Abstract
OBJECTIVES:
We aimed to summarize the most significant and impactful publications describing the pharmacotherapeutic care of critically ill patients in 2023.
DATA SOURCES:
PubMed/MEDLINE and the Clinical Pharmacy and Pharmacology Pharmacotherapy Literature Update.
STUDY SELECTION:
Randomized controlled trials and prospective studies of adult critically ill patients assessing a pharmacotherapeutic intervention and reporting clinical endpoints published between January 1, 2023, and December 31, 2023, were eligible for inclusion in this article.
DATA EXTRACTION:
Articles from a systematic search and the Clinical Pharmacy and Pharmacology Pharmacotherapy Literature Update were included. An a priori defined three-round modified Delphi process was employed to achieve consensus on the most impactful publications based on the following considerations: 1) overall contribution to scientific knowledge and 2) novelty to the literature.
DATA SYNTHESIS:
The systematic search and Clinical Pharmacy and Pharmacology Pharmacotherapy Literature Update returned a total of 1202 articles, of which 1164 were excluded. The remaining 38 articles underwent a three-round modified Delphi process. In each round, articles were independently scored based on overall contribution to scientific knowledge and novelty to the literature. Included articles are summarized and their impact discussed. Article topics included hydrocortisone for severe community-acquired pneumonia, inhaled amikacin for prevention of ventilator-associated pneumonia, methylene blue for septic shock, restrictive vs. liberal fluid management for sepsis-induced hypotension, andexanet alfa for major bleeding associated with factor Xa inhibitors, and early administration of four-factor prothrombin complex concentrate in patients with trauma at risk for massive transfusion.
CONCLUSIONS:
This review provides a summary and perspective on the potential impact of the most relevant articles in 2023 describing advances in the pharmacotherapeutic care of critically ill patients.
Keywords: critical bleeding, critical care, critically ill, pharmacotherapy, pneumonia, septic shock
KEY POINTS
Question: What were the most impactful publications related to critical care pharmacotherapy in 2023?
Findings: This systematic search and modified Delphi process identified five randomized controlled trials and a single-arm prospective trial. These articles spanned a number of domains including sepsis and septic shock resuscitation, treatment and prevention of pneumonia, and management of hemorrhage.
Meaning: This review provides a focused update on and analysis of the most impactful articles describing advancements in the pharmacotherapeutic management of critically ill adult patients published in 2023.
A rise in critical care publications poses challenges for timely implementation of evidence-based medicine, making it difficult for clinicians to evaluate emerging literature without becoming overwhelmed (1–3). Strategies including journal surveillance, interaction with social media communities, and continuing education allow clinicians to engage with the scientific literature while managing information overload (4, 5). The Clinical Pharmacy and Pharmacology Literature Update (CPPLU) working group of the Society of Critical Care Medicine (SCCM), Section of Clinical Pharmacy and Pharmacology, reviews major critical care journals and disseminates monthly summaries of the most impactful articles to various sections of the Society, and provides an annual summary and perspective of these articles (6–16). We aimed to summarize and evaluate the impact of the most relevant publications related to critical care pharmacotherapy in 2023.
METHODS
We performed a systematic search of PubMed/MEDLINE from January 1, 2023, to December 31, 2023, to capture articles related to critical care pharmacotherapy. Search criteria were similar to those used in previous reviews, excluding systematic reviews, and meta-analyses (Appendix 1, http://links.lww.com/CCX/B404) (15, 16). Monthly critical care pharmacotherapy literature updates produced by the CPPLU were reviewed to include any articles not captured in the systematic search. Two independent reviewers (B.M., P.K.G.) assessed each title and abstract for inclusion based on predefined criteria including: 1) randomized controlled trial (RCT) or prospective study design, 2) critically ill adult patient population, 3) assessment of a pharmacotherapeutic intervention, and 4) reporting of clinical endpoints. A full-text review was subsequently performed to exclude remaining articles that did not fulfill inclusion criteria. Eligible articles were entered into a survey (Appendix 2, http://links.lww.com/CCX/B404).
An a priori defined three-round modified Delphi process was performed consisting of a Qualtrics (Provo, UT) survey including article titles and links to full-text files. A multiprofessional panel of reviewers (n = 15) chosen to represent diverse critical care professions and specialties as well as geographic regions independently assessed and scored each article on a 5-point scale according to overall contribution to scientific knowledge (morbidity/expense) and novelty to the literature. Articles scoring at or above the median were included in the subsequent round until the six articles most relevant to critical care pharmacotherapy were chosen. The process for article identification and selection is consistent with previous reviews (15, 16). Selected studies were summarized and analyzed for applicability to critical care practice.
RESULTS
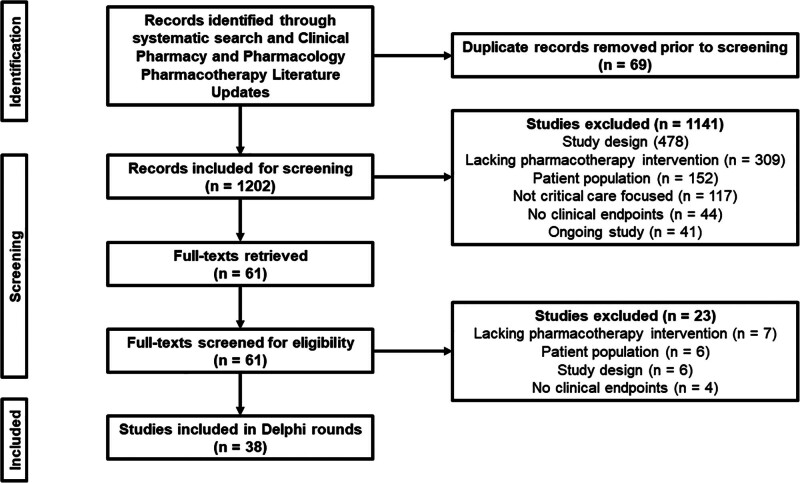
The systematic search and CPPLU returned 1202 articles, with 1141 excluded during title and abstract review and 23 excluded following full-text review. The remaining 38 articles were included in the modified Delphi process (Fig. 1). The results of the modified Delphi process are presented in eTables 1–3 (http://links.lww.com/CCX/B404). After three rounds, six studies with the highest median ranking were selected for inclusion.
Figure 1.
Flow diagram of article screening.
DISCUSSION
Hydrocortisone in Severe Community-Acquired Pneumonia
The Community-Acquired Pneumonia: Evaluation of Corticosteroids (CAPE-COD) trial was a multicenter, double-blind, randomized controlled superiority trial investigating the impact of early hydrocortisone therapy on 28-day mortality in adult patients admitted to the ICU with severe community-acquired pneumonia (sCAP) (17). Diagnosis of sCAP was based on clinical and radiographic criteria, with severity defined by: invasive or noninvasive mechanical ventilation (MV), high-flow nasal cannula (HFNC) or nonrebreather oxygen mask with a ratio of Pao2:Fio2 less than 300 and Fio2 greater than or equal to 50%, or a Pulmonary Severity Index (PSI) greater than 130. Exclusion criteria included influenza pneumonia, septic shock, or suspected aspiration. Patients were randomized to continuous infusion hydrocortisone 200 mg/d (n = 400) or placebo (n = 395) for 4 days, tapered over a total of 8 or 14 days or stopped early if discharged from the ICU (median 5 d; interquartile range [IQR], 3–8 d). Treatment with hydrocortisone was associated with an absolute reduction in 28-day mortality of 5.7% (6.2% vs. 11.9%; 95% CI, –9.6% to –1.7%; p = 0.006) and decreased need for invasive MV (hazard ratio [HR], 0.69; 95% CI, 0.50–0.94) and vasopressors (HR, 0.59; 95% CI, 0.43–0.82). A subgroup analysis based on C-reactive protein (CRP) suggests that CRP greater than 15 mg/dL may further identify patients most likely to benefit from corticosteroid therapy, supporting results of previous studies (18). Median daily insulin requirements were greater in the hydrocortisone group (35.5 vs. 20.5 U; 95% CI, 4.0–13.8; p < 0.001). There were no differences in hospital-acquired infection or gastrointestinal bleeding.
Pneumonia confers a significant risk of morbidity and mortality in the ICU (19). Inflammation, impaired gas exchange, and respiratory failure associated with sCAP may be attenuated with corticosteroid therapy. Two prior meta-analyses of corticosteroids in pneumonia suggested improved time to clinical stabilization and decreased mortality, respectively; however, the latter was deemed to have a high risk of bias (20, 21). Previous studies have evaluated a variety of corticosteroid agents, doses (30 mg to 1 mg/kg/d methylprednisolone equivalent), and durations (4–9 d) while demonstrating improvements in surrogate markers (e.g., MV-free days, clinical stability, length of stay [LOS]) (20). While baseline characteristics and comorbidities seen in previous studies were similar to those in CAPE-COD, few specifically enrolled patients with sCAP.
Although terminated early, CAPE-COD is the largest trial demonstrating a mortality benefit with corticosteroid therapy in patients with sCAP and corroborates findings from previous trials and meta-analyses. The larger study size and higher severity of illness in CAPE-COD (22.2% intubated, 63.8% HFNC or noninvasive MV, 47.5% PSI class V) may explain the mortality difference observed. Based on rapid initiation of hydrocortisone in CAPE-COD (within 15 hr of ICU admission), benefits of corticosteroids in sCAP may be dependent on timing of initiation. Limitations of the trial include a nonstandardized approach to antimicrobial management and lower than anticipated mortality in both groups. Additionally, while a continuous infusion of hydrocortisone was used, intermittent dosing is often used in clinical practice. Finally, immunocompromised patients represented 6.4% of patients in CAPE-COD; therefore, it is unclear if benefits of corticosteroid therapy translate to this population where further immunosuppression may be detrimental. A notable takeaway from this trial is the importance of identifying patients most likely to benefit from corticosteroid therapy. Overall, results from CAPE-COD support early hydrocortisone therapy in sCAP, and this is further reflected in recent clinical practice guideline updates from SCCM that endorse the administration of steroids to adult patients with severe pneumonia (22).
Inhaled Amikacin to Prevent Ventilator-Associated Pneumonia
The Inhaled Amikacin vs. Placebo to Prevent Ventilator-Associated Pneumonia (AMIKINHAL) trial was a multicenter, double-blind, randomized controlled superiority trial evaluating inhaled amikacin for prevention of ventilator-associated pneumonia (VAP) among adult critically ill patients receiving MV for greater than or equal to 72 hours. Exclusion criteria included MV greater than 96 hours, diagnosis of VAP before enrollment, systemic aminoglycosides, Kidney Disease: Improving Global Outcomes stage 2 or 3 acute kidney injury, glomerular filtration rate less than 30 mL/min, or scheduled extubation within 24 hours. Patients received inhaled amikacin 20 mg/kg of ideal body weight (n = 417) or an equivalent volume of 0.9% sodium chloride (NaCl) (n = 430) daily for 3 days; 77.5% were on systemic antibiotics at randomization. VAP within 28 days (positive bacterial culture plus at least two of: leukocytes ≥ 10,000/mL or ≤ 4,000/mL, fever, or purulent secretions with new infiltrate on chest radiograph) was lower in the amikacin group (14.8% vs. 22.1%; difference in restricted mean survival time to VAP 1.5 d; 95% CI, 0.6–2.5 d; p = 0.004). Enterobacterales (27.8%) and Staphylococcus aureus (20.7%) were the primary isolates. First episode of VAP per 1000 MV days (16 vs. 23; risk ratio [RR], 0.68; 95% CI, 0.49–0.94) and occurrence of a ventilator-associated condition (increase of 20% in Fio2 or 3 cm H2O of positive end-expiratory pressure sustained for at least 2 d after a period of stability; 32.8% vs. 39.5%; HR, 0.79; 95% CI, 0.64–0.99) were lower in the amikacin group. There were no significant differences in systemic antibiotic exposure, duration of MV to day 28, ICU or hospital LOS, ICU or hospital mortality, or safety outcomes.
Approximately 10% of intubated patients will develop VAP, with the highest risk in the days after intubation (23–25). VAP prolongs the duration of MV and ICU and hospital LOS and is associated with an attributable mortality of 13% (25). Previous studies investigating prophylactic inhaled antibiotics (e.g., colistin, ceftazidime) for VAP prevention have demonstrated mixed results. These studies were largely conducted in surgical or trauma ICUs, while AMIKINHAL included 86.4% medical admissions. Past studies also enrolled patients with a greater severity of illness and used longer durations of prophylactic therapy (1–2 wk or until extubation) but required similar durations of MV before enrollment (26–30). Due to conflicting results, risk of adverse drug effects, and emergence of resistant bacteria, the practice of utilizing inhaled antibiotics for VAP prevention has not been widely implemented.
In the AMIKINHAL trial, frequency of VAP within 28 days was reduced, although this time frame may not be adequate to assess emergence of resistance. Limitations of the trial include use of inhaled 0.9% NaCl as a placebo, which may have increased the risk of VAP in the control group. In addition, the requirement of positive quantitative bacterial cultures for diagnosis of VAP potentially excluded patients with clinical VAP and raises concerns that amikacin may have limited diagnostic yield. Although this study was not powered for patient-centered outcomes, there was no improvement in these measures despite lower rates of VAP, which is a key consideration when evaluating this intervention for application in clinical practice. Current guidelines do not recommend prophylactic inhaled antibiotics for prevention of VAP (31). Larger trials are needed to determine the safety and efficacy of early initiation of inhaled prophylactic antibiotics for VAP and impact on patient-centered outcomes.
Early Restrictive or Liberal Fluid Management for Sepsis-Induced Hypotension
The Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis (CLOVERS) trial was a multicenter, randomized, unblinded superiority trial that evaluated the impact of two resuscitation strategies in patients with sepsis-induced hypotension (32). Adult patients with suspected or proven infection and systolic blood pressure less than 100 mm Hg or mean arterial pressure less than 65 mm Hg (or receiving vasopressors) after administration of 1–3 L of fluid within 4 hours of enrollment were eligible. Exclusion criteria included fluid overload or nonsepsis severe volume depletion. Patients were randomized to a restrictive fluid (RF) with early vasopressor (n = 782) or liberal fluid (LF; n = 781) protocol for 24 hours (complete protocols available with full text of article) (32). Median volume of pre-randomization fluid was similar (2050 mL; IQR, 1450–2450 mL). ICU admission occurred in 65.6% of patients, and 19.4% were on vasopressors at enrollment. The trial was stopped early for futility. All-cause mortality was similar at 90 days (14.0% vs. 14.9%; absolute difference [AD], –0.9%; 95% CI, –4.4% to 2.6%; p = 0.61). Total IV fluids administered differed by 2134 mL at 24 hours (1267 vs. 3400 mL; 95% CI, –2318 to –1949 mL). Vasopressors were used 21.8% more frequently (59.0% vs. 37.2%; 95% CI, 16.9–26.6%), 1.4 hours earlier (95% CI, –2.0 to –0.8 hr), and for 4.2 hours longer (95% CI, 3.3–5.2 hr) in the RF group. There were no significant differences in other secondary efficacy outcomes, subgroup analyses, or adverse events.
Millions of people are affected by sepsis every year, with hospital mortality estimates of 15–27% (33, 34). Early resuscitation is a key component of sepsis management, and fluids are a mainstay of therapy (35). Current guidelines recommend a minimum of 30 mL/kg of crystalloids within the first 3 hours of resuscitation, with subsequent fluid administration guided by dynamic measures (36). A LF resuscitation strategy may cause volume overload and increased need for medical intervention, while a strategy favoring vasopressor use may increase the risk of side effects (37). Approximately 25% of patients may be fluid-responsive at 2 hours from onset of septic shock, although volume status and responsiveness can be difficult to assess (38). LF and RF resuscitation strategies were previously compared in ICU patients with septic shock and a higher disease severity with no significant difference in 90-day mortality (39).
While the CLOVERS trial did not find a significant difference in outcomes comparing resuscitation strategies, the findings support those of previous studies that found no increased risk of adverse events with a RF strategy. The trial also provides additional evidence of the safety of peripheral administration of vasopressors up to 72 hours (63.2% of patients requiring vasopressors, 1% rate of line-related extravasation complications). Adherence to the study protocol was 96.3%, but the unblinded study design may have introduced bias in selection, treatment, or reporting. The trial had limited representation or reporting on the prevalence of relevant subgroups that may benefit from a specific resuscitation strategy (e.g., end-stage renal disease). Additionally, some crossover between study arms may have limited treatment effect. The 2134 mL difference in fluid administration at 24 hours is greater than the difference seen in the Conservative versus LIberal Approach to Fluid Therapy of Septic Shock in Intensive Care (CLASSIC) trial (813 mL), but a patient population with a lower overall severity of illness and a protocol duration of only 24 hours may have limited the magnitude of treatment effect (32, 39). The optimal amount of fluid to administer for sepsis-induced hypotension remains uncertain and the results of this trial should not change universal resuscitation practices outside of specific clinical situations (e.g., evidence of fluid overload). Future investigations may benefit from enrolling patients at greatest risk for fluid overload.
Early Adjunctive Methylene Blue in Patients With Septic Shock: A Randomized Controlled Trial
This was a single center, double blind, RCT evaluating time to vasopressor discontinuation with early adjunctive methylene blue (MB) in adult patients with septic shock (defined by Sepsis-3 criteria) (40). Exclusion criteria included norepinephrine initiation greater than 24 hours before enrollment, high probability of death within 48 hours, burn injury, history of glucose-6-phosphate dehydrogenase deficiency, or recent (4 wk) intake of selective serotonin reuptake inhibitors. Patients received MB 100 mg IV over 6 hours (n = 45) or placebo (n = 46) daily for three doses. All patients received antimicrobial therapy and hydrocortisone within 3 hours of septic shock diagnosis. At enrollment, median norepinephrine doses were 0.45 µg/kg/min (MB) and 0.37 µg/kg/min (placebo), 76.9% were on vasopressin, and median time from shock diagnosis to study intervention was 8 hours in both groups. Time to vasopressor discontinuation was 29.4 hours shorter in the MB group (69 vs. 94 hr; 95% CI, 15.4–50.7 hr; p < 0.001). Patients receiving MB had a higher chance of shock reversal (HR, 2.7; 95% CI, 1.5–5.0; p = 0.0007), 1.0 more vasopressor-free days (p = 0.008), lower cumulative fluid balance (741 mL; 95% CI, 293–1188 mL; p = 0.001), and shorter ICU (1.5 d; 95% CI, 0.08–2.5 d; p = 0.039) and hospital (2.7 d; 95% CI, 0.3–4.6 d; p = 0.027) LOS, but there was no difference in all-cause mortality at 28 days (33.3% vs. 45.7%; RR, 0.76; 95% CI, 0.55–1.05; p = 0.23).
Adjunctive therapies such as vasopressin and corticosteroids have been associated with reduced catecholamine requirements and shorter time to shock reversal in septic shock. Studies suggest early implementation of adjuncts and minimization of catecholamines may reduce catecholamine-related complications or mortality in select patients (41–45). Historically, the use of nonselective nitric oxide synthase (NOS) inhibitors increased mortality in septic shock, and MB, a selective inhibitor of inducible NOS, has been reserved as salvage therapy (46). Several small trials have evaluated the effects of MB on hemodynamic parameters in patients with septic shock, with two previous RCTs demonstrating an improvement in hemodynamic parameters that were underpowered to assess patient-centered outcomes (47, 48).
This study is the largest to evaluate MB in septic shock. Supportive care principles (e.g., use of steroids, fluid responsiveness assessment) were uniform and well-described, and enrolled patients had a high severity of illness. Study size and single-center design limit generalizability as well as power to evaluate other patient-centered outcomes. While the study was blinded, urine discoloration likely unmasked the treatment arms and could have imparted bias. Despite promising results in this trial, due to the small study size and limited additional evidence, MB is likely best reserved as an adjunct agent for refractory shock, subject to expert discussion of risk and benefits in specific cases. Given these limitations, larger, multicenter studies are needed to validate these findings and determine the impact of MB on morbidity and mortality in septic shock, as well as optimal dosing and duration.
Efficacy and Safety of Early Administration of Four-Factor Prothrombin Complex Concentrate in Patients With Trauma at Risk of Massive Transfusion: The PROCOAG Randomized Clinical Trial
PROCOAG was a pragmatic, randomized, double-blind, placebo-controlled, multicenter superiority trial evaluating the impact of four-factor prothrombin complex concentrate (4F-PCC) 25 U/kg (n = 164) vs. placebo (n = 160) on 24-hour blood product consumption (BPC) and thromboembolic events (TEs) in adult trauma patients at risk of massive transfusion (MT) (49). Included patients were transfused greater than or equal to 1 U packed RBCs (PRBCs) and had an Assessment of Blood Consumption score of greater than or equal to 2 or deemed at risk of MT (≥ 3 U PRBC within the first hour or ≥ 10 U PRBC within the first 24 hr) by clinical assessment. Exclusion criteria included traumatic cardiac arrest, devastating injuries (expected survival < 1 hr from admission), and preinjury anticoagulant use. Patients received restricted fluid expansion and early transfusion with PRBC:fresh frozen plasma (FFP) ratios between 1:1 and 2:1, tranexamic acid within 3 hours, and fibrinogen and platelet supplementation for predefined parameters. Median Injury Severity Score (ISS) was 36 (94.1% with ISS > 15) and 69.7% required a procedure for hemostasis. Median times from injury to arrival and arrival to treatment were 105 and 35 minutes, respectively. BPC was not different between 4F-PCC and placebo (12 U [5–19 U] vs. 11 U [6–19 U]; AD, 0.2; 95% CI, –2.99 to 3.33; p = 0.72). There was no difference in consumption of individual components, time to correction of coagulopathy, or mortality at 24 hours or 28 days. Patients in the 4F-PCC group experienced 11% more TE by day 28 (35% vs. 24%; 95% CI, 1–21%; p = 0.03).
Blood product transfusion is a vital component of the initial resuscitation of major trauma patients with hemorrhage, although transfusion of large volumes poses risk (50). Interventions that reduce blood product transfusion may reduce fluid overload, risk of multiple organ failure, and transfusion reactions. Previous observational studies evaluating 4F-PCC plus FFP compared with FFP alone found decreased PRBC and FFP transfusion requirements and lower mortality with 4F-PCC without an increased risk of thrombosis (51, 52). These studies primarily focused on patients with evidence of coagulopathy while only 66.9% and 25.7% of patients in PROCOAG were classified as having coagulopathy (prothrombin time ratio [PTr > 1.2] and severe coagulopathy [PTr > 1.5]), which may have contributed to the difference in observed outcomes. A recent meta-analysis of nine studies (six evaluating 4F-PCC, three evaluating three-factor prothrombin complex concentrate [PCC]) found no overall effect of PCC administration on mortality and rates of TE (53).
The results of the PROCOAG trial do not support the routine use of 4F-PCC in trauma patients at risk of MT. Limitations of this trial include a higher rate of administration of TXA in the placebo arm, a difference in time from arrival to FFP transfusion between groups, and lack of patient-centered outcomes and routine viscoelastic testing to guide intervention, thus predisposing patients without coagulopathy to potential risks of treatment. Clinical application of this intervention may be best reserved for patients with objective evidence of coagulopathy to optimize benefit and limit risk; while U.S. guidelines do not comment on adjunct use of PCC, European guidelines recommend reserving PCC for patients with evidence of a functional coagulation deficiency (54, 55). Future studies should identify specific trauma phenotypes that could demonstrate a benefit from PCC without an increased risk of TE.
Final Study Report of Andexanet Alfa for Major Bleeding With Factor Xa Inhibitors
This study reports final safety and efficacy outcomes from Andexanet Alfa, a Novel Antidote to the Anticoagulation Effects of Factor Xa Inhibitors (ANNEXA-4), a multicenter, prospective, single-group cohort study, with secondary analyses examining relationships between anti-factor Xa (FXa) activity, mortality, and hemostatic efficacy (56). Adults with acute major bleeding (hemodynamic compromise, hemoglobin decrease ≥ 2 g/dL or hemoglobin ≤ 8 g/dL, or bleeding in a critical area or organ) within 18 hours of administration of FXa inhibitors (apixaban, rivaroxaban, edoxaban, or enoxaparin (≥ 1 mg/kg)) were eligible. Patients on low dose FXa inhibitors for coronary or peripheral vascular disease were excluded. All patients (n = 479) received andexanet alfa as a bolus followed by a 2-hour infusion; low vs. high dose regimens were determined by the FXa inhibitor received, dose, and timing of last dose. Primary sites of bleeding were intracranial hemorrhage (ICH) and gastrointestinal. Median decrease in anti-FXa activity from baseline was 93% (95% CI, 94–93%), 94% (95% CI, 95–93%), 71% (95% CI, 82–65%), and 75% (95% CI, 79–67%) in the apixaban, rivaroxaban, edoxaban, and enoxaparin groups, respectively. Excellent or good hemostatic efficacy at 12 hours occurred in 80% of patients (95% CI, 75–84) with no difference across subgroups. TE occurred in 10.4% of patients, 3.3% after reinitiation of prophylactic parenteral anticoagulation but none occurring after restarting oral anticoagulation. Reduction in anti-FXa activity from baseline was associated with improved hemostatic efficacy in patients with ICH (area under the receiver operating curve, 0.62; 95% CI, 0.54–0.70) and reduced mortality in patients younger than 75 years old (adjusted p = 0.022). Hemostatic efficacy was correlated with survival in all patients (p < 0.001).
This final analysis of the ANNEXA-4 trial includes the largest number of patients to date and evaluates both efficacy and safety data as well as the relationship between anti-FXa activity levels, mortality, and hemostatic efficacy (57, 58). Limitations of this trial include an open-label design and lack of comparator group, exclusion of patients scheduled to undergo surgery within 12 hours and patients with large ICH, or planned administration of blood products (e.g., PCC, FFP). This trial provides further evidence of FXa agent reversal and hemostatic efficacy. Additionally, the trial demonstrates an association between FXa activity and hemostatic efficacy in patients with ICH as well as between FXa activity and mortality in patients younger than 75 years old (this was not found in patients age > 75 yr, potentially due to age-related confounders in comorbidities or management). Considering clinical application of study results, the safety data from ANNEXA-4 reinforces the need for prompt resumption of anticoagulation when feasible since all TE occurred before resuming oral anticoagulation. Results from the ANNEXA-I trial comparing andexanet alfa to usual care in patients with ICH taking oral FXa inhibitors support hemostatic efficacy of andexanet alfa while also demonstrating an increased risk of TEs compared with usual care (59).
CONCLUSIONS
This review provides a summary and analysis of studies relevant to critical care pharmacotherapy published in 2023. Utilizing a systematic search and a modified Delphi process, six articles were selected that represented the most novel and significant contributions to the critical care pharmacotherapy literature.
ACKNOWLEDGMENTS
We acknowledge members of the Clinical Pharmacy and Pharmacology Pharmacotherapy Literature Update for their contributions.
Supplementary Material
Footnotes
Dr. Makic is an editor of critical care texts for Elsevier and chair of clinical practice alerts. The remaining authors have disclosed that they do not have any potential conflicts of interest.
All authors made substantial contributions to the conception and drafting of this work and have approved this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Contributor Information
Janhavi Athale, Email: athale.janhavi@mayo.edu.
Robert A. Balk, Email: robert_balk@rush.edu.
Michael L. Behal, Email: michlbehal@outlook.com.
Judah E. Brown, Email: ywx9002@nyp.org.
Tyler Chanas, Email: tyler.chanas@ecuhealth.org.
Roxana Dumitru, Email: jhx9002@nyp.org.
Dalton C. Gifford, Email: dalton.gifford@uky.edu.
Benjamin Hohlfelder, Email: hohlfeb@ccf.org.
Honey M. Jones, Email: honey_jones@med.unc.edu.
Mary Beth F. Makic, Email: marybeth.makic@cuanschutz.edu.
Alicia J. Sacco, Email: sacco.alicia@mayo.edu.
Benjamin J. Sines, Email: benjamin_sines@med.unc.edu.
Payal K. Gurnani, Email: pkgurnani@houstonmethodist.org.
REFERENCES
- 1.Tsutsumi Y, Tsuchiya A, Kawahara T: Publication hyper-inflation in the field of intensive care. Intensive Care Med 2023; 49:706–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu N, Liao C, Hsu C: Proportions and trends of critical care trials in leading general medical journals, 1970-2022. Crit Care 2023; 27:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wyatt JC, Sullivan F: Keeping up: Learning in the workplace. BMJ 2005; 331:1129–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maggio LA, Artino AR, Jr: Staying up to date and managing information overload. J Grad Med Educ 2018; 10:597–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamtchum-Tatuene J, Zafack JG: Keeping up with the medical literature: Why, how, and when? Stroke 2021; 52:e746–e748 [DOI] [PubMed] [Google Scholar]
- 6.Turck CJ, Frazee E, Kram B, et al. ; Critical Care Pharmacotherapy Literature Update Group: Major publications in the critical care pharmacotherapy literature: February 2012 through February 2013. Am J Health Syst Pharm 2014; 71:68–77 [DOI] [PubMed] [Google Scholar]
- 7.Rech MA, Day SA, Kast JM, et al. ; Critical Care Pharmacotherapy Literature Update Group: Major publications in the critical care pharmacotherapy literature: January-December 2013. Am J Health Syst Pharm 2015; 72:224–236 [DOI] [PubMed] [Google Scholar]
- 8.Day SA, Cucci M, Droege ME, et al. : Major publications in the critical care pharmacotherapy literature: January-December 2014. Am J Health Syst Pharm 2015; 72:1974–1985 [DOI] [PubMed] [Google Scholar]
- 9.Wong A, Erdman M, Hammond DA, et al. : Major publications in the critical care pharmacotherapy literature in 2015. Am J Health Syst Pharm 2017; 74:295–311 [DOI] [PubMed] [Google Scholar]
- 10.Horner D, Altshuler D, Droege C, et al. : Major publications in the critical care pharmacotherapy literature: January-December 2016. J Crit Care 2018; 43:327–339 [DOI] [PubMed] [Google Scholar]
- 11.Hammond DA, Baumgartner L, Cooper C, et al. : Major publications in the critical care pharmacotherapy literature: January-December 2017. J Crit Care 2018; 45:239–246 [DOI] [PubMed] [Google Scholar]
- 12.Sikora Newsome A, Bissell BD, Burry LD, et al. : Major publications in the critical care pharmacotherapy literature in 2018. J Crit Care 2019; 52:200–207 [DOI] [PubMed] [Google Scholar]
- 13.Smith Condeni M, Basting AT, Cotello PG, et al. : Major publications in the critical care pharmacotherapy literature: 2019. J Crit Care 2021; 62:197–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bissell BD, Campbell J, Collins R, et al. : Major publications in the critical care pharmacotherapy literature: 2020. Crit Care Explor 2021; 3:e0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wieruszewski PM, Brickett LM, Dayal L, et al. : Major publications in the critical care pharmacotherapy literature: 2021. Crit Care Explor 2022; 4:e0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gurnani PK, Barlow B, Boling B, et al. : Major publications in the critical care pharmacotherapy literature: 2022. Crit Care Explor 2023; 5:e0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dequin P, Meziani F, Quenot J, et al. ; CRICS-TriGGERSep Network: Hydrocortisone in severe community-acquired pneumonia. N Engl J Med 2023; 388:1931–1941 [DOI] [PubMed] [Google Scholar]
- 18.Torres A, Sibila O, Ferrer M, et al. : Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: A randomized clinical trial. JAMA 2015; 313:677–686 [DOI] [PubMed] [Google Scholar]
- 19.Nair GB, Niederman MS: Updates on community acquired pneumonia management in the ICU. Pharmacol Ther 2021; 217:107663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Briel M, Spoorenberg SMC, Snijders D, et al. ; Ovidius Study Group: Corticosteroids in patients hospitalized with community-acquired pneumonia: Systematic review and individual patient data metaanalysis. Clin Infect Dis 2018; 66:346–354 [DOI] [PubMed] [Google Scholar]
- 21.Stern A, Skalsky K, Avni T, et al. : Corticosteroids for pneumonia. Cochrane Database Syst Rev 2017; 12:CD007720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaudhuri D, Nei AM, Rochwerg B, et al. : 2024 focused update: Guidelines on use of corticosteroids in sepsis, acute respiratory distress syndrome, and community-acquired pneumonia. Crit Care Med 2024; 52:e219–e233 [DOI] [PubMed] [Google Scholar]
- 23.Magill SS, O’Leary E, Janelle SJ, et al. ; Emerging Infections Program Hospital Prevalence Survey Team: Changes in prevalence of health care-associated infections in US hospitals. N Engl J Med 2018; 379:1732–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Eldridge N, Metersky ML, et al. : National trends in patient safety for four common conditions, 2005-2011. N Engl J Med 2014; 370:341–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalil AC, Metersky ML, Klompas M, et al. : Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016; 63:e61–e111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rouby JJ, Poete P, de Lassale EM, et al. : Prevention of gram negative nosocomial bronchopneumonia by intratracheal colistin in critically ill patients. Histologic and bacteriologic study. Intensive Care Med 1994; 20:187–192 [DOI] [PubMed] [Google Scholar]
- 27.Wood GC, Boucher BA, Croce MA, et al. : Aerosolized ceftazidime for prevention of ventilator-associated pneumonia and drug effects on the proinflammatory response in critically ill trauma patients. Pharmacotherapy 2002; 22:972–982 [DOI] [PubMed] [Google Scholar]
- 28.Claridge JA, Edwards NM, Swanson J, et al. : Aerosolized ceftazidime prophylaxis against ventilator-associated pneumonia in high-risk trauma patients: Results of a double-blind randomized study. Surg Infect (Larchmt) 2007; 8:83–90 [DOI] [PubMed] [Google Scholar]
- 29.Karvouniaris M, Makris D, Zygoulis P, et al. : Nebulised colistin for ventilator-associated pneumonia prevention. Eur Respir J 2015; 46:1732–1739 [DOI] [PubMed] [Google Scholar]
- 30.Lode H, Hoffken G, Kemmerich B, et al. : Systemic and endotracheal antibiotic prophylaxis of nosocomial pneumonia in ICU. Intensive Care Med 1992; 18:S24–S27 [DOI] [PubMed] [Google Scholar]
- 31.Klompas M, Branson R, Cawcutt K, et al. : Strategies to prevent ventilator-associated pneumonia, ventilator-associated events, and nonventilator hospital-acquired pneumonia in acute-care hospitals: 2022 Update. Infect Control Hosp Epidemiol 2022; 43:687–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shapiro NI, Douglas IS, Brower RG, et al. ; National Heart, Lung, and Blood Institute Prevention and Early Treatment of Acute Lung Injury Clinical Trials Network: Early restrictive or liberal fluid management for sepsis-induced hypotension. N Engl J Med 2023; 388:499–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rhee C, Dantes R, Epstein L, et al. ; CDC Prevention Epicenter Program: Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. JAMA 2017; 318:1241–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fleischmann-Struzek C, Mellhammar L, Rose N, et al. : Incidence and mortality of hospital- and ICU-treated sepsis: Results from an updated and expanded systematic review and meta-analysis. Intensive Care Med 2020; 46:1552–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rivers E, Nguyen B, Havstad S, et al. ; Early Goal-Directed Therapy Collaborative Group: Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345:1368–1377 [DOI] [PubMed] [Google Scholar]
- 36.Evans L, Rhodes A, Alhazzani W, et al. : Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Crit Care Med 2021; 49:e1063–e1143 [DOI] [PubMed] [Google Scholar]
- 37.Kelm DJ, Perrin JT, Cartin-Ceba R, et al. : Fluid overload in patients with severe sepsis and septic shock treated with early goal-directed therapy is associated with increased acute need for fluid-related medical interventions and hospital death. Shock 2015; 43:68–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernandez G, Ospina-Tascon GA, Damiani LP, et al. ; The ANDROMEDA SHOCK Investigators and the Latin America Intensive Care Network (LIVEN): Effect of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28-day mortality among patients with septic shock: The ANDROMEDA-SHOCK randomized clinical trial. JAMA 2019; 321:654–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyhoff TS, Hjortrup PB, Wetterslev J, et al. ; CLASSIC Trial Group: Restriction of intravenous fluid in ICU patients with septic shock. N Engl J Med 2022; 386:2459–2470 [DOI] [PubMed] [Google Scholar]
- 40.Ibarra-Estrada M, Kattan E, Aguilera-Gonzalez P, et al. : Early adjunctive methylene blue in patients with septic shock: A randomized controlled trial. Crit Care 2023; 27:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russell JA, Walley KR, Singer J, et al. ; VASST Investigators: Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 2008; 358:877–887 [DOI] [PubMed] [Google Scholar]
- 42.Annane D, Renault A, Brun-Buisson C, et al. ; CRICS-TRIGGERSEP Network: Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med 2018; 378:809–818 [DOI] [PubMed] [Google Scholar]
- 43.Sacha GL, Lam SW, Wang L, et al. : Association of catecholamine dose, lactate, and shock duration at vasopressin initiation with mortality in patients with septic shock. Crit Care Med 2022; 50:614–623 [DOI] [PubMed] [Google Scholar]
- 44.Hammond DA, Ficek OA, Painter JT, et al. : Prospective open-label trial of early concomitant vasopressin and norepinephrine therapy versus initial norepinephrine monotherapy in septic shock. Pharmacotherapy 2018; 38:531–538 [DOI] [PubMed] [Google Scholar]
- 45.Ragoonanan D, Allen B, Cannon C, et al. : Comparison of early versus late initiation of hydrocortisone in patients with septic shock in the ICU setting. Ann Pharmacother 2022; 56:264–270 [DOI] [PubMed] [Google Scholar]
- 46.Lopez A, Lorente JA, Steingrub J, et al. : Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: Effect on survival in patients with septic shock. Crit Care Med 2004; 32:21–30 [DOI] [PubMed] [Google Scholar]
- 47.Memis D, Karamanlioglu B, Yuksel M, et al. : The influence of methylene blue infusion on cytokine levels during severe sepsis. Anaesth Intensive Care 2022; 30:755–762 [DOI] [PubMed] [Google Scholar]
- 48.Kirov MY, Evgenov OV, Evgenov NV, et al. : Infusion of methylene blue in human septic shock: A pilot, randomized, controlled study. Crit Care Med 2001; 29:1860–1867 [DOI] [PubMed] [Google Scholar]
- 49.Bouzat P, Charbit J, Abback P, et al. ; PROCOAG Study Group: Efficacy and safety of early administration of 4-factor prothrombin complex concentrate in patients with trauma at risk of massive transfusion: The PROCOAG randomized clinical trial. JAMA 2023; 329:1367–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore FA, Moore EE, Sauaia A: Blood transfusion. An independent risk factor for postinjury multiple organ failure. Arch Surg 1997; 132:620–624; discussion 624–625 [PubMed] [Google Scholar]
- 51.Zeeshan M, Hamidi M, Feinstein AJ, et al. : Four-factor prothrombin complex concentrate is associated with improved survival in trauma-related hemorrhage: A nationwide propensity-matched analysis. J Trauma Acute Care Surg 2019; 87:274–281 [DOI] [PubMed] [Google Scholar]
- 52.Jehan F, Aziz H, O’Keeffe T, et al. : The role of four-factor prothrombin complex concentrate in coagulopathy of trauma: A propensity matched analysis. J Trauma Acute Care Surg 2018; 85:18–24 [DOI] [PubMed] [Google Scholar]
- 53.Hannadjas I, James A, Davenport R, et al. : Prothrombin complex concentrate (PCC) for treatment of trauma-induced coagulopathy: Systematic review and meta-analyses. Crit Care 2023; 27:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cannon JW, Khan MA, Raja AS, et al. : Damage control resuscitation in patients with severe traumatic hemorrhage: A practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg 2017; 82:605–617 [DOI] [PubMed] [Google Scholar]
- 55.Rossaint R, Afshari A, Bouillon B, et al. : The European guideline on management of major bleeding and coagulopathy following trauma: Sixth edition. Crit Care 2023; 27:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Milling TJ, Jr, Middledorp S, Xu L, et al. : Final study report of andexanet alfa for major bleeding with factor Xa inhibitors. Circulation 2023; 147:1026–1038 [DOI] [PubMed] [Google Scholar]
- 57.Connolly SJ, Milling TJ, Jr, Eikelboom JW: Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med 2016; 65:279–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Connolly SJ, Crowther M, Eikelboom JW, et al. ; ANNEXA-4 Investigators: Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med 2019; 380:1326–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Connolly SJ, Sharma M, Cohen AT, et al. ; ANNEXA-I Investigators: Andexanet for factor Xa inhibitor-associated acute intracerebral hemorrhage. N Engl J Med 2024; 390:1745–1755 [DOI] [PubMed] [Google Scholar]