Abstract
Purpose of Review:
We review the latest screening and diagnostic techniques, and the most recent recommendations on the management of hydroxychloroquine retinopathy.
Recent Findings:
Hydroxychloroquine (HCQ) has been shown to cause retinal toxicity in a dose-dependent fashion. Early diagnosis is critical as the resultant retinopathy is not reversible. New imaging modalities, such as adaptive optics (AO), microperimetry, and retro-mode imaging, may show promise in the timely diagnosis of HCQ retinopathy.
Summary:
Automated visual fields and spectral-domain optical coherence tomography (SD-OCT) are the primary tests used in routine screening for HCQ retinopathy, but fundus autofluorescence (FAF) and multifocal electroretinogram (mfERG) have also been shown to be useful. A baseline ophthalmologic examination is recommended in all patients beginning long-term hydroxychloroquine therapy within the first year of starting therapy. Automated visual fields and SD-OCT should be included during this baseline exam in patients with pre-existing macular conditions. Afterwards, annual screening can be deferred for the first 5 years of HCQ treatment unless the patient has a major risk factor.
Keywords: Hydroxychloroquine retinopathy, Hydroxychloroquine toxicity, Plaquenil retinopathy, Plaquenil toxicity, Bull’s Eye retinopathy, Bull’s Eye maculopathy
Hydroxychloroquine (HCQ) was first developed in the 1960s as an antimalarial drug, but it is now seldom used for that purpose due to widespread resistance.[1] It use has expanded to include autoimmune conditions due to its immunomodulatory properties.
Indications :
Hydroxychloroquine most common indication is the treatment of rheumatologic conditions such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and mixed connective tissue disorders.[2–4] In fact, it is estimated that over 50% of patients with SLE are treated with HCQ due to its demonstrated survival benefit, reduced incidence of diabetes, dyslipidemia, thrombosis and other cardiovascular complications, and its antithrombotic effect in patients with antiphospholipid antibody syndrome [2–7]. HCQ is also under investigation for treatment of several autoimmune dermatologic conditions and a range of infectious diseases, and an adjunct to a variety of chemotherapy agents in oncology. [8–10]
Recently, during the coronavirus disease 2019 (COVID-19) pandemic, HCQ was recommended by the Centers for Disease Control and Prevention (CDC) in hospitalized patients with mild-moderate COVID-19, based on early preclinical studies. In vitro studies demonstrated that HCQ had activity against the virus and many retrospective studies showed improved morbidity and/or mortality among patients hospitalized with COVID-19. [11–14] Subsequent large-scale randomized control trials (RCTs) failed to demonstrate a benefit in patients treated with HCQ and the recommendation was eventually withdrawn [15–17].
Contraindications:
Despite a relatively benign safety profile with documented safe use in pregnancy and in children, there are contraindications for the use of HCQ: hypersensitivity to HCQ or related compounds and pre-existing maculopathy. [18] Notably, HCQ is often continued during pregnancy in patients with SLE and its use has been associated with improved maternal and neonatal outcomes. [19]
Dosing:
Historically, HCQ was dosed based on ideal body weight (IBW), however, recent studies suggest that actual body weight (ABW) based dosing may better prevent systemic and retinal toxicity which are especially likely to occur in patients with compromised hepatic or renal function. [20– 22].
When used in autoimmune disorders, the duration of HCQ therapy is not clearly defined, and it is not uncommon for patients to take HCQ for decades or even for the remainder of their lives. Most patients require chronic suppressive therapy with a combination of disease modifying drugs with the goal of long-term disease control. [23]
Systemic Toxicity:
Mild adverse reactions to HCQ are relatively common, especially on drug initiation. [6] Gastrointestinal symptoms (which are often self-limited and usually resolve upon drug cessation) including nausea, vomiting, or diarrhea can affect up to one-third of patients. Cutaneous hyperpigmentation occurs in approximately one-fourth, and myopathy is a common, though rarely clinically significant, side-effect.[24] HCQ has been associated with hypoglycemia, which can be severe in patients taking antidiabetic medications. [25]
More severe HCQ-related toxicity is very rare. Cases of hepatic and renal failure as well as bone marrow suppression have been reported [6, 26]. Cardiac side effects, including cardiomyopathy and conduction anomalies such as prolonged Q-T interval, were a concern during the COVID-19 pandemic, but several prospective studies have demonstrated that these effects rarely result in adverse cardiac events. [27, 28]
Hydroxychloroquine-Induced Retinal Toxicity:
Mechanism
Numerous studies and animal models have attempted to characterize the mechanisms by which HCQ exerts its effects on the body, but a complete understanding of the pathophysiology of HCQ retinopathy remains elusive. HCQ is highly lipophilic, with a large volume of distribution, and long half-life, allowing the drug to easily penetrate cell membranes and lysosomes, disrupt toll-like receptor pathways and interfere with cyclic GMP-AMP mediators of gene expression [6, 29–35]. Other drug effects include inhibition of enzymes, cytokine release, cell signaling, apoptosis, antigen presentation, and the ability to directly inhibit natural killer cells [36–49].
The mechanisms for HCQ’s toxic effects on the retina are also incompletely elucidated, but may include inhibition of all-trans retinol recycling in the retinal pigment epithelium (RPE) via organic anion transporting polypeptide 1A2 (OATP1A2)[50, 51]; impaired phagocytosis of photoreceptor outer segments, disrupted lysosome function and autophagy in RPE cells[52]; autophagy-lysosomal defects leading to alterations in sphingolipid metabolism and early damage in the inner retinal neurons and retinal ganglion cells [53]; excess Ca2+ influx by stimulation of the transient receptor potential melastatin 2 (TRPM2) cation channel and led subsequent retinal oxidative toxicity[54].
Inter-individual variability and comorbid disease states may also play a role in the retinal toxicity of HCQ by affecting pharmacokinetics. The long half-life of 40–50 days can be as short as 5 days in some contexts and can differ significantly in individuals with severe COVID-19 infection [55–57]. SLE could confer additional risk for HCQ retinopathy over other indications for HCQ use [58].
Recent case reports showing retinopathy within 2 years of initiation– even as early as two months– suggest additional mechanisms and risk factors for HCQ toxicity, and even suggest the need for supplementary screening tests beyond those currently recommended for some patients [59, 60].
Risk Factors
The most recent American Academy of Ophthalmology (AAO) guidelines regarding HCQ retinopathy were published in 2016. According to those guidelines, major risk factors for HCQ retinopathy include HCQ dose >5.0 mg/kg ABW or chloroquine dose >2.3 mg/kg ABW, duration of use >5 years, renal impairment calculated by reduced glomerular filtration rate (GFR), tamoxifen use, and macular disease [21, 61]. Isolated drusen should not be seen as a contraindication to initiation of HCQ therapy.
The duration of HCQ use is perhaps the most widely appreciated risk factor for retinopathy among physicians. Although a few cases of early HCQ retinal toxicity have been reported since the updated 2016 guidelines, the risk is still considered insignificant before 5 years of continuous use [21, 58, 60, 61].
A. Dosing Risk Factors:
Another major consideration is the daily dosing of HCQ and cumulative lifetime dose of the drug, and the 2016 AAO recommendations were updated to incorporate updated practices based on new evidence. Traditionally, HCQ was dosed according to the ideal body weight (IBW) of the patient; however, multiple retrospective reviews demonstrated that patients received daily doses in excess to those recommended by AAO [62–64]. The 2016 guidelines recommended a change to using actual body weight (ABW) for safer and more accurate dosing [21, 61].
Notably, a recent small case-control study showed that elevated blood levels of HCQ were not associated with an increased risk of retinal toxicity, and thus should not be used as an indication to reduce dosages [65].
B. Ocular Risk Factors:
Early HCQ retinopathy screening should be initiated in patients with pre-existing maculopathy based on a presumed increase in risk for HCQ retinopathy [22, 61]. No study has demonstrated increased incidence of HCQ retinopathy in patients with pre-existing maculopathy, but the 2016 AAO clinical guidelines recommend a baseline examination with SD-OCT and 10–2 visual field testing for non-Asian patients (24–2 in Asian populations) within the first year of initial treatment [61].
C. Systemic Risk Factors:
Currently, only renal failure, as defined by GFR, tamoxifen use, and pharmacogenomic factors are considered major systemic risk factors for HCQ retinopathy. Age was previously considered a major risk factor in the 2011 AAO guidelines, but this was removed in the 2016 revision. This exclusion has been supported by a 2021 study showing no association between HCQ retinopathy and age or sex [66].
Renal dysfunction, specifically, a reduction in GFR of 50%, has been associated with a doubling of the rate of HCQ retinopathy [21]. Additionally, tamoxifen use has been shown to directly lead to retinal toxicity, as well as predispose or potentiate the mechanisms of HCQ toxicity [67–69]. The association between renal dysfunction, tamoxifen use and HCQ retinopathy has been redemonstrated in more recent studies as well [65].
In addition to these systemic factors, pharmacogenomic variables likely explain part of the apparent idiosyncratic nature of HCQ retinopathy. Previous studies have demonstrated that pathologic variants of the gene ABCA4 may predispose to retinopathy, while other variants of the same gene are thought to be protective [70, 71]. Further evidence of pharmacogenomic contributions to HCQ retinopathy comes from a recent case-control study which showed that the geographical origin of patients (patients from the West Indies and sub-Saharan Africa) was associated with higher risk of retinopathy [65].
Epidemiology
Though the study HCQ retinopathy epidemiology is difficult due in large part to the lack of large multi-center, generalizable data, the results of the Melles and Marmor study, the largest epidemiological study to date, remain the primary source of accurate information regarding the incidence of this disease [21]. According to this study, the overall prevalence of HCQ retinopathy is 7.5%. When stratifying by duration, the incidence is less than 1% at 5 years and less than 2% at 10 years. However, the incidence increases to around 20% after 20 years of use for those taking 4.0–5.0 mg/kg ABW/day and can exceed 50% at 20 years for those taking greater than 5.0 mg/kg ABW/day.
Interestingly, the clinical presentations of HCQ retinopathy vary by race and geographic origin. In a 2015 study, patients of European descent were more likely to exhibit a classic parafoveal pattern of deficits, while Asian patients were more likely to manifest an extramacular or pericentral pattern of disease with abnormalities near the vascular arcades [72]. 55% of Asian patients vs. 2% of Caucasian patients will develop a peripheral pericentral toxicity pattern instead of the well-known parafoveal pattern [73]. Black and Hispanic patients had predominantly parafoveal deficits, but pericentral/mixed deficits occurred more commonly in these races than in Caucasians [72]. A 2020 case control study showed that patients from the West Indies and sub-Saharan Africa had an increased risk of HCQ retinopathy (OR 8.7, P = 0.007) [65]. Future studies should use large, diverse datasets from multiple centers to further characterize differences in rates of HCQ retinopathy among different populations.
Clinical Features
Though patients taking HCQ with detectable retinopathy are frequently asymptomatic, symptomatic patients typically present with color deficits, missing central vision, difficulty reading, reduced or blurred vision, glare, flashing lights, and metamorphopsia [61, 74, 75]. Symptomatic patients have detectable abnormalities in the RPE that can evolve into a complete bull’s eye maculopathy on clinical exam. Early signs of bull’s eye maculopathy can be seen on fundus exam as macular edema and/or bilateral granular depigmentation of the RPE in the macula. Over time, this can progress to concentric rings of hypo- and hyperpigmentation surrounding the fovea, creating the classic “bull’s eye”. In late stages, attenuation of retinal arterioles and optic disc pallor can also be evident [74–76]. Figure 1. These changes are generally irreversible and are usually accompanied by severe vision loss and occasionally complicated by cystoid macular edema (CME), epiretinal membrane (ERM), and other sequelae [76].
Figure 1.
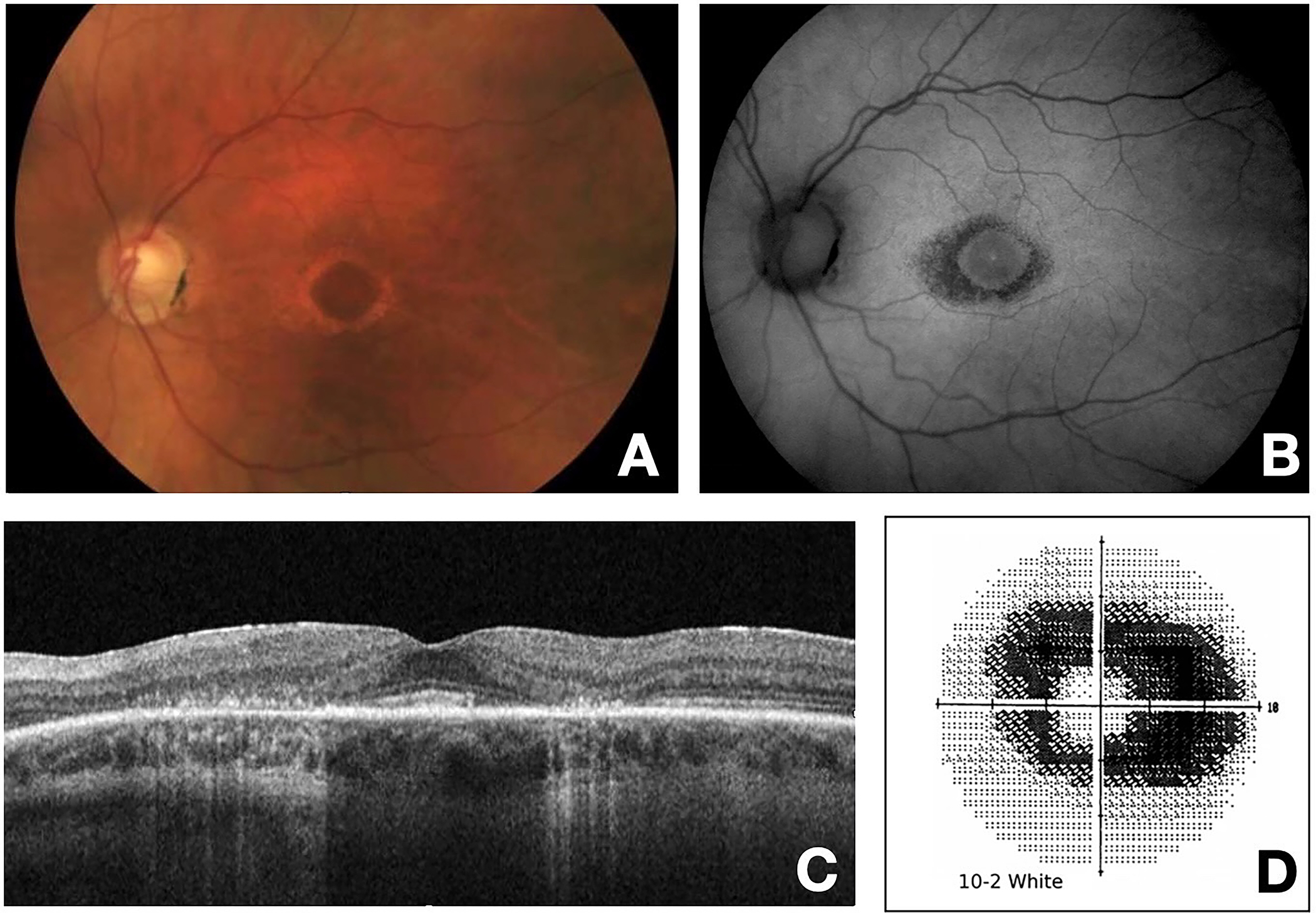
A. Color fundus photo of the left eye demonstrating “Bull’s Eye” maculopathy. Paracentral depigmentation of the retinal pigment epithelium (RPE) that spares the central fovea can be visualized. B. Fundus autofluorescence (FAF) photo of the left eye showing parafoveal hypo autofluorescence due to the low level RPE metabolic activity with underlying local atrophy with secondary photoreceptor loss. C. Optical coherence tomography (OCT) of the macula showing significant loss of the parafoveal photoreceptor inner segment/outer segment (IS/OS) junction and thinning of the outer retina with RPE irregularity. D. Humphrey visual field central 10–2 white-on-white pattern demonstrates dense paracentral scotoma.
Differential Diagnosis
The appearance of hydroxychloroquine maculopathy on exam, while characteristic, is not specific. The bull’s eye maculopathy seen in Figure 1 corresponds to foveal and parafoveal damage to the RPE [77]. The differential diagnosis for a finding of bull’s-eye maculopathy includes Stargardt’s disease, cone/rod and cone dystrophies, age-related macular degeneration, benign concentric annular dystrophy, neuronal ceroid lipofuscinosis, fenestrated sheen macular dystrophy, central areolar choroidal dystrophy, chronic macular hole, and macular telangiectasia [78]. Summary of the differential diagnosis for bull’s-eye maculopathy can be found in Table 1.
Table 1.
Differential Diagnosis of Bull’s Eye Maculopathy
| Diagnosis | Clinical Characteristics |
|---|---|
| HCQ Retinopathy | • Typically presents after 5 years of HCQ use |
| Stargardt Disease | • VA loss usually in childhood/early adulthood • DFE reveals characteristic round, yellow white pisciform flecks in the posterior pole |
| Rod/Cone Dystrophy | • Presentation in first two decades of life • Presents with central scotomas and photophobia |
| Age-related Macular Degeneration | • Incidence higher in populations >70 • DFE reveals presence of Drusen |
| Benign Concentric Annular Macular Dystrophy | • Presents in childhood • Typically will have family history |
| Batten Disease | • Typically presents with rapid vision loss early in life • Associated with intellectual and motor deterioration and seizures |
| Fenestrated Sheen Macular Dystrophy | • Characterized by a yellow sheen with red fenestrations that is limited to the macula |
| Central Areolar Choroidal Dystrophy | • Macular changes in third and fifth decades • Progress gradually leading to severe vision loss between the fourth and seventh decades |
| Chronic Macular Hole | • Sharp border between the depigmented macular area and the surrounding normally pigmented RPE • HCQ retinopathy has relatively indistinct borders |
| Macular Telangiectasia | • Presence of ectatic vessels • Evidence of venules diving at a right angle into deeper retinal layer |
Stargardt disease is the most common hereditary macular dystrophy. It is associated with loss of visual acuity typically starting in childhood or early adulthood. On fundoscopy, it can be differentiated from HCQ retinopathy by the characteristic round, yellow white pisciform flecks (due to lipofuscin A2E deposits) seen in the posterior pole.
Cone dystrophy and rod/cone dystrophy refer to a group of disorders that affect the cones and rods and cones, respectively. These dystrophies manifest with decreased visual acuity with central scotomas, reduced color vision, and photophobia, which help differentiate them from HCQ toxicity. These patients tend to present in the first two decades of life [79].
Age-related macular degeneration (AMD) is the leading cause of blindness in the industrialized world. It is characterized by the accumulation of lipid and protein deposits (drusen), along with progressive degeneration of photoreceptors and adjacent tissues [80]. Advanced disease may involve parafoveal areas of retinal pigment epithelium (RPE) loss which can occasionally appear as a bull’s-eye maculopathy. AMD can be differentiated from HCQ toxicity by the presence of drusen as well as patient demographics. The pathophysiology of AMD is multifactorial in nature, but age is a primary risk factor. The prevalence of age-related macular degeneration sharply increases in those 75 years or older.
Benign concentric annular macular dystrophy (BCAMD) is an extremely rare, autosomal dominant macular dystrophy that presents in childhood with peripheral vision loss and decreased night vision [81]. These patients often have relatively preserved visual acuity compared to other focal RPE dystrophies. Family history of BCAMD and presentation in childhood aid in diagnosis.
Neuronal ceroid lipofuscinosis (NCL), also known as Batten disease is a member of a group of neurodegenerative disorders characterized by lysosomal accumulation of auto fluorescent lipopigments [82]. It can be easily differentiated from HCQ toxicity clinically as the most common form, juvenile neuronal ceroid lipofuscinosis (JNCL), presents with rapid vision loss early in life due to retinal degeneration. This is followed by progressive intellectual and motor deterioration and seizures and is invariably fatal by the third decade of life.
Fenestrated sheen macular dystrophy (FSMD) is a rare, slowly progressive, autosomal dominant macular dystrophy characterized by a yellow sheen with red fenestrations that is limited to the macula. Patients develop progressive annular hypopigmentation around the area of the sheen at the level of the RPE [83]. Eventually, a hyperpigmented area appears centrally giving rise to a “bull’s eye”-appearing lesion. Family history and the characteristic sheen are key in the diagnosis.
Central areolar choroidal dystrophy (CACD) is an incredibly rare hereditary dystrophy of the macula. It presents as a well-circumscribed lesion of the macula with loss of retinal and choroidal tissue. The RPE, choriocapillaris, and neurosensory retina are absent in dystrophy with some cases of CACD displaying a bull’s eye appearance of pigment epithelial atrophy. Family history and clinical picture play a role in diagnosis as macular changes usually begin between the third and fifth decades and progress gradually leading to severe vision loss between the fourth and seventh decades [84].
Chronic macular hole should be considered in the differential diagnosis of bull’s eye retinopathy as selective depigmentation of the RPE may occur under the cuff of subretinal fluid that surrounds a chronic macular hole. The pigmentation of the RPE underlying the macular hole is usually preserved and corresponds to the center of the bull’s eye pattern [85]. Unlike the bull’s eye maculopathy associated with HCQ retinopathy which has relatively indistinct borders, chronic macular hole generally has a sharp border between the depigmented macular area and the surrounding normally pigmented RPE.
Macular telangiectasia (MacTel) is a multifactorial macular disease characterized by abnormalities of capillaries of the fovea/perifoveal region and associated with atrophy of foveal photoreceptors which can progress to cystic slit-like changes and cavitations in all retinal layers. It is divided into 3 main groups with type 2 being the most common. The term “MacTel” usually refers specifically to type 2. Eyes with MacTel have a bull’s-eye retinopathy which can be differentiated from others by the presence of ectatic vessels with evidence of venules diving at a right angle into deeper retinal layer [78].
Screening and Monitoring
Current practice in the United States is guided by the 2016 AAO guidelines, which recommend the use of both automated visual fields and SD-OCT for routine screening. Fundus autofluorescence (FAF) and multifocal electroretinogram (mfERG) were also noted as useful screening tests [86]. While visual fields are potentially more sensitive, they are also subjective and thus variably reliable. SD-OCT is preferred as it is widely available, objective, highly specific, and considered sensitive to visually significant levels of toxicity. Given the variation in disease presentation in Asian vs non-Asian eyes, wider-angle scans or scans directed across the vascular arcade region are particularly important for these populations. The Royal College of Ophthalmologists (RCOphth) 2020 clinical guidelines support the inclusion of (FAF) along with SD-OCT in primary screening, reserving the use of automated visual fields for patients with a structural abnormality on imaging [61].
The AAO and RCOphth agree that at least one objective test (SD-OCT/FAF) should confirm findings on subjective testing before a diagnosis of HCQ retinopathy is made. The groups recommend that all patients beginning long-term HCQ therapy undergo baseline ophthalmologic examination within the first year of starting therapy in order to assess for any pre-existing ocular conditions, specifically of the macula, and to establish a record of the fundus appearance and functional status. Visual fields and SD-OCT are not necessary at this baseline exam unless other abnormalities are present that might affect screening tests. [86]
With proper dosing, the risk of HCQ retinopathy is low within the first decade of treatment, and annual screening can be deferred for the first 5 years of HCQ treatment unless the patient has a major risk factor: HCQ dose >5.0 mg/kg ABW, chloroquine dose >2.3 mg/kg ABW, macular disease, GFR of less than 60ml/min/1.73m2, or tamoxifen use. Patients with any of these risk factors should be screened annually after beginning HCQ treatment [61].
Since initial damage can be recognized as a distinct, focal interruption of the photoreceptor outer segment, screening should aim to uncover previously unrecognized areas of retinopathy, rather than looking for gradual or chronic changes.
Spectral-domain optical coherence tomography (SD-OCT) is one of the most widely performed imaging modalities for the diagnosis and monitoring of macular disease. In hydroxychloroquine toxicity, there are parafoveal and, more commonly in Asian populations, peripheral thickness and volume changes that can be visualized on SD-OCT. These changes often present in the inferior/inferotemporal retina with the parafoveal retina more noticeably affected. Though, as previously alluded to, wide-angle SD-OCT imaging or SD-OCT scans through arcades are required to detect pericentral disease often seen in patients of Asian descent. In addition to outer nuclear layer thinning, morphological changes that can be seen on SD-OCT in hydroxychloroquine retinopathy include disruption of the photoreceptor layer, disruption of the IS/OS junction, loss of space between ellipsoid zone and interdigitation zone, loss of interdigitation zone, retinal pigment epithelium (RPE) loss and accumulation of debris and increased choroidal reflectance secondary to RPE loss [86].
Central visual field testing is frequently used in the screening of hydroxychloroquine retinopathy. It has the benefit of being a functional test and is very sensitive in reliable patients. However, it is a subjective test and its efficacy as a screening tool relies on the patient. 10–2 visual field testing has high resolution within the macula and is excellent for non-Asian patients, while wider test patterns (24–2 or 30–2) are needed for Asian patients in whom toxicity often manifests beyond the macula. Even a single central spot of reduced sensitivity in a 24–2 or 30–2 should be taken seriously, given the decreased sensitivity compared to 10–2 fields [61]. Uncertain visual field changes should trigger re-testing or evaluation with objective testing, such as SD-OCT.
Fundus autofluorescence (FAF) is an imaging technique that uses a monochromatic light source (typically 488nm) to elicit the auto-reflectance properties of lipofuscin within the RPE. In hydroxychloroquine retinopathy, there is often early hyperfluorescence indicating RPE stress, and later hypofluorescence indicating RPE loss [61]. The distribution of disease may be parafoveal or pericentral, as described in the SD-OCT section. Fundus autofluorescence can reveal early photoreceptor damage as findings of FAF may precede retinal thinning on SD-OCT. It is also valuable in that it gives a topographic view of damage across the posterior fundus.
Multifocal electroretinography (mfERG) is an objective electrodiagnostic test that involves the projection of an array of hexagonal light sources onto the retina. It has been shown to be sensitive and specific in the screening of hydroxychloroquine retinopathy however it is a costly test, and its availability is limited. Findings may include amplitude reduction, prolonged implicit time, ring response reduction or ring ratios greater than normal limits, as well as color difference plots indicating decreased response time [61, 86].
Tests not recommended for screening
Fundus examination and photography are useful for baseline examination when a patient initiates HCQ treatment as tests to screen for pre-existing macular conditions. However, for yearly screening for HCQ retinopathy, macula OCT and visual fields are more sensitive modalities and are recommended over fundus examination or photography. Other tests not recommended for yearly screening include time-domain optical coherence tomography, fluorescein angiography, full-field electroretinogram, Amsler grid, color vision testing, and electrooculogram [61]. While color vision changes can be found in HCQ retinopathy, regular color vision screening is not recommended in patients as changes in color vision have not been shown to be sensitive or specific. Further discussion can be found in Table 2.
Table 2.
Summary of Screening Recommendations.
| Tests Recommended for Screening | Reasoning | Tests Not Recommended for Screening | Reasoning |
|---|---|---|---|
| Spectral Domain Optical Coherence Tomography (SD-OCT) | Retinal thickness and volume changes secondary to retinopathy can be visualized on SD-OCT | Fundus Examination and Photography | Photoreceptor damage is detectable with other techniques such as SD-OCT and FAF well before visible changes in the fundus |
| Fundus Autofluorescence (FAF) | FAF can reveal early photoreceptor damage | Time-Domain Optical Coherence Tomography | The resolution is insufficient to detect early toxicity |
| 10–2 Automated Visual Field Testing (non-Asian populations) | Functional test that is very sensitive in reliable patients | Fluorescein Angiography | RPE defects may be visualized but later in the course of toxicity compared to other techniques |
| 24–2 or 30–2 Automated Visual Field Testing (Asian populations) | Even a single central spot of reduced sensitivity in a 24–2 or 30–2 should be taken seriously, given the decreased sensitivity compared to 10–2 fields | Full-Field Electroretinogram | Abnormalities may be seen in only very late toxicity. It may be useful to judge the extent of damage beyond the macula |
| Multifocal Electroretinogram (mERG) | It has been shown to be sensitive and specific in the screening of hydroxychloroquine retinopathy | Amsler Grid | Other techniques such as HVF testing are more reliable in the screening of scotomas |
| Color Vision Testing | Color vision errors may occur but are not sensitive or specific | ||
| Electrooculogram | The electrooculogram has not been shown to be a reliable screening test. |
Testing under investigation:
Adaptive optics (AO), microperimetry, and retro-mode imaging all show promise in the diagnosis of HCQ retinopathy [87]. Adaptive optics allows direct visualization of individual photoreceptor cells and studies involving eyes with HCQ retinopathy have shown that structural disruption of photoreceptors seen on AO corresponds to ellipsoid zone (EZ) defects on SD-OCT. Another study showed that these changes on AO can be seen even before EZ changes are detectable on SD-OCT. However, AO equipment is not readily accessible, and the imaging is difficult to perform, analyze, and interpret.
Microperimetry assesses the pointwise retinal sensitivity in the macula by integrating computerized threshold perimetry with real-time fundus imaging. Studies have evaluated microperimetry in the detection of HCQ retinal toxicity and showed inferior sensitivity but superior specificity when compared with mfERG. With this high specificity, microperimetry has been suggested as an ancillary test to exclude the diagnosis of hydroxychloroquine retinopathy if other screening tests provided conflicting results. However, microperimetry is not widely available limiting its practicality [87].
Retro-mode imaging enables visualization of the outer retina using laser ophthalmoscopy. It has been shown that changes in parafoveal reflectance can be detected in patients with hydroxychloroquine retinopathy with high specificity. However, retro-mode imaging is limited in availability and thus its use remains currently impractical [87].
Management of HCQ Retinopathy
Hydroxychloroquine retinopathy is not reversible, and cellular damage appears to continue for a period even following drug discontinuation, though this is typically mild if the toxicity is recognized before there is RPE damage [88]. Yet, there is no therapy shown to be effective in treating or reducing the risk of developing HCQ retinopathy.
Patients taking hydroxychloroquine with age-related maculopathy or macular dystrophies are sometimes advised to avoid sun exposure and supplement lutein and zeaxanthin (which are foveal protectants). The value of these recommendations has yet to be demonstrated.
Primary prevention is currently the best approach in the management of HCQ toxicity. Once signs of retinopathy are recognized, the decision to stop medication should be made in conjunction with the patient and the prescribing physician so that systemic control of disease is optimized.
Conclusion
Hydroxychloroquine is known to cause irreversible retinal toxicity in a dose-dependent fashion and given the lack of treatments, primary prevention is the best strategy to avoid visual complications. Patients and prescribing physicians need to be mindful of this risk and the importance of regular screening. It is our hope that this review will further inform the screening and examination of patients taking HCQ prior to the presentation of visual symptoms.
Funding
NIH Center Core Grant P30EY014801Research to Prevent Blindness – Unrestricted Grant
Grant:
NIH Center Core Grant P30EY014801Research to Prevent Blindness – Unrestricted Grant
Footnotes
Competing interests
Not applicable
Declarations:
Ethical Approval
Not applicable
Availability of data and materials
Not applicable
References
- 1.Gabourel JD (1963) Effects of hydroxychloroquine on the growth of mammalian cells in vitro. J Pharmacol Exp Ther 141:122–130 [PubMed] [Google Scholar]
- 2.Alarcón GS, McGwin G, Bertoli AM, Fessler BJ, Calvo-Alén J, Bastian HM, Vilá LM, Reveille JD, LUMINA Study Group (2007) Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L). Ann Rheum Dis 66:1168–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pons-Estel GJ, Alarcón GS, McGwin G, Danila MI, Zhang J, Bastian HM, Reveille JD, Vilá LM, Lumina Study Group (2009) Protective effect of hydroxychloroquine on renal damage in patients with lupus nephritis: LXV, data from a multiethnic US cohort. Arthritis Rheum 61:830–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willis R, Seif AM, McGwin G, et al. (2012) Effect of hydroxychloroquine treatment on pro-inflammatory cytokines and disease activity in SLE patients: data from LUMINA (LXXV), a multiethnic US cohort. Lupus 21:830–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fanouriakis A, Kostopoulou M, Alunno A, et al. (2019) 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 78:736–745 [DOI] [PubMed] [Google Scholar]
- 6.Schrezenmeier E, Dörner T (2020) Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol 16:155–166 [DOI] [PubMed] [Google Scholar]
- 7.Petri M (2011) Use of hydroxychloroquine to prevent thrombosis in systemic lupus erythematosus and in antiphospholipid antibody-positive patients. Curr Rheumatol Rep 13:77–80 [DOI] [PubMed] [Google Scholar]
- 8.Ochsendorf FR (2010) Use of antimalarials in dermatology. J Dtsch Dermatol Ges 8:829–844; quiz 845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poklepovic A, Gewirtz DA (2014) Outcome of early clinical trials of the combination of hydroxychloroquine with chemotherapy in cancer. Autophagy 10:1478–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plantone D, Koudriavtseva T (2018) Current and Future Use of Chloroquine and Hydroxychloroquine in Infectious, Immune, Neoplastic, and Neurological Diseases: A Mini-Review. Clin Drug Investig 38:653–671 [DOI] [PubMed] [Google Scholar]
- 11.Yao X, Ye F, Zhang M, et al. (2020) In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin Infect Dis 71:732–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg ES, Dufort EM, Udo T, et al. (2020) Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State. JAMA 323:2493–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geleris J, Sun Y, Platt J, et al. (2020) Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med 382:2411–2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavalcanti AB, Zampieri FG, Rosa RG, et al. (2020) Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N Engl J Med 383:2041–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO Solidarity Trial Consortium, Pan H, Peto R, et al. (2021) Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med 384:497–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Self WH, Semler MW, Leither LM, et al. (2020) Effect of Hydroxychloroquine on Clinical Status at 14 Days in Hospitalized Patients With COVID-19: A Randomized Clinical Trial. JAMA 324:2165–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.RECOVERY Collaborative Group, Horby P, Mafham M, et al. (2020) Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med 383:2030–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rainsford KD, Parke AL, Clifford-Rashotte M, Kean WF (2015) Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology 23:231–269 [DOI] [PubMed] [Google Scholar]
- 19.Leroux M, Desveaux C, Parcevaux M, Julliac B, Gouyon JB, Dallay D, Pellegrin JL, Boukerrou M, Blanco P, Lazaro E (2015) Impact of hydroxychloroquine on preterm delivery and intrauterine growth restriction in pregnant women with systemic lupus erythematosus: a descriptive cohort study. Lupus 24:1384–1391 [DOI] [PubMed] [Google Scholar]
- 20.Marmor MF, Kellner U, Lai TYY, Lyons JS, Mieler WF, American Academy of Ophthalmology (2011) Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology 118:415–422 [DOI] [PubMed] [Google Scholar]
- 21.Melles RB, Marmor MF (2014) The Risk of Toxic Retinopathy in Patients on Long-term Hydroxychloroquine Therapy. JAMA Ophthalmology 132:1453–1460 [DOI] [PubMed] [Google Scholar]
- 22.Hydroxychloroquine sulfate 200mg Film-coated Tablets - Summary of Product Characteristics (SmPC) - (emc). https://www.medicines.org.uk/emc/product/1764/smpc. Accessed 29 Jun 2023
- 23.Marmor MF (2012) Comparison of screening procedures in hydroxychloroquine toxicity. Arch Ophthalmol 130:461–469 [DOI] [PubMed] [Google Scholar]
- 24.Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA (2010) Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis 69:20–28 [DOI] [PubMed] [Google Scholar]
- 25.Shojania K, Koehler BE, Elliott T (1999) Hypoglycemia induced by hydroxychloroquine in a type II diabetic treated for polyarthritis. J Rheumatol 26:195–196 [PubMed] [Google Scholar]
- 26.Galvañ VG, Oltra MR, Rueda D, Esteban MJ, Redón J (2007) Severe acute hepatitis related to hydroxychloroquine in a woman with mixed connective tissue disease. Clin Rheumatol 26:971–972 [DOI] [PubMed] [Google Scholar]
- 27.Gasperetti A, Biffi M, Duru F, et al. (2020) Arrhythmic safety of hydroxychloroquine in COVID-19 patients from different clinical settings. Europace euaa216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mercuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, Zimetbaum PJ, Gold HS (2020) Risk of QT Interval Prolongation Associated With Use of Hydroxychloroquine With or Without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 5:1036–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ducharme J, Farinotti R (1996) Clinical pharmacokinetics and metabolism of chloroquine. Focus on recent advancements. Clin Pharmacokinet 31:257–274 [DOI] [PubMed] [Google Scholar]
- 30.Furst DE (1996) Pharmacokinetics of hydroxychloroquine and chloroquine during treatment of rheumatic diseases. Lupus 5 Suppl 1: S11–15 [PubMed] [Google Scholar]
- 31.Ponticelli C, Moroni G (2017) Hydroxychloroquine in systemic lupus erythematosus (SLE). Expert Opin Drug Saf 16:411–419 [DOI] [PubMed] [Google Scholar]
- 32.Lamphier M, Zheng W, Latz E, et al. (2014) Novel small molecule inhibitors of TLR7 and TLR9: mechanism of action and efficacy in vivo. Mol Pharmacol 85:429–440 [DOI] [PubMed] [Google Scholar]
- 33.Kuznik A, Bencina M, Svajger U, Jeras M, Rozman B, Jerala R (2011) Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol 186:4794–4804 [DOI] [PubMed] [Google Scholar]
- 34.Gardet A, Pellerin A, McCarl C-A, Diwanji R, Wang W, Donaldson D, Franchimont N, Werth VP, Rabah D (2019) Effect of in vivo Hydroxychloroquine and ex vivo Anti-BDCA2 mAb Treatment on pDC IFNα Production From Patients Affected With Cutaneous Lupus Erythematosus. Front Immunol 10:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.An J, Woodward JJ, Lai W, Minie M, Sun X, Tanaka L, Snyder JM, Sasaki T, Elkon KB (2018) Inhibition of Cyclic GMP-AMP Synthase Using a Novel Antimalarial Drug Derivative in Trex1-Deficient Mice. Arthritis Rheumatol 70:1807–1819 [DOI] [PubMed] [Google Scholar]
- 36.Wozniacka A, Lesiak A, Boncela J, Smolarczyk K, McCauliffe DP, Sysa-Jedrzejowska A (2008) The influence of antimalarial treatment on IL-1beta, IL-6 and TNF-alpha mRNA expression on UVB-irradiated skin in systemic lupus erythematosus. Br J Dermatol 159:1124–1130 [DOI] [PubMed] [Google Scholar]
- 37.Lesiak A, Narbutt J, Kobos J, Kordek R, Sysa-Jedrzejowska A, Norval M, Wozniacka A (2009) Systematic administration of chloroquine in discoid lupus erythematosus reduces skin lesions via inhibition of angiogenesis. Clin Exp Dermatol 34:570–575 [DOI] [PubMed] [Google Scholar]
- 38.Zeidi M, Kim HJ, Werth VP (2019) Increased Myeloid Dendritic Cells and TNF-α Expression Predicts Poor Response to Hydroxychloroquine in Cutaneous Lupus Erythematosus. J Invest Dermatol 139:324–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith CK, Kaplan MJ (2015) The role of neutrophils in the pathogenesis of systemic lupus erythematosus. Curr Opin Rheumatol 27:448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith CK, Vivekanandan-Giri A, Tang C, et al. (2014) Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol 66:2532–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cepika A-M, Soldo Jureša D, Morović Vergles J, Malenica B, Santak M, Kapitanović S, Mayer M, Anić B, Sentić M, Gagro A (2012) Decrease in circulating DNA, IL-10 and BAFF levels in newly-diagnosed SLE patients after corticosteroid and chloroquine treatment. Cell Immunol 276:196–203 [DOI] [PubMed] [Google Scholar]
- 42.Levy JMM, Towers CG, Thorburn A (2017) Targeting autophagy in cancer. Nat Rev Cancer 17:528–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Humbert M, Bertolino P, Forquet F, Rabourdin-Combe C, Gerlier D, Davoust J, Salamero J (1993) Major histocompatibility complex class II-restricted presentation of secreted and endoplasmic reticulum resident antigens requires the invariant chains and is sensitive to lysosomotropic agents. Eur J Immunol 23:3167–3172 [DOI] [PubMed] [Google Scholar]
- 44.Silva JC da, Mariz HA, Rocha LF da, Oliveira PSS de, Dantas AT, Duarte ALBP, Pitta I da R, Galdino SL, Pitta MG, da R (2013) Hydroxychloroquine decreases Th17-related cytokines in systemic lupus erythematosus and rheumatoid arthritis patients. Clinics (Sao Paulo) 68:766–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao L, Ma H, Jiang Z, Jiang Y, Ma N (2014) Immunoregulation therapy changes the frequency of interleukin (IL)-22+CD4+ T cells in systemic lupus erythematosus patients. Clinical & Experimental Immunology 177:212–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shin MS, Lee N, Kang I (2011) Effector T cell subsets in systemic lupus erythematosus: update focusing on Th17 cells. Curr Opin Rheumatol 23:444–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sailler L, Puissant B, Méliani P, et al. (2007) Blood concentrations of hydroxychloroquine and its desethyl derivative correlate negatively with the percentage of CD45RO+ cells among CD4+ lymphocytes in hydroxychloroquine-treated lupus patients. Ann N Y Acad Sci 1108:41–50 [DOI] [PubMed] [Google Scholar]
- 48.Spada R, Rojas JM, Barber DF (2015) Recent findings on the role of natural killer cells in the pathogenesis of systemic lupus erythematosus. J Leukoc Biol 98:479–487 [DOI] [PubMed] [Google Scholar]
- 49.Fox RI (1993) Mechanism of action of hydroxychloroquine as an antirheumatic drug. Semin Arthritis Rheum 23:82–91 [DOI] [PubMed] [Google Scholar]
- 50.Yusuf IH, Sharma S, Luqmani R, Downes SM (2017) Hydroxychloroquine retinopathy. Eye (Lond) 31:828–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu C, Zhu L, Chan T, Lu X, Shen W, Madigan MC, Gillies MC, Zhou F (2016) Chloroquine and Hydroxychloroquine Are Novel Inhibitors of Human Organic Anion Transporting Polypeptide 1A2. J Pharm Sci 105:884–890 [DOI] [PubMed] [Google Scholar]
- 52.Rosenthal AR, Kolb H, Bergsma D, Huxsoll D, Hopkins JL (1978) Chloroquine retinopathy in the rhesus monkey. Invest Ophthalmol Vis Sci 17:1158–1175 [PubMed] [Google Scholar]
- 53.Mondal K, Porter H, Cole J, et al. (2022) Hydroxychloroquine Causes Early Inner Retinal Toxicity and Affects Autophagosome-Lysosomal Pathway and Sphingolipid Metabolism in the Retina. Mol Neurobiol 59:3873–3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ertuğrul A, Özkaya D, Nazıroğlu M (2023) Curcumin attenuates hydroxychloroquine-mediated apoptosis and oxidative stress via the inhibition of TRPM2 channel signalling pathways in a retinal pigment epithelium cell line. Graefes Arch Clin Exp Ophthalmol 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tett SE, Cutler DJ, Day RO, Brown KF (1989) Bioavailability of hydroxychloroquine tablets in healthy volunteers. Br J Clin Pharmacol 27:771–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Munster T, Gibbs JP, Shen D, et al. (2002) Hydroxychloroquine concentration-response relationships in patients with rheumatoid arthritis. Arthritis Rheum 46:1460–1469 [DOI] [PubMed] [Google Scholar]
- 57.Zahr N, Urien S, Llopis B, et al. (2021) Pharmacokinetics and pharmacodynamics of hydroxychloroquine in hospitalized patients with COVID-19. Therapie 76:285–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu HY, Cramarossa G, Pope JE (2023) Systemic Lupus Erythematosus May Be a Risk Factor for Antimalarial-Induced Retinopathy Compared With Other Rheumatologic Diseases. ACR Open Rheumatol 5:173–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeltsch BM, Sarraf D, Madjdpour D, Hanson JVM, Pfiffner FK, Koller S, Berger W, Barthelmes D, Al-Sheikh M (2023) Rapid onset hydroxychloroquine toxicity. Retin Cases Brief Rep. 10.1097/ICB.0000000000001393 [DOI] [PubMed] [Google Scholar]
- 60.Stern EM, Johnson JS, Mazzulla DA (2017) Highly Accelerated Onset of Hydroxychloroquine Macular Retinopathy. Ochsner J 17:280–283 [PMC free article] [PubMed] [Google Scholar]
- 61.Marmor MF, Kellner U, Lai TYY, Melles RB, Mieler WF, American Academy of Ophthalmology (2016) Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy (2016 Revision). Ophthalmology 123:1386–1394 These are the most recent guidelines on the screening and management of hydroxychloroquine toxicity put out by the American Academy of Ophthalmology. [DOI] [PubMed] [Google Scholar]
- 62.Browning DJ, Lee C, Rotberg D (2014) The impact of different algorithms for ideal body weight on screening for hydroxychloroquine retinopathy in women. Clin Ophthalmol 8:1401–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walvick MD, Walvick MP, Tongson E, Ngo CH (2011) Hydroxychloroquine: lean body weight dosing. Ophthalmology 118:2100; author reply 2101 [DOI] [PubMed] [Google Scholar]
- 64.Wolfe F, Marmor MF (2010) Rates and predictors of hydroxychloroquine retinal toxicity in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res (Hoboken) 62:775–784 [DOI] [PubMed] [Google Scholar]
- 65.Lenfant T, Salah S, Leroux G, et al. (2020) Risk factors for hydroxychloroquine retinopathy in systemic lupus erythematosus: a case–control study with hydroxychloroquine blood-level analysis. Rheumatology (Oxford) keaa157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abdelbaky MSE, El Mamoun TA, Mabrouk FI, Hassan RM (2021) Frequency and risk factors for hydroxychloroquine retinopathy among patients with systemic lupus erythematosus. Egypt J Intern Med 33:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nayfield SG, Gorin MB (1996) Tamoxifen-associated eye disease. A review. J Clin Oncol 14:1018–1026 [DOI] [PubMed] [Google Scholar]
- 68.Kim LA, Amarnani D, Gnanaguru G, Tseng WA, Vavvas DG, D’Amore PA (2014) Tamoxifen Toxicity in Cultured Retinal Pigment Epithelial Cells Is Mediated by Concurrent Regulated Cell Death Mechanisms. Invest Ophthalmol Vis Sci 55:4747–4758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Toimela T, Tähti H, Salminen L (1995) Retinal pigment epithelium cell culture as a model for evaluation of the toxicity of tamoxifen and chloroquine. Ophthalmic Res 27 Suppl 1:150–153 [DOI] [PubMed] [Google Scholar]
- 70.Shroyer NF, Lewis RA, Lupski JR (2001) Analysis of the ABCR (ABCA4) gene in 4-aminoquinoline retinopathy: is retinal toxicity by chloroquine and hydroxychloroquine related to Stargardt disease? Am J Ophthalmol 131:761–766 [DOI] [PubMed] [Google Scholar]
- 71.Grassmann F, Bergholz R, Mändl J, Jägle H, Ruether K, Weber BH (2015) Common synonymous variants in ABCA4 are protective for chloroquine induced maculopathy (toxic maculopathy). BMC Ophthalmol 15:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Melles RB, Marmor MF (2015) Pericentral retinopathy and racial differences in hydroxychloroquine toxicity. Ophthalmology 122:110–116 [DOI] [PubMed] [Google Scholar]
- 73.Corradetti G, Violanti S, Au A, Sarraf D (2019) Wide field retinal imaging and the detection of drug associated retinal toxicity. International Journal of Retina and Vitreous 5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gbinigie K, Frie K (2020) Should chloroquine and hydroxychloroquine be used to treat COVID-19? A rapid review. BJGP Open 4:bjgpopen20X101069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yam JCS, Kwok AKH (2006) Ocular toxicity of hydroxychloroquine. Hong Kong Med J 12:294–304 [PubMed] [Google Scholar]
- 76.Kellner S, Weinitz S, Farmand G, Kellner U (2014) Cystoid macular oedema and epiretinal membrane formation during progression of chloroquine retinopathy after drug cessation. Br J Ophthalmol 98:200–206 [DOI] [PubMed] [Google Scholar]
- 77.Bull’s-eye maculopathy due to hydroxychloroquine toxicity. https://webeye.ophth.uiowa.edu/eyeforum/atlas/pages/Hydroxychloroquine-toxicity/index.htm. Accessed 29 Jun 2023 [Google Scholar]
- 78.Fernandez Schultis S, Nguyen C (2017) Macular Telangiectasia: A cause of bull’s eye maculopathy. New Front Ophthalmol. 10.15761/NFO.1000178 [DOI] [Google Scholar]
- 79.Cone Dystrophy - Symptoms, Causes, Treatment | NORD. https://rarediseases.org/rare-diseases/cone-dystrophy/. Accessed 29 Jun 2023 [Google Scholar]
- 80.Fleckenstein M, Keenan TDL, Guymer RH, Chakravarthy U, Schmitz-Valckenberg S, Klaver CC, Wong WT, Chew EY (2021) Age-related macular degeneration. Nat Rev Dis Primers 7:31. [DOI] [PubMed] [Google Scholar]
- 81.Macular dystrophy, concentric annular - About the Disease - Genetic and Rare Diseases Information Center. https://rarediseases.info.nih.gov/diseases/9887/macular-dystrophy-concentric-annular. Accessed 29 Jun 2023 [Google Scholar]
- 82.Neuronal ceroid lipofuscinosis - About the Disease - Genetic and Rare Diseases Information Center. https://rarediseases.info.nih.gov/diseases/10739/neuronal-ceroid-lipofuscinosis/. Accessed 29 Jun 2023 [Google Scholar]
- 83.Polk TD, Gass JD, Green WR, Novak MA, Johnson MW (1997) Familial internal limiting membrane dystrophy. A new sheen retinal dystrophy. Arch Ophthalmol 115:878–885 [DOI] [PubMed] [Google Scholar]
- 84.Schachat AP, Wilkinson CP, Hinton DR, Sadda SR, Wiedemann P (2017) Ryan’s Retina, 6th ed. Elsevier - Health Sciences Division, Philadelphia, PA [Google Scholar]
- 85.Scott IU, Flynn HW, Smiddy WE (1998) Bull’s-eye maculopathy associated with chronic macular hole. Arch Ophthalmol 116:1116–1117 [PubMed] [Google Scholar]
- 86.Hydroxychloroquine and Chloroquine Retinopathy: Recommendations on Monitoring. The Royal College of Ophthalmologists. These are the most recent guidelines on the screening and management of hydroxychloroquine toxicity put out by the Royal College of Ophthalmology. [Google Scholar]
- 87.Yusuf IH, Charbel Issa P, Ahn SJ (2022) Novel imaging techniques for hydroxychloroquine retinopathy. Front Med (Lausanne) 9:1026934 This study includes a discussion of imaging modalities that may prove to be useful in the early diagnosis of hydroxychloroquine retinopathy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marmor MF, Hu J (2014) Effect of disease stage on progression of hydroxychloroquine retinopathy. JAMA Ophthalmol 132:1105–1112 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable


