Abstract
Background
The key endpoints for the assessment of the effect of maintenance therapy for metastatic colorectal cancer (mCRC) are survival and quality-of-life outcomes. We aimed to compare dermatology-related quality of life (DRQOL) in patients with RAS wild-type (wt) mCRC treated with fluorouracil and folinic acid (FU/FA) + panitumumab (Pmab) versus FU/FA alone as maintenance therapy after folinic acid, fluorouracil and oxaliplatin + Pmab induction.
Patients and methods
The phase II randomized PanaMa (AIO KRK 0212; NCT01991873) trial included 387 patients at 70 community/academic sites in Germany. For this prespecified secondary analysis, DRQOL outcomes were assessed using the Functional Assessment of Cancer Therapy-epidermal growth factor receptor inhibitor (FACT-EGFRI), Dermatology Life Quality Index (DLQI), and Skindex-16 questionnaires at every second cycle of therapy until disease progression/death.
Results
At least one DRQOL questionnaire was completed by a total of 310/377 (82%) patients who received induction therapy, and by 216/248 (87%) patients who were randomized and received maintenance therapy. Patients who experienced skin toxicity according to the National Cancer Institute (NCI)-Common Terminology Criteria for Adverse Events (CTCAE) during induction therapy had significantly worse DRQOL according to all three measures, compared to those who did not [i.e. Skindex-16, mean difference at cycle 2 −12.87; 95% confidence interval (CI) −20.01 to −5.73; P < 0.001]. During maintenance therapy, significantly improved recovery was observed in all DRQOL measures for patients receiving FU/FA, compared to those receiving additional Pmab (i.e. Skindex-16, mean difference at cycle 6 −16.53; 95% CI −22.68 to −10.38; P < 0.001).
Conclusions
In this secondary analysis of a phase II randomized clinical trial, patient-reported DRQOL outcomes correlated with skin toxicity according to NCI-CTCAE during induction therapy. Maintenance therapy with FU/FA + Pmab was associated with deteriorated DRQOL versus FU/FA alone in patients with RAS wt mCRC.
Key words: colorectal cancer, quality of life, patient-reported outcomes, anti-EGFR, maintenance therapy
Highlights
-
•
We investigated the longitudinal impact of additional maintenance therapy with Pmab on DRQOL.
-
•
During induction therapy, skin toxicity as NCI-CTCAE correlated with DRQOL outcomes.
-
•
Maintenance therapy with additional Pmab was inferior in terms of DRQOL outcomes according to multiple validated measures.
-
•
Choice of QOL questionnaires in clinical trial design should take into account expected drug toxicity profiles.
Introduction
In patients with left-sided, RAS wild-type (wt) metastatic colorectal cancer (mCRC), doublet combination chemotherapy with fluorouracil and folinic acid (FU/FA) plus either oxaliplatin (folinic acid, fluorouracil and oxaliplatin [FOLFOX]) or irinotecan (FOLFIRI) in addition to an anti-epidermal growth factor receptor (EGFR) antibody (i.e. cetuximab or panitumumab; Pmab) is a standard-of-care first-line therapy.1,2 In patients for whom secondary resection of metastases is not an option and who respond well to systemic therapy (i.e. at least stable disease or better), continuous doublet chemotherapy is often hampered due to cumulative toxicities, especially in oxaliplatin-based regimens,3,4 underlining the need for maintenance strategies in this patient population.1,5, 6, 7, 8 Regarding efficacy endpoints, available analyses of VALENTINO and PanaMa trials suggest that maintenance therapy after induction therapy with FOLFOX plus Pmab might ideally be continued with FU/FA plus Pmab.1,9, 10, 11 Of note, the PanaMa trial randomized patients after a 3-month induction therapy with FOLFOX plus Pmab in a 1 : 1 fashion to receive maintenance therapy with either FU/FA plus Pmab or FU/FA alone.10
Besides efficacy endpoints, patient-reported outcomes (PROs) of health-related quality of life (HRQOL) are endpoints of increasing importance.12, 13, 14 However, adequate evaluation of HRQOL endpoints may be confined by the choice of the appropriate HRQOL questionnaire. As such, previous HRQOL analyses of patients treated in the PanaMa trial using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30)15 described significant recovery of HRQOL dimensions during maintenance therapy, after initial deterioration during induction therapy. However, no significant differences in HRQOL were detected between the randomized treatment (i.e. FU/FA plus Pmab versus FU/FA) arms of the trial.16
Therefore, taking the specific profile of the investigated drug (panitumumab) into account, this prespecified secondary analysis of the trial aims to evaluate if classic toxicity assessments by the National Cancer Institute (NCI)-Common Terminology Criteria for Adverse Events (CTCAE) are mirrored by dermatology-related quality of life (DRQOL) assessment using Functional Assessment of Cancer Therapy-epidermal growth factor receptor inhibitor (FACT-EGFRI),17,18 Dermatology Life Quality Index (DLQI),19 and Skindex-16.20 Secondly, this investigation aimed to explore whether DRQOL was affected by the respective randomized treatment sequences in the trial (i.e. use of Pmab during the maintenance therapy part). To answer these questions, DRQOL was analyzed across all patients during the 3 months of induction therapy and stratified by treatment arm during the subsequent maintenance therapy.
Patients and methods
Study design and participants
This investigation represents a pre-planned analysis of the multi-center, open-label, randomized phase II PanaMa trial (NCT01991873). Patients with RAS wt mCRC and disease control (stable disease or partial or complete remission) after first-line induction therapy with six cycles of FOLFOX plus Pmab were randomly assigned to receive maintenance treatment with FU/FA ± Pmab. The primary endpoint was progression-free survival (time from random assignment until progression or death). Detailed methods of the study, along with efficacy and safety results, have been reported previously.10 Briefly, eligible patients had RAS wt mCRC (KRAS and NRAS exons 2-4), an Eastern Cooperative Oncology Group (ECOG) performance status of 0-1, no previous chemotherapy for metastatic disease, with the exception of one application of FOLFOX in patients in need of treatment while waiting for the result of RAS testing, measurable disease based on Response Evaluation Criteria in Solid tumors (RECIST) version 1.1 criteria, and adequate organ function. Key exclusion criteria included untreated central nervous system lesions and a <6-month interval after end of adjuvant treatment for colorectal cancer. The study protocol and its amendments were approved by an independent institutional review board or ethics committee at each study site. The study was conducted in compliance with Good Clinical Practice guidelines and the Declaration of Helsinki. All patients provided written, informed consent. The trial is registered with ClinicalTrials.gov (NCT01991873).
PROs of DRQOL and statistical analysis
DRQOL was assessed using three distinct questionnaires: the FACT-EGFRI,17,18 DLQI,19 and Skindex-16.20 These instruments were selected due to their validity and reliability in capturing the dermatologic symptoms and quality of life.
The FACT-EGFRI is a 22-item questionnaire that comprises several domains, including physical well-being, social/family well-being, emotional well-being, and functional well-being. It includes a specific subscale addressing skin-related symptoms.17,18 An increase in skin-related symptoms is represented by a decrease in the score. The DLQI is a widely used 10-item questionnaire designed to assess the impact of skin conditions on quality of life, encompassing domains such as symptoms and feelings, daily activities, leisure, work or school, personal relationships, and treatment.19 An increase in skin-related symptoms is represented by an increase in the score. The Skindex-16 is a 16-item questionnaire focusing on the effects of skin diseases on emotions, functioning, and symptoms.20 An increase in skin-related symptoms is represented by an increase in the score. Source data were captured by printed forms that were filled before the respective treatment appointment by patients. The data were transferred electronically into the electronic case report form for every second cycle until disease progression. All patients who received at least one dose of induction or maintenance therapy and completed at least one DRQOL assessment were included into this analysis. DRQOL outcomes were assessed separately for the induction and maintenance study treatment phase. Analysis was carried out according to the presence of dermatologic toxicities and allocated to treatment for induction and maintenance therapy, respectively.
Completion and compliance rates were summarized overall and at baseline, cycle 6 for induction phase, and cycle 6 of maintenance therapy for the maintenance phase, respectively. Completion rate was defined as the number of patients in the DRQOL analysis population who completed at least one item of the DRQOL assessment divided by the number of patients in the DRQOL analysis population. Compliance rate was defined as the number of patients who completed at least one item divided by the number of eligible patients who were expected to complete the HRQOL assessment at the respective time point.
For the induction phase, baseline was defined as DRQOL evaluation before the start of induction therapy, while for the maintenance phase baseline was defined as DRQOL outcomes before the start of maintenance therapy. DRQOL outcomes were mean changes in FACT-EGFRI, DLQI, and Skindex-16 scores from baseline to every second cycle of treatment.
To evaluate the consistency of the patient-reported DRQOL and the investigator-assessed skin toxicity by NCI-CTCAE grading during induction therapy, DRQOL was compared in patients with or without skin toxicity, and by NCI-CTCAE grade (grades 0-3). During maintenance therapy, DRQOL was compared in patients by treatment arm. Fisher’s exact test was used to compare two categorical variables. Kruskal–Wallis one-way analysis of variance was used to compare differences among independent groups of samples. Multiple linear regression analysis was used to test for impact of baseline characteristics and toxicity endpoints on DRQOL outcomes.
All tests were two sided, and P values <0.05 were considered statistically significant. Statistical analyses were carried out using the SPSS 29 software program (SPSS, Chicago, IL), and R, version 4.3.2 (R Foundation for Statistical Computing).
Results
Patient characteristics
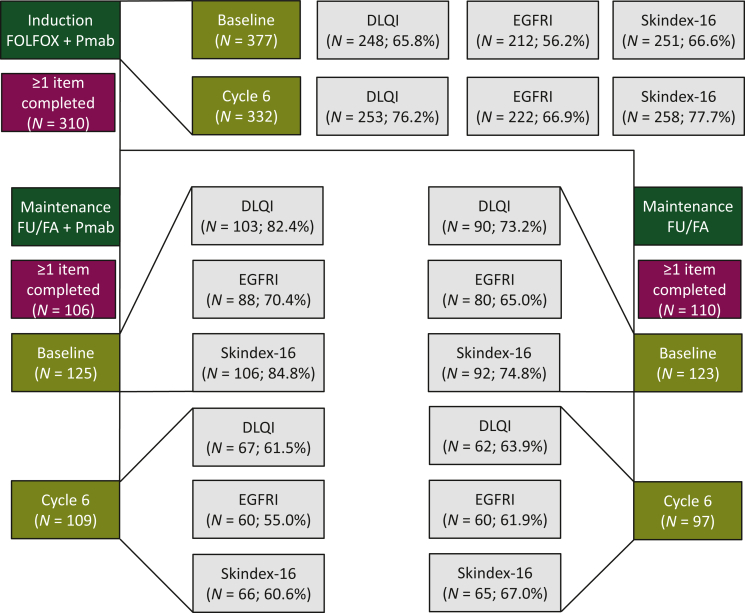
Of 377 patients in the induction group (safety set), 310 (82.2%) patients completed at least one DRQOL assessment and were included in the DRQOL induction analysis population (Figure 1, green and burgundy boxes). Completion rates for the three DRQOL questionnaires ranged from 56.2% (EGFRI) to 66.6% (Skindex-16) at baseline (before cycle 1 of induction therapy) and 66.9% (EGFRI) to 77.7% (Skindex-16) at cycle 6 of induction therapy (Figure 1, olive and gray boxes).
Figure 1.
CONSORT diagram of the study population. Compliance rates (burgundy boxes), and DLQI, FACT-EGFRI and Skindex-16 questionnaire completion rates (gray boxes) are shown for the induction (top panel, olive boxes; baseline and cycle 6 of induction therapy) and maintenance (bottom panel, olive boxes; baseline and cycle 6 of maintenance therapy) population.
DLQI, Dermatology Life Quality Index; FACT-EGFRI, Functional Assessment of Cancer Therapy-epidermal growth factor receptor inhibitor; FU/FA, fluorouracil and folinic acid; Pmab, panitumumab.
Of 248 randomized (and treated in the maintenance part of trial) patients, 106/125 (84.8%) receiving FU/FA plus Pmab maintenance and 110/123 (89.4%) receiving FU/FA maintenance completed at least one DRQOL assessment and were included in the DRQOL maintenance population (n = 216; 87%, Figure 1, green and burgundy boxes). Completion rates for the three DRQOL questionnaires ranged from 65.0% (EGFRI) to 84.8% (Skindex-16) at baseline (before cycle 1 of maintenance therapy) and 55.0% (EGFRI) to 67.0% (Skindex-16) at cycle 6 of induction therapy (Figure 1, olive and gray boxes).
DRQOL outcomes during induction therapy
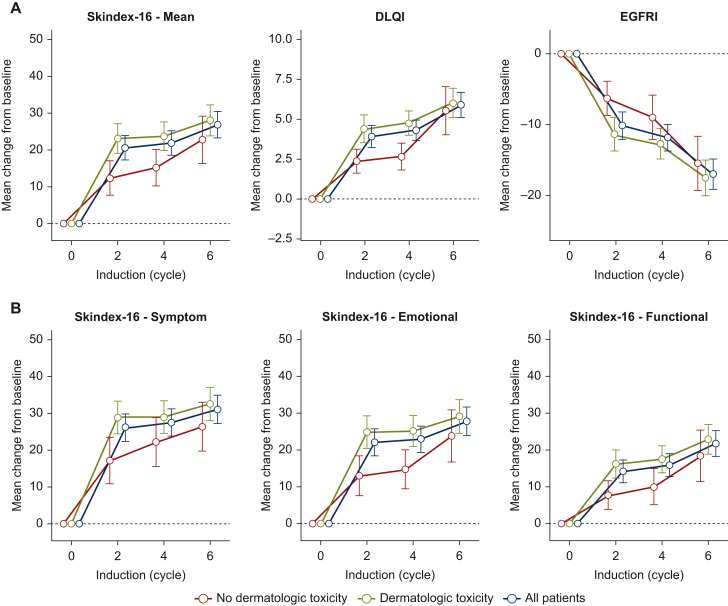
Between baseline (before start of cycle 1 of induction therapy) and cycle 6 of induction therapy, all patients showed significant deterioration in all mean DRQOL scores [Skindex-16, mean change 26.78; 95% confidence interval (CI) 23.24-30.32; P < 0.001; DLQI, mean change 5.90; 95% CI 5.11-6.69; P < 0.001; FACT-EGFRI, mean change –16.99; 95% CI −19.14 to −14.84; P < 0.001, Table 1, first panel; Figure 2A], including Skindex-16 subdomains (Figure 2B).
Table 1.
DRQOL scores during induction therapy
| DRQOL questionnaire | Change from baseline to cycle 6 (induction) |
Baseline (induction) |
Mean difference at cycle 2 (induction) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients (N) | Mean difference | 95% CI (lower, upper) | Two-Sided p | Skin toxicity during induction | Patients (N) | Mean score | Std. deviation | Std. error mean | Two-sided P value | Patients (N) | Mean difference | 95% CI (lower, upper) | Two-sided P value | |||
| Skindex-16 | 258.00 | 26.78 | 23.24 | 30.32 | 0.000 | Yes | 195.00 | 2.95 | 11.08 | 0.79 | 0.020 | 224.00 | −12.87 | −20.01 | −5.73 | 0.000 |
| No | 56.00 | 0.78 | 3.59 | 0.48 | 68.00 | |||||||||||
| Skindex-16: Symptom | 258.00 | 31.07 | 27.22 | 34.93 | 0.000 | Yes | 195.00 | 3.38 | 12.20 | 0.87 | 0.084 | 224.00 | −13.66 | −21.85 | −5.46 | 0.001 |
| No | 56.00 | 1.34 | 5.84 | 0.78 | 68.00 | |||||||||||
| Skindex-16: Emotional | 258.00 | 27.81 | 23.95 | 31.67 | 0.000 | Yes | 195.00 | 3.35 | 12.66 | 0.91 | 0.036 | 224.00 | −14.20 | −22.08 | −6.32 | 0.000 |
| No | 56.00 | 0.98 | 4.95 | 0.66 | 68.00 | |||||||||||
| Skindex-16: Functional | 257.00 | 21.80 | 18.25 | 25.35 | 0.000 | Yes | 194.00 | 2.04 | 9.61 | 0.69 | 0.005 | 224.00 | −10.47 | −15.68 | −5.25 | 0.000 |
| No | 56.00 | 0.06 | 0.45 | 0.06 | 68.00 | |||||||||||
| DLQI | 253.00 | 5.90 | 5.11 | 6.69 | 0.000 | Yes | 194.00 | 0.53 | 1.46 | 0.10 | 0.004 | 222.00 | −2.38 | −3.50 | −1.25 | 0.000 |
| No | 54.00 | 0.17 | 0.50 | 0.07 | 67.00 | |||||||||||
| FACT-EGFRI | 222.00 | −16.99 | −19.14 | −14.84 | 0.000 | Yes | 168.00 | 69.51 | 6.28 | 0.48 | 0.007 | 184.00 | 6.58 | 2.39 | 10.76 | 0.002 |
| No | 44.00 | 71.05 | 1.84 | 0.28 | 55.00 | |||||||||||
First panel: mean changes in DRQOL scores from baseline to cycle 6 of induction therapy. Second panel: mean DRQOL scores at baseline by skin toxicity status (bold: patients experiencing skin toxicity during induction therapy). Third panel: mean differences in DRQOL scores at cycle 2 of induction therapy between patients who experienced skin toxicity during induction therapy versus those who did not.
CI, confidence interval; DLQI, Dermatology Life Quality Index; DRQOL, dermatology-related quality of life; FACT-EGFRI, Functional Assessment of Cancer Therapy-epidermal growth factor receptor inhibitor.
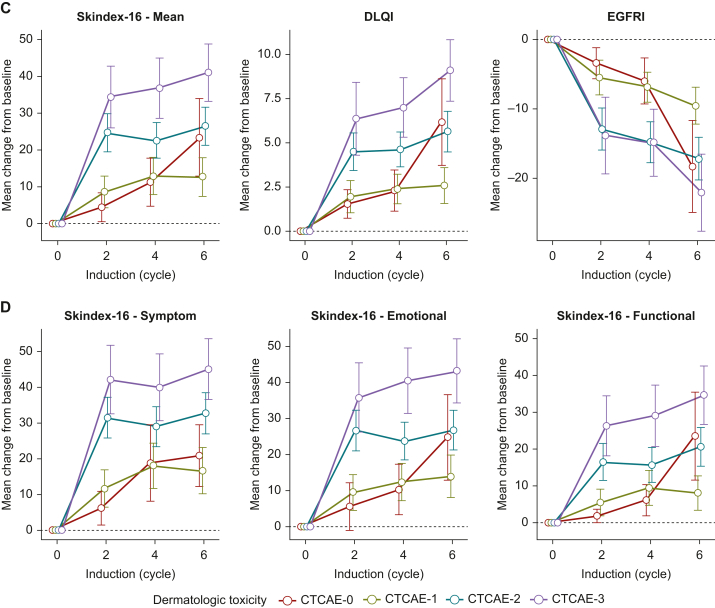
Figure 2.
DRQOL outcomes during induction therapy. Mean changes of (A) Skindex-16, DLQI, and EGFRI scores and (B) Skindex-16 Symptom, Emotional, and Functional subdomains are shown from baseline (before start of induction therapy) for all patients (blue), patients with dermatologic toxicity (olive), and patients without dermatologic toxicity (red) at cycles 2, 4, and 6 of induction therapy. (C) Skindex-16, DLQI, and EGFRI scores and (D) Skindex-16 Symptom, Emotional, and Functional subdomains are shown from baseline (before start of induction therapy) for patients with dermatologic toxicity by CTCAE-grades 0 (red), 1 (olive), 2 (teal), and 3 (violet) at cycles 2, 4, and 6 of induction therapy. Whiskers mark 95% CI.
CI, confidence interval; CTCAE, Common Terminology Criteria for Adverse Events; DLQI, Dermatology Life Quality Index; DRQOL, dermatology-related quality of life; EGFRI, epidermal growth factor receptor inhibitor.
DRQOL outcomes in patients with documented skin toxicity according to NCI-CTCAE assessment
At baseline (before start of cycle 1 of induction therapy), patients who subsequently experienced skin toxicity as an adverse event already presented significantly worse mean DRQOL scores, i.e. Skindex-16 [mean 2.95, standard deviation (SD) 11.08, including Skindex-16 Emotional and Functional subdomains], DLQI (mean 0.53, SD 1.46), and FACT-EGFRI (mean 69.51, SD 6.28), compared to those who did not (Skindex-16 mean 0.78, SD 3.59, P = 0.020; DLQI mean 0.17, SD 0.50, P = 0.004; FACT-EGFRI mean 71.05, SD 1.84, P = 0.007, Table 1, second panel). Across skin toxicity grades 0-3, the distribution of all DRQOL scores, except DLQI, did not show significant differences at baseline (before start of cycle 1 of induction therapy; Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.103628, first panel). Of note, differences between groups remained under the minimal clinically important difference threshold for all three questionnaires.
During cycles 2 and 4 of induction therapy, patients who experienced skin toxicity continued to present significantly deteriorated mean DRQOL scores, compared to those who did not (i.e. Skindex-16 at cycle 2, mean difference −12.87, 95% CI −18.69 to −7.05, P < 0.001; DLQI at cycle 2, mean difference −2.38, 95% CI −3.50 to −1.25, P < 0.001; FACT-EGFRI at cycle 2, mean difference 6.58, 95% CI 3.40-9.76, P < 0.001; Table 1, third panel; Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103628, first and second panel; Figure 2A), including Skindex-16 subdomains (Table 1, third panel; Figure 2B). In contrast, at cycle 6, this trend did not reach statistical significance (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103628, third panel).
During all cycles of induction therapy, the distribution of all DRQOL scores were significantly different across skin toxicity grades 0-3 (Figure 2C and D; Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.103628, second to fourth panel). Stepwise pairwise comparisons of skin toxicity grades 0-3 revealed significantly worsened outcomes across all cycles of induction and DRQOL questionnaires between skin toxicity grades 1 and 2 (Figure 2C and D; Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.103628 second to fourth panel).
Multiple linear regression including baseline characteristics and toxicity endpoints at cycle 6 of induction therapy significantly predicted Skindex-16 and DLQI outcomes [Skindex-16, F (18, 180) = 2.02, R2 = 0.17, P = 0.011]. Strongest significant individual predictors for favorable Skindex-16 outcomes were absence of skin toxicity as an adverse event during induction (t = 2.59, P = 0.010) and no prior surgery for primary tumor (t = −2.92, P = 0.004; Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2024.103628).
DRQOL outcomes during maintenance therapy
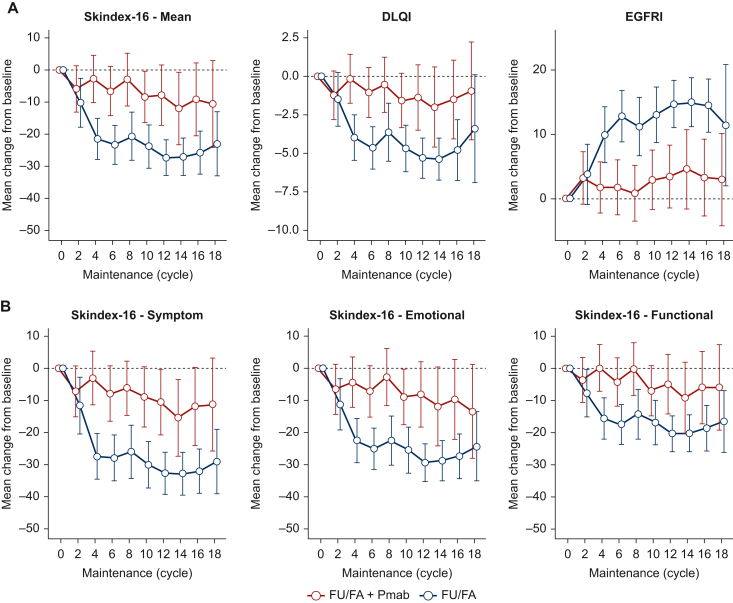
Between baseline (before start of cycle 1 of maintenance therapy) and cycle 6 of maintenance therapy, all randomized patients presented significant improvement in all mean DRQOL scores (Skindex-16, mean change −14.84, 95% CI −19.82 to −9.86, P < 0.001; DLQI, mean change −2.77, 95% CI −3.87 to −1.66, P < 0.001; FACT-EGFRI, mean change 7.32, 95% CI 4.28-10.36, P < 0.001; Table 2, first panel; Figure 3A), including Skindex-16 subdomains (Table 2, first panel; Figure 3B).
Table 2.
DRQOL scores during maintenance therapy
| DRQOL questionnaire | Change from baseline to cycle 6 (maintenance) |
Treatment arm | Baseline (maintenance) |
Mean difference at cycle 6 (maintenance) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients (N) | Mean difference | 95% CI (lower, upper) | Two-sided P value | Patients (N) | Mean score | Std. deviation | Std. error mean | Two-sided P value | Patients (N) | Mean difference | 95% CI (lower, upper) | Two-sided P value | ||||
| Skindex-16 | 131.00 | −14.84 | −19.82 | −9.86 | 0.000 | FU/FA+Pmab | 106.00 | 28.15 | 26.11 | 2.54 | 0.962 | 66.00 | −16.53 | −22.68 | −10.38 | 0.000 |
| FU/FA | 92.00 | 28.33 | 26.48 | 2.76 | 65.00 | |||||||||||
| Skindex-16: Symptom | 131.00 | −17.78 | −23.55 | −12.01 | 0.000 | FU/FA+Pmab | 106.00 | 33.77 | 28.20 | 2.74 | 0.919 | 66.00 | −19.54 | −26.98 | −12.09 | 0.000 |
| FU/FA | 92.00 | 34.19 | 30.74 | 3.20 | 65.00 | |||||||||||
| Skindex-16: Emotional | 131.00 | −15.96 | −21.27 | −10.65 | 0.000 | FU/FA+Pmab | 106.00 | 29.35 | 28.63 | 2.78 | 0.873 | 66.00 | −17.22 | −23.67 | −10.77 | 0.000 |
| FU/FA | 92.00 | 29.99 | 28.25 | 2.94 | 65.00 | |||||||||||
| Skindex-16: Functional | 131.00 | −10.77 | −15.64 | −5.90 | 0.000 | FU/FA+Pmab | 106.00 | 21.58 | 25.62 | 2.50 | 0.988 | 66.00 | −13.18 | −19.37 | −7.00 | 0.000 |
| FU/FA | 92.00 | 21.52 | 26.08 | 2.72 | 65.00 | |||||||||||
| DLQI | 129.00 | −2.77 | −3.87 | −1.66 | 0.000 | FU/FA+Pmab | 103.00 | 5.87 | 5.91 | 0.58 | 0.933 | 67.00 | −3.53 | −4.86 | −2.20 | 0.000 |
| FU/FA | 90.00 | 5.94 | 5.69 | 0.60 | 62.00 | |||||||||||
| FACT-EGFRI | 120.00 | 7.32 | 4.28 | 10.36 | 0.000 | FU/FA+Pmab | 88.00 | 53.76 | 13.20 | 1.41 | 0.875 | 60.00 | 11.39 | 7.57 | 15.21 | 0.000 |
| FU/FA | 80.00 | 54.11 | 15.30 | 1.71 | 60.00 | |||||||||||
First panel: mean changes in DRQOL scores from baseline to cycle 6 of maintenance therapy. Second panel: mean DRQOL scores at baseline by treatment arm (bold: FU/FA + Pmab arm). Third panel: mean differences in DRQOL scores at cycle 6 of maintenance therapy between the two treatment arms (FU/FA + Pmab versus FU/FA).
CI, confidence interval; DLQI, Dermatology Life Quality Index; DRQOL, dermatology-related quality of life; FACT-EGFRI, Functional Assessment of Cancer Therapy-epidermal growth factor receptor inhibitor; FU/FA, fluorouracil, folinic acid; Pmab, Panitumumab.
Figure 3.
DRQOL outcomes during maintenance therapy. Mean changes of (A) Skindex-16, DLQI, and EGFRI scores and (B) Skindex-16 Symptom, Emotional, and Functional subdomains are shown from baseline (before start of maintenance therapy) for patients who received FU/FA plus Pmab (red) or FU/FA (blue) for every second cycle at cycles 2-16 of maintenance therapy. Whiskers mark 95% CI.
CI, confidence interval; DLQI, Dermatology Life Quality Index; DRQOL, dermatology-related quality of life; EGFRI, epidermal growth factor receptor inhibitor; FU/FA, fluorouracil and folinic acid; Pmab, panitumumab.
At baseline (before start of cycle 1 of maintenance therapy), there were no numerical or significant differences in any mean DRQOL scores between both maintenance treatment arms (Table 2, second panel).
Of note, at cycle 6 of maintenance therapy, patients who received FU/FA + Pmab maintenance had significantly worse mean DRQOL scores, compared to those who did not (Skindex-16, mean difference −16.53, 95% CI −22.68 to −10-38, P < 0.001; DLQI, mean difference −3.53, 95% CI −4.86 to −2.20, P < 0.001; FACT-EGFRI, mean difference 11.39, 95% CI 7.57-15.21, P < 0.001; Table 2, third panel; Figure 3A), including Skindex-16 subdomains (Table 2, third panel; Figure 3B).
Multiple linear regression including baseline characteristics and toxicity endpoints significantly predicted all DRQOL measures, i.e. Skindex-16 scores at cycle 6 of maintenance [F (18, 105) = 2.93, R2 = 0.33, P < 0.001]. Strongest significant individual predictors for favorable Skindex-16 outcomes were FU/FA arm (t = −5.04, P < 0.001) and low ECOG performance status (t = 3.09, P = 0.003; Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2024.103628).
Discussion
This prespecified analysis of the PanaMa (AIO KRK 0212) trial aimed to investigate the impact of maintenance therapy with FU/FA with or without Pmab on DRQOL in patients with RAS wt mCRC. This investigation was specifically planned with the knowledge that classic quality-of-life assessment by EORTC QLQ-C30 rarely reports substantial differences when chemotherapy-based regimes are compared,11,21, 22, 23, 24 and also anticipating that Pmab with its classic side-effects nevertheless might invoke higher rates of toxicity in the trial. The PanaMa trial therefore provides a unique opportunity to demonstrate that PRO tools assess the specific side-effects of Pmab and also to evaluate the evolution of DRQOL during both induction and maintenance therapy in this specific patient population.
Findings from this analysis revealed several additional insights into the relationship between treatment regimens and DRQOL. During induction therapy, a global deterioration of mean DRQOL was observed, as assessed by the Skindex-16, DLQI, and FACT-EGFRI questionnaires. Of note, patients who developed skin toxicity presented worse DRQOL scores throughout the induction phase. However, at cycle 6 of induction therapy, the trend of deteriorated DRQOL was neither clinically meaningful nor statistically significant, suggesting a potential adaptation or improved management of skin toxicity over time.18,25,26 Analysis of skin toxicity by NCI-CTCAE grading (grades 0-3) revealed a strong and significant correlation of DRQOL outcomes and skin toxicity grading across all questionnaires and all cycles of induction therapy. Of note, stepwise pairwise comparisons identified most prominent differences between grades 1 and 2, while differences in DRQOL remained negligible between grades 0 and 1. Multiple linear regression analyses identified the absence of skin toxicity and no prior surgery for the primary tumor as predictors for favorable DRQOL outcomes during induction therapy. These results suggest positive correlation between skin toxicity as an adverse event and decreased DRQOL as a PRO. Despite limited evidence in this setting, a poor correlation of adverse events and PROs from the EORTC QLQ-CR30 was described in the QUACK trial.27
By contrast, the maintenance therapy phase demonstrated a positive impact on DRQOL, with all patients experiencing significant improvements in Skindex-16, DLQI, and FACT-EGFRI scores. This improvement signifies a relief from the dermatologic symptoms that may have occurred during induction therapy. However, a noteworthy observation was made concerning patients receiving FU/FA plus Pmab maintenance, who exhibited significantly worse DRQOL scores at cycle 6 compared to those receiving FU/FA alone, indicating that the addition of Pmab might contribute to inferior DRQOL in this context. This suggests that despite a certain relief in treatment side-effect burden and maybe some adaptation, continued use of EGFR antibodies during maintenance therapy impacts on patients and this impact can be measured by DRQOL.
While this study demonstrates that assessing DRQOL in a comprehensive manner, using validated questionnaires such as Skindex-16, DLQI, and FACT-EGFRI, provides a valid tool to capture the impact of Pmab-specific side-effects (i.e. skin toxicity) on PROs, it remains important to note that these observations were not made with the general HRQOL as assessed by the EORTC QLQ-C30 from patients treated in the PanaMa trial.16 This clear lack of internal consistency may suggest that compromised DRQOL does not necessarily impact on global HRQOL. This counterintuitive finding might be potentially based on the knowledge that EGFR-related toxicity is associated with favorable outcome and patients experience EGFR-related skin toxicity not solely as an unwished side-effect.28, 29, 30, 31, 32, 33, 34, 35, 36 Previous studies looking into correlation of PROs with CTCAE ratings had varying outcomes depending on clinical setting, PRO, and CTCAE rating examined.37 Different outcomes of DRQOL and HRQOL in this analysis may underline the importance of including adequate PRO questionnaires, to capture the impact of trial-specific toxicity profiles on quality of life. Acknowledging the significance of capturing the patient perspective alongside clinician-based reporting, the NCI has created a patient-reported version of the CTCAE (PRO-CTCAE).38
The limitations of this analysis include its retrospective nature and the potential for selection bias, as not all patients completed DRQOL assessments at every time point. While compliance rates were comparably high at 82.2% (induction group) and 84.8%-89.4% (maintenance group), a significant variance in completion rates was noted, ranging from 55.0% to 84.8%. Of note, Skindex-16 showed the highest, and EGFRI the lowest completion rates during all of the time points assessed. These findings suggest additional burden of high amounts of questionnaires for patients, with a reduction in questionnaires and the time points they are applied potentially leading to sustained completion rates. Furthermore, the analysis focused on RAS wt mCRC patients receiving Pmab, and the results may not be generalizable to other patient populations.
This prespecified secondary analysis of the PanaMa trial suggests that specifically skin-focused PROs adequately capture dermatologic side-effects—consistent with investigators-assessed toxicity—which might be missed by more general quality-of-life questionnaires. Of note, in the PanaMa trial, these skin-focused assessments reported inferior DRQOL with FU/FA plus Pmab during maintenance as compared to FU/FA alone. These results emphasize the growing importance of PROs, the need for different outcome assessments, and also the necessity to implement HRQOL questionnaires with specific profiles to adequately assess side-effects from a patient’s perspective.
Disclosure
AB: stock and other ownership interests: BioNTech SE; honoraria: Amgen, AstraZeneca, Merck. Research funding: Amgen (Inst); travel, accommodations, expenses: Amgen. MK: consulting or advisory role: Amgen; travel, accommodations, expenses: Amgen. UG: stock and other ownership interests: BioNTech SE; honoraria: Boehringer Ingelheim, Amgen, AstraZeneca, Bristol Myers Squibb, MSD Oncology, Sanofi Aventis GmbH, Fujifilm, Novartis, Celltrion; consulting or advisory role: Amgen, MSD Oncology; research funding: Ipsen (Inst), MacroGenics (Inst); travel, accommodations, expenses: Boehringer Ingelheim, GlaxoSmithKline. LM: travel, accommodations, expenses: Octapharma, Pierre Fabre. LFvW: honoraria: Pierre Fabre, Lilly. IJ: advisory roles/honoraria for talks/travel expenses: Roche, BMS, Merck KGaA, Pierre Fabre, Roche, Servier, Taiho Pharma, Takeda Pharmaceutical. AHSA: honoraria: MSD; travel, accommodations, expenses: Merck, BMS GmbH and Co. KG. AK: honoraria: Taiho Pharmaceutical, Amgen, Servier; travel, accommodations, expenses: medac, Amgen, Servier. AS: honoraria: Roche, Servier, Taiho Pharmaceutical; consulting or advisory role: Bristol Myers Squibb/Pfizer, Novocure; travel, accommodations, expenses: Amgen, Roche, Lilly, Pfizer. EG: consulting or advisory role: MSD, Bristol Myers Squibb, AstraZeneca/Daiichi Sankyo, Pfizer. SK: employment: University Hospital Essen; honoraria: Bristol Myers Squibb, MSD Oncology, AstraZeneca, Merck Serono, Amgen, Roche, Servier, Amgen, Lilly, Sanofi/Aventis, Novartis, Pierre Fabre; consulting or advisory role: Roche, Merck Serono, Amgen, MSD Oncology, Sanofi, Bristol Myers Squibb, Lilly, Servier, AstraZeneca, Janssen-Cilag, Novartis, Pierre Fabre, Incyte; research funding: Merck Serono, Bristol Myers Squibb, Celgene, Lilly, Servier, Roche/Genentech; travel, accommodations, expenses: Merck Serono, Lilly, Amgen, Sanofi, Roche, Pierre Fabre, BMS; other relationship: Sanofi, Amgen, Merck Serono, Bristol Myers Squibb, Roche, Lilly. KH: honoraria: Roche, Taiho; consulting or advisory role: Bristol Myers Squibb (BMS), Servier; travel, accommodations, expenses: Lilly, Amgen, Servier, Merck; MK, consulting or advisory role: Amgen; travel, accommodations, expenses: Amgen. SF, stock; other ownership interests: AbbVie, BMS/Pfizer, Johnson & Johnson/Janssen, Merck. VH: honoraria: Roche, Amgen, Sanofi, Merck, Servier, Pfizer, Pierre Fabre, AstraZeneca, MSD, Seagen; consulting or advisory role: Merck, Amgen, Roche, MSD, Bristol Myers Squibb, MSD Oncology, Novartis, Pierre Fabre, TERUMO, GlaxoSmithKline, Servier/Pfizer, AstraZeneca, OncoSil, Nordic Bioscience; research funding: Merck (Inst), Amgen (Inst), Roche (Inst); travel, accommodations, expenses: Merck. SS: honoraria: Merck KGaA, Roche, Amgen, Servier, MSD, Pfizer, Pierre Fabre, Bristol Myers Squibb GmbH, Nordic Bioscience, AstraZeneca; consulting or advisory role: Merck KGaA, Roche, Amgen, Pierre Fabre, MSD, Astrazeneca, Servier, Glaxosmithkline, Terumo, Nordic Bioscience, Seagen; research funding: Pierre Fabre (Inst), Roche Molecular Diagnostics (Inst), Merck Serono (Inst), Amgen (Inst); travel, accommodations, expenses: Merck KGaA, Roche, Sanofi, Bayer, Sirtex Medical, Amgen, Lilly, Takeda, Pierre Fabre, AstraZeneca. TT: research funding: Amgen; travel, accommodations, expenses: Ipsen, Takeda, OMT, AbbVie, Novartis, MSD, Sanofi/Aventis, Amgen, Johnson and Johnson/Janssen. DPM: honoraria: Merck Serono, Amgen, Servier, Bristol Myers Squibb, Taiho Pharmaceutical, Merck Sharp and Dohme, Pierre Fabre, Onkowissen, Sanofi, Lilly, AstraZeneca/MedImmune, Incyte, Takeda; consulting or advisory role: Merck Serono, Amgen, Merck Sharp and Dohme, Roche, Servier, Incyte, Bristol Myers Squibb, Pierre Fabre, Lilly, Cor2Ed, IQVIA, Onkowissen; research funding: Amgen (Inst), Servier (Inst); travel, accommodations, expenses: Amgen, Merck Serono, Servier. All other authors have declared no conflicts of interest.
Acknowledgements
We thank all patients and their families who agreed to take part in the trial. We also thank the investigators and the study teams who participated in the PanaMa trial.
Funding
This work was supported by AIO Studien gGmbH, Berlin, Germany (legal funder/sponsor of the trial); Amgen Inc (Thousand Oaks, CA) supported the trial with study medication and a research grant to the AIO Studien gGmbH (no grant number).
Supplementary Data
References
- 1.Cervantes A., Adam R., Rosello S., et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(1):10–32. doi: 10.1016/j.annonc.2022.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Arnold D., Lueza B., Douillard J.Y., et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28(8):1713–1729. doi: 10.1093/annonc/mdx175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andre T., Meyerhardt J., Iveson T., et al. Effect of duration of adjuvant chemotherapy for patients with stage III colon cancer (IDEA collaboration): final results from a prospective, pooled analysis of six randomised, phase 3 trials. Lancet Oncol. 2020;21(12):1620–1629. doi: 10.1016/S1470-2045(20)30527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andre T., Tournigand C., Mineur L., et al. Phase II study of an optimized 5-fluorouracil-oxaliplatin strategy (OPTIMOX2) with celecoxib in metastatic colorectal cancer: a GERCOR study. Ann Oncol. 2007;18(1):77–81. doi: 10.1093/annonc/mdl336. [DOI] [PubMed] [Google Scholar]
- 5.Quidde J., Hegewisch-Becker S., Graeven U., et al. Quality of life assessment in patients with metastatic colorectal cancer receiving maintenance therapy after first-line induction treatment: a preplanned analysis of the phase III AIO KRK 0207 trial. Ann Oncol. 2016;27(12):2203–2210. doi: 10.1093/annonc/mdw425. [DOI] [PubMed] [Google Scholar]
- 6.Tournigand C., Cervantes A., Figer A., et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer - a GERCOR study. J Clin Oncol. 2006;24(3):394–400. doi: 10.1200/JCO.2005.03.0106. [DOI] [PubMed] [Google Scholar]
- 7.Pietrantonio F., Morano F., Corallo S., et al. Maintenance therapy with panitumumab alone vs panitumumab plus fluorouracil-leucovorin in patients with RAS wild-type metastatic colorectal cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2019;5(9):1268–1275. doi: 10.1001/jamaoncol.2019.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goey K.K.H., Elias S.G., van Tinteren H., et al. Maintenance treatment with capecitabine and bevacizumab versus observation in metastatic colorectal cancer: updated results and molecular subgroup analyses of the phase 3 CAIRO3 study. Ann Oncol. 2017;28(9):2128–2134. doi: 10.1093/annonc/mdx322. [DOI] [PubMed] [Google Scholar]
- 9.Raimondi A., Nichetti F., Stahler A., et al. Optimal maintenance strategy following FOLFOX plus anti-EGFR induction therapy in patients with RAS wild type metastatic colorectal cancer: an individual patient data pooled analysis of randomised clinical trials. Eur J Cancer. 2023;190 doi: 10.1016/j.ejca.2023.112945. [DOI] [PubMed] [Google Scholar]
- 10.Modest D.P., Karthaus M., Fruehauf S., et al. Panitumumab plus fluorouracil and folinic acid versus fluorouracil and folinic acid alone as maintenance therapy in RAS wild-type metastatic colorectal cancer: the randomized PANAMA trial (AIO KRK 0212) J Clin Oncol. 2022;40(1):72–82. doi: 10.1200/JCO.21.01332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raimondi A., Di Maio M., Morano F., et al. Health-related quality of life in patients with RAS wild-type metastatic colorectal cancer treated with panitumumab-based first-line treatment strategy: a pre-specified secondary analysis of the Valentino study. Eur J Cancer. 2020;135:230–239. doi: 10.1016/j.ejca.2020.04.048. [DOI] [PubMed] [Google Scholar]
- 12.Gong J., Wu D., Chuang J., Tuli R., Simard J., Hendifar A. Moving beyond conventional clinical trial end points in treatment-refractory metastatic colorectal cancer: a composite quality-of-life and symptom control end point. Clin Ther. 2017;39(11):2135–2145. doi: 10.1016/j.clinthera.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Schuurhuizen C., Braamse A.M.J., Konings I., et al. Does severe toxicity affect global quality of life in patients with metastatic colorectal cancer during palliative systemic treatment? A systematic review. Ann Oncol. 2017;28(3):478–486. doi: 10.1093/annonc/mdw617. [DOI] [PubMed] [Google Scholar]
- 14.Osoba D., Bezjak A., Brundage M., et al. Analysis and interpretation of health-related quality-of-life data from clinical trials: basic approach of The National Cancer Institute of Canada Clinical Trials Group. Eur J Cancer. 2005;41(2):280–287. doi: 10.1016/j.ejca.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Aaronson N.K., Ahmedzai S., Bergman B., et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 16.Ballhausen A., Karthaus M., Fruehauf S., et al. Health-related quality of life in patients with RAS wild-type metastatic colorectal cancer treated with fluorouracil and folinic acid with or without panitumumab as maintenance therapy: a prespecified secondary analysis of the PanaMa (AIO KRK 0212) trial. Eur J Cancer. 2023;190 doi: 10.1016/j.ejca.2023.112955. [DOI] [PubMed] [Google Scholar]
- 17.Wagner L.I., Berg S.R., Gandhi M., et al. The development of a Functional Assessment of Cancer Therapy (FACT) questionnaire to assess dermatologic symptoms associated with epidermal growth factor receptor inhibitors (FACT-EGFRI-18) Support Care Cancer. 2013;21(4):1033–1041. doi: 10.1007/s00520-012-1623-4. [DOI] [PubMed] [Google Scholar]
- 18.Wong S.F., Unger J.M., Wade J.L., 3rd, et al. A prospective study to validate the functional assessment of cancer therapy (FACT) for epidermal growth factor receptor inhibitor (EGFRI)-induced dermatologic toxicities FACT-EGFRI 18 questionnaire: SWOG S1013. J Patient Rep Outcomes. 2020;4(1):54. doi: 10.1186/s41687-020-00220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finlay A.Y., Khan G.K. Dermatology Life Quality Index (DLQI) - a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 20.Chren M.M., Lasek R.J., Sahay A.P., Sands L.P. Measurement properties of Skindex-16: a brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg. 2001;5(2):105–110. doi: 10.1007/BF02737863. [DOI] [PubMed] [Google Scholar]
- 21.Liposits G., Eshoj H.R., Moller S., et al. Quality of life in vulnerable older patients with metastatic colorectal cancer receiving palliative chemotherapy-the randomized NORDIC9-study. Cancers (Basel) 2021;13(11):2604. doi: 10.3390/cancers13112604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertaut A., Touchefeu Y., Blanc J., et al. Health-related quality of life analysis in metastatic colorectal cancer patients treated by second-line chemotherapy, associated with either cetuximab or bevacizumab: the PRODIGE 18 randomized phase II study. Clin Colorectal Cancer. 2022;21(2):e49–e61. doi: 10.1016/j.clcc.2021.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi K., Ando M., Ooki A., et al. Quality of life analysis in patients with RAS wild-type metastatic colorectal cancer treated with first-line cetuximab plus chemotherapy. Clin Colorectal Cancer. 2017;16(2):e29–e37. doi: 10.1016/j.clcc.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Kosmala R., Fokas E., Flentje M., et al. Quality of life in rectal cancer patients with or without oxaliplatin in the randomised CAO/ARO/AIO-04 phase 3 trial. Eur J Cancer. 2021;144:281–290. doi: 10.1016/j.ejca.2020.11.029. [DOI] [PubMed] [Google Scholar]
- 25.Chren M.M., Sahay A.P., Bertenthal D.S., Sen S., Landefeld C.S. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127(6):1351–1357. doi: 10.1038/sj.jid.5700740. [DOI] [PubMed] [Google Scholar]
- 26.Basra M.K., Salek M.S., Camilleri L., Sturkey R., Finlay A.Y. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology. 2015;230(1):27–33. doi: 10.1159/000365390. [DOI] [PubMed] [Google Scholar]
- 27.Ooki A., Morita S., Tsuji A., et al. Disagreement between patient- and physician-reported outcomes on symptomatic adverse events as poor prognosis in patients treated with first-line cetuximab plus chemotherapy for unresectable metastatic colorectal cancer: results of Phase II QUACK trial. Cancer Med. 2020;9(24):9419–9430. doi: 10.1002/cam4.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holch J.W., Held S., Stintzing S., et al. Relation of cetuximab-induced skin toxicity and early tumor shrinkage in metastatic colorectal cancer patients: results of the randomized phase 3 trial FIRE-3 (AIO KRK0306) Ann Oncol. 2020;31(1):72–78. doi: 10.1016/j.annonc.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Cunningham D., Humblet Y., Siena S., et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351(4):337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 30.Saltz L.B., Meropol N.J., Loehrer PJ Sr, Needle M.N., Kopit J., Mayer R.J. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22(7):1201–1208. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 31.Lenz H.J., Van Cutsem E., Khambata-Ford S., et al. Multicenter phase II and translational study of cetuximab in metastatic colorectal carcinoma refractory to irinotecan, oxaliplatin, and fluoropyrimidines. J Clin Oncol. 2006;24(30):4914–4921. doi: 10.1200/JCO.2006.06.7595. [DOI] [PubMed] [Google Scholar]
- 32.Jonker D.J., O'Callaghan C.J., Karapetis C.S., et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357(20):2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 33.Van Cutsem E., Lenz H.J., Kohne C.H., et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33(7):692–700. doi: 10.1200/JCO.2014.59.4812. [DOI] [PubMed] [Google Scholar]
- 34.Van Cutsem E., Siena S., Humblet Y., et al. An open-label, single-arm study assessing safety and efficacy of panitumumab in patients with metastatic colorectal cancer refractory to standard chemotherapy. Ann Oncol. 2008;19(1):92–98. doi: 10.1093/annonc/mdm399. [DOI] [PubMed] [Google Scholar]
- 35.Hecht J.R., Patnaik A., Berlin J., et al. Panitumumab monotherapy in patients with previously treated metastatic colorectal cancer. Cancer. 2007;110(5):980–988. doi: 10.1002/cncr.22915. [DOI] [PubMed] [Google Scholar]
- 36.Dienstmann R., Brana I., Rodon J., Tabernero J. Toxicity as a biomarker of efficacy of molecular targeted therapies: focus on EGFR and VEGF inhibiting anticancer drugs. Oncologist. 2011;16(12):1729–1740. doi: 10.1634/theoncologist.2011-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atkinson T.M., Ryan S.J., Bennett A.V., et al. The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): a systematic review. Support Care Cancer. 2016;24(8):3669–3676. doi: 10.1007/s00520-016-3297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basch E., Reeve B.B., Mitchell S.A., et al. Development of the National Cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE) J Natl Cancer Inst. 2014;106(9) doi: 10.1093/jnci/dju244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.