Abstract
β‐barrel membrane proteins in the mitochondrial outer membrane are crucial for mediating the metabolite exchange between the cytosol and the mitochondrial intermembrane space. In addition, the β‐barrel membrane protein subunit Tom40 of the translocase of the outer membrane (TOM) is essential for the import of the vast majority of mitochondrial proteins encoded in the nucleus. The sorting and assembly machinery (SAM) in the outer membrane is required for the membrane insertion of mitochondrial β‐barrel proteins. The core subunit Sam50, which has been conserved from bacteria to humans, is itself a β‐barrel protein. The β‐strands of β‐barrel precursor proteins are assembled at the Sam50 lateral gate forming a Sam50‐preprotein hybrid barrel. The assembled precursor β‐barrel is finally released into the outer mitochondrial membrane by displacement of the nascent β‐barrel, termed the β‐barrel switching mechanism. SAM forms supercomplexes with TOM and forms a mitochondrial outer‐to‐inner membrane contact site with the mitochondrial contact site and cristae organizing system (MICOS) of the inner membrane. SAM shares subunits with the ER‐mitochondria encounter structure (ERMES), which forms a membrane contact site between the mitochondrial outer membrane and the endoplasmic reticulum. Therefore, β‐barrel membrane protein biogenesis is closely connected to general mitochondrial protein and lipid biogenesis and plays a central role in mitochondrial maintenance.
Keywords: Mco6, Mdm10, mitochondria, outer membrane, SAM, Sam35, Sam37, Sam50, sorting and assembly machinery, β‐barrel protein
Mitochondrial β‐barrel precursors are synthesized in the cytosol and imported by the translocase of the outer membrane (TOM). The sorting and assembly machinery (SAM) inserts β‐barrel membrane proteins from the intermembrane space into the outer membrane. The endosymbiotic origin of mitochondria explains the conservation of the membrane insertion mechanism and of the essential core subunit Sam50 from bacteria to humans.

Abbreviations
- BAM
bacterial β‐barrel assembly machinery
- ER
endoplasmic reticulum
- ERMES
endoplasmic reticulum‐mitochondria encounter structure
- IM
inner membrane
- IMS
mitochondrial intermembrane space
- Mco6
Mdm10 complex protein of 6 kDa
- Mdm10/12/34
mitochondrial distribution and morphology proteins
- MICOS
mitochondrial contact site and cristae organizing system
- MIM
mitochondrial import machinery
- Mmm1
maintenance of mitochondrial morphology protein 1
- MTCH2
mitochondrial carrier homolog 2
- OM
outer membrane
- POTRA
polypeptide‐transport‐associated domain
- SAM
mitochondrial sorting and assembly machinery
- SEC translocase
bacterial translocase of the secretory system
- TIM
translocase of the inner mitochondrial membrane
- TOM
translocase of the outer mitochondrial membrane
β‐Barrel membrane proteins
Mitochondria are eukaryotic organelles descended from a Gram‐negative α‐proteobacterial ancestor in an ancient endosymbiotic event. Over the course of evolution, mitochondria have integrated with the eukaryotic host cell in many ways, but retained essential key features of the bacterial ancestor. These features include many metabolic enzymes such as the citric acid cycle, the respiratory chain, and the ATP synthase. Another essential ancestral feature is the presence of membrane integral β‐barrel proteins in the mitochondrial outer membrane, which are found in no other eukaryotic host cell membrane with the exception of other endosymbiosis‐derived organelles like plastids [1, 2, 3]. Almost all integral membrane proteins of the Gram‐negative bacterial outer membrane are β‐barrels with an even number of β‐strands [4]. Early in mitochondrial evolution, one of these β‐barrel proteins evolved the function of translocating proteins from the cytosol across the outer membrane into the mitochondria. This process is essential to retain mitochondrial function while genes encoding mitochondrial proteins were transferred from the mitochondrial genome to the nucleus. Presently, the vast majority (~ 99%) of mitochondrial proteins are encoded in the nucleus and translated in the cytosol. All of these precursor proteins that are imported across the outer membrane are translocated by the β‐barrel protein core subunit Tom40 of the translocase of the outer mitochondrial membrane (TOM) [5]. The second essential outer membrane β‐barrel protein is Sam50, which is required for inserting β‐barrel proteins, like Tom40 and the outer membrane metabolite channeling β‐barrel of the porin/VDAC (voltage‐dependent anion channel) family, into the outer mitochondrial membrane (Table 1). Due to the crucial nature of β‐barrel proteins, this bacterial feature was universally retained in all mitochondria and a significant proportion of the mitochondrial outer membrane proteome is still made up of β‐barrels [6, 7].
Table 1.
β‐barrel membrane protein substrates of the mitochondrial sorting and assembly machinery (SAM).
| Eukaryotic group | Substrate name | Number of β‐strands |
|---|---|---|
| Fungi (S. cerevisiae) | Sam50 | 16 |
| Tom40 | 19 | |
| Por1 | 19 | |
| Por2 | 19 | |
| Mdm10 | 19 | |
| Mammals (H. sapiens) | SAMM50 | 16 |
| TOMM40 | 19 | |
| VDAC1 | 19 | |
| VDAC2 | 19 | |
| VDAC3 | 19 |
The machineries for β‐barrel membrane protein biogenesis were discovered in mitochondria and Gram‐negative bacteria. In mitochondria, it is named the sorting and assembly machinery (SAM), or topogenesis of mitochondrial outer membrane β‐barrel proteins (TOB) complex [8, 9], and in bacteria the β‐barrel assembly machinery (BAM) [10, 11]. Similar to most mitochondrial proteins, all mitochondrial β‐barrel membrane proteins are encoded in the nucleus and translated in the cytosol. These β‐barrel precursor proteins are imported across the outer mitochondrial membrane into the intermembrane space by the TOM complex. Intermembrane space transfer chaperones guide the β‐barrel preproteins to the SAM complex, which inserts them into the outer membrane (Fig. 1) [12]. In Gram‐negative bacteria, β‐barrel membrane proteins are transported across the inner bacterial membrane by the SEC complex. Periplasmic chaperones support the transfer to the BAM complex, which inserts the β‐barrel precursor proteins into the outer bacterial membrane (Fig. 1) [13, 14, 15, 16, 17]. The core mitochondrial SAM complex subunit, Sam50, belongs to the Omp85 protein superfamily and is homologous to the bacterial core subunit BamA of the BAM complex [8, 10, 18, 19]. The conservation of the machineries for the membrane insertion of β‐barrel proteins from bacteria to mitochondria explains why mitochondrial β‐barrel proteins are translocated across the outer mitochondrial membrane before they are inserted from the intermembrane space side into the outer membrane. The SAM complexes consist of the membrane integral β‐barrel subunits Sam50 and Mdm10, the α‐helical outer membrane protein Mco6, and the peripheral cytosol‐facing subunits Sam35 and Sam37 in yeast (Table 2) [9, 20, 21, 22, 23, 24]. This review focuses predominantly on mitochondrial β‐barrel membrane protein biogenesis in yeast, as this is by far the best‐analyzed organism to date. In addition to SAMM50, animals contain two to three cytosolically exposed metaxins (MTX1, MTX2, and MTX3), which are related to Sam35 and Sam37 (Table 2) [25, 26, 27]. Recent efforts including high‐resolution structures have greatly advanced our understanding of mitochondrial β‐barrel membrane protein biogenesis at a molecular level. The roles of peripheral SAM subunits have been further elucidated and the role of Mco6 as a novel SAM subunit was recently identified. Another emerging concept of the SAM complex, in addition to its role in β‐barrel biogenesis, is its role as a hub of interactions between mitochondrial outer and inner membrane complexes.
Fig. 1.
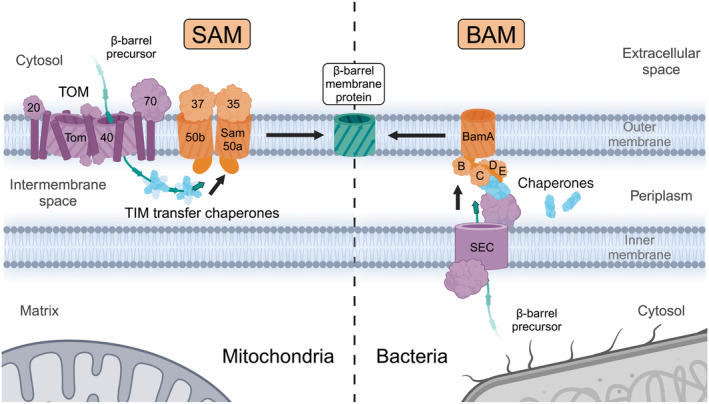
β‐barrel membrane protein biogenesis in mitochondria and Gram‐negative bacteria. Mitochondrial β‐barrels are synthesized in the eukaryotic cytosol, translocated across the outer membrane by the TOM complex, and guided by the TIM transfer chaperones across the intermembrane space to the SAM complex, where they are inserted into the outer membrane. Bacterial β‐barrels are synthesized in the cytosol, translocated across the inner membrane by the SEC translocase, chaperoned through the periplasm by a system of chaperones, and inserted into the outer membrane by the BAM complex. Although mitochondrial and bacterial β‐barrels are synthesized in opposite compartments related to the outer membrane, they are conservatively inserted into the outer membrane from the intermembrane space/periplasmic side by the homologous Omp85 family proteins Sam50 and BamA respectively. BAM, β‐barrel assembly machinery; SAM, sorting and assembly machinery; TIM, translocase of the inner mitochondrial membrane; TOM, translocase of the outer membrane.
Table 2.
Protein components of the mitochondrial sorting and assembly machinery (SAM).
| Eukaryotic group | Name | Alias | kDa |
|---|---|---|---|
| Fungi (S. cerevisiae) | Sam50 | Tob55, Omp85 | 54 |
| Sam37 | Mas37, Tob37, Tom37 | 37 | |
| Sam35 | Tob38, Tom38 | 37 | |
| Mdm10 | 56 | ||
| Mco6 | 6 | ||
| Mammals (H. sapiens) | SAMM50 | 52 | |
| Metaxin‐1 | MTX1 | 51 a | |
| Metaxin‐2 | MTX2 | 30 a | |
| Metaxin‐3 | MTX3 | 35 a |
Canonical sequence (Isoform 1).
Mechanism of mitochondrial β‐barrel membrane protein biogenesis
Mitochondrial β‐barrel membrane proteins begin their biogenesis in the cytosol where they are post‐translationally chaperoned by the cytosolic heat shock proteins Hsp70 and Hsp40 and targeted to the TOM complex receptors Tom20 and Tom70 (Fig. 1) [28, 29, 30, 31]. The targeting signal to bind the receptors and traverse the TOM complex is a β‐hairpin composed of two adjacent antiparallel β‐strands [9, 32]. As all membrane β‐strands are amphipathic in nature, the β‐hairpin can be regarded as a structure resembling the characteristics of the classical amphipathic (α‐helical) mitochondrial presequence and as such likely binds to similar sites on the TOM receptors. The receptors are not strictly required, as artificial β‐barrel proteins can assemble in mitochondria independently of the receptors [33]. Once the β‐barrel precursor arrives at the TOM complex, it binds to residues within the Tom40 channel, which drives the translocation across the outer membrane (Fig. 1) [34]. As precursors cross the intermembrane space from TOM to SAM, TIM transfer chaperone complexes Tim9/10 and Tim8/13 prevent their aggregation [35, 36, 37]. The TIM transfer chaperones form hexameric circular complexes with alternating subunits. Each subunit contains intramolecular disulfide bonds and extended N‐ and C‐terminal α‐helices with conserved hydrophobic residues pointing into the cleft in between the helices (Fig. 2) [38, 39]. The interactions involve many loose precursor associations within the hydrophobic clefts of the TIM transfer chaperones such that precursors can be bound but also be efficiently released as they are membrane‐inserted without any external energy input [36, 40].
Fig. 2.
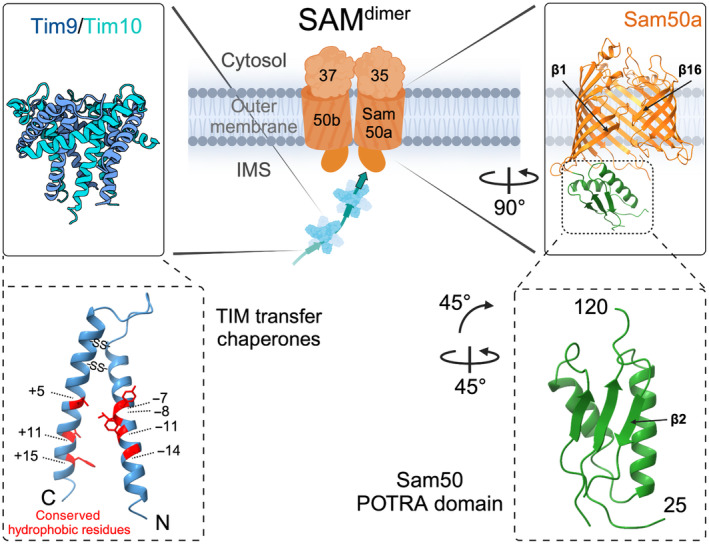
Translocase of the inner mitochondrial membrane (TIM) transfer chaperone and Sam50 POTRA domain structures. The TIM transfer chaperones Tim9/Tim10 (and Tim8/13) form an alternating hexameric structure. Each subunit contains intramolecular disulfide bonds and conserved hydrophobic residues (in positions + and ‐ with respect to the residues involved in disulfide bond formation) in their N‐ and C‐terminal helices that form a precursor binding cleft. Sam50 contains one N‐terminal POTRA domain in the intermembrane space (IMS). POTRA β‐strand 2 is available for β‐strand augmentation by the substrate and thus contributes to β‐barrel membrane protein assembly. POTRA, polypeptide‐transport‐associated domain.
The conserved β‐signal within the C‐terminal β‐strand of mitochondrial β‐barrel membrane proteins targets the precursor proteins to the SAM complex (Fig. 3, bottom) [41]. With the help of the Sam50 cytosolic loop 6 containing the conserved IRGF motif, the β‐signal binds in an antiparallel orientation to the N‐terminal β‐strand of Sam50 (Fig. 3 left panel on the top) [42]. Sam50 and the homologous bacterial BamA have 16 β‐strands each with weakly interacting terminal β‐strands (fewer inter‐strand hydrogen bonds) unlike other β‐barrel membrane proteins that do not function as β‐barrel insertases (Fig. 3 right panel on the top). The dynamics of these terminal β‐strands allows Sam50 to readily bind the β‐signal of incoming β‐barrel precursors. Both open and closed states of Sam50/BamA have been observed in cryo‐EM structures, by intramolecular crosslinking and molecular dynamics simulations [42, 43, 44, 45, 46]. The next step after SAM binding is insertion of the precursor into the membrane. Both Sam50 and BamA are hydrophobically mismatched with the membrane near their terminal β‐strands and therefore locally thin/distort the membrane to facilitate protein insertion [47, 48, 49, 50, 51]. This is a common strategy by membrane insertases to make the thermodynamics of insertion more favorable [52]. It is unclear how many β‐strands are inserted into the membrane at once, but it seems likely that β‐hairpins are inserted into the membrane sequentially from C‐ to N terminus [42, 53, 54]. Therefore, the hydrophilic loops of each β‐hairpin are likely shielded from the membrane by the interior of the Sam50 barrel and loop 6 as the β‐hairpin is inserted.
Fig. 3.
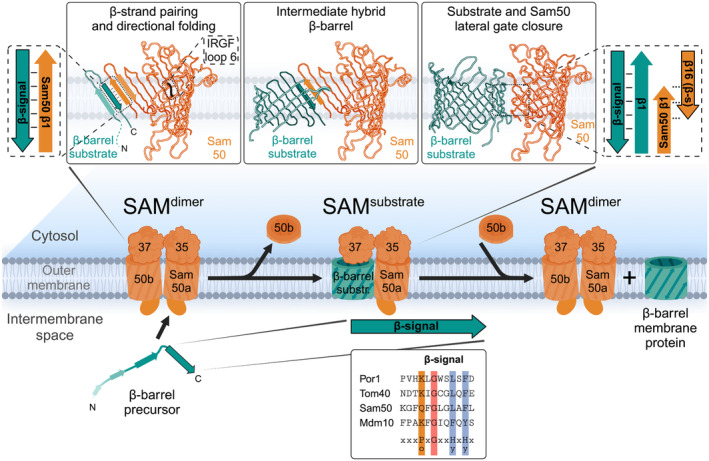
Membrane insertion of β‐barrel precursors at the SAM complex by β‐barrel switching. The last C‐terminal β‐strand of the substrate, which contains a conserved β‐signal, binds to the SAMdimer complex by pairing with the first β‐strand of Sam50a. While the β‐signal remains bound to Sam50a, additional β‐strands are sequentially inserted into the membrane from C‐ to N terminus, likely as β‐hairpins. This is termed the hybrid barrel stage. The growing substrate β‐barrel displaces Sam50b. Once the substrate barrel is fully folded, its terminal β‐strands pair, replacing the β‐signal pairing with Sam50a and closing the lateral gates of both β‐barrels. The newly assembled β‐barrel precursor is released into the outer membrane and switched by Sam50b to re‐form the SAMdimer complex. Hy, hydrophobic residues; Po, polar residues; SAM, sorting and assembly machinery.
During insertion of sequential β‐hairpins, a hybrid barrel is formed in the plane of the outer membrane between the β‐barrel precursor and Sam50 with a strong interaction (hydrogen‐bond pairing) between the Sam50 N‐terminal β‐strand with the C‐terminal precursor β‐strand (Figs 3 and 4, middle panels) [42, 54]. In contrast, the C‐terminal β‐strands of Sam50 and the assembling N‐terminal β‐strands of the substrate curve similar to the mature barrel structures, such that they associate loosely with each other (without hydrogen‐bond pairing) (Figs 3 and 4, middle panels) [42, 54]. The hybrid barrel model was initially suggested in the bacterial field based on the observation that BamA lateral gate opening is important for its function [45] and that a folded passenger domain of an autotransporter secreted in Escherichia coli requires the combined hybrid barrels of the autotransporter β‐barrel domain and the BamA barrel to accommodate translocation of the folded domain [55, 56]. The model was proven in mitochondria by extensive crosslinking experiments [42] and recently validated by the Tom40 precursor‐SAM structure for mitochondria [54] and precursor‐BAM structures for bacteria [57, 58].
Fig. 4.
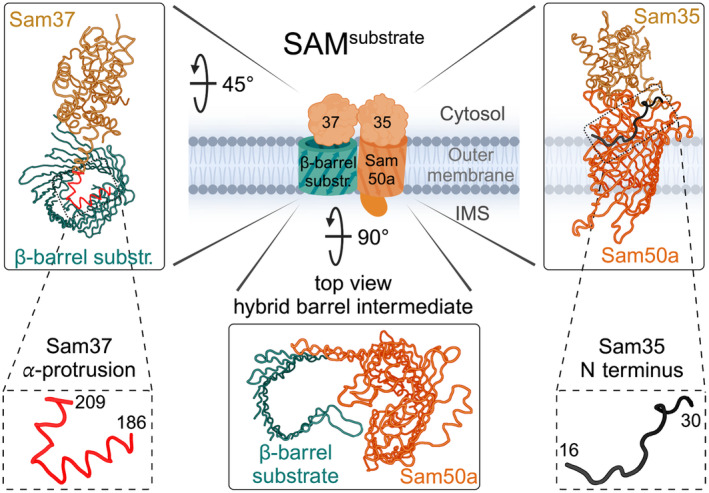
Role of peripheral cytosolic subunits Sam35 and Sam37. Sam35 sits atop Sam50a, interacting with the barrel wall with its N terminus. Sam35 assists in substrate binding to Sam50a. Sam37 is similar to Sam35 in structure and its ability to bind mitochondrial β‐barrels from the cytosolic side. Sam37 contains an α‐protrusion which is able to bind into the lumen of the growing β‐barrel substrate, thereby stabilizing the β‐barrel during its formation.
It is feasible that the formation of the hydrogen bonds between the first and the last β‐strand of the substrate and reformation of the lateral gate of Sam50 is sufficient to disrupt the hybrid barrel interaction between the substrate and Sam50, such that β‐barrel biogenesis can proceed without external energy input [59].
Eukaryotic specific subunits for β‐barrel membrane protein biogenesis
Even though the β‐barrel membrane insertion machineries SAM in mitochondria and BAM in bacteria operate by a similar core mechanism due to the conserved subunits, Sam50 and BamA, there are also fundamental differences. The additional mitochondrial SAM complex subunits are either peripheral subunits on the cytosolic face of the SAM complex (Sam35 and Sam37) or integral outer membrane proteins (Mdm10 and Mco6) and have no homology to bacterial BAM subunits. In contrast, all additional bacterial BAM subunits (BamB to BamE) are periplasmic proteins peripherally associated together with BamA [13, 14, 16, 17]. It is tempting to speculate that the BAM machinery evolved to operate without extracellular proteins to protect the BAM machinery from the extracellular environment, while the mitochondrial machinery could evolve with cytosolic subunits on the opposing face of the outer membrane and with an α‐helical outer membrane protein, since these are common in the mitochondrial outer membrane (Fig. 1).
Sam50 and BamA contain polypeptide‐transport‐associated (POTRA) domains located in the intermembrane space or periplasm respectively. POTRA domains adopt a three‐stranded β‐sheet structure overlaid with a pair of antiparallel helices with β‐α‐α‐β‐β arrangement (Fig. 2 right) [60, 61]. The POTRA domain β‐strand 2 is available for β‐strand augmentation by substrate proteins, and thus, the POTRA domains were suggested to be crucial for targeting the β‐barrel substrates to SAM and BAM [62, 63]. Even though numerous POTRA domains can be deleted in the bacterial system, the presence of a single POTRA domain is essential for BamA function [64]. In contrast, the single POTRA domain of mitochondrial Sam50 is not essential, but likely supports β‐barrel protein assembly and substrate release [41, 65].
The two additional SAM complex core subunits Sam35 and Sam37 are structurally similar peripheral membrane proteins that cap different mitochondrial β‐barrel membrane proteins (Sam50, Mdm10, or growing substrate barrels). Sam35 was discovered shortly after Sam50, but Sam37 was identified with a proposed function as a mitochondrial import receptor long before this [66]. These peripheral subunits do not have strong sequence conservation across species, but are generally present throughout. In some species, Sam37 even has a transmembrane segment that makes it an integral outer membrane protein [43]. Sam35 and Sam37 have a glutathione‐S‐transferase (GST)‐like fold and are related to metaxins (MTX1, MTX2, and MTX3) in humans that are also known to be involved in β‐barrel biogenesis together with human SAMM50 (Table 2) [25, 27, 43].
Sam35 (Tom38, Tob38) is the other essential mitochondrial SAM subunit and caps the β‐barrel protein Sam50 from the cytosolic face [21, 23, 24, 43, 46, 54, 67]. The N terminus of Sam35 is essential and associates with the cytosolic rim at the C terminus of the Sam50 barrel (Fig. 4, right) [46]. This suggests that Sam35 is involved in Sam50 lateral gate opening, which is consistent with the crucial role of Sam35 for β‐signal binding at the SAM complex [41, 68]. Although bacteria do not have a Sam35 homolog, the BAM complex contains a second essential subunit BamD associated on the opposing periplasmic side. However, BamD does not play a catalytic role in outer membrane protein assembly, but rather functions to regulate the activity of BamA by substrate binding [69, 70].
Mitochondrial Sam37 (Mas37, Tom37, and Tob37) is not essential for cell viability but it is crucial for efficient β‐barrel protein biogenesis [9]. Sam37 is found in three different SAM complex variants: (a) the SAMdimer complex with two copies of Sam50 (named Sam50a and Sam50b) associated with their lateral gates facing each other (Fig. 3) [46] (b) the SAMMdm10 complex with the β‐barrel proteins Sam50a and Mdm10 (Fig. 5) [46] (c) the SAMsubstrate complex with Sam50a and the β‐barrel protein substrate (Figs 3, 4, 5) [54, 67]. Sam37 caps the dynamically associated β‐barrel component of each of these complexes from the cytosolic side of the outer membrane.
Fig. 5.
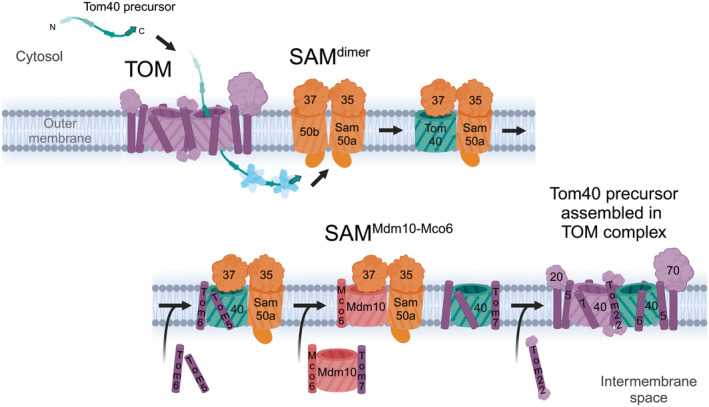
Tom40 assembly at SAM. Tom40 follows the general β‐barrel insertion pathway in terms of binding to Sam50a via a β‐signal and forming a hybrid barrel structure (upper panel). However, unlike other β‐barrels, Tom40 assembles with small α‐helical transmembrane proteins while bound to the SAM complex (lower panel). Tom5 and Tom6 assemble with the Tom40 precursor while still bound to SAM. Release of Tom40 requires another β‐barrel protein, Mdm10, which displaces the Tom40 substrate at SAM via interactions with Sam50a and Sam37. During the displacement process, Mdm10 delivers another small translocase of the outer membrane (TOM) protein, Tom7, to Tom40‐5‐6. Mdm10 is itself stabilized by an additional small membrane protein, Mco6. The final core TOM subunit, Tom22, is assembled with Tom40‐5‐6‐7, which allows for dimerization and formation of the mature TOM complex.
Upon engagement of the β‐barrel substrate with the SAM complex, the substrate β‐signal binds to the N‐terminal β‐strand of Sam50a and thereby displaces Sam50b. This mechanism of SAMdimer to SAMsubstrate complex conversion for mitochondrial β‐barrel biogenesis was termed β‐barrel switching (Fig. 3) [46]. Sam37 stabilizes the growing barrel of the substrate from the cytosolic side and contains an α‐helical protrusion that can insert into the substrate β‐barrel lumen (Fig. 4, left). Thereby, Sam37 and its α‐helical protrusion enhance the efficiency of mitochondrial β‐barrel membrane protein biogenesis and substrate release [9, 54, 68]. Thus, Sam37 is required for the efficient barrel closure of the substrate and functions as the molecular cooper of the SAM complex.
In contrast to bacterial β‐barrel proteins with an even number of β‐strands, the major mitochondrial β‐barrel proteins of the porin/VDAC and Tom40 families contain 19 β‐strands with an α‐helical N terminus, which can fold into the lumen of the barrel (Table 1) [71, 72, 73, 74]. These α‐helical N termini of the β‐barrel substrates are crucial for barrel formation and efficient substrate release from the SAM complex [54]. The lipid composition of the mitochondrial outer membrane also greatly contributes to an efficient assembly of β‐barrel membrane proteins. Cardiolipin, phosphatidylcholine, or phosphatidylethanolamine depletion greatly reduces the biogenesis of β‐barrel proteins [75, 76, 77].
Taken together, Sam35 is essential for the engagement of the β‐barrel protein substrate with Sam50. Sam37, the α‐helical N‐termini of mitochondrial β‐barrel proteins, and the physical properties of the lipid bilayer are crucial for the formation of β‐barrels in the mitochondrial outer membrane.
Assembly of the TOM complex
The main mitochondrial entry gate for precursor proteins in the outer membrane, the TOM complex, consists of the β‐barrel protein Tom40 and six α‐helical membrane proteins: the receptors Tom20, Tom22, and Tom70, and the small proteins Tom5, Tom6, and Tom7 (Fig. 5). The structurally best‐characterized stoichiometry of the TOM core complex consists of two copies of the β‐barrel protein Tom40 and two copies each of the α‐helical subunits Tom5, Tom6, Tom7, and Tom22. The two Tom40 β‐barrels are tethered by the α‐helical transmembrane segments of the Tom22 proteins, while the small α‐helical Tom proteins Tom5, Tom6, and Tom7 specifically associate individually with the Tom40 barrels [71, 74, 78, 79, 80].
Different assembly intermediate structures of Tom40 in the SAMsubstrate complex were obtained. In the hybrid barrel intermediate structure with 12 membrane‐inserted β‐strands, the N‐terminal β‐strand 1 of Tom40 is found in the lumen of the growing barrel [54]. The Sam37 α‐helical protrusion, which dynamically projects into the substrate β‐barrel, assists in assembling Tom40 (Fig. 4) [9, 54]. After all the other β‐strands are inserted into the membrane, the N‐terminal α‐helical domain of Tom40 displaces β‐strand 1 from the Tom40 lumen allowing it to hydrogen bond with the last β‐strand, closing the barrel and completing the folding process. The small TOM proteins, Tom5 and Tom6, assemble with Tom40 before release from the SAM complex [81, 82]. It can be speculated that the association of Tom5 stabilizes the Tom40 β‐barrel domain and thus increases the efficiency of Tom40 β‐barrel formation at the SAM complex. In the SAMsubstrate structures with a completely folded β‐barrel of Tom40, the structure of Tom40 already resembles its conformation in the mature TOM complex [67, 71, 74]. Moreover, in the SAMTom40‐5‐6 structure, Tom5 and Tom6 are associated with Tom40 like in the assembled TOM complex [67, 71, 74]. The isolation of the stable SAMTom40‐5‐6 intermediate indicates that folding of the β‐barrel and release from SAM are distinct steps for Tom40 [67].
The release of the Tom40‐Tom5‐Tom6 intermediate from the SAM complex is greatly enhanced by another outer membrane β‐barrel protein called Mdm10 (mitochondrial distribution and morphology protein 10) together with the α‐helical outer membrane protein Mco6 (Mdm10 complex protein of 6 kDa) (Fig. 5) [20, 22, 83]. Mco6 forms a complex with Mdm10, which is related to Tom40, and is thought to stabilize the β‐barrel protein [20, 84]. The Mdm10‐Mco6 complex displaces the Tom40‐Tom5‐Tom6 intermediate from the SAM complex by β‐barrel switching and the SAMMdm10‐Mco6 complex is formed [20, 46, 83]. The Mdm10‐Mco6 interaction with SAM represents a subpopulation of the SAM complexes [20]. After the release of the Tom40‐Tom5‐Tom6 intermediate from SAM, it assembles with Tom7 and with Tom22. Mdm10 binds Tom7 on the opposite side of its β‐barrel domain with respect to Mco6; however, in the SAMMdm10‐Mco6 complex, the binding site for Tom7 is occupied by Sam50 [20]. Therefore, it seems likely that Tom7 is directly transferred from Mdm10 to Tom40 concomitantly during the β‐barrel switching process (Fig. 5) [85, 86]. Mdm10 and Mco6 are likely also involved in the assembly of Tom22 with Tom40, since mdm10 and mco6 mutants display a reduced assembly of Tom22 and, in addition, a diminished protein level of Tom22 [20, 22]. One can speculate that a Tom7‐Mdm10‐Mco6 complex is specifically required for the release of the Tom40‐Tom5‐Tom6 intermediate to coordinate the transfer of the phospholipid which binds to the Tom40 lateral gate with the assembly of Tom7 and Tom22 [71]. The SAMMdm10‐Mco6 complex is likely in an equilibrium with other SAM complexes, ERMES, and β‐barrel substrates.
In summary, the β‐barrel switching mechanism for the biogenesis of the TOM complex involves the displacement of Sam50b from the SAMdimer complex by the Tom40 precursor (Fig. 3) and the reverse displacement of the Tom40‐Tom5‐Tom6 precursor intermediate by Mdm10‐Mco6 (Fig. 5).
Interactions of the SAM complex
The SAM complex interacts with several other protein complexes of mitochondria (Fig. 6), but the most important interaction for β‐barrel biogenesis is that with the TOM complex. The TOM‐SAM supercomplex is formed by interactions between the cytosolic domains of Tom22 and Sam37 (Fig. 6) [87, 88]. Although not strictly essential, the formation of a TOM‐SAM supercomplex allows for the efficient coupling of β‐barrel translocation steps from translocation across the outer membrane via TOM—to insertion into the membrane via SAM. The TIM transfer chaperones do not seem to directly interact with SAM but merely prevent the aggregation of hydrophobic precursor segments as they cross the aqueous intermembrane space. Novel SAM complex/subunit interactions continue to be identified, such as the recent finding that the metaxins in human cells also link mitochondria with microtubule motor assemblies [89].
Fig. 6.
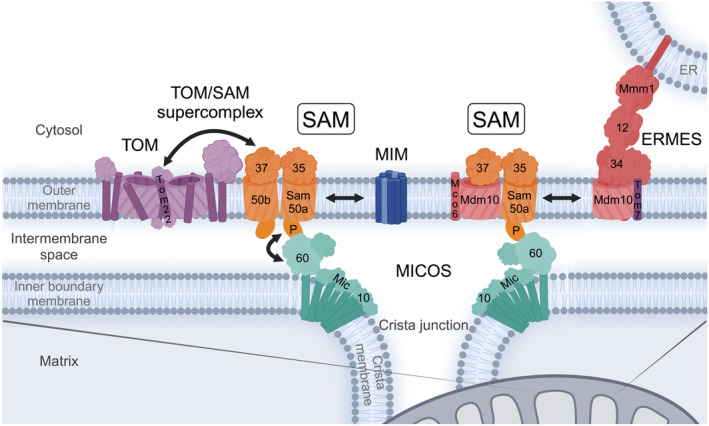
Interactions of the sorting and assembly machinery (SAM) complex with other mitochondrial protein machineries. SAM forms a supercomplex with translocase of the outer membrane (TOM) through interactions of Sam37 and Tom22. This couples β‐barrel substrate translocation across and insertion into the outer membrane. SAM interaction with the α‐helical outer membrane insertase mitochondrial import machinery (MIM) facilitates small TOM subunit assembly with Tom40 at the SAM complex. Mdm10 is a subunit of both SAM and ERMES to regulate mitochondrial protein and lipid biogenesis. The Sam50 POTRA domain interacts with the MICOS complex at cristae junctions in the inner membrane and forms a mitochondrial contact site between the outer and the inner mitochondrial membranes. All of these interactions place SAM in a central position with regard to protein and lipid biogenesis in mitochondria. ERMES, endoplasmic reticulum‐mitochondria encounter structure; MICOS, mitochondrial contact site and cristae organizing system.
The SAM complex also interacts with the mitochondrial import complex (MIM), which facilitates insertion of α‐helical proteins into the outer membrane in yeast (Fig. 6) [90, 91, 92]. MIM consists of the two α‐helical outer membrane proteins Mim1 and Mim2, which are thought to form a channel for the insertion of α‐helical outer membrane protein substrates [93, 94, 95, 96]. SAM interacts with MIM in order to coordinate the assembly of α‐helical and β‐barrel membrane proteins [65, 90, 94]. Specifically, the interaction couples Tom5 and Tom6 insertion via MIM with the biogenesis of Tom40 at SAM (Fig. 5). Moreover, SAM subunits are also required for α‐helical protein insertion of the TOM core subunit Tom22 and the small TOM proteins Tom5, Tom6, and Tom7 [97].
In addition to these functional interactions for coupling precursor protein import (TOM) or α‐helical protein assembly (MIM), SAM also interacts with the mitochondrial contact site and cristae organizing system (MICOS) in the inner membrane forming the mitochondrial intermembrane space bridging complex (MIB) [98, 99, 100, 101, 102]. This mitochondrial membrane contact site is formed between the POTRA domain of the outer membrane protein Sam50 and the intermembrane space domain of the inner membrane protein Mic60 (Fcj1, Mitofilin) of the MICOS complex (Fig. 6) [103, 104]. Depletion of Mic60 impairs the assembly of β‐barrel membrane proteins [103]. The homo‐oligomeric MICOS core subunit Mic10 is thought to bend the inner membrane via two transmembrane segments connected by a short charged loop in the matrix to form V‐shaped structures that induce the inner membrane curvature to form crista junctions [105, 106]. The diameter of the crista junction is likely fixed by an antiparallel tetrameric assembly of the Mic60 intermembrane space domain, which connects the opposing sides of the crista junction membrane [107].
Even though the SAM complex does not directly interact with the endoplasmic reticulum‐mitochondria encounter structure (ERMES), it shares a subunit, Mdm10, that binds to either complex (Fig. 6) [108]. ERMES is responsible for physically connecting mitochondria and the ER and for transferring lipids between them [109, 110, 111]. ERMES consists of an α‐helical ER membrane protein named Mmm1 (maintenance of mitochondrial morphology 1) and two cytosolic proteins Mdm12 and Mdm34. All three ERMES proteins contain a synaptotagmin‐like mitochondrial lipid‐binding protein (SMP) domain, which form a conduit of hydrophobic cavities for the transfer of lipids between the organelles [112, 113]. The β‐barrel protein Mdm10 anchors ERMES at the mitochondrial outer membrane. Mdm10 is in an equilibrium between the SAM and ERMES complexes and Tom7 can only bind to free Mdm10 or Mdm10 within the ERMES complex, but not in the SAMMdm10‐Mco6 complex [20, 85, 86]. Interestingly, the Mco6 homolog Emr1 of fission yeast (Schizosaccharomyces pombe) was found to regulate the number of ERMES foci, consistent with the role of Mco6 to stabilize Mdm10 [20, 114]. Tom7 overexpression increases the abundance of SAM complexes lacking Mdm10 and concomitantly enhances the β‐barrel assembly of porin [86]. This is consistent with the observation that precursors do not bind to the SAMMdm10‐Mco6 complex since Mdm10 overexpression blocks substrate binding [83]. Conversely, TOM7 deletion reduces the assembly of porin but enhances the efficiency of Tom40 assembly due to the increased availability of the assembly factor Mdm10 [85, 86]. This intricate mechanism of balancing the activities of SAM and ERMES by the TOM subunit Tom7 likely regulates the protein and lipid biogenesis of the mitochondrial outer membrane in relation to the assembled TOM complex.
All interactions considered, SAM is a central complex in mitochondrial protein and lipid biogenesis pathways through its main role as insertase for β‐barrel membrane proteins, its cooperation with the biogenesis of α‐helical outer membrane proteins (MIM), its connection to the general import pore (TOM), the sharing of the Mdm10 subunit and its regulation by Tom7 with the ER‐mitochondria lipid exchange contact site (ERMES), and its structural role for establishing mitochondrial outer‐to‐inner membrane contact sites with MICOS. It remains to be determined whether the MICOS interaction evolved simply to tether the two mitochondrial membranes or whether the close proximity of SAM and MICOS provides additional functional advantages.
Conclusion and open questions
Ongoing interest in β‐barrel membrane protein biogenesis has produced a large step forward in recent years in our understanding of the basic mechanisms from both the mitochondrial and the bacterial fields. Before the β‐barrel assembly machineries were discovered in mitochondria (SAM) and bacteria (BAM), it had been assumed that β‐barrel membrane proteins preform in the cytosol/periplasm to insert directly into the outer membrane [115, 116]. It is now clear that the biogenesis of native β‐barrel membrane proteins requires the essential Sam50/BamA, which form 16‐stranded β‐barrels with a dynamic lateral gate in their respective outer membranes. The opening of the lateral gate is crucial so that the C‐terminal β‐signal‐strand of the precursors can bind to the N‐terminal β‐strand of Sam50/BamA to start the formation of a hybrid barrel. After formation of the hydrogen bonds between the first and the last β‐strands of the substrate, the folded β‐barrel can be released into the outer membrane. This process has been well studied in yeast and also in bacteria.
However, there are many open questions, especially for the assembly of β‐barrel proteins in metazoan/human mitochondria. How do the metaxin proteins support the β‐barrel membrane protein biogenesis compared with yeast Sam35 and Sam37 even though they are not stably associated with SAMM50? How is the TOM complex assembled in metazoan/human mitochondria? In humans, a mitochondrial carrier homolog 2 (MTCH2) was identified as a crucial protein required for the insertion of α‐helical outer membrane proteins [117]. Does MTCH2 also functionally and physically interact with SAM? How is the biogenesis of β‐barrel membrane proteins regulated under different metabolic and growth conditions?
Taken together, it is good to see that the mitochondrial and the bacterial fields managed to agree on a general mechanism for β‐barrel membrane protein biogenesis consistent with the finding that mitochondrial β‐barrel membrane proteins can be assembled in bacteria and vice versa [118, 119, 120]. Yet, much work remains to understand the intricate mechanisms for the biogenesis of this fascinating class of essential membrane proteins.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
IG, JVB, NP, and NW conceived the manuscript and conceptualized the figures and tables. IG wrote the manuscript draft and JVB created the draft of the images and tables. All authors wrote the manuscript.
Acknowledgements
Figures were created with BioRender.com. The structures shown were adapted for illustration purposes from PDB codes: 3DXR (Fig. 2 left panels) [121], 7BTX (Fig. 3 right and Fig. 4 left and right panels) [46], 7E4H (Fig. 2 right panels) [67], 7VKU (Fig. 3 left and middle and Fig. 4 middle panels) [54]. IG was supported by an Alexander von Humboldt Foundation Research Fellowship. This work was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) PF 202/9‐1—project ID: 394024777 (NP); WI 4506/1‐1—project ID: 406757425 (NW); SFB 1381—project ID: 403222702 (NW); Germany's Excellence Strategy (CIBSS EXC‐2189—project ID: 390939984, NP and NW). Open Access funding enabled and organized by Projekt DEAL.
Iniyan Ganesan and Jon V. Busto contributed equally to this article.
References
- 1. Day PM, Inoue K and Theg SM (2019) Chloroplast outer membrane β‐barrel proteins use components of the general import apparatus. Plant Cell 31, 1845–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gross LE, Klinger A, Spies N, Ernst T, Flinner N, Simm S, Ladig R, Bodensohn U and Schleiff E (2021) Insertion of plastidic β‐barrel proteins into the outer envelopes of plastids involves an intermembrane space intermediate formed with Toc75‐V/OEP80. Plant Cell 33, 1657–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roumia AF, Theodoropoulou MC, Tsirigos KD, Nielsen H and Bagos PG (2020) Landscape of eukaryotic transmembrane beta barrel proteins. J Proteome Res 19, 1209–1221. [DOI] [PubMed] [Google Scholar]
- 4. Franklin MW, Nepomnyachyi S, Feehan R, Ben‐Tal N, Kolodny R and Slusky JS (2018) Evolutionary pathways of repeat protein topology in bacterial outer membrane proteins. Elife 7, e40308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Araiso Y, Imai K and Endo T (2022) Role of the TOM complex in protein import into mitochondria: structural views. Annu Rev Biochem 91, 679–703. [DOI] [PubMed] [Google Scholar]
- 6. Burri L, Vascotto K, Gentle IE, Chan NC, Beilharz T, Stapleton DI, Ramage L and Lithgow T (2006) Integral membrane proteins in the mitochondrial outer membrane of Saccharomyces cerevisiae . FEBS J 273, 1507–1515. [DOI] [PubMed] [Google Scholar]
- 7. Morgenstern M, Stiller SB, Lübbert P, Peikert CD, Dannenmaier S, Drepper F, Weill U, Höß P, Feuerstein R, Gebert M et al. (2017) Definition of a high‐confidence mitochondrial proteome at quantitative scale. Cell Rep 19, 2836–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Paschen SA, Waizenegger T, Stan T, Preuss M, Cyrklaff M, Hell K, Rapaport D and Neupert W (2003) Evolutionary conservation of biogenesis of β‐barrel membrane proteins. Nature 426, 862–866. [DOI] [PubMed] [Google Scholar]
- 9. Wiedemann N, Kozjak V, Chacinska A, Schönfisch B, Rospert S, Ryan MT, Pfanner N and Meisinger C (2003) Machinery for protein sorting and assembly in the mitochondrial outer membrane. Nature 424, 565–571. [DOI] [PubMed] [Google Scholar]
- 10. Voulhoux R, Bos MP, Geurtsen J, Mols M and Tommassen J (2003) Role of a highly conserved bacterial protein in outer membrane protein assembly. Science 299, 262–265. [DOI] [PubMed] [Google Scholar]
- 11. Wu T, Malinverni J, Ruiz N, Kim S, Silhavy TJ and Kahne D (2005) Identification of a multicomponent complex required for outer membrane biogenesis in Escherichia coli . Cell 121, 235–245. [DOI] [PubMed] [Google Scholar]
- 12. Höhr AIC, Straub SP, Warscheid B, Becker T and Wiedemann N (2015) Assembly of β‐barrel proteins in the mitochondrial outer membrane. Biochim Biophys Acta 1853, 74–88. [DOI] [PubMed] [Google Scholar]
- 13. Diederichs KA, Buchanan SK and Botos I (2021) Building better barrels – β‐barrel biogenesis and insertion in bacteria and mitochondria. J Mol Biol 433, 166894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Doyle MT and Bernstein HD (2024) Molecular machines that facilitate bacterial outer membrane protein biogenesis. Annu Rev Biochem 93, 211–231. [DOI] [PubMed] [Google Scholar]
- 15. Horne JE and Radford SE (2022) Roll out the barrel! Outer membrane tension resolves an unexpected folding intermediate. Cell 185, 1107–1109. [DOI] [PubMed] [Google Scholar]
- 16. Lundquist K, Billings E, Bi M, Wellnitz J and Noinaj N (2021) The assembly of β‐barrel membrane proteins by BAM and SAM. Mol Microbiol 115, 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tomasek D and Kahne D (2021) The assembly of β‐barrel outer membrane proteins. Curr Opin Microbiol 60, 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gentle I, Gabriel K, Beech P, Waller R and Lithgow T (2003) The Omp85 family of proteins is essential for outer membrane biogenesis in mitochondria and bacteria. J Cell Biol 164, 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kozjak V, Wiedemann N, Milenkovic D, Lohaus C, Meyer HE, Guiard B, Meisinger C and Pfanner N (2003) An essential role of Sam50 in the protein sorting and assembly machinery of the mitochondrial outer membrane. J Biol Chem 278, 48520–48523. [DOI] [PubMed] [Google Scholar]
- 20. Busto JV, Ganesan I, Mathar H, Steiert C, Schneider EF, Straub SP, Ellenrieder L, Song J, Stiller SB, Lübbert P et al. (2024) Role of the small protein Mco6 in the mitochondrial sorting and assembly machinery. Cell Rep 43, 113805. [DOI] [PubMed] [Google Scholar]
- 21. Ishikawa D, Yamamoto H, Tamura Y, Moritoh K and Endo T (2004) Two novel proteins in the mitochondrial outer membrane mediate β‐barrel protein assembly. J Cell Biol 166, 621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meisinger C, Rissler M, Chacinska A, Szklarz LKS, Milenkovic D, Kozjak V, Schönfisch B, Lohaus C, Meyer HE, Yaffe MP et al. (2004) The mitochondrial morphology protein Mdm10 functions in assembly of the preprotein translocase of the outer membrane. Dev Cell 7, 61–71. [DOI] [PubMed] [Google Scholar]
- 23. Milenkovic D, Kozjak V, Wiedemann N, Lohaus C, Meyer HE, Guiard B, Pfanner N and Meisinger C (2004) Sam35 of the mitochondrial protein sorting and assembly machinery is a peripheral outer membrane protein essential for cell viability. J Biol Chem 279, 22781–22785. [DOI] [PubMed] [Google Scholar]
- 24. Waizenegger T, Habib SJ, Lech M, Mokranjac D, Paschen SA, Hell K, Neupert W and Rapaport D (2004) Tob38, a novel essential component in the biogenesis of β‐barrel proteins of mitochondria. EMBO Rep 5, 704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Armstrong LC, Komiya T, Bergman BE, Mihara K and Bornstein P (1997) Metaxin is a component of a preprotein import complex in the outer membrane of the mammalian mitochondrion. J Biol Chem 272, 6510–6518. [DOI] [PubMed] [Google Scholar]
- 26. Huynen MA, Mühlmeister M, Gotthardt K, Guerrero‐Castillo S and Brandt U (2016) Evolution and structural organization of the mitochondrial contact site (MICOS) complex and the mitochondrial intermembrane space bridging (MIB) complex. Biochim Biophys Acta 1863, 91–101. [DOI] [PubMed] [Google Scholar]
- 27. Kozjak‐Pavlovic V, Ross K, Benlasfer N, Kimmig S, Karlas A and Rudel T (2007) Conserved roles of Sam50 and metaxins in VDAC biogenesis. EMBO Rep 8, 576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jores T, Lawatscheck J, Beke V, Franz‐Wachtel M, Yunoki K, Fitzgerald JC, Macek B, Endo T, Kalbacher H, Buchner J et al. (2018) Cytosolic Hsp70 and Hsp40 chaperones enable the biogenesis of mitochondrial β‐barrel proteins. J Cell Biol 217, 3091–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Keil P, Weinzierl A, Kiebler M, Dietmeier K, Söllner T and Pfanner N (1993) Biogenesis of the mitochondrial receptor complex. Two receptors are required for binding of MOM38 to the outer membrane surface. J Biol Chem 268, 19177–19180. [PubMed] [Google Scholar]
- 30. Krimmer T, Rapaport D, Ryan MT, Meisinger C, Kassenbrock CK, Blachly‐Dyson E, Forte M, Douglas MG, Neupert W, Nargang FE et al. (2001) Biogenesis of porin of the outer mitochondrial membrane involves an import pathway via receptors and the general import pore of the tom complex. J Cell Biol 152, 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Model K, Meisinger C, Prinz T, Wiedemann N, Truscott KN, Pfanner N and Ryan MT (2001) Multistep assembly of the protein import channel of the mitochondrial outer membrane. Nat Struct Biol 8, 361–370. [DOI] [PubMed] [Google Scholar]
- 32. Jores T, Klinger A, Groß LE, Kawano S, Flinner N, Duchardt‐Ferner E, Wöhnert J, Kalbacher H, Endo T, Schleiff E et al. (2016) Characterization of the targeting signal in mitochondrial β‐barrel proteins. Nat Commun 7, 12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moitra A, Tiku V and Rapaport D (2024) Yeast mitochondria can process de novo designed β‐barrel proteins. FEBS J 291, 292–307. [DOI] [PubMed] [Google Scholar]
- 34. Shiota T, Imai K, Qiu J, Hewitt VL, Tan K, Shen H‐H, Sakiyama N, Fukasawa Y, Hayat S, Kamiya M et al. (2015) Molecular architecture of the active mitochondrial protein gate. Science 349, 1544–1548. [DOI] [PubMed] [Google Scholar]
- 35. Hoppins SC and Nargang FE (2004) The Tim8‐Tim13 complex of Neurospora crassa functions in the assembly of proteins into both mitochondrial membranes. J Biol Chem 279, 12396–12405. [DOI] [PubMed] [Google Scholar]
- 36. Weinhäupl K, Lindau C, Hessel A, Wang Y, Schütze C, Jores T, Melchionda L, Schönfisch B, Kalbacher H, Bersch B et al. (2018) Structural basis of membrane protein chaperoning through the mitochondrial intermembrane space. Cell 175, 1365–1379.e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wiedemann N, Frazier AE and Pfanner N (2004) The protein import machinery of mitochondria. J Biol Chem 279, 14473–14476. [DOI] [PubMed] [Google Scholar]
- 38. Beverly KN, Sawaya MR, Schmid E and Koehler CM (2008) The Tim8–Tim13 complex has multiple substrate binding sites and binds cooperatively to Tim23. J Mol Biol 382, 1144–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Webb CT, Gorman MA, Lazarou M, Ryan MT and Gulbis JM (2006) Crystal structure of the mitochondrial chaperone TIM9•10 reveals a six‐bladed α‐propeller. Mol Cell 21, 123–133. [DOI] [PubMed] [Google Scholar]
- 40. Sučec I, Wang Y, Dakhlaoui O, Weinhäupl K, Jores T, Costa D, Hessel A, Brennich M, Rapaport D, Lindorff‐Larsen K et al. (2020) Structural basis of client specificity in mitochondrial membrane‐protein chaperones. Sci Adv 6, eabd0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kutik S, Stojanovski D, Becker L, Becker T, Meinecke M, Krüger V, Prinz C, Meisinger C, Guiard B, Wagner R et al. (2008) Dissecting membrane insertion of mitochondrial β‐barrel proteins. Cell 132, 1011–1024. [DOI] [PubMed] [Google Scholar]
- 42. Höhr AIC, Lindau C, Wirth C, Qiu J, Stroud DA, Kutik S, Guiard B, Hunte C, Becker T, Pfanner N et al. (2018) Membrane protein insertion through a mitochondrial β‐barrel gate. Science 359, eaah6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Diederichs KA, Ni X, Rollauer SE, Botos I, Tan X, King MS, Kunji ERS, Jiang J and Buchanan SK (2020) Structural insight into mitochondrial β‐barrel outer membrane protein biogenesis. Nat Commun 11, 3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Doerner PA and Sousa MC (2017) Extreme dynamics in the BamA β‐barrel seam. Biochemistry 56, 3142–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Noinaj N, Kuszak AJ, Balusek C, Gumbart JC and Buchanan SK (2014) Lateral opening and exit pore formation are required for BamA function. Structure 22, 1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Takeda H, Tsutsumi A, Nishizawa T, Lindau C, Busto JV, Wenz L‐S, Ellenrieder L, Imai K, Straub SP, Mossmann W et al. (2021) Mitochondrial sorting and assembly machinery operates by β‐barrel switching. Nature 590, 163–169. [DOI] [PubMed] [Google Scholar]
- 47. Albrecht R, Schütz M, Oberhettinger P, Faulstich M, Bermejo I, Rudel T, Diederichs K and Zeth K (2014) Structure of BamA, an essential factor in outer membrane protein biogenesis. Acta Crystallogr D Biol Crystallogr 70, 1779–1789. [DOI] [PubMed] [Google Scholar]
- 48. Iadanza MG, Schiffrin B, White P, Watson MA, Horne JE, Higgins AJ, Calabrese AN, Brockwell DJ, Tuma R, Kalli AC et al. (2020) Distortion of the bilayer and dynamics of the BAM complex in lipid nanodiscs. Commun Biol 3, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu J and Gumbart JC (2020) Membrane thinning and lateral gating are consistent features of BamA across multiple species. PLoS Comput Biol 16, e1008355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Noinaj N, Kuszak AJ, Gumbart JC, Lukacik P, Chang H, Easley NC, Lithgow T and Buchanan SK (2013) Structural insight into the biogenesis of β‐barrel membrane proteins. Nature 501, 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schiffrin B, Calabrese AN, Higgins AJ, Humes JR, Ashcroft AE, Kalli AC, Brockwell DJ and Radford SE (2017) Effects of periplasmic chaperones and membrane thickness on BamA‐catalyzed outer‐membrane protein folding. J Mol Biol 429, 3776–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wu X and Rapoport TA (2021) Translocation of proteins through a distorted lipid bilayer. Trends Cell Biol 31, 473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stubenrauch C, Belousoff MJ, Hay ID, Shen H‐H, Lillington J, Tuck KL, Peters KM, Phan M‐D, Lo AW, Schembri MA et al. (2016) Effective assembly of fimbriae in Escherichia coli depends on the translocation assembly module nanomachine. Nat Microbiol 1, 1–8. [DOI] [PubMed] [Google Scholar]
- 54. Takeda H, Busto JV, Lindau C, Tsutsumi A, Tomii K, Imai K, Yamamori Y, Hirokawa T, Motono C, Ganesan I et al. (2023) A multipoint guidance mechanism for β‐barrel folding on the SAM complex. Nat Struct Mol Biol 30, 176–187. [DOI] [PubMed] [Google Scholar]
- 55. Bernstein HD (2015) Looks can be deceiving: recent insights into the mechanism of protein secretion by the autotransporter pathway. Mol Microbiol 97, 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Doyle MT and Bernstein HD (2021) BamA forms a translocation channel for polypeptide export across the bacterial outer membrane. Mol Cell 81, 2000–2012.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Doyle MT, Jimah JR, Dowdy T, Ohlemacher SI, Larion M, Hinshaw JE and Bernstein HD (2022) Cryo‐EM structures reveal multiple stages of bacterial outer membrane protein folding. Cell 185, 1143–1156.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shen C, Chang S, Luo Q, Chan KC, Zhang Z, Luo B, Xie T, Lu G, Zhu X, Wei X et al. (2023) Structural basis of BAM‐mediated outer membrane β‐barrel protein assembly. Nature 617, 185–193. [DOI] [PubMed] [Google Scholar]
- 59. Hagan CL, Kim S and Kahne D (2010) Reconstitution of outer membrane protein assembly from purified components. Science 328, 890–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kim S, Malinverni JC, Sliz P, Silhavy TJ, Harrison SC and Kahne D (2007) Structure and function of an essential component of the outer membrane protein assembly machine. Science 317, 961–964. [DOI] [PubMed] [Google Scholar]
- 61. Sánchez‐Pulido L, Devos D, Genevrois S, Vicente M and Valencia A (2003) POTRA: a conserved domain in the FtsQ family and a class of β‐barrel outer membrane proteins. Trends Biochem Sci 28, 523–526. [DOI] [PubMed] [Google Scholar]
- 62. Habib SJ, Waizenegger T, Niewienda A, Paschen SA, Neupert W and Rapaport D (2006) The N‐terminal domain of Tob55 has a receptor‐like function in the biogenesis of mitochondrial β‐barrel proteins. J Cell Biol 176, 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ma X, Wang Q, Li Y, Tan P, Wu H, Wang P, Dong X, Hong L and Meng G (2019) How BamA recruits OMP substrates via poly‐POTRAs domain. FASEB J 33, 14690–14702. [DOI] [PubMed] [Google Scholar]
- 64. Bos MP, Robert V and Tommassen J (2007) Functioning of outer membrane protein assembly factor Omp85 requires a single POTRA domain. EMBO Rep 8, 1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stroud DA, Becker T, Qiu J, Stojanovski D, Pfannschmidt S, Wirth C, Hunte C, Guiard B, Meisinger C, Pfanner N et al. (2011) Biogenesis of mitochondrial β‐barrel proteins: the POTRA domain is involved in precursor release from the SAM complex. Mol Biol Cell 22, 2823–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gratzer S, Lithgow T, Bauer RE, Lamping E, Paltauf F, Kohlwein SD, Haucke V, Junne T, Schatz G and Horst M (1995) Mas37p, a novel receptor subunit for protein import into mitochondria. J Cell Biol 129, 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang Q, Guan Z, Qi L, Zhuang J, Wang C, Hong S, Yan L, Wu Y, Cao X, Cao J et al. (2021) Structural insight into the SAM‐mediated assembly of the mitochondrial TOM core complex. Science 373, 1377–1381. [DOI] [PubMed] [Google Scholar]
- 68. Chan NC and Lithgow T (2008) The peripheral membrane subunits of the SAM complex function codependently in mitochondrial outer membrane biogenesis. Mol Biol Cell 19, 126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hart EM, Gupta M, Wühr M and Silhavy TJ (2020) The gain‐of‐function allele bamAE470K bypasses the essential requirement for BamD in β‐barrel outer membrane protein assembly. Proc Natl Acad Sci USA 117, 18737–18743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lee J, Sutterlin HA, Wzorek JS, Mandler MD, Hagan CL, Grabowicz M, Tomasek D, May MD, Hart EM, Silhavy TJ et al. (2018) Substrate binding to BamD triggers a conformational change in BamA to control membrane insertion. Proc Natl Acad Sci USA 115, 2359–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Araiso Y, Tsutsumi A, Qiu J, Imai K, Shiota T, Song J, Lindau C, Wenz L‐S, Sakaue H, Yunoki K et al. (2019) Structure of the mitochondrial import gate reveals distinct preprotein paths. Nature 575, 395–401. [DOI] [PubMed] [Google Scholar]
- 72. Bayrhuber M, Meins T, Habeck M, Becker S, Giller K, Villinger S, Vonrhein C, Griesinger C, Zweckstetter M and Zeth K (2008) Structure of the human voltage‐dependent anion channel. Proc Natl Acad Sci USA 105, 15370–15375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hiller S, Garces RG, Malia TJ, Orekhov VY, Colombini M and Wagner G (2008) Solution structure of the integral human membrane protein VDAC‐1 in detergent micelles. Science 321, 1206–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tucker K and Park E (2019) Cryo‐EM structure of the mitochondrial protein‐import channel TOM complex at near‐atomic resolution. Nat Struct Mol Biol 26, 1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Becker T, Horvath SE, Böttinger L, Gebert N, Daum G and Pfanner N (2013) Role of phosphatidylethanolamine in the biogenesis of mitochondrial outer membrane proteins. J Biol Chem 288, 16451–16459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gebert N, Joshi AS, Kutik S, Becker T, McKenzie M, Guan XL, Mooga VP, Stroud DA, Kulkarni G, Wenk MR et al. (2009) Mitochondrial cardiolipin involved in outer‐membrane protein biogenesis: implications for Barth syndrome. Curr Biol 19, 2133–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schuler M‐H, Di Bartolomeo F, Böttinger L, Horvath SE, Wenz L‐S, Daum G and Becker T (2015) Phosphatidylcholine affects the role of the sorting and assembly machinery in the biogenesis of mitochondrial β‐barrel proteins. J Biol Chem 290, 26523–26532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bausewein T, Mills DJ, Langer JD, Nitschke B, Nussberger S and Kühlbrandt W (2017) Cryo‐EM structure of the TOM core complex from Neurospora crassa . Cell 170, 693–700.e7. [DOI] [PubMed] [Google Scholar]
- 79. Guan Z, Yan L, Wang Q, Qi L, Hong S, Gong Z, Yan C and Yin P (2021) Structural insights into assembly of human mitochondrial translocase TOM complex. Cell Discov 7, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang W, Chen X, Zhang L, Yi J, Ma Q, Yin J, Zhuo W, Gu J and Yang M (2020) Atomic structure of human TOM core complex. Cell Discov 6, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Becker T, Guiard B, Thornton N, Zufall N, Stroud DA, Wiedemann N and Pfanner N (2010) Assembly of the mitochondrial protein import channel. Mol Biol Cell 21, 3106–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Thornton N, Stroud DA, Milenkovic D, Guiard B, Pfanner N and Becker T (2010) Two modular forms of the mitochondrial sorting and assembly machinery are involved in biogenesis of α‐helical outer membrane proteins. J Mol Biol 396, 540–549. [DOI] [PubMed] [Google Scholar]
- 83. Yamano K, Tanaka‐Yamano S and Endo T (2010) Mdm10 as a dynamic constituent of the TOB/SAM complex directs coordinated assembly of Tom40. EMBO Rep 11, 187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Flinner N, Ellenrieder L, Stiller SB, Becker T, Schleiff E and Mirus O (2013) Mdm10 is an ancient eukaryotic porin co‐occurring with the ERMES complex. Biochim Biophys Acta 1833, 3314–3325. [DOI] [PubMed] [Google Scholar]
- 85. Meisinger C, Wiedemann N, Rissler M, Strub A, Milenkovic D, Schönfisch B, Müller H, Kozjak V and Pfanner N (2006) Mitochondrial protein sorting: differentiation of β‐barrel assembly by Tom7‐mediated segregation of Mdm10. J Biol Chem 281, 22819–22826. [DOI] [PubMed] [Google Scholar]
- 86. Yamano K, Tanaka‐Yamano S and Endo T (2010) Tom7 regulates Mdm10‐mediated assembly of the mitochondrial import channel protein Tom40. J Biol Chem 285, 41222–41231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Qiu J, Wenz L‐S, Zerbes RM, Oeljeklaus S, Bohnert M, Stroud DA, Wirth C, Ellenrieder L, Thornton N, Kutik S et al. (2013) Coupling of mitochondrial import and export translocases by receptor‐mediated Supercomplex formation. Cell 154, 596–608. [DOI] [PubMed] [Google Scholar]
- 88. Wenz L‐S, Ellenrieder L, Qiu J, Bohnert M, Zufall N, van der Laan M, Pfanner N, Wiedemann N and Becker T (2015) Sam37 is crucial for formation of the mitochondrial TOM–SAM supercomplex, thereby promoting β‐barrel biogenesis. J Cell Biol 210, 1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhao Y, Song E, Wang W, Hsieh C‐H, Wang X, Feng W, Wang X and Shen K (2021) Metaxins are core components of mitochondrial transport adaptor complexes. Nat Commun 12, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Becker T, Pfannschmidt S, Guiard B, Stojanovski D, Milenkovic D, Kutik S, Pfanner N, Meisinger C and Wiedemann N (2008) Biogenesis of the mitochondrial TOM complex: Mim1 promotes insertion and assembly of signal‐anchored receptors. J Biol Chem 283, 120–127. [DOI] [PubMed] [Google Scholar]
- 91. Hulett JM, Lueder F, Chan NC, Perry AJ, Wolynec P, Likić VA, Gooley PR and Lithgow T (2008) The transmembrane segment of Tom20 is recognized by Mim1 for docking to the mitochondrial TOM complex. J Mol Biol 376, 694–704. [DOI] [PubMed] [Google Scholar]
- 92. Popov‐Čeleketić J, Waizenegger T and Rapaport D (2008) Mim1 functions in an oligomeric form to facilitate the integration of Tom20 into the mitochondrial outer membrane. J Mol Biol 376, 671–680. [DOI] [PubMed] [Google Scholar]
- 93. Dimmer KS, Papić D, Schumann B, Sperl D, Krumpe K, Walther DM and Rapaport D (2012) A crucial role for Mim2 in the biogenesis of mitochondrial outer membrane proteins. J Cell Sci 125, 3464–3473. [DOI] [PubMed] [Google Scholar]
- 94. Doan KN, Grevel A, Mårtensson CU, Ellenrieder L, Thornton N, Wenz L‐S, Opaliński Ł, Guiard B, Pfanner N and Becker T (2020) The mitochondrial import complex MIM functions as main translocase for α‐helical outer membrane proteins. Cell Rep 31, 107567. [DOI] [PubMed] [Google Scholar]
- 95. Krüger V, Becker T, Becker L, Montilla‐Martinez M, Ellenrieder L, Vögtle F‐N, Meyer HE, Ryan MT, Wiedemann N, Warscheid B et al. (2017) Identification of new channels by systematic analysis of the mitochondrial outer membrane. J Cell Biol 216, 3485–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Vitali DG, Drwesh L, Cichocki BA, Kolb A and Rapaport D (2020) The biogenesis of mitochondrial outer membrane proteins show variable dependence on import factors. iScience 23, 100779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Stojanovski D, Guiard B, Kozjak‐Pavlovic V, Pfanner N and Meisinger C (2007) Alternative function for the mitochondrial SAM complex in biogenesis of α‐helical TOM proteins. J Cell Biol 179, 881–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Harner M, Körner C, Walther D, Mokranjac D, Kaesmacher J, Welsch U, Griffith J, Mann M, Reggiori F and Neupert W (2011) The mitochondrial contact site complex, a determinant of mitochondrial architecture. EMBO J 30, 4356–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hoppins S, Collins SR, Cassidy‐Stone A, Hummel E, DeVay RM, Lackner LL, Westermann B, Schuldiner M, Weissman JS and Nunnari J (2011) A mitochondrial‐focused genetic interaction map reveals a scaffold‐like complex required for inner membrane organization in mitochondria. J Cell Biol 195, 323–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ott C, Ross K, Straub S, Thiede B, Götz M, Goosmann C, Krischke M, Mueller MJ, Krohne G, Rudel T et al. (2012) Sam50 functions in mitochondrial intermembrane space bridging and biogenesis of respiratory complexes. Mol Cell Biol 32, 1173–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rabl R, Soubannier V, Scholz R, Vogel F, Mendl N, Vasiljev‐Neumeyer A, Körner C, Jagasia R, Keil T, Baumeister W et al. (2009) Formation of cristae and crista junctions in mitochondria depends on antagonism between Fcj1 and Su e/g. J Cell Biol 185, 1047–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. von der Malsburg K, Müller JM, Bohnert M, Oeljeklaus S, Kwiatkowska P, Becker T, Loniewska‐Lwowska A, Wiese S, Rao S, Milenkovic D et al. (2011) Dual role of Mitofilin in mitochondrial membrane organization and protein biogenesis. Dev Cell 21, 694–707. [DOI] [PubMed] [Google Scholar]
- 103. Bohnert M, Wenz L‐S, Zerbes RM, Horvath SE, Stroud DA, von der Malsburg K, Müller JM, Oeljeklaus S, Perschil I, Warscheid B et al. (2012) Role of mitochondrial inner membrane organizing system in protein biogenesis of the mitochondrial outer membrane. Mol Biol Cell 23, 3948–3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zerbes RM, Bohnert M, Stroud DA, von der Malsburg K, Kram A, Oeljeklaus S, Warscheid B, Becker T, Wiedemann N, Veenhuis M et al. (2012) Role of MINOS in mitochondrial membrane architecture: cristae morphology and outer membrane interactions differentially depend on Mitofilin domains. J Mol Biol 422, 183–191. [DOI] [PubMed] [Google Scholar]
- 105. Barbot M, Jans DC, Schulz C, Denkert N, Kroppen B, Hoppert M, Jakobs S and Meinecke M (2015) Mic10 oligomerizes to bend mitochondrial inner membranes at cristae junctions. Cell Metab 21, 756–763. [DOI] [PubMed] [Google Scholar]
- 106. Bohnert M, Zerbes RM, Davies KM, Mühleip AW, Rampelt H, Horvath SE, Boenke T, Kram A, Perschil I, Veenhuis M et al. (2015) Central role of Mic10 in the mitochondrial contact site and cristae organizing system. Cell Metab 21, 747–755. [DOI] [PubMed] [Google Scholar]
- 107. Bock‐Bierbaum T, Funck K, Wollweber F, Lisicki E, von der Malsburg K, von der Malsburg A, Laborenz J, Noel JK, Hessenberger M, Jungbluth S et al. (2022) Structural insights into crista junction formation by the Mic60‐Mic19 complex. Sci Adv 8, eabo4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Meisinger C, Pfannschmidt S, Rissler M, Milenkovic D, Becker T, Stojanovski D, Youngman MJ, Jensen RE, Chacinska A, Guiard B et al. (2007) The morphology proteins Mdm12/Mmm1 function in the major β‐barrel assembly pathway of mitochondria. EMBO J 26, 2229–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kojima R, Endo T and Tamura Y (2016) A phospholipid transfer function of ER‐mitochondria encounter structure revealed in vitro. Sci Rep 6, 30777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS and Walter P (2009) An ER‐mitochondria tethering complex revealed by a synthetic biology screen. Science 325, 477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wozny MR, Di Luca A, Morado DR, Picco A, Khaddaj R, Campomanes P, Ivanović L, Hoffmann PC, Miller EA, Vanni S et al. (2023) In situ architecture of the ER–mitochondria encounter structure. Nature 618, 188–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Jeong H, Park J, Jun Y and Lee C (2017) Crystal structures of Mmm1 and Mdm12–Mmm1 reveal mechanistic insight into phospholipid trafficking at ER‐mitochondria contact sites. Proc Natl Acad Sci USA 114, E9502–E9511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kawano S, Tamura Y, Kojima R, Bala S, Asai E, Michel AH, Kornmann B, Riezman I, Riezman H, Sakae Y et al. (2017) Structure–function insights into direct lipid transfer between membranes by Mmm1–Mdm12 of ERMES. J Cell Biol 217, 959–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Rasul F, Zheng F, Dong F, He J, Liu L, Liu W, Cheema JY, Wei W and Fu C (2021) Emr1 regulates the number of foci of the endoplasmic reticulum‐mitochondria encounter structure complex. Nat Commun 12, 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Schleiff E, Silvius JR and Shore GC (1999) Direct membrane insertion of voltage‐dependent Anion‐selective Channel protein catalyzed by mitochondrial Tom20. J Cell Biol 145, 973–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Tamm LK, Arora A and Kleinschmidt JH (2001) Structure and assembly of β‐barrel membrane proteins. J Biol Chem 276, 32399–32402. [DOI] [PubMed] [Google Scholar]
- 117. Guna A, Stevens TA, Inglis AJ, Replogle JM, Esantsi TK, Muthukumar G, Shaffer KCL, Wang ML, Pogson AN, Jones JJ et al. (2022) MTCH2 is a mitochondrial outer membrane protein insertase. Science 378, 317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ulrich T and Rapaport D (2015) Biogenesis of beta‐barrel proteins in evolutionary context. Int J Med Microbiol 305, 259–264. [DOI] [PubMed] [Google Scholar]
- 119. Walther DM, Papic D, Bos MP, Tommassen J and Rapaport D (2009) Signals in bacterial β‐barrel proteins are functional in eukaryotic cells for targeting to and assembly in mitochondria. Proc Natl Acad Sci USA 106, 2531–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Walther DM, Bos MP, Rapaport D and Tommassen J (2010) The mitochondrial porin, VDAC, has retained the ability to be assembled in the bacterial outer membrane. Mol Biol Evol 27, 887–895. [DOI] [PubMed] [Google Scholar]
- 121. Baker MJ, Webb CT, Stroud DA, Palmer CS, Frazier AE, Guiard B, Chacinska A, Gulbis JM and Ryan MT (2009) Structural and functional requirements for activity of the Tim9–Tim10 complex in mitochondrial protein import. Mol Biol Cell 20, 769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]


