Abstract
The objective of this study was to design, code, and test the feasibility, acceptability, and preliminary efficacy of a digital therapeutic self-management tool for pediatric inflammatory bowel disease (IBD). The Self-Management Assistance for Recommended Treatment (SMART) portal development involved an iterative co-design process with a series of focus group/interview sessions with key stakeholders. Subsequently, a pilot, single-arm, open-label trial was conducted with 22 patients; medication adherence was the primary outcome. Usage data for the SMART portal were good, with patients demonstrating better engagement than parents. Results from the trial demonstrated improvement in medication adherence (M = 24%–31%; t = 7.94, P < 0.05) and self-management barriers as well as trends in health-related quality of life and symptoms. The SMART portal is a feasible digital therapeutic self-management tool for pediatric IBD that demonstrated preliminary efficacy in this pilot trial. Large, controlled trials are needed to definitively determine the clinical efficacy of this tool.
Keywords: e-Health, inflammatory bowel disease, pediatric, self-management, technology
Poor self-management is a pervasive problem resulting in poor health outcomes and treatment nonadherence (1), increased risk of relapse (2), and a $100–300 billion increase in health care costs in the United States (1,3). In inflammatory bowel disease (IBD), 88% of adolescents demonstrate significant medication nonadherence, with approximately 40%–50% missed doses (4). These data are alarming given that the risk of relapse is 5.5 times greater in nonadherent adult patients (2) and health care costs are 3.7 times higher for nonadherent pediatric patients (5).
Prior research has documented several barriers to self-management in IBD, including forgetting, interference with activities, not having medication available, and oppositional behavior (6), and clinical trials have demonstrated the efficacy of behavioral interventions targeting these barriers (7,8). However, access to this treatment remains a significant barrier for these patients. Efforts to address self-management, which are primarily clinic-based, are inadequate due to: (1) time constraints of brief and/or infrequent clinic visits, (2) exclusive use of educational approaches, which are insufficient for behavior change (9,10), and (3) limited number of clinicians qualified in self-management behavior intervention. Thus, patients are not receiving critically needed evidence-based self-management care, and a more accessible approach is necessary.
Digital therapeutics are ideal for addressing pediatric self-management due to their accessibility and appeal. Unfortunately, there are no existing technological resources tailored to self-management needs in pediatric IBD. Available generic technologies are also limited in approach and have often failed to engage all stakeholders, resulting in rejection or lack of sustained use by stakeholders (11).
To address this gap in self-management care provision in pediatric IBD, we developed the “Self-Management Assistance for Recommended Treatment (SMART)” portal, the first digital therapeutic self-management tool for patients with IBD, their parents, and clinicians. The SMART portal incorporates evidence-based assessment and intervention components that target the specific barriers that are unique to individual patients. Following an iterative development process for the SMART portal, we conducted a pilot and feasibility test to determine the feasibility and preliminary efficacy of the SMART portal intervention on clinical outcomes, specifically treatment adherence, health-related quality of life (HRQOL), and symptom reduction. We hypothesized that the SMART portal intervention would demonstrate adequate feasibility based on usage data and user feedback and preliminary efficacy for clinical endpoints in this pilot trial.
METHODS
Development of the SMART Portal
The SMART portal development phase involved an iterative co-design process that was conducted via a series of focus group/interview sessions with key stakeholders including patients (age 9–18) and their parents, gastroenterologists, nurses, psychologists, and social workers. The initial concept of the digital therapeutic IBD self-management application was presented and feedback was solicited from each of these groups separately. Subsequently, an alpha prototype was designed and coded. Confirmatory interviews were then conducted with the end user groups. Any additional modifications were then made and a fully functional digital therapeutic tool was deployed for testing in an 8-week feasibility pilot clinical trial.
This tool was comprised of 16 intervention modules (Fig. 1; IBD Education, Medication Adherence, and Goal Setting not pictured), 2 of which were developed for parents to complete as appropriate. Modules were designed to be completed within approximately 20 minutes. An automated algorithm was developed to use responses on an assessment battery, consisting of 26 questions designed for this study to identify barriers to self-management. It was completed by both patient participants and parents, to assign as many treatment modules as indicated in a manner that targeted individualized patient self-management needs. Module assignments were given to participants with instructions to complete them at their own pace. Modules were accessible by both patient participants and parents. Although modules were only assigned as indicated by the algorithm to participants in a personalized treatment approach, participants maintained access to unassigned modules.
FIGURE 1.
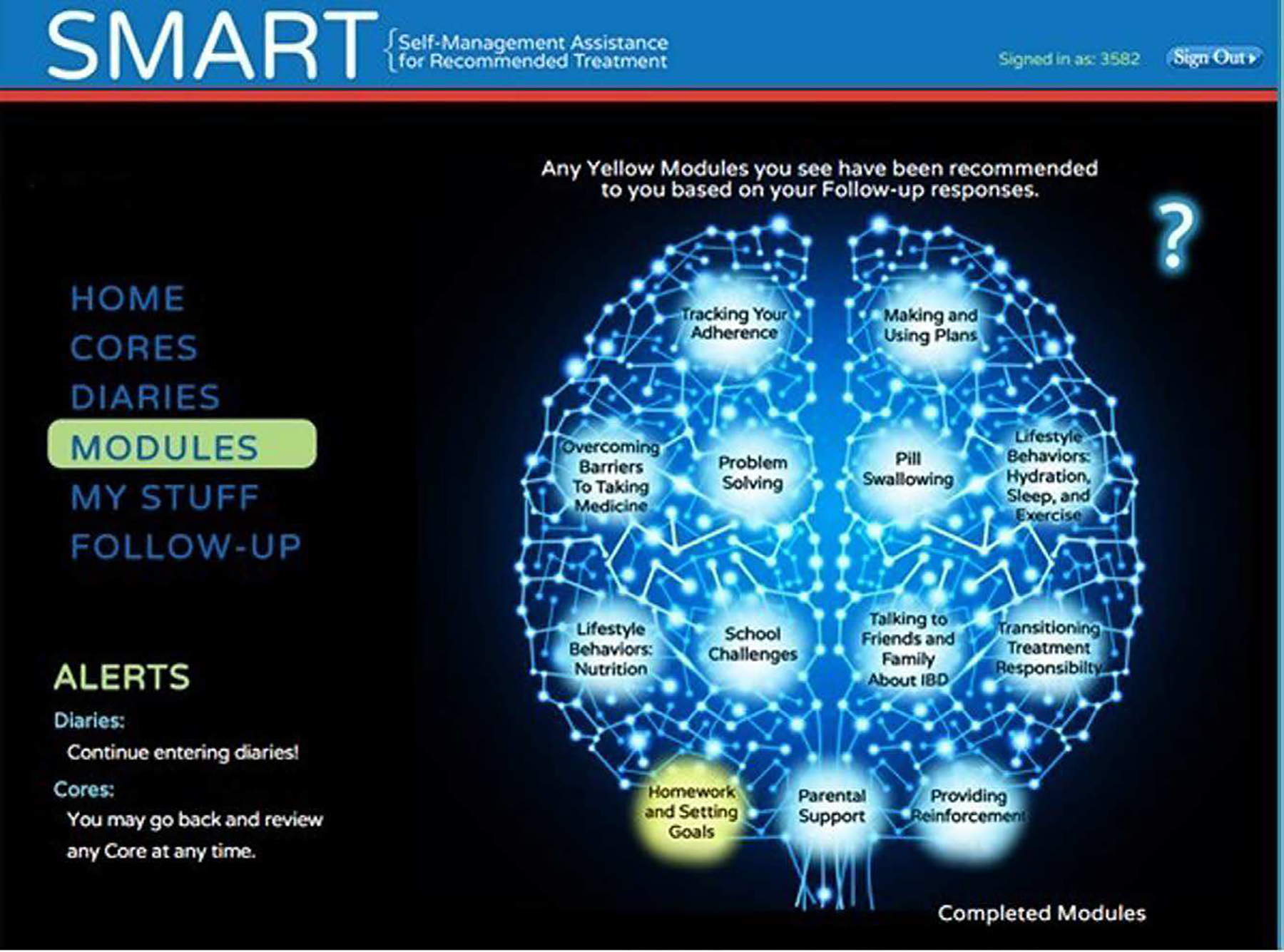
Sample module screenshot.
Study Design and Participants
This was a single-arm open-label trial in which established patients (ie, not second opinion) with a confirmed diagnosis of Crohn disease or ulcerative colitis (collectively IBD) were recruited from a multidisciplinary IBD Center from June 2017 through November 2018. Additional inclusion criteria were: age 9–18 years inclusive; prescription of at least 1 oral medication used to treat IBD (including over the counter supplements such as vitamin D, iron, etc); access to the internet whether public (eg, library) or private (eg, home, personal); English fluency for patient and caregiver. Approval by the Institutional Review Board was obtained prior to recruitment. Written informed consent and assent were obtained by participants and their parents/caregivers. Participants completed baseline and post-treatment (ie, 8 weeks after baseline) assessments and their recommended treatment plans during the course of the study. See Figure 2 for participant flow chart. The final sample was comprised of 22 patients (62% male) with IBD (95% Crohn disease), ages 9–18 (M = 14.1). The majority of participants were White (95%), and non-Hispanic (95%). Participants were compensated for their participation.
FIGURE 2.
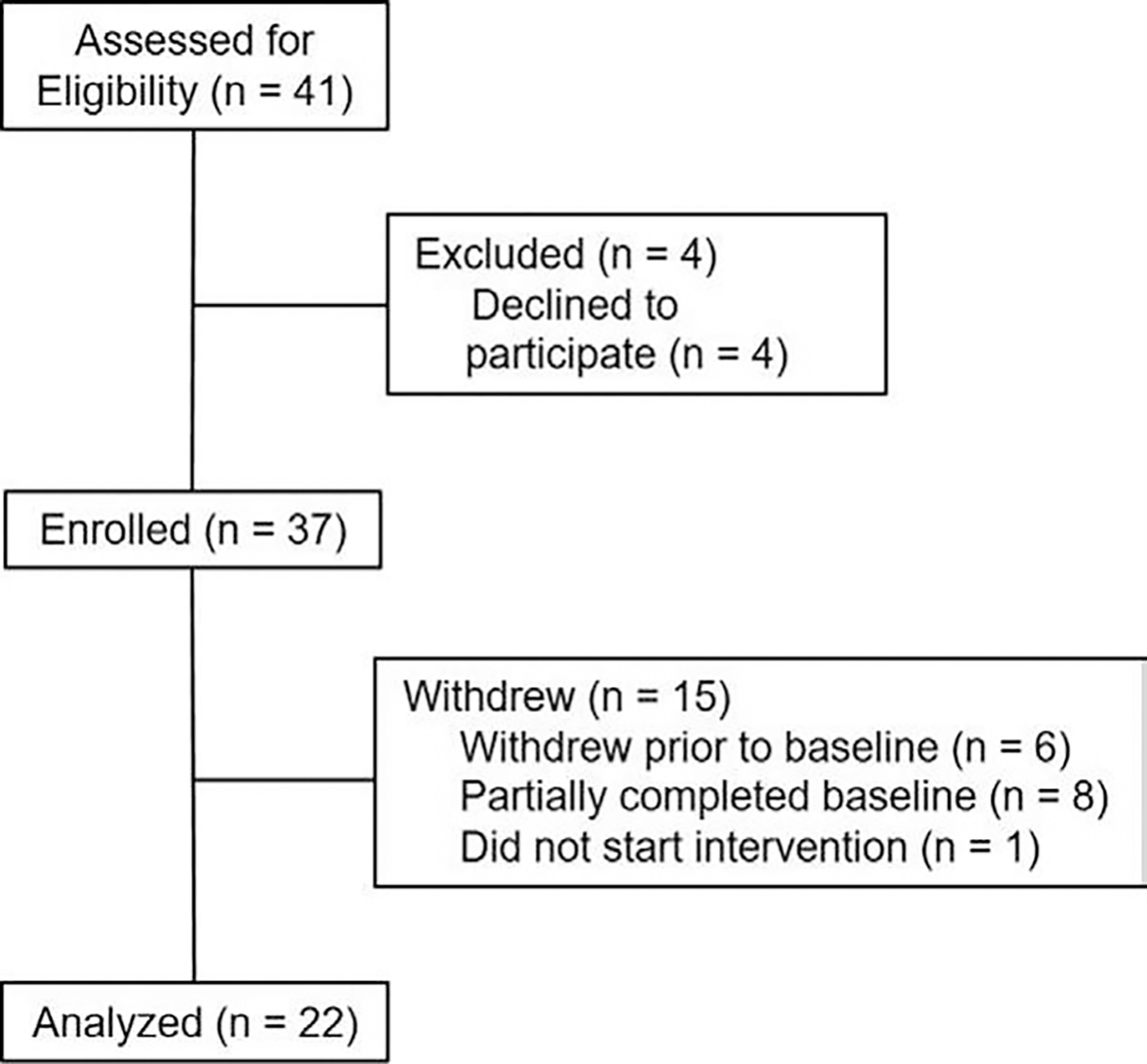
Participant flow chart.
Measures
Demographic data were provided by caregivers at baseline. Treatment integrity was maintained via automated electronic delivery of the intervention, which precluded human error or variation in intervention delivery.
Daily Diary
Participants completed a diary consisting of 10 items each day during the 8-week intervention period. These items assessed stool frequency, consistency, blood content, nocturnal stools, abdominal pain, activity limitations, overall well-being, and progress toward goals. Participants were able to view trends in diary data via graphical feedback in the SMART portal such that relationships could be observed (eg, progress toward goals and activity limitations).
Adherence
Adherence was defined as the percent of prescribed medication taken. Pill counts of all IBD medication prescribed to the patient were completed by patients and/or caregivers via the portal at assessment time points. Pill counts are an accurate and clinically feasible measure of adherence, and research has shown no significant difference in values obtained via a health care provider versus patient or parent (12). Only 1 participant was prescribed an injection-based medication, and this was assessed in the same manner as pill counts.
Pediatric Quality of Life Inventory (PedsQL 4.0)
The PedsQL 4.0 is a 23-item self-report assessment of children’s HRQOL across 4 areas of functioning: physical, emotional, social, and school (13). The PedsQL 4.0 has both patient- and caregiver-report forms and can be used in children ages 2–18 years. Respondents rate, on an anchored 5-point scale, how much of a problem each item has been during the past month. The PedsQL 4.0 is a well-established reliable and valid measure of HRQOL used extensively in pediatric research.
IMPACT-III Questionnaire
This is a 35-item patient-report pediatric IBD-specific measure of HRQOL completed by patients (14). The IMPACT-III Questionnaire (IMPACT-III) has demonstrated good reliability (α = 0.90, test-retest estimates = 0.90 (14)) and validity. Respondents rate the extent to which he/she is affected by a particular issue (eg, stomach pain) across 6 factors of QOL.
Parent and Adolescent Medication Barriers Scales
The Parent Medication Barriers Scale (PMBS; 16-items) and the Adolescent Medication Barriers Scale (AMBS; 17-items) assess perceived barriers to medication taking (15). Each item is rated on a 5-point Likert-like scale from 1 = “strongly disagree” to 5 = “strongly agree.” These measures have strong internal consistency (α = 0.87) and stability over time (15). Four factor-analytically derived subscales include: Disease Frustration/Adolescent Issues, Regimen Adaptation/Cognitive, Ingestion Issues, and Parent Reminder. Parents completed the PMBS; patients completed the AMBS.
Patient-Reported Symptoms
The Partial Harvey Bradshaw Index (PHBI) (16) is a 3-item measure used to assess disease symptoms in pediatric Crohn disease patients. Scores are summed and range 0–12, with higher scores representing greater disease severity. The Pediatric Ulcerative Colitis Activity Index (17) is a 6-item measure used to assess disease symptoms in pediatric ulcerative colitis and indeterminate colitis patients. Scores are summed and range 0–85, with higher scores representing more severe disease. Patient participants completed these items.
Data Analyses
Although this trial was aimed at determining feasibility and preliminary efficacy, we exceeded the recommended sample size (n = 20) for a pilot trial designed to inform a larger efficacy trial with 80% power to detect a medium effect (18). IBM SPSS Statistics (Chicago, IL) statistical software was used for descriptive analyses of the data and 2-tailed pairwise-dependent samples t tests (P < 0.05) for intervention outcome data. Differences in the following variables were tested in a series of within-subject pairwise comparisons at baseline and post-treatment: (1) medication adherence, (2) PMBS/AMBS total scores and subscale scores, (3) PedsQL total scores and subscale scores for caregiver and patient forms, and (4) disease symptoms.
RESULTS
Feasibility and Acceptability
Our usage data revealed that all participants completed at least 1 assigned intervention module (ie, all participants were not assigned all the same modules as the algorithm individually tailored the intervention), with the highest completion rates by participants focused on IBD education (100%), medication adherence (93%), goal setting (77%), action plans (71%), and nutrition (100%). The 2 parent modules (29%) had the lowest completion rates. Participants were assigned an average of 6 modules and the overall completion rate was 75%. Given that our primary intervention target is the patient, and our usage data by patients is superior to that which has been published to date (19), the SMART portal appears to be a feasible and acceptable digital therapeutic tool for pediatric IBD.
SMART Portal Intervention
Participants were prescribed an average of 2 medications, with a range of 1–4. Results of the intervention revealed a 24%–31% increase from baseline in adherence (t = 7.94, P < 0.05) across medications assessed. In addition, there was a reduction in self-management barriers as reflected in improvement on the Parent Medication Barriers Scale Total score (t = −3.70, P < 0.01), Disease Frustration scale (t = −3.53, P < 0.01), and Ingestion Issues scale (t = −5.48, P < 0.001), and a trend on the Regimen Adaptation scale (t = −2.09, P = 0.05). Correlations between adherence and module completion rate were nonsignificant (all P’s > 0.05), which may be a function of the pilot nature of this study. Patients also reported improved, albeit nonsignificant, IBD-specific, and generic HRQOL on the IMPACT General Well-being scale (P = 0.27), PedsQL Emotional Functioning (P = 0.31), Social Functioning (P = 0.74), and Psychological Health Summary scales (P = 0.58). We also observed a small, albeit nonsignificant, improvement in symptoms reported by patients on the PHBI (P = 0.84) and a nonsignificant reduction in number of stools from M = 2.83 to M = 0.5 (P > 0.05).
Participants also provided post-intervention feedback that supports the use of the SMART portal. Participants broadly reported, and gave highest ratings (ie, 3 or 4 on 0–4 scale) regarding their ease of using the SMART portal, likelihood of repeated use, comprehension of content, trust of information, satisfaction with intervention experience, and the portal’s esthetic design, method of intervention delivery, ability to keep their interest and attention, and convenience of use. Parents felt the modules targeting parent needs/concerns helped them support their children to manage their IBD more effectively. Although the short-term nature of this pilot study precluded use of data by clinicians in patient care, they provided critical feedback in developing the patient pathway and a clinician pathway for future use. Clinicians felt the ability to access patient data (eg, adherence, pain, stools, activity) would be extraordinarily useful as these data are not available via other resources prior to clinical encounters.
DISCUSSION
The purpose of this study was to iteratively develop and test the feasibility, acceptability, and preliminary efficacy of the SMART portal to promote better self-management in pediatric IBD. Results of the 8-week open-label single-arm trial indicated that our usage data were similar to and, in some cases, better than health app usage in general. Indeed, in a national survey of 934 individuals (19), 65.5% reported using a health app at least once daily and 44.4% reported using their apps for 1–10 minutes. With treatment module usage data for patients exceeding this, the SMART portal demonstrated appropriate patient engagement. In contrast, parent engagement was lower than expected. Nevertheless, as patients were the primary intervention target, the feasibility of the SMART portal was good overall.
The SMART portal also demonstrated beneficial impact on medication adherence and health outcomes in this pilot study. Adherence to medication improved significantly as a result of the SMART portal with an average of 24%–31% increase, consistent with what prior research has shown with in-person and telehealth behavioral interventions targeting medication adherence (7,8,20); however, this is the first study demonstrating this effect using a personalized digital therapeutic tool without provider interaction of some kind. Additionally, we found significant reduction in self-management barriers. This is important because it indicates that the SMART portal mitigates the issues/barriers that lead to poorer adherence. Finally, although nonsignificant, the observed improvement in HRQOL, patient-reported symptoms, and stool frequency are notable. These outcomes often take longer to change; thus, the short-term nature of this pilot study may not have allowed for observable changes to occur. Alternatively, a brief intervention may not have a strong enough impact on these outcomes, suggesting that sustained intervention may be necessary. Future research including long-term follow-up is needed to fully understand the impact of this intervention. Taken together, these findings suggest that the SMART portal offers a personalized and more accessible tool than current approaches to self-management which rely on qualified personnel and availability to provide ongoing in-person treatment.
Limitations to this pilot study include modest sample size, high proportion of patients with Crohn disease, and no control arm. Larger scale testing involving an adequately powered randomized controlled trial and greater inclusion of patients with ulcerative colitis is needed to definitively determine the impact of the SMART portal. Additionally, the sample lacked racial diversity, with the vast majority of participants describing themselves as White and non-Hispanic. Future trials evaluating the efficacy of the SMART portal will need to enroll a more diverse sample to ensure our attempts to build a culturally sensitive digital therapeutic tool were successful. There was higher than anticipated withdraw from the study as well. However, only 1 participant completed the assessment battery, was able to access the intervention, and then withdrew. Thus, the withdrawal rate is most likely a reflection of the desire to complete assessments rather than usability of the SMART portal. Finally, all participants were prescribed an oral medication or supplement; thus, generalizability at this pilot stage of testing is limited. However, the self-management strategies in the intervention module likely apply to all types of treatment regimens, though further research is needed to determine this. These limitations notwithstanding, this pilot study offers initial support for the utility and potential positive impact of the SMART portal to support self-management in pediatric IBD.
What Is Known
Self-management is a significant problem in pediatric inflammatory bowel disease (IBD) and is associated with increased risk of relapse and health care costs.
Patients generally do not receive appropriate self-management intervention support.
Digital therapeutics can expand access to care, but tools need to be developed and tested.
What Is New
We developed the first self-management digital therapeutic for adolescents with IBD.
A pilot study demonstrated good usage, with improvement in adherence and self-management barriers and trends in health-related quality of life and symptoms.
Results indicate preliminary efficacy of the Self-Management Assistance for Recommended Treatment (SMART) portal and warrant continued evaluation of this digital therapeutic.
Sources of Funding:
This study was supported by a grant (R21 HD074842) from the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NIH/NICHD).
Footnotes
The authors report no conflicts of interest.
Clinicaltrials.gov number: NCT01966744.
REFERENCES
- 1.Rapoff MA. Adherence to Pediatric Medical Regimens. 2nd ed. New York, NY: Springer; 2010. [Google Scholar]
- 2.Kane S, Huo D, Aikens J, Hanauer S. Medication nonadherence and the outcomes of patients with quiescent ulcerative colitis. Am J Med 2003;114:39–43. [DOI] [PubMed] [Google Scholar]
- 3.DiMatteo MR. The role of effective communication with children and their families in fostering adherence to pediatric regimens. Patient Educ Couns 2004;55:339–44. [DOI] [PubMed] [Google Scholar]
- 4.Hommel KA, Davis CM, Baldassano RN. Objective versus subjective assessment of oral medication adherence in pediatric inflammatory bowel disease. Inlamm Bowel Dis 2009;15:589–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hommel KA, McGrady ME, Peugh J, et al. Longitudinal patterns of medication nonadherence and associated health care costs. Inlamm Bowel Dis 2017;23:1577–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hommel KA, Baldassano RN. Brief report: barriers to treatment adherence in pediatric inflammatory bowel disease. J Pediatr Psychol 2010;35:1005–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hommel KA, Herzer M, Ingerski LM, Hente E, Denson LA. Individually tailored treatment of medication nonadherence. J Pediatr Gastroenterol Nutr 2011;53:435–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hommel KA, Hente EA, Odell S, et al. Evaluation of a group-based behavioral intervention to promote adherence in adolescents with inflammatory bowel disease. Eur J Gastroenterol Hepatol 2012;24:64–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sluijs EMF, McMinn AM, Griffin SJ. Effectiveness of interventions to promote physical activity in children and adolescents: systematic review of controlled trials. BMJ 2007;335:703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA 2002;288:2469–75. [DOI] [PubMed] [Google Scholar]
- 11.Evers KE, Prochaska JM, Prochaska JO, Driskell M-M, Cummins CO, Velicer WF. Strengths and weaknesses of health behavior change programs on the internet. J Health Psychol 2003;8:63–70. [DOI] [PubMed] [Google Scholar]
- 12.Pieper KB, Rapoff MA, Purviance MR, Lindsley CB. Improving compliance with prednisone therapy in pediatric patients with rheumatic disease. Arthritis Care Res 1989;2:132–5. [DOI] [PubMed] [Google Scholar]
- 13.Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care 1999;37:126–39. [DOI] [PubMed] [Google Scholar]
- 14.Otley A, Smith C, Nicholas D, et al. The IMPACT questionnaire: a valid measure of health-related quality of life in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2002;35:557–63. [DOI] [PubMed] [Google Scholar]
- 15.Simons LE, McCormick ML, Devine K, Blount RL. Medication barriers predict adolescent transplant recipients’ adherence and clinical outcomes at 18-month follow-up. J Pediatr Psychol 2010;35:1038–48. [DOI] [PubMed] [Google Scholar]
- 16.Markowitz J, Grancher K, Kohn N, Lesser M, Daum F. A multicenter trial of 6-mercaptopurine and prednisone in children with newly diagnosed Crohn’s disease. Gastroenterology 2000;119:895–902. [DOI] [PubMed] [Google Scholar]
- 17.Turner D, Otley AR, Mack D, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology 2007;133:423–32. [DOI] [PubMed] [Google Scholar]
- 18.Bell ML, Whitehead AL, Julious SA. Guidance for using pilot studies to inform the design of intervention trials with continuous outcomes. Clin Epidemiol 2018;10:153–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krebs P, Duncan DT. Health app use among US mobile phone owners: a National Survey. JMIR mHealth uHealth 2015;3:e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hommel KA, Hente E, Herzer M, Ingerski LM, Denson LA. Telehealth behavioral treatment for medication nonadherence: a pilot and feasibility study. Eur J Gastroenterol Hepatol 2013;25:469–73. [DOI] [PMC free article] [PubMed] [Google Scholar]


