Abstract
Background
Gallstones and alcohol account for more than 80% of acute pancreatitis. Cholecystectomy is the definitive treatment for gallstones. Laparoscopic cholecystectomy is the preferred route for performing cholecystectomy. The timing of laparoscopic cholecystectomy after an attack of acute biliary pancreatitis is controversial.
Objectives
To compare the benefits and harms of early versus delayed laparoscopic cholecystectomy in people with acute biliary pancreatitis. For mild acute pancreatitis, we considered 'early' laparoscopic cholecystectomy to be laparoscopic cholecystectomy performed within three days of onset of symptoms. We considered all laparoscopic cholecystectomies performed beyond three days of onset of symptoms as 'delayed'. For severe acute pancreatitis, we considered 'early' laparoscopic cholecystectomy as laparoscopic cholecystectomy performed within the index admission. We considered all laparoscopic cholecystectomies performed in a later admission as 'delayed'.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, issue 12), MEDLINE, EMBASE, Science Citation Index Expanded, and trial registers until January 2013.
Selection criteria
We included randomised controlled trials, irrespective of language or publication status, comparing early versus delayed laparoscopic cholecystectomy for people with acute biliary pancreatitis.
Data collection and analysis
Two authors independently assessed trials for inclusion and independently extracted data. We planned to analyse data with both the fixed‐effect and the random‐effects models using Review Manager 5 (RevMan 2011). We calculated the risk ratio (RR), or mean difference (MD) with 95% confidence intervals (CI) based on an intention‐to‐treat analysis.
Main results
We identified one trial comparing early versus delayed laparoscopic cholecystectomy for people with mild acute pancreatitis. Fifty participants with mild acute gallstone pancreatitis were randomised either to early laparoscopic cholecystectomy (within 48 hours of admission irrespective of whether the abdominal symptoms were resolved or the laboratory values had returned to normal) (n = 25), or to delayed laparoscopic cholecystectomy (surgery after resolution of abdominal pain and after the laboratory values had returned to normal) (n = 25). This trial is at high risk of bias. There was no short‐term mortality in either group. There was no significant difference between the groups in the proportion of participants who developed serious adverse events (RR 0.33; 95% CI 0.01 to 7.81). Health‐related quality of life was not reported in this trial. There were no conversions to open cholecystectomy in either group. The total hospital stay was significantly shorter in the early laparoscopic cholecystectomy group than in the delayed laparoscopic cholecystectomy group (MD ‐2.30 days; 95% CI ‐4.40 to ‐0.20). This trial reported neither the number of work‐days lost nor the costs. We did not identify any trials comparing early versus delayed laparoscopic cholecystectomy after severe acute pancreatitis.
Authors' conclusions
There is no evidence of increased risk of complications after early laparoscopic cholecystectomy. Early laparoscopic cholecystectomy may shorten the total hospital stay in people with mild acute pancreatitis. If appropriate facilities and expertise are available, early laparoscopic cholecystectomy appears preferable to delayed laparoscopic cholecystectomy in those with mild acute pancreatitis. There is currently no evidence to support or refute early laparoscopic cholecystectomy for people with severe acute pancreatitis. Further randomised controlled trials at low risk of bias are necessary in people with mild acute pancreatitis and severe acute pancreatitis.
Keywords: Humans; Acute Disease; Cholecystectomy, Laparoscopic; Cholecystectomy, Laparoscopic/adverse effects; Gallstones; Gallstones/complications; Gallstones/surgery; Pancreatitis; Pancreatitis/etiology; Pancreatitis/surgery; Randomized Controlled Trials as Topic; Time Factors
Plain language summary
Early or delayed removal of gallbladder by key‐hole surgery after a sudden episode of gallstone‐related pancreatitis
Review question
There is considerable controversy regarding how long one should wait after a sudden attack of acute gallstone pancreatitis before removing the gallbladder.
We set out to answer this question by performing a thorough search of the literature for studies which compared the different times at which laparoscopic cholecystectomy was performed. We included only randomised controlled trials (studies which can help avoid arriving at wrong conclusions if designed and conducted appropriately). We searched the literature for all studies reported until January 2013. Two authors independently assessed trials for inclusion and independently extracted data to minimise errors. We considered 'early' laparoscopic cholecystectomy as laparoscopic cholecystectomy performed within three days of onset of symptoms. We considered all laparoscopic cholecystectomies performed beyond three days of onset of symptoms as 'delayed'. For severe acute pancreatitis, we considered 'early' laparoscopic cholecystectomy as laparoscopic cholecystectomy performed within the same admission as the sudden attack of pancreatitis. We considered all laparoscopic cholecystectomies performed in a subsequent admission as 'delayed'.
Background
The pancreas is an abdominal organ that secretes several digestive juices which help in the digestion of food. It also lodges the insulin‐secreting cells which maintain the blood sugar levels. Acute pancreatitis is a sudden inflammatory process in the pancreas which might involve nearby organs or may have an effect on other organ systems including blood circulation. Depending upon the presence of organ failure (such as kidneys, lungs or blood circulation) and the presence of local complications such as fluid collection around the pancreas, pancreatitis can be classified as severe acute pancreatitis or mild acute pancreatitis. People with severe pancreatitis have organ failure or local complications, or both, while those with mild pancreatitis do not have such features. The two main causes of acute pancreatitis are gallstones and alcohol, accounting for more than 80% of acute pancreatitis. Removal of the gallbladder (cholecystectomy) is the definitive treatment for prevention of further attacks of acute gallstone pancreatitis if the person is suitable for surgery. Laparoscopic removal (key‐hole surgery) of the gallbladder is the currently preferred method of cholecystectomy with more than 99% of patients recovering completely without any major ill health.
Study characteristics
We identified one trial comparing early versus delayed laparoscopic cholecystectomy for people with mild acute pancreatitis. Out of the 50 participants included in this trial, 25 underwent early laparoscopic cholecystectomy while the remaining 25 underwent delayed laparoscopic cholecystectomy. All 50 participants were alive at the end of the trial. There was no significant difference between the two groups in the proportion of participants who developed complications. Health‐related quality of life was not reported in this trial. There were no conversions to open cholecystectomy in either group. The total hospital stay was shorter by approximately two days in the early laparoscopic cholecystectomy group than in the delayed laparoscopic cholecystectomy group. The trial did not report the number of work‐days lost or the costs. We did not identify any trials comparing early versus delayed laparoscopic cholecystectomy after severe acute pancreatitis.
Key results
Based on the observations in the one trial included in this review, there appears to be no evidence of increased risk of complications after early laparoscopic cholecystectomy. Early laparoscopic cholecystectomy may shorten the total hospital stay in people with mild acute pancreatitis. If appropriate facilities and expertise are available, early laparoscopic cholecystectomy appears preferable to delayed laparoscopic cholecystectomy in people with mild acute pancreatitis. There is currently no evidence to support or refute early laparoscopic cholecystectomy for people with severe acute pancreatitis. Further well‐designed randomised controlled trials are necessary in people with mild acute pancreatitis and severe acute pancreatitis.
Quality of the evidence
The one trial identified is at high risk of bias, i.e. there was potential to arrive at wrong conclusions because of the way that the study was designed and conducted.
Summary of findings
Summary of findings for the main comparison. Early versus delayed laparoscopic cholecystectomy for mild acute pancreatitis.
| Early versus delayed laparoscopic cholecystectomy for mild acute pancreatitis | |||||
| Patient or population: people with mild acute gallstone pancreatitis Settings: secondary or tertiary care Intervention: early laparoscopic cholecystectomy (within 3 days) Comparison: delayed laparoscopic cholecystectomy (beyond 3 days) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Delayed laparoscopic cholecystectomy (beyond 72 hours) | Early laparoscopic cholecystectomy (within 72 hours) | ||||
| Serious adverse events | 40 per 1000 | 13 per 1000 (0 to 312) | RR 0.33 (0.01 to 7.81) | 50 (1 study) | ⊕⊝⊝⊝ very low1,2,3,4 |
| Hospital stay | The mean hospital stay in the control groups was 5.8 days | The mean hospital stay in the intervention groups was 2.3 lower (4.4 to 0.2 lower) | 49 (1 study) | ⊕⊕⊝⊝ low1,2 | |
| *The basis for the assumed risk is the control group risk. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 The trial was at high risk of bias. 2 There were too few trials to assess publication bias. 3 Overlaps 1 and 0.75 or 1.25. 4 The total number of events was fewer than 300.
Background
Description of the condition
The pancreas is an abdominal organ that secretes several digestive enzymes into the pancreatic ductal system that empties into the small bowel. It also lodges the Islets of Langerhans, which secrete several hormones including insulin (NCBI 2011). Acute pancreatitis is a sudden inflammatory process in the pancreas, with variable involvement of adjacent organs or other organ systems (Bradley 1993). Depending upon the presence of organ failure (such as kidneys, lungs or blood circulation) and the presence of local complications such as necrosis (destruction with liquefaction of tissues), an abscess (collection of pus) or pseudocyst (circumscribed collection of fluid without a cellular lining of the collection), pancreatitis can be classified as acute severe pancreatitis or acute mild pancreatitis (Bradley 1993). People with severe pancreatitis have organ failure or local complications, or both, while those with mild pancreatitis do not have such features (Bradley 1993).
There are regional variations in the incidence of first attacks of pancreatitis ranging from 10 per 100,000 in England to 44 per 100,000 in the USA (Spanier 2008). In European countries other than England, such as Germany, Sweden, Norway, Denmark, Netherlands and Finland, the incidence of first attacks of pancreatitis ranges between 15 and 37 per 100,000 (Omdal 2011; Sandzen 2009; Spanier 2008). The main reason for the differences in the incidence of first attacks of pancreatitis is considered to be the differences in alcohol consumption (Spanier 2008). There has been an increase in the incidence of pancreatitis worldwide (Spanier 2008). The two main causes of acute pancreatitis are gallstones and alcohol, accounting for more than 80% of acute pancreatitis (Spanier 2008). Gallstones can cause temporary obstruction at the ampulla of Vater, which is a channel shared by the bile duct and pancreatic duct, resulting in increased pressure within the pancreas leading on to enzyme activation within the pancreas and acute pancreatitis (Wang 2009).
Removal of the gallbladder (cholecystectomy) is the definitive and effective treatment for prevention of further attacks of acute gallstone pancreatitis if the patient is suitable for surgery. Current British Society of Gastroenterology guidelines, based on a low level of evidence (BSG 2005), state that anyone with biliary pancreatitis (gallstone‐related pancreatitis) should undergo cholecystectomy during the same hospital admission, unless a clear plan has been made for definitive treatment within the next two weeks.
Laparoscopic removal (key‐hole surgery) of the gallbladder is the currently preferred method of cholecystectomy (Ballal 2009; Dolan 2009; Harboe 2011). The standard laparoscopic procedure involves inflating the abdomen with carbon dioxide (pneumoperitoenum), introducing cameras and instruments through four small incisions (two of about 1 cm and two of about 0.5 cm) and removing the gallbladder. Variations include lifting the anterior abdominal wall (front of the tummy) rather than inflating the tummy and using fewer ports and smaller incisions (Gurusamy 2010; Gurusamy 2012a; Ma 2011). The major complications related to laparoscopic cholecystectomy include injury to bile duct (0.3%) (Giger 2011) and other rare complications such as injury to the bowel (Gossage 2010; Marakis 2007), and injury to major blood vessels (Marakis 2007), resulting in an overall mortality of 0.2% (Giger 2011).
Description of the intervention
There is no universally accepted definition of 'early' laparoscopic cholecystectomy. In people with mild acute gallstone pancreatitis, we consider any laparoscopic cholecystectomy performed within three days after onset of pancreatitis as early laparoscopic cholecystectomy. The reason for choosing three days is that this allows time for the clinicians to make the diagnosis of mild pancreatitis and organise the laparoscopic cholecystectomy. We considered laparoscopic cholecystectomy performed after three days as 'delayed' laparoscopic cholecystectomy.
In people with severe acute gallstone pancreatitis, we consider any laparoscopic cholecystectomy performed during the same admission as 'early' laparoscopic cholecystectomy. This is because the patients may be at high risk of anaesthetic and surgical complications until recovery from systemic organ failure. In these people, we considered laparoscopic cholecystectomy performed in a later admission as 'delayed' laparoscopic cholecystectomy.
How the intervention might work
Delaying laparoscopic cholecystectomy exposes the person to a risk of potentially fatal recurrent acute pancreatitis (BSG 2005). On the other hand, considering that pancreatitis is a systemic disorder, the delay by 72 hours allows them to recover fully prior to laparoscopic cholecystectomy in the case of people with mild pancreatitis. In the case of severe pancreatitis, the delay may allow the inflammation to settle down completely before the laparoscopic cholecystectomy.
Why it is important to do this review
As mentioned previously, current British Society of Gastroenterology guidelines state that anyone with biliary pancreatitis should undergo cholecystectomy (open or laparoscopic) during the same hospital admission, unless a clear plan has been made for definitive treatment within the next two weeks. This is based on a low level of evidence (BSG 2005). It is not clear whether delaying surgery after diagnosis of mild acute gallstone pancreatitis is beneficial or harmful to these patients. Ito 2008 showed in a retrospective cohort study that even a delay of two weeks after discharge exposes the patients to complications of gallstones including recurrent pancreatitis. Wilson 2010 performed a literature review of early versus delayed cholecystectomy after acute pancreatitis and concluded that people with mild gallstone pancreatitis should have cholecystectomy during the index admission within 48 hours of arrival, and that those with more severe disease should undergo the procedure at a later date, which could even be weeks or months after the pancreatitis episode, depending on the clinical circumstances. There has been no Cochrane review assessing whether laparoscopic cholecystectomy should be performed early or be delayed in people with acute gallstone pancreatitis.
Objectives
To compare the benefits and harms of early versus delayed laparoscopic cholecystectomy in people with acute biliary pancreatitis.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised clinical trials, irrespective of blinding, language, publication status, or sample size. We excluded quasi‐randomised studies or other study designs.
Types of participants
People eligible to undergo laparoscopic cholecystectomy for acute pancreatitis.
Types of interventions
Early versus delayed laparoscopic cholecystectomy for acute pancreatitis. The definitions and justification for the use of the terms have been stated. We did not include other definitions by individual authors.
Types of outcome measures
We considered all the outcomes measured up to six months after the onset of acute pancreatitis. We anticipated that any differences in the treatments would be evident within this time frame.
Primary outcomes
All‐cause mortality.
Other serious adverse events, defined as any event that would increase mortality, is life‐threatening, requires inpatient hospitalisation, results in a persistent or significant disability, or any important medical event which might have jeopardised the participant or required intervention to prevent it (ICH‐GCP 1996).
Overall quality of life (using any validated measurement scale such as EQ5D or SF‐36). If quality of life was reported at multiple time points in the same trial, we planned to use the latest time point within six months after onset of acute pancreatitis.
Secondary outcomes
Conversion to open cholecystectomy (because of inability to complete the operation laparoscopically or because of injury to important structures requiring open operation).
Total hospital stay (includes the length of hospital stay for treatment of pancreatitis and performing laparoscopic cholecystectomy).
Number of work‐days lost.
Costs.
Search methods for identification of studies
Electronic searches
We searched the following databases from 1987 until January 2013:
the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (2012, Issue 12) (Appendix 1);
MEDLINE (Appendix 2);
EMBASE (Appendix 3);
Science Citation Index Expanded (Appendix 4) (Royle 2003).
Searching other resources
We searched the references of the identified trials for relevant studies. We searched the WHO ICTRP (World Health Organization International Clinical Trials Registry Platform) (http://apps.who.int/trialsearch/). The register includes ISRCTN Register and NIH ClinicalTrials.gov Register among other registers.
Data collection and analysis
We performed the systematic review following the instructions given in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011).
Selection of studies
Two authors (KG and MN) identified trials for inclusion independently of each other. We have listed the excluded studies with the reasons for exclusion (Characteristics of excluded studies table). We resolved any differences through discussion.
Data extraction and management
Both authors independently extracted the following data.
Year and language of publication.
Country.
Year of conduct of the trial.
Inclusion and exclusion criteria.
Sample size.
Outcomes (described above).
Risk of bias (described below).
Source of funding.
Conflicts of interest.
We sought any unclear or missing information by contacting the authors of the individual trials. If there was any doubt whether the trials shared the same participants, either completely or partially (by identifying common authors and centres), we planned to contact the authors of the trials to clarify whether the trial report had been duplicated. We resolved any differences in opinion through discussion.
Assessment of risk of bias in included studies
We followed the instructions given in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011). According to empirical evidence (Kjaergard 2001; Moher 1998; Schulz 1995; Wood 2008), the risk of bias of the trials was assessed based on the following bias risk domains.
Sequence generation
Low risk of bias: the method used was either adequate (e.g., computer‐generated random numbers, table of random numbers) or unlikely to introduce confounding.
Uncertain risk of bias: there was insufficient information to assess whether the method used was likely to introduce confounding.
High risk of bias: the method used (e.g., quasi‐randomised studies) was improper and likely to introduce confounding. Such studies were excluded.
Allocation concealment
Low risk of bias: the method used (e.g., central allocation) was unlikely to induce bias in the final observed effect.
Uncertain risk of bias: there was insufficient information to assess whether the method used was likely to induce bias in the estimate of effect.
High risk of bias: the method used (e.g., open random allocation schedule) was likely to induce bias in the final observed effect.
Blinding of participants, personnel, and outcome assessors
Low risk of bias: blinding was performed adequately, or the outcome measurement was not likely to be influenced by lack of blinding.
Uncertain risk of bias: there was insufficient information to assess whether the type of blinding used was likely to induce bias in the estimate of effect.
High risk of bias: no blinding or incomplete blinding, and the outcome or the outcome measurement was likely to be influenced by lack of blinding.
We anticipated that blinding of participants was unethical as this involves exposure of both groups to a sham operation. We assessed the trials to be at high risk of bias for outcomes such as severe adverse events other than mortality, quality of life, and hospital stay. Blinding of personnel and outcome assessors is possible and we considered all the outcomes other than mortality to be at high risk of bias if there was lack of blinding of personnel or outcome assessors.
Incomplete outcome data
Low risk of bias: the underlying reasons for missing data were unlikely to make treatment effects depart from plausible values, or proper methods had been employed to handle missing data.
Uncertain risk of bias: there was insufficient information to assess whether the missing data mechanism in combination with the method used to handle missing data was likely to induce bias in the estimate of effect.
High risk of bias: the crude estimate of effects (e.g., complete case estimate) will clearly be biased due to the underlying reasons for missing data, and the methods used to handle missing data were unsatisfactory.
Selective outcome reporting
Low risk of bias: the trial protocol was available and all of the trial's prespecified outcomes that were of interest in the review have been reported, or similar; if the trial protocol was not available, all the primary outcomes in this review likely to be measured in such a trial were reported.
Uncertain risk of bias: there was insufficient information to assess whether the magnitude and direction of the observed effect was related to selective outcome reporting.
High risk of bias: not all of the trial's prespecified primary outcomes had been reported, or similar.
We considered trials which were classified as low risk of bias in all the above domains as low bias‐risk trials.
Measures of treatment effect
For dichotomous variables, we calculated the risk ratio (RR) with a 95% confidence interval (CI). Risk ratio calculations do not include trials in which no events occurred in either group, whereas risk difference calculations do. We planned to report the risk difference if the results using this association measure changed the conclusions when compared with risk ratio. For continuous variables, we calculated the mean difference (MD) with a 95% CI for outcomes such as hospital stay and planned to calculate the standardised mean difference (SMD) with 95% CI for quality of life (where different scales might be used).
Unit of analysis issues
The units of analysis were individual participants with acute gallstone pancreatitis who were eligible for laparoscopic cholecystectomy.
Dealing with missing data
We performed an intention‐to‐treat analysis (Newell 1992) whenever possible. We planned to impute data for binary outcomes using various scenarios such as good outcome analysis, bad outcome analysis, 'best‐case' scenario, and 'worst‐case' scenario (Gurusamy 2009).
For continuous outcomes, we used available‐case analysis. We impute the standard deviation from P values, standard error or confidence intervals according to the instructions given in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011), and we planned to use the median for the meta‐analysis when the mean was not available. If it was not possible to calculate the standard deviation from the P value or the CIs, we planned to impute the standard deviation as the highest standard deviation in the other trials included under that outcome, fully recognising that this form of imputation will decrease the weight of the study for calculation of MDs and bias the effect estimate to no effect in the case of SMDs (Higgins 2011).
Assessment of heterogeneity
We explored heterogeneity by the Chi² test with significance set to a P value of 0.10, and measured the quantity of heterogeneity by the I² statistic (Higgins 2002). We also planned to use overlapping of CIs in the forest plot to determine heterogeneity.
Assessment of reporting biases
We planned to use visual asymmetry in a funnel plot to explore reporting bias (Egger 1997; Macaskill 2001) in the presence of at least 10 trials. We planned to perform the linear regression approach described by Egger 1997 to determine funnel plot asymmetry.
Data synthesis
We planned to perform the meta‐analyses using the software package Review Manager 5 (RevMan 2011) and following the recommendations of The Cochrane Collaboration (Higgins 2011). We planned to use both a random‐effects model (DerSimonian 1986) and a fixed‐effect model (DeMets 1987) for meta‐analyses. In the case of discrepancy between the two models we planned to report both results; otherwise we planned to report the results of the fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses.
Trials at low risk of bias compared to trials at high risk of bias.
Participants with mild acute gallstone pancreatitis compared to those with severe acute gallstone pancreatitis.
We planned to use the test for subgroup differences.
Sensitivity analysis
We planned to perform a sensitivity analysis by imputing data for binary outcomes using various scenarios such as good outcome analysis, bad outcome analysis, 'best‐case' scenario, and 'worst‐case' scenario (Gurusamy 2009). We planned to perform a sensitivity analysis by excluding the trials in which the mean or the standard deviation were imputed.
Summary of findings table
We have presented the results of all the available outcomes in Table 1.
Results
Description of studies
Results of the search
We identified a total of 451 references through the electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (n = 25), MEDLINE (n = 114), EMBASE (n = 130), Science Citation Index Expanded (n = 172), and WHO ICTRP (n = 10). We have shown the flow of references in Figure 1. We excluded 81 duplicates and 366 clearly irrelevant references through reading titles and abstracts. Four references were retrieved for further assessment. Of these, two were full texts and two were records from trial registers. Of the full text reports, one reference was a completed trial which has been included in this review (Aboulian 2010) and one reference was the protocol of an ongoing trial (Bouwense 2012). Of the trial records, one trial suffered from a lack of participants and was not reported (ISRCTN42476855), and another was an ongoing trial (NCT01687959). No references were identified through scanning the reference list of the identified randomised trial. In total, one publication describing one randomised trial fulfilled the inclusion criteria.
1.
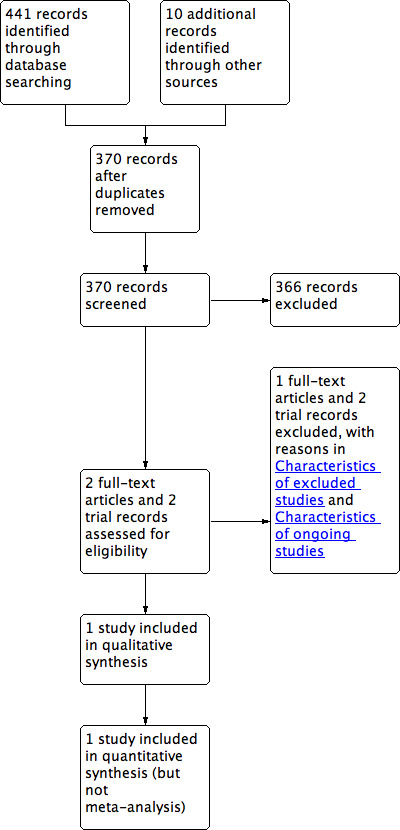
Study flow diagram.
Included studies
Fifty‐one participants with mild acute gallstone pancreatitis were randomised to early laparoscopic cholecystectomy (within 48 hours of admission irrespective of whether the abdominal symptoms were resolved or the laboratory values returned to normal) or to delayed laparoscopic cholecystectomy (surgery after resolution of abdominal pain and after the laboratory values had returned to normal). One person in the delayed laparoscopic cholecystectomy group did not undergo surgery because the pancreatitis was deemed to be due to hyperlipidaemia. One participant developed myocardial infarction in the delayed laparoscopic cholecystectomy group and the trial authors excluded them from the analysis. We included this participant in mortality and serious adverse events results but could not include them for hospital stay. In total, we included 50 participants (25 with early laparoscopic cholecystectomy and 25 with delayed laparoscopic cholecystectomy) for mortality and serious adverse events and 49 participants (25 with early laparoscopic cholecystectomy and 24 with delayed laparoscopic cholecystectomy) for length of hospital stay.
The average age of the participants included in the trial was 37 years. The proportion of women included in this trial was 10%.
Risk of bias in included studies
The risk of bias in the trial is summarised in Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Appropriate methods of random sequence generation and allocation concealment were used in this trial (Aboulian 2010).
Blinding
It is impossible to blind participants unless sham surgery was performed in both groups, which may be unethical considering that the participants may be exposed to unnecessary risks during the sham surgery. It was not clear whether the surgeons who performed the operation and the outcome assessors were aware of the participant groups (Aboulian 2010).
Incomplete outcome data
One person was excluded after randomisation because the aetiology of acute pancreatitis was considered to be hypertriglyceridaemia. This participant was excluded from the calculation of hospital stay in the delayed laparoscopic cholecystectomy group (the participant had developed myocardial infarction and did not undergo laparoscopic cholecystectomy) (Aboulian 2010). The exclusion of this participant is considered unlikely to affect the conclusions of the trial or this review.
Selective reporting
The trial reported all the outcomes that are likely to be reported in such a trial (Aboulian 2010). We therefore considered the trial to be at low risk of bias regarding this domain.
Effects of interventions
See: Table 1
The results are summarised in the Table 1.
Primary outcomes
All‐cause mortality
There was no mortality in either group at 30 days.
Other serious adverse events (other complications)
One participant in the delayed laparoscopic cholecystectomy group developed myocardial infarction and laparoscopic cholecystectomy was not performed. This was the only patient who developed a serious adverse event in either group. There was no significant difference between the groups (risk ratio (RR) 0.33; 95% confidence interval (CI) 0.01 to 7.81) (Analysis 1.1).
1.1. Analysis.
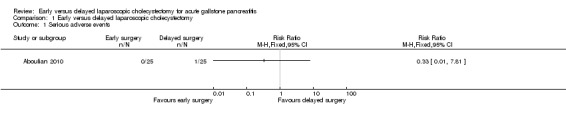
Comparison 1 Early versus delayed laparoscopic cholecystectomy, Outcome 1 Serious adverse events.
Quality of life
Health‐related quality of life was not reported in this trial.
Secondary outcomes
Conversion to open cholecystectomy
There were no conversions to open cholecystectomy in either group.
Total hospital stay
The total hospital stay was significantly shorter in the early laparoscopic cholecystectomy group than in the delayed laparoscopic cholecystectomy group (mean difference (MD) ‐2.30 days; 95% CI ‐4.40 to ‐0.20) (Analysis 1.2).
1.2. Analysis.
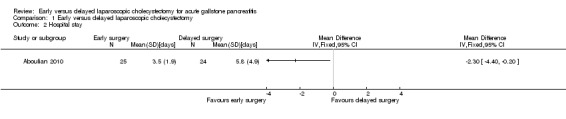
Comparison 1 Early versus delayed laparoscopic cholecystectomy, Outcome 2 Hospital stay.
Number of work‐days lost
This outcome was not reported in this trial.
Costs
This outcome was not reported in this trial.
Other information
Variation in the effect measures and model used
Since only one trial was included in this review, we did not assess whether there were differences between the random‐effects model and the fixed‐effect model. The conclusions of the review did not change for binary outcomes when risk difference rather than risk ratio was used.
Subgroup analysis
We did not perform subgroup analysis because only one trial was included in this review.
Sensitivity analysis
We did not perform sensitivity analysis since all participants were included for the binary outcomes, and there were no post‐randomisation drop‐outs in the only trial included in this review (Aboulian 2010). Although we had imputed the standard deviation from the confidence interval for the total hospital stay, we did not perform a sensitivity analysis since this was the only trial included in this review.
Reporting bias
We did not explore reporting bias by using the funnel plot because of insufficient studies.
Discussion
Summary of main results
This review compared early versus delayed laparoscopic cholecystectomy for people with acute gallstone pancreatitis. We defined early laparoscopic cholecystectomy differently in the mild and severe acute gallstone pancreatitis. In mild pancreatitis, we defined early laparoscopic cholecystectomy as that performed within three days of onset of symptoms. The three days was chosen arbitrarily as different authors define early laparoscopic cholecystectomy differently. The rationale for choosing three days for defining early acute pancreatitis is that this allows adequate time for the surgery to be organised. Approximately 20% of participants with mild acute pancreatitis have common bile duct stones (Aboulian 2010). The common bile duct stones have to be treated as well as the gallbladder stones. Various strategies include magnetic resonance cholangiopancreatography (magnetic resonance imaging (MRI) to diagnose common bile duct stones) followed by endoscopic sphincterotomy (endoscopic removal of common bile duct stones) for people with common bile duct stones and intra‐operative cholangiography (cholangiography performed during laparoscopic cholecystectomy) followed by intra‐operative laparoscopic common bile duct exploration and laparoscopic cholecystectomy along with intra‐operative or post‐operative endoscopic sphincterotomy. The arbitrary three day period allows time for the diagnosis and treatment of common bile duct stones by pre‐operative endoscopic sphincterotomy. Endoscopic sphincterotomy appears to decrease the complications associated with acute pancreatitis in people presenting with biliary obstruction (Tse 2012). Intra‐operative endoscopic sphincterotomy is at least as effective, has fewer complications (including pancreatitis), and is more cost‐effective than pre‐operative endoscopic sphincterotomy in people with common bile duct stones (Gurusamy 2011; Gurusamy 2012b) (although the people included in the meta‐analysis did not have pancreatitis when they were undergoing the procedure) and may be one of the strategies used to perform the operation within three days of onset of symptoms. In the trial included in this review, the people with common bile duct stones were identified during an intra‐operative cholangiogram and treated with post‐operative endoscopic sphincterotomy (Aboulian 2010).The alternative to endoscopic sphincterotomy in dealing with common bile duct stones is laparoscopic common bile duct exploration (LBCDE) although LBCDE is not the surgeons' preferred way of dealing with common bile duct stones (Ludwig 2001; Bingener 2006; Spelsberg 2009). The reasons for this preference include the lack of availability of equipment for LCBDE, the time taken for LCBDE, and the lack of skills to perform LCBDE (Bingener 2006). Thus various strategies are available for dealing with common bile duct stones within the three days. The three day period also allows differentiation between mild and severe pancreatitis as severe pancreatitis becomes apparent within 48 hours in most patients (and hence the reason for repeating the clinical scoring system in 48 hours) (Ranson 1974; Ranson 1977). Any systemic deterioration of patients with mild acute pancreatitis will be noted in this time period and the surgery can be postponed to a suitable time when the anaesthetic risk becomes low.
There is no evidence for an increase in the serious adverse events by performing early laparoscopic cholecystectomy in the only trial included in this review (Aboulian 2010) and there does not appear to be any reason why serious adverse events should be higher in the presence of mild pancreatitis (people with systemic illness will generally be classified as severe pancreatitis by most of the clinical scoring systems). On the other hand, early laparoscopic cholecystectomy appears to decrease the hospital stay by about two days (Aboulian 2010). In the absence of any evidence for a difference in the serious adverse events, the longer hospital stay in the delayed laparoscopic cholecystectomy group is probably due to the time it takes for the symptoms to settle down completely and all the blood parameters suggestive of pancreatitis to return to normal levels. The only trial included in this review did not compare early laparoscopic cholecystectomy with delayed laparoscopic cholecystectomy in a second admission. It is not clear whether the total hospital day would be different between early laparoscopic cholecystectomy and delayed laparoscopic cholecystectomy in a second admission. This delayed laparoscopic cholecystectomy in a second admission however has the danger of exposing the patients to recurrent symptoms due to gallstones. While there is no evidence from randomised controlled trials on this issue of complications during the waiting period, a significant proportion of patients return with biliary complications during the first year of the index admission when the surgery is not performed in the index admission (Sandzen 2009). At least 60% of people who did not undergo cholecystectomy in the index admission were readmitted with biliary complications in the first year compared to 5% of people who underwent cholecystectomy in the index admission. Considering that the inpatient stay of people with mild acute biliary pancreatitis who did not undergo cholecystectomy during the index admission is about four to six days (Sandzen 2009), adding early laparoscopic cholecystectomy is unlikely to prolong the hospital stay significantly. In addition, the number of work days lost is likely to be lower if the laparoscopic cholecystectomy is performed in the index admission compared to laparoscopic cholecystectomy in a second admission. However, it is difficult to identify whether there will be any difference to the work days lost if delayed laparoscopic cholecystectomy was performed in the index admission since this outcome was not reported in the trial included in this review (Aboulian 2010). Quality of life and costs were also not reported in this trial. These outcomes should be measured in the future trials.
In the case of severe pancreatitis, there are no randomised controlled trials comparing early versus delayed laparoscopic cholecystectomy in people with severe acute pancreatitis. In open cholecystectomy, early cholecystectomy (within six weeks of index admission) resulted in increased complication rates and length of hospital stay in an observational study (Nealon 2004). Delayed laparoscopic cholecystectomy may decrease the conversion to open cholecystectomy since the inflammation and fluid collections associated with severe pancreatitis are likely to settle down or become well‐defined pseudocysts during the waiting time allowing surgical management of pseudocysts, which can be performed laparoscopically when expertise is available (Palanivelu 2007). However, delayed laparoscopic cholecystectomy is not without problems. The problems of recurrence of biliary symptoms (Ito 2008) along with the hospital stay related to the second admission exist and currently there is no evidence that the complications or conversion to open cholecystectomy are lower with delayed laparoscopic cholecystectomy than early laparoscopic cholecystectomy. A significant proportion of fluid collections after acute pancreatitis disappear spontaneously and endoscopic drainage is necessary only in the patients who have persistent fluid collections. Waiting for more than six weeks increases the proportion of people in whom the pseudocysts resolve spontaneously and a wait‐and‐watch policy has been suggested as an alternative treatment for asymptomatic pseudocysts associated with acute pancreatitis (Kim 2012).Thus, the timing of laparoscopic cholecystectomy in people with severe pancreatitis appears to be a major dilemma for surgeons and those undergoing laparoscopic cholecystectomy for acute severe pancreatitis. In the absence of any clinical trials comparing the different management strategies, it is impossible to conclude whether it is preferable to perform early or delayed laparoscopic cholecystectomy for acute severe pancreatitis.
Overall completeness and applicability of evidence
The only trial included in this review compared early laparoscopic cholecystectomy and delayed laparoscopic cholecystectomy performed in the index admission. The review is therefore applicable only to this comparison in people with mild acute gallstone pancreatitis.
Quality of the evidence
The quality of the evidence was low or very low. However, it must be noted that this is the best available evidence currently. The search of trial register shows that there are two ongoing trials on this topic. Until the information from these trials becomes available, this will be the best available evidence on this topic.
Potential biases in the review process
The review includes periods when it was not mandatory to register clinical trials for publication in journals. We were not able to assess publication bias because of the inclusion of one trial in this trial. We have imputed the standard deviation from the 95% confidence intervals. In addition, the length of total hospital stay may not be normally distributed. These factors may have introduced bias.
Agreements and disagreements with other studies or reviews
This is the first systematic review on this topic which includes only randomised controlled trials. We agree with the trial authors that early laparoscopic cholecystectomy decreases hospital stay compared to delayed laparoscopic cholecystectomy in people with mild acute pancreatitis.
Authors' conclusions
Implications for practice.
There is currently no evidence for an increase in the complications after early laparoscopic cholecystectomy after mild acute pancreatitis. Early laparoscopic cholecystectomy may shorten the total hospital stay in people with mild acute pancreatitis. If appropriate facilities and expertise are available, early laparoscopic cholecystectomy appears preferable to delayed laparoscopic cholecystectomy in people with mild acute pancreatitis. There is currently no evidence to support or refute early laparoscopic cholecystectomy for people with severe acute pancreatitis.
Implications for research.
Further randomised controlled trials at low risk of bias are necessary in people with mild acute pancreatitis and severe acute pancreatitis.
Acknowledgements
To the Cochrane Upper Gastro‐intestinal and Pancreatic Diseases Group.
Peer reviewers: Eduardo Villatoro, Sarah Rhodes, Marilyn Walsh.
This project was funded by the National Institute for Health Research. Disclaimer of the Department of Health: 'The views and opinions expressed in the review are those of the authors and do not necessarily reflect those of the National Institute for Health Research (NIHR), National Health Services (NHS), or the Department of Health'.
Appendices
Appendix 1. CENTRAL search strategy
#1 laparoscop* OR coelioscop* OR celioscop* OR peritoneoscop* #2 cholecystectom* #3 MeSH descriptor Cholecystectomy, Laparoscopic explode all trees #4 (( #1 AND #2 ) OR #3) #5 MeSH descriptor Pancreatitis explode all trees #6 pancreatiti* #7 (#5 OR #6) #8 (#4 AND #7)
Appendix 2. MEDLINE search strategy
(((laparoscop* OR coelioscop* OR celioscop* OR peritoneoscop*) AND (cholecystectom*)) OR “cholecystectomy, laparoscopic”[MeSH]) AND (pancreatiti* OR "Pancreatitis"[Mesh]) AND ((randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]))
Appendix 3. EMBASE search strategy
1. (laparoscop$ or coelioscop$ or celioscop$ or peritoneoscop$).af. 2. exp Laparoscopic surgery/ 3. 1 or 2 4. cholecystectom$.af. 5. exp Cholecystectomy/ 6. 4 or 5 7. pancreatiti$.af. 8. exp Pancreatitis/ 9. 7 and 8 10. 3 and 6 and 9 11. Clinical trial/ 12. Randomized controlled trial/ 13. Randomization/ 14. Single‐Blind Method/ 15. Double‐Blind Method/ 16. Cross‐Over Studies/ 17. Random Allocation/ 18. Placebo/ 19. Randomi?ed controlled trial$.tw. 20. Rct.tw. 21. Random allocation.tw. 22. Randomly allocated.tw. 23. Allocated randomly.tw. 24. (allocated adj2 random).tw. 25. Single blind$.tw. 26. Double blind$.tw. 27. ((treble or triple) adj blind$).tw. 28. Placebo$.tw. 29. Prospective study/ 30. or/11‐29 31. Case study/ 32. Case report.tw. 33. Abstract report/ or letter/ 34. or/31‐33 35. 30 not 34 36. 10 and 35
Appendix 4. Science Citation Index Expanded search strategy
#1 TS=(laparoscop* OR coelioscop* OR celioscop* OR peritoneoscop*) #2 TS=(cholecystectom*) #3 TS=(pancreatiti*) #4 TS=(random* OR rct* OR crossover OR masked OR blind* OR placebo* OR meta‐analysis OR systematic review* OR meta‐analys*) #5 #4 AND #3 AND #2 AND #1
Data and analyses
Comparison 1. Early versus delayed laparoscopic cholecystectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serious adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Hospital stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aboulian 2010.
| Methods | Randomised clinical trial | |
| Participants | Country: USA. Number randomised: 51. Post‐randomisation drop‐outs: 1 (1.9%). Revised sample size: 49. Average age: 37 years. Women: 5 (10%). Inclusion criteria All adults between the age of 18 and 100 with mild gallstone pancreatitis. A subject was classified as having gallstone pancreatitis if they had the following:
The classification of mild pancreatitis was defined by the presence of the following:
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: early laparoscopic cholecystectomy (n = 25). Further details: surgery within 48 hours of admission and potentially before normalisation of laboratory values and resolution of abdominal symptoms. Group 2: delayed laparoscopic cholecystectomy (n = 25). Further details: surgery after resolution of abdominal pain and normalisation of laboratory values. | |
| Outcomes | The outcomes reported were mortality, serious adverse events, conversion to open cholecystectomy and hospital stay. | |
| Notes | One participant in the delayed laparoscopic cholecystectomy group did not undergo surgery because the cause of pancreatitis was deemed to be hypertriglyceridaemia. This person developed myocardial infarction and the authors excluded him from the analysis. We included this person in all the outcomes except hospital stay. Attempts were made to contact the authors in January 2013. Source of funding: Not stated. Conflicts of interest: Not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random assignment was performed by drawing a sealed, unlabeled, unordered envelope from a container by an independent party immediately after informed consent was obtained". |
| Allocation concealment (selection bias) | Low risk | Quote: "Random assignment was performed by drawing a sealed, unlabeled, unordered envelope from a container by an independent party immediately after informed consent was obtained". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: It is not possible to blind the participants and healthcare providers unless sham surgery was provided, which may be unethical. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Although one participant who developed myocardial infarction was not included in the analysis of the hospital stay, inclusion of this participant is likely to increase rather than decrease the difference. |
| Selective reporting (reporting bias) | Low risk | Comment: All important outcomes expected to be measured in such a trial were reported. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ISRCTN42476855 | Although reported in the trial registry, this trial suffered from lack of participants and hence was not reported (author replies). |
Characteristics of ongoing studies [ordered by study ID]
Bouwense 2012.
| Trial name or title | Pancreatitis of biliary origin, optimal timing of cholecystectomy (PONCHO) |
| Methods | Randomised controlled trial |
| Participants | Patients with acute biliary pancreatitis |
| Interventions | Early laparoscopic cholecystectomy (within 72 hours after recovery of a first episode of mild biliary pancreatitis versus delayed laparoscopic cholecystectomy (25 to 30 days after recovery of pancreatitis) |
| Outcomes | Mortality and readmissions |
| Starting date | 1st January 2011 |
| Contact information | Djamila Boerma (d.boerma@antoniusziekenhuis.nl) |
| Notes | It is unlikely that this trial will be included for the analysis since the intervention is performed after recovery. However, some participants may meet the inclusion criteria and we will try to get the information on such people. |
NCT01687959.
| Trial name or title | Timing of laparoscopic cholecystectomy after endoscopic retrograde cholangiography for acute biliary pancreatitis |
| Methods | Randomised controlled trial |
| Participants | Patients with acute biliary pancreatitis |
| Interventions | Early laparoscopic cholecystectomy (within 72 hours after endoscopic sphincterotomy versus delayed laparoscopic cholecystectomy (after 6 weeks) |
| Outcomes | Mortality and morbidity of laparoscopic cholecystectomy |
| Starting date | September 2012 |
| Contact information | Mustafa Hasbahceci, Department of General Surgery; Bezmialem Vakif university Istanbul, Turkey, 34093 |
| Notes | Some patients with mild acute pancreatitis and all patients with acute severe pancreatitis will meet the inclusion criteria for this review. |
Differences between protocol and review
We included number of work‐days lost and costs as secondary outcomes.
Contributions of authors
KS Gurusamy wrote the review, assessed the trials for inclusion and extracted data from the included trial. M Nagendran independently assessed the trials for inclusion and extracted data from the included trial. BR Davidson critically commented on the review.
Sources of support
Internal sources
None, Not specified.
External sources
-
National Insitute for Health Research (NIHR), UK.
NIHR is the health research wing of the UK Government. It part funds Dr K Gurusamy's salary and funds all the materials needed for the preparation of this review.
Declarations of interest
None
New
References
References to studies included in this review
Aboulian 2010 {published data only}
- Aboulian A, Chan T, Yaghoubian A, Kaji AH, Putnam B, Neville A, et al. Early cholecystectomy safely decreases hospital stay in patients with mild gallstone pancreatitis: a randomized prospective study. Annals of Surgery 2010;251(4):615‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ISRCTN42476855 {unpublished data only}
- ISRCTN42476855. The timings of cholecystectomy in acute pancreatitis. http://www.controlled‐trials.com/ISRCTN42476855 (accessed 31 January 2013). [ISRCTN42476855]
References to ongoing studies
Bouwense 2012 {published data only}
- Bouwense SA, Besselink MG, Brunschot S, Bakker OJ, Santvoort HC, Schepers NJ, et al. Pancreatitis of biliary origin, optimal timing of cholecystectomy (PONCHO trial): study protocol for a randomized controlled trial. Trials 2012;13(1):225. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01687959 {unpublished data only}
- Timing of laparoscopic cholecystectomy after endoscopic retrograde cholangiography for acute biliary pancreatitis. Ongoing study September 2012.
Additional references
Ballal 2009
- Ballal M, David G, Willmott S, Corless DJ, Deakin M, Slavin JP. Conversion after laparoscopic cholecystectomy in England. Surgical Endoscopy 2009;23(10):2338‐44. [DOI] [PubMed] [Google Scholar]
Bingener 2006
- Bingener J, Schwesinger WH. Management of common bile duct stones in a rural area of the United States: results of a survey. Surgical Endoscopy 2006;20(4):577‐9. [DOI] [PubMed] [Google Scholar]
Bradley 1993
- Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11‐13, 1992. Archives of Surgery 1993;128(5):586‐90. [DOI] [PubMed] [Google Scholar]
BSG 2005
- British Society of Gastroenterology. UK guidelines for the management of acute pancreatitis. Gut 2005;54 Suppl 3:iii1‐9. [PUBMED: 15831893] [DOI] [PMC free article] [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Dolan 2009
- Dolan JP, Diggs BS, Sheppard BC, Hunter JG. The national mortality burden and significant factors associated with open and laparoscopic cholecystectomy: 1997‐2006. Journal of Gastrointestinal Surgery 2009;13(12):2292‐301. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giger 2011
- Giger U, Ouaissi M, Schmitz SF, Krahenbuhl S, Krahenbuhl L. Bile duct injury and use of cholangiography during laparoscopic cholecystectomy. British Journal of Surgery 2011;98(3):391‐6. [DOI] [PubMed] [Google Scholar]
Gossage 2010
- Gossage JA, Forshaw MJ. Prevalence and outcome of litigation claims in England after laparoscopic cholecystectomy. International Journal of Clinical Practice 2010;64(13):1832‐5. [DOI] [PubMed] [Google Scholar]
Gurusamy 2009
- Gurusamy KS, Gluud C, Nikolova D, Davidson BR. Assessment of risk of bias in randomized clinical trials in surgery. British Journal of Surgery 2009;96(4):342‐9. [DOI] [PubMed] [Google Scholar]
Gurusamy 2010
- Gurusamy KS, Samraj K, Ramamoorthy R, Farouk M, Fusai G, Davidson BR. Miniport versus standard ports for laparoscopic cholecystectomy. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD006804.pub2] [DOI] [PubMed] [Google Scholar]
Gurusamy 2011
- Gurusamy K, Sahay SJ, Burroughs AK, Davidson BR. Systematic review and meta‐analysis of intraoperative versus preoperative endoscopic sphincterotomy in patients with gallbladder and suspected common bile duct stones. British Journal of Surgery 2011;98(7):908‐16. [DOI] [PubMed] [Google Scholar]
Gurusamy 2012a
- Gurusamy KS, Koti R, Samraj K, Davidson BR. Abdominal lift for laparoscopic cholecystectomy. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD006574.pub3] [DOI] [PubMed] [Google Scholar]
Gurusamy 2012b
- Gurusamy K, Wilson E, Burroughs AK, Davidson BR. Intra‐operative vs pre‐operative endoscopic sphincterotomy in patients with gallbladder and common bile duct stones: cost‐utility and value‐of‐information analysis. Applied Health Economics and Health Policy 2012;10(1):15‐29. [DOI] [PubMed] [Google Scholar]
Harboe 2011
- Harboe KM, Bardram L. The quality of cholecystectomy in Denmark: outcome and risk factors for 20,307 patients from the national database. Surgical Endoscopy 2011;25(5):1630‐41. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Colloboration, 2011. Available from www.cochrane‐handbook.org.
ICH‐GCP 1996
- International Conference on Harmonisation of Technical Requiements for Registration of Pharmaceuticals for Human Use. Code of Federal Regulation & ICH Guidelines. Media: Parexel Barnett, 1996. [Google Scholar]
Ito 2008
- Ito K, Ito H, Whang EE. Timing of cholecystectomy for biliary pancreatitis: do the data support current guidelines?. Journal of Gastrointestinal Surgery 2008;12(12):2164‐70. [DOI] [PubMed] [Google Scholar]
Kim 2012
- Kim KO, Kim TN. Acute pancreatic pseudocyst: incidence, risk factors, and clinical outcomes. Pancreas 2012;41(4):577‐81. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Ludwig 2001
- Ludwig K, Kockerling F, Hohenberger W, Lorenz D. Surgical therapy in cholecysto‐/choledocholithiasis. Results of a Germany‐wide questionnaire sent to 859 clinics with 123,090 cases of cholecystectomy. Der Chirurg 2001;72(10):1171‐8. [DOI] [PubMed] [Google Scholar]
Ma 2011
- Ma J, Cassera MA, Spaun GO, Hammill CW, Hansen PD, Aliabadi‐Wahle S. Randomized controlled trial comparing single‐port laparoscopic cholecystectomy and four‐port laparoscopic cholecystectomy. Annals of Surgery 2011;254(1):22‐7. [DOI] [PubMed] [Google Scholar]
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. [DOI] [PubMed] [Google Scholar]
Marakis 2007
- Marakis GN, Pavlidis TE, Ballas K, Aimoniotou E, Psarras K, Karvounaris D, et al. Major complications during laparoscopic cholecystectomy. International Surgery 2007;92(3):142‐6. [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
NCBI 2011
- NCBI. MeSH. NLM Controlled Vocabulary. Pancreas. http://www.ncbi.nlm.nih.gov/mesh/68010179 (accessed on 08/03/2012) 2011.
Nealon 2004
- Nealon WH, Bawduniak J, Walser EM. Appropriate timing of cholecystectomy in patients who present with moderate to severe gallstone‐associated acute pancreatitis with peripancreatic fluid collections. Annals of Surgery 2004;239(6):741‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI] [PubMed] [Google Scholar]
Omdal 2011
- Omdal T, Dale J, Lie SA, Iversen KB, Flaatten H, Ovrebo K. Time trends in incidence, etiology, and case fatality rate of the first attack of acute pancreatitis. Scandinavian Journal of Gastroenterology 2011;46(11):1389‐98. [DOI] [PubMed] [Google Scholar]
Palanivelu 2007
- Palanivelu C, Senthilkumar K, Madhankumar MV, Rajan PS, Shetty AR, Jani K, et al. Management of pancreatic pseudocyst in the era of laparoscopic surgery‐‐experience from a tertiary centre. Surgical Endoscopy 2007;21(12):2262‐7. [DOI] [PubMed] [Google Scholar]
Ranson 1974
- Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surgery, Gynecology and Obstetrics 1974;139(1):69‐81. [PubMed] [Google Scholar]
Ranson 1977
- Ranson JH, Pasternack BS. Statistical methods for quantifying the severity of clinical acute pancreatitis. Journal of Surgical Research 1977;22(2):79‐91. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Sandzen 2009
- Sandzen B, Rosenmuller M, Haapamaki MM, Nilsson E, Stenlund HC, Oman M. First attack of acute pancreatitis in Sweden 1988 ‐ 2003: incidence, aetiological classification, procedures and mortality ‐ a register study. BMC Gastroenterology 2009;9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Spanier 2008
- Spanier BW, Dijkgraaf MG, Bruno MJ. Epidemiology, aetiology and outcome of acute and chronic pancreatitis: An update. Best Practice & Research. Clinical Gastroenterology 2008;22(1):45‐63. [DOI] [PubMed] [Google Scholar]
Spelsberg 2009
- Spelsberg FW, Nusser F, Huttl TK, Obeidat FW, Lang RA, Jauch KW, et al. Management of cholecysto‐ and choledocholithiasis‐‐survey and analysis of 16 615 cholecystectomies and common bile duct explorations in Bavaria. Zentralblatt fur Chirurgie 2009;134(2):120‐6. [DOI] [PubMed] [Google Scholar]
Tse 2012
- Tse F, Yuan Y. Early routine endoscopic retrograde cholangiopancreatography strategy versus early conservative management strategy in acute gallstone pancreatitis. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD009779.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2009
- Wang GJ, Gao CF, Wei D, Wang C, Ding SQ. Acute pancreatitis: etiology and common pathogenesis. World Journal of Gastroenterology 2009;15(12):1427‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 2010
- Wilson CT, Moya MA. Cholecystectomy for acute gallstone pancreatitis: early vs delayed approach. Scandinavian Journal of Surgery 2010;99(2):81‐5. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]


