Molecule Fitting (SMF) structure, uniquely and completely defines the data analysis. The results are completely contained in a Single Molecule Data (SMD) structure. SMITE is designed to make lowest-level tools just as easy to use as the higher-level application-specific classes. All tools make use of the SMF and SMD structures. SMITE is organized into a set of namespaces that group similar tools and concepts. The namespace +smi contains the highest level tools that will be the most common entry point for processing SMLM and SPT data sets.
Code coverage includes mature SMLM data analysis techniques (applying gain and offset corrections to raw data, finding localizations, thresholding localizations based on various criteria, frame connection and drift correction), SMLM/SPT simulations, sophisticated SPT analyses, post-processing clustering and statistical analyses (e.g., diffusion analysis and hidden Markov models for characterizing dimers in SPT results), a variety of visualizations, experimental point spread function creation and characterization, all sprinkled with various examples of usage. Interaction with these tools is via GUIs or scripting. See Figure 1 for several examples of SMITE GUIs.
Figure 1:
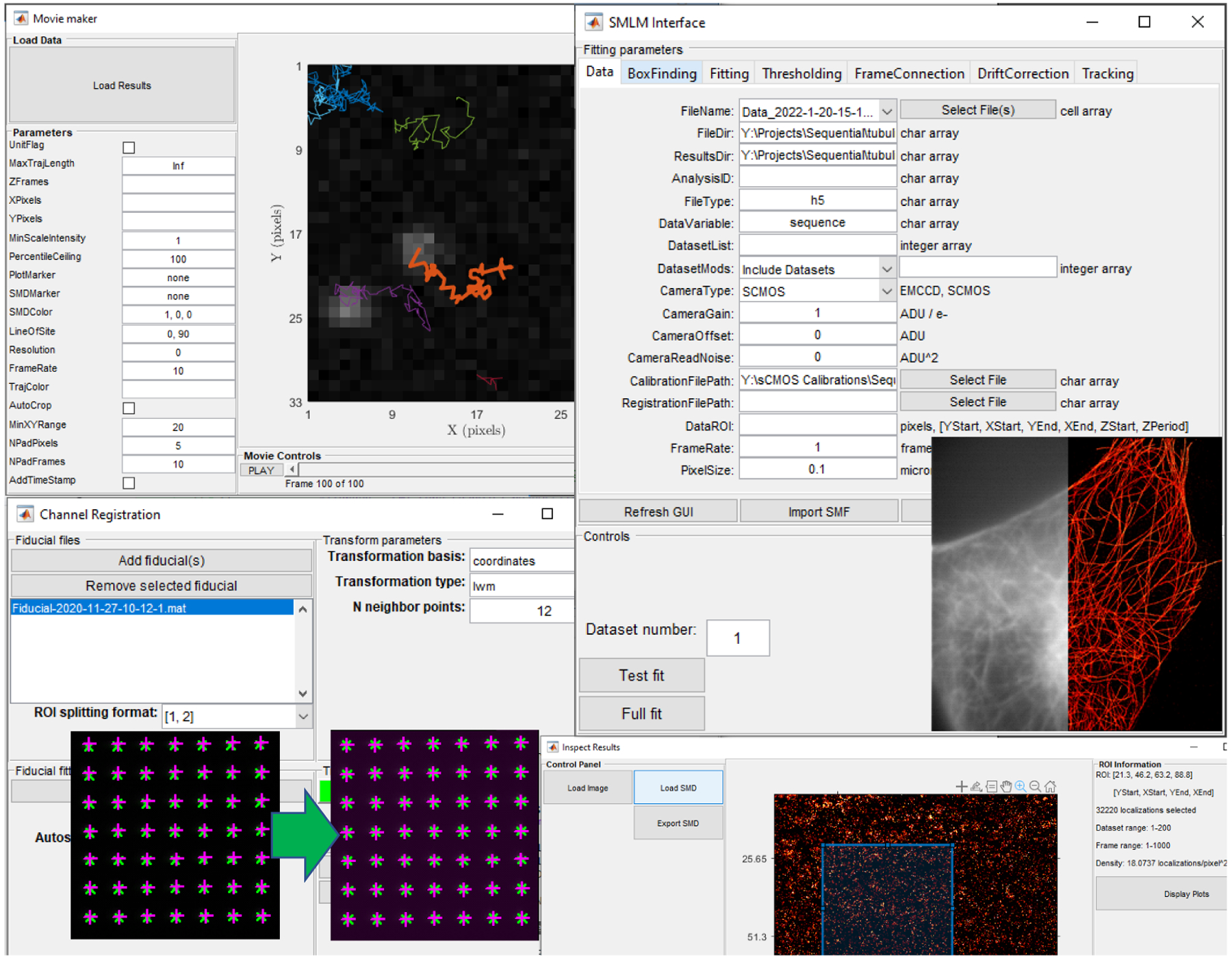
SMITE GUIs for (upper left) making movies from SPT trajectories, (upper right) SMLM analysis, (lower left) channel registration, and (lower right) inspection of results contained in SMD structures.
SMITE is a tool designed to be used by researchers and upper level students interested in fluorescence single molecule imaging and applications. Some of the algorithms have already been published: 2D Gaussian blob maximum likelihood estimate (Smith et al., 2010), frame connection (Schodt & Lidke, 2021), drift correction (Wester et al., 2021), Bayesian grouping of localizations (Fazel et al., 2022), diffusion estimation (Relich et al., 2016). However, this is the first time that they have been integrated together, sharing common data structures. Applications are described in (Bailey et al., 2022; Franco Nitta et al., 2021; Mazloom Farsibaf et al., 2021; Schodt et al., 2023). Typical raw image data can be found in (Pallikkuth, Martin, et al., 2018). A summary of the namespaces and classes in SMITE can be found in the online documentation at https://github.com/LidkeLab/smite/blob/main/doc/SMITEclasses.md.
SMAP (Ries, 2020), an alternative MATLAB integrated SMLM/SPT code, is GUI oriented, while SMITE was designed to be more focused on scripting (although many GUIs are available as well) in order to make batch processing extremely simple. SMITE, in addition, is designed to operate with HDF5 (Hierarchical Data Format) files which efficiently store very large datasets, while SMAP preferentially works with TIFF formatted files. Both SMITE and SMAP work with separate software to control instruments, MATLAB Instrument Control (MIC) (Pallikkuth, Meddens, et al., 2018) and Micro-Manager (Edelstein et al., 2014), respectively.
Acknowledgements
This work was supported by NIH grants K12GM088021 (ASERT-IRACDA program), NCI P30CA118100, NCI R01CA248166, NIGMS R01GM109888, NIGMS R21GM104691, NIGMS R21GM132716, NIGMS R35GM126934, NCRR RR024438, NIGMS 1R01GM140284, NIBIB 1R21EB019589, NIGMS 5P50GM085273 (New Mexico Spatiotemporal Modeling Center); NSF grants 0954836 and 2039517; DOE grant DE-SC0019267; support from the University of New Mexico Office of the Vice President for Research Program for Enhancing Research Capacity; and supported by grants from NVIDIA and utilized an NVIDIA A6000 GPU.
References
- Bailey EM, Salazar-Cavazos E, Grattan RM, Wester MJ, Schodt DJ, Rojo J, Lidke KA, & Lidke DS (2022). Quantification of protein phosphorylation using single molecule pull-down (SiMPull). Journal of Visualized Experiments, 184, e63665: 1–20. 10.3791/63665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, Davidson MW, Lippincott-Schwartz J, & Hess HF (2006). Imaging Intracellular Fluorescent Proteins at Nanometer Resolution. Science, 313(5793), 1642–1645. 10.1126/science.1127344 [DOI] [PubMed] [Google Scholar]
- Edelstein AD, Tsuchida MA, Amodaj N, Pinkard H, Vale RD, & Stuurman N (2014). Advanced methods of microscope control using Manager software. Journal of Biological Methods, 1(2), e11. 10.14440/jbm.2014.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazel M, & Wester MJ (2022). Analysis of Super-resolution Single Molecule Localization Microscopy Data: a tutorial. AIP Advances, 12(1), 010701–1–010701–29. 10.1063/5.0069349 [DOI] [Google Scholar]
- Fazel M, Wester MJ, Schodt DJ, Restrepo Cruz S, Strauss S, Schueder F, Schlichthaerle T, Gillette JM, Lidke DS, Rieger B, Jungmann R, & Lidke KA (2022). High-Precision Estimation of Emitter Positions using Bayesian Grouping of Localizations. Nature Communications, 13(7152), 1–11. 10.1038/s41467-022-34894-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco Nitta C, Green EW, Jhamba ED, Keth JM, Ortiz-Caraveo I, Grattan RM, Schodt DJ, Gibson AC, Rajput A, Lidke KA, Wilson BS, Steinkamp MP, & Lidke DS (2021). EGFR transactivates RON to drive oncogenic crosstalk. eLife, 10, e63678: 1–27. 10.7554/eLife.63678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell SW (2007). Far-Field Optical Nanoscopy. Science, 316(5828), 1153–1158. 10.1126/science.1137395 [DOI] [PubMed] [Google Scholar]
- Lidke KA, Rieger B, Jovin TM, & Heintzmann R (2005). Superresolution by localization of quantum dots using blinking statistics. Optics Express, 13(18), 7052–7062. 10.1364/OPEX.13.007052 [DOI] [PubMed] [Google Scholar]
- Mazloom Farsibaf H, Farzam F, Fazel M, Wester MJ, Meddens MBM, & Lidke KA (2021). Comparing lifeact and phalloidin for super-resolution imaging of actin in fixed cells. PLoS ONE, 16(1), e0246138. 10.1371/journal.pone.0246138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallikkuth S, Martin C, Farzam F, Edwards JS, Lakin MR, Lidke DS, & Lidke KA (2018). Supporting data for Sequential Super-Resolution Imaging using DNA Strand Displacement [Data set]. In University of New Mexico Digital Repository. University of New Mexico. 10.25827/CS2A-DH13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallikkuth S, Meddens M, Fazel M, Farsibaf H, Farzam F, Wester M, & Lidke K (2018). A MATLAB-based Instrument Control Package for Fluorescence Imaging. Biophysical Journal, 114(3, Supplement 1), 532a. 10.1016/j.bpj.2017.11.2912 [DOI] [Google Scholar]
- Relich PK, Olah MJ, Cutler PJ, & Lidke KA (2016). Estimation of the diffusion constant from intermittent trajectories with variable position uncertainties. Physical Review E, 93(4), 042401. 10.1103/PhysRevE.93.042401 [DOI] [PubMed] [Google Scholar]
- Ries J (2020). SMAP: a modular super-resolution microscopy analysis platform for SMLM data. Nature Methods, 17, 370–372. 10.1038/s41592-020-0938-1 [DOI] [PubMed] [Google Scholar]
- Rust MJ, Bates M, & Zhuang X (2006). Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nature Methods, 3(10), 793–796. 10.1038/nmeth929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schodt DJ, Farzam F, Liu S, & Lidke KA (2023). Automated multi-target super-resolution microscopy with trust regions. Biomedical Optics Express, 14(1), 429–440. 10.1364/BOE.477501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schodt DJ, & Lidke KA (2021). Spatiotemporal Clustering of Repeated Super-Resolution Localizations via Linear Assignment Problem. Frontiers in Bioinformatics, 1, 57. 10.3389/fbinf.2021.724325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Tauzin LJ, Baiyasi R, Wang W, Moringo N, Shuang B, & Landes CF (2017). Single Particle Tracking: From Theory to Biophysical Applications. Chemical Reviews, 117(11), 7331–7376. 10.1021/acs.chemrev.6b00815 [DOI] [PubMed] [Google Scholar]
- Smith CS, Joseph N, Rieger B, & Lidke KA (2010). Fast, single-molecule localization that achieves theoretically minimum uncertainty. Nature Methods, 7(5). 10.1038/nmeth.1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Linde S, Loschberger A, Klein T, Heidbreder M, Wolter S, Heilemann M, & Sauer M (2011). Direct stochastic optical reconstruction microscopy with standard fluorescent probes. Nature Protocols, 16(6(7)), 991–1009. 10.1038/nprot.2011.336 [DOI] [PubMed] [Google Scholar]
- Wester MJ, Schodt DJ, Mazloom-Farsibaf H, Fazel M, Pallikkuth S, & Lidke KA (2021). Robust, fiducial-free drift correction for super-resolution imaging. Scientific Reports, 11(23672), 1–14. 10.1038/s41598-021-02850-7 [DOI] [PMC free article] [PubMed] [Google Scholar]


