Abstract
Introduction
Autoimmune hemolytic anemia (AIHA) occurs in 0.7–5.6% of patients undergoing hematopoietic stem cell transplantation, especially from unrelated or haploidentical donor or after lympho-depleted transplant; the majority of cases are represented by warm AIHA, occurring in a full donor chimerism setting. Standard treatments (corticosteroids, intravenous immunoglobulin, splenectomy, rituximab, cyclophosphamide, plasma exchange) lead to lower response rates than those reported in primary AIHA. Daratumumab use has been proposed in many autoimmune conditions (immune thrombocytopenic purpura, aplastic anemia, thrombotic thrombocytopenic purpura, systemic lupus erythematosus, multiple sclerosis), but only few reports have been published on its use for post-HSCT AIHA, mainly in pediatric patients.
Case Presentation
We report the successful use of daratumumab in a 68-year-old patient, suffering from post-HSCT AIHA. Five months after Rh-mismatched HSCT, the patient was diagnosed with anti-D AIHA. After first-line treatment (oral prednisone, rituximab, and plasma exchange) failure, being still transfusion-dependent with symptomatic anemia, he underwent treatment with daratumumab, achieving both clinical and laboratory responses.
Discussion
Daratumumab may represent a safe and effective alternative to conventional immunosuppressive therapy, and it deserves further investigations.
Keywords: ABO blood group, Autoimmune hemolytic anemia transplantation
A 68-year-old man was diagnosed with therapy-related acute myeloid leukemia in February 2021. He received induction chemotherapy with daunorubicin/cytarabine liposome (100 units/m2 days 1, 3, 5) obtaining a complete remission; the patient then received two consolidation cycles with the same drug (65 units/m2 days 1, 3) as a bridging therapy to hematopoietic stem cell transplantation (HSCT). Notably, induction was complicated by prolonged cytopenia and the patient was extensively supported with red blood cell concentrates and platelet pools.
Patient subsequently underwent allogeneic HSCT in October 2021. His HSCT-specific comorbidity index was 5 (obesity, diabetes, previous thyroid cancer), blood group was A Rh-negative, and his Cytomegalovirus (CMV) serostatus was positive. Graft source was peripheral blood from a 0 Rh-positive, CMV-negative, male-matched unrelated donor. The administered conditioning regimen was TBF (reduced busulfan dose for comorbidity: thiotepa 5 mg/kg/day on days −7 and −6, busulfan 3.2 mg/kg as a single daily dose on days −5 and −4, fludarabine 50 mg/m2/day on days −5 to −3). Graft-versus-host disease (GVHD) prophylaxis consisted of 2 days (+3 and +5) of posttransplant cyclophosphamide followed by cyclosporine A from day 0 (progressively tapered from day +100) and mycophenolate mofetil from day 0 to day +30.
The posttransplant course was unremarkable achieving neutrophils and platelet engraftment on days +34 and +38, respectively, and bone marrow blood chimerism demonstrated 99% donor cells since day +28, with no signs of GVHD. In December 2021, the patient developed asymptomatic SARS-CoV-2 infection treated with monoclonal antibody (bamlanivimab/etesevimab); in February 2022, he developed CMV reactivation (maximum DNAemia 16,000 copies/mL), treated with oral valganciclovir first, then with foscarnet.
In March 2022, 5 months after transplant, the patient presented at the outpatient clinic lamenting asthenia, dyspnea, and hyperchromic urines. Scleral icterus was evident on eye examination. Laboratory exams revealed severe normochromic anemia (hemoglobin 6.6 g/dL, MCV 86.1 fL), slightly reduced reticulocyte count (44×109/L, range 50–100), increased total bilirubin (3.0 mg/dL, 2.1 unconjugated), and increased LDH (497 UI/L). Haptoglobin was suppressed, there was no evidence of schistocyte in peripheral blood smear, active parvovirus B19 or CMV infections were excluded, and a passenger lymphocyte syndrome caused by the minor AB0 discrepancy seemed very unlikely given the onset time of the hemolysis and the full donor chimerism; direct antiglobulin test (DAT) was positive (IgG 3+), and indirect antiglobulin test showed an anti-D specificity. Anti-D IgG titer was 2,048 (it was assessed negative before HSCT).
Bone marrow examination showed no sign of disease relapse, increased cellularity with erythroid hyperplasia, and a full donor chimerism. Therefore, we excluded pure red cell aplasia and confirmed the diagnosis of anti-D autoimmune hemolytic anemia.
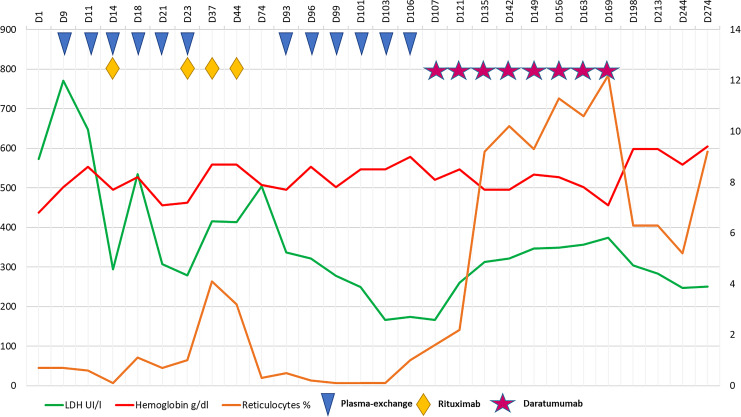
First-line treatment consisted of oral prednisone 1 mg/kg/day plus anti-CD20 antibody rituximab administered at 375 mg/m2/week × 4 weeks associated with therapeutic plasma exchange to decrease antibody titer more rapidly (a total of 6 sessions were performed with three times a week schedule; Fig. 1). One month after last rituximab administration, the patient remained transfusion-dependent with symptomatic anemia and persistence of hemolysis markers. Anti-D titer at first declined to 512 and then rebounded to 2,048, with a DAT IgG 3+.
Fig. 1.
Laboratory exams and treatments for AIHA. AIHA, autoimmune hemolytic anemia.
At this point, daratumumab, obtained for compassionate use, was started at the dose of 16 mg/kg per week IV, for a total of 8 doses. After the second administration, antibody titer dropped to 512; steroid was progressively tapered. In parallel, to manage iron overload due to the high transfusion burden, chelation therapy with iv deferoxamine was started. We observed a progressive increase in hemoglobin levels during treatment, with reduction in biochemical hemolysis markers and marked reticulocytosis (172×109/L, 7.4%). Two weeks after the last dose of daratumumab, our patient received his last red blood cell transfusion.
Three months after treatment, he is still transfusion independent, totally asymptomatic, with median hemoglobin levels of 9.5 g/dL, persistent mild reticulocytosis, and no biochemical evidence of hemolysis; DAT remains positive, but anti-D titer is lower than 32. Bone marrow examination confirms a slight increase in erythroid line and no evidence of disease recurrence.
AIHA accounts for most cases of post-HSCT autoimmune cytopenia, with a variable incidence from 0.7% to 5.6%, occurring at a median of 5–10 months post-HSCT [1]. Most patients who develop autoimmune hemolysis in the posttransplant setting experience warm AIHA: the diagnostic approach is similar to primary AIHA [2], although a prompt diagnosis can be delayed by the common occurrence of post-HSCT anemia.
Risk factors associated with post-HSCT AIHA occurrence are recipient characteristics (pediatric age, nonmalignant disorders), transplant variables (unrelated or haploidentical donor, lympho-depleted or umbilical-cord-blood HSCT), and posttransplant complications (GVHD or infections). A particularly high incidence (19–21%) has been reported in infants with severe combined immune deficiency undergoing haploidentical HSCT. In the present case, potential risk factors were represented by the use of an unrelated donor and possibly by CMV reactivation [3, 4].
The Rh blood group system is one of the most polymorphic and immunogenic systems known in humans [5]. Even if Rh antigens appear early during erythropoietic differentiation, anti-D antibody binding capacity progressively increases during maturation, reaching the maximum in late-stage erythropoiesis and mature erythrocytes [6].
Among the non-ABO red cell antigen systems, the Rh is most frequently implicated in the development of posttransplant AIHA, especially for those patients who had received several transfusions before HSCT [4, 7]. Altogether, few data regarding D-mismatched HSCT are available, reporting worse outcomes when an anti-D titer is detected pre- or posttransplant [8].
As in our patient, the majority of reported cases of AIHA occur in the context of full donor chimerism, suggesting that the auto-antibodies are derived from donor plasma cells. However, it cannot be ruled out that the anti-D antibodies may be a product of residual recipient plasma cells, thus representing an alloantibody, reflecting a pretransplant alloimmunization, when the patient was extensively exposed to platelet concentrates from multiple donors.
Treatment of post-HSCT AIHA is not standardized. Current evidence suggests more frequent refractoriness to standard treatment [4, 9] as compared with primary AIHA. Traditional therapeutic options include corticosteroids, intravenous immunoglobulin, splenectomy, rituximab, cyclophosphamide, and plasma exchange, which lead to lower response rates than those reported in primary forms. New agents are increasingly been tested as sirolimus, bortezomib, abatacept, daratumumab, and complement inhibitors [10, 11].
Daratumumab is a human IgG1κ monoclonal antibody directed against CD38 (expressed at high levels on plasma cells), originally designed for treatment of multiple myeloma, but potentially targeting also nonmalignant autoantibody-producing plasma cells: its use has been proposed off-label in immune thrombocytopenic purpura, aplastic anemia, thrombotic thrombocytopenic purpura, systemic lupus erythematosus, and multiple sclerosis [1, 12]. To the best of our knowledge, only few case reports have been published on the use of daratumumab (16 mg/kg per week IV for 6 weeks), in post-HSCT AIHA management mainly in pediatric patients receiving transplant for non-oncologic indications [13–17], where it can represent an effective and life-saving option.
Moreover, safety profile seems acceptable, as transitory depression of humoral immunity is the most frequent adverse effect reported, manageable with immunoglobulin substitution therapy. On the other side, long-term responses, the incidence of infections, and potential long-term toxicity cannot be evaluated because of generally short follow-up.
In the case here described, this drug proved to be a reliable option to treat a potential life-threating complication, refractory to all other therapies. The complexity of post-HSCT immune-mediated anemia requires close collaboration between transfusion medicine, hematology, and transplantation services. Detailed knowledge about recipient/donor blood group phenotypes beyond conventional ABO incompatibility may be useful to define appropriate management and minimize risk of posttransplant autoimmune cytopenia. In this setting, daratumumab may represent a safer and more effective alternative to conventional immunosuppressive drugs, and it deserves further investigations.
Acknowledgments
We thank the patient for allowing us to report her case and the “Centro di ricerca sulle cellule staminali emopoietiche e le terapie cellulari” – Università Cattolica del Sacro Cuore, Rome, for the support.
Statement of Ethics
This study protocol was reviewed, and the need for approval was waived by the Ethic Committee of Università Cattolica del Sacro Cuore, Rome. Written informed consent was obtained from the patient for publication in compliance with the Helsinki Declaration.
Conflict of Interest Statement
The authors declare no relevant conflict of interests nor competing interests.
Funding Sources
None.
Author Contributions
Filippo Frioni, Elisabetta Metafuni, Simona Sica, Luciana Teofili, and Patrizia Chiusolo designed the work. Sabrina Giammarco, Nicola Piccirillo, Giuseppina Massini, and Elisabetta Metafuni performed research. Filippo Frioni, Elisabetta Metafuni, and Patrizia Chiusolo analyzed data. Maria Assunta Limongiello, Claudio Pellegrino, Federica Sorà, and Francesco Autore treated the patient. Filippo Frioni and Elisabetta Metafuni made the figure. Filippo Frioni, Elisabetta Metafuni, and Pastrizia Chiusolo wrote the paper. All coauthors reviewed critically the manuscript and gave their final approval.
Funding Statement
None.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1. González-Vicent M, Sanz J, Fuster JL, Cid J, de Heredia CD, Morillo D, et al. Autoimmune Hemolytic Anemia (AIHA) following allogeneic Hematopoietic Stem Cell Transplantation (HSCT): a retrospective analysis and a proposal of treatment on behalf of the Grupo Español De Trasplante de Medula Osea en Niños (GETMON) and the Grupo Español de Trasplante Hematopoyetico (GETH). Transfus Med Rev. 2018;32(3):179–85. [DOI] [PubMed] [Google Scholar]
- 2. Jäger U, Barcellini W, Broome CM, Gertz MA, Hill A, Hill QA, et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: recommendations from the first international consensus meeting. Blood Rev. 2020:41:100648. [DOI] [PubMed] [Google Scholar]
- 3. Kruizinga MD, Van Tol MJD, Bekker V, Netelenbos T, Smiers FJ, Bresters D, et al. Risk factors, treatment, and immune dysregulation in autoimmune cytopenia after allogeneic hematopoietic stem cell transplantation in pediatric patients. Biol Blood Marrow Transplant. 2018;24(4):772–8. [DOI] [PubMed] [Google Scholar]
- 4. Wang M, Wang W, Abeywardane A, Adikarama M, McLornan D, Raj K, et al. Autoimmune hemolytic anemia after allogeneic hematopoietic stem cell transplantation: analysis of 533 adult patients who underwent transplantation at king’s college hospital. Biol Blood Marrow Transplant. 2015;21(1):60–6. [DOI] [PubMed] [Google Scholar]
- 5. Westhoff CM. The Rh blood group system in review: a new face for the next decade. Transfusion. 2004;44(11):1663–73. [DOI] [PubMed] [Google Scholar]
- 6. Southcott MJG, Tanner MJA, Anstee DJ. The expression of human blood group antigens during erythropoiesis in a cell culture system: presented in part as an abstract at the 39th American society of hematology meeting, december 5-9, 1997 (blood 90:175b, 1997 [abstr, suppl 1, part 2]). Blood. 1999;93(12):4425–35. [PubMed] [Google Scholar]
- 7. Franchini M, Gandini G, Aprili G. Non-ABO red blood cell alloantibodies following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2004;33(12):1169–72. [DOI] [PubMed] [Google Scholar]
- 8. Cid J, Lozano M, Klein HG, Flegel WA. Matching for the D antigen in haematopoietic progenitor cell transplantation: definition and clinical outcomes. Blood Transfus. 2014;12(3):301–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sanz J, Arango M, Carpio N, Montesinos, P, Moscardó, F, Martín, G, et al. Autoimmune cytopenias after umbilical cord blood transplantation in adults with hematological malignancies: a single-center experience. Bone Marrow Transplant. 2014;49(8):1084–8. [DOI] [PubMed] [Google Scholar]
- 10. Barcellini W, Fattizzo B, Zaninoni A. Management of refractory autoimmune hemolytic anemia after allogeneic hematopoietic stem cell transplantation: current perspectives. J Blood Med. 2019;10:265–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Migdady Y, Pang Y, Kalsi SS, Childs R, Arai S. Post–hematopoietic stem cell transplantation immune-mediated anemia: a literature review and novel therapeutics. Blood Adv. 2022;6(8):2707–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Szlasa W, Czarny J, Sauer N, Rakoczy K, Szymańska N, Stecko J, et al. Targeting CD38 in neoplasms and non-cancer diseases. Cancers. 2022;14(17):4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schuetz C, Hoenig M, Moshous D, Weinstock C, Castelle M, Bendavid M, et al. Daratumumab in life-threatening autoimmune hemolytic anemia following hematopoietic stem cell transplantation. Blood Adv. 2018;2(19):2550–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blennerhassett R, Sudini L, Gottlieb D, Bhattacharyya A. Post-allogeneic transplant Evans syndrome successfully treated with daratumumab. Br J Haematol. 2019;187(2):e48–51. [DOI] [PubMed] [Google Scholar]
- 15. Tolbert VP, Goldsby R, Huang J, Shimano K, Melton A, Willert J, et al. Daratumumab is effective in the treatment of refractory post-transplant autoimmune hemolytic anemia: a pediatric case report. Blood. 2016;128(22):4819. [Google Scholar]
- 16. Driouk L, Schmitt R, Peters A, Heine S, Girschick HJ, Strahm B, et al. Daratumumab therapy for post-HSCT immune-mediated cytopenia: experiences from two pediatric cases and review of literature. Mol Cell Pediatr. 2021;8(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Even-Or E, Naser Eddin A, Shadur B, Dinur Schejter Y, Najajreh M, Zelig O, et al. Successful treatment with daratumumab for post-HSCT refractory hemolytic anemia. Pediatr Blood Cancer. 2020;67(1):e28010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.