Abstract
Gut microbiota changes and brain-gut-axis (BGA) dysregulation are common in people with Parkinson’s Disease (PD). Probiotics and prebiotics are emerging as a potential therapeutic approach for PD patients. The aim of this paper was to assess the neurological and gastroenterological effects in PD patients with constipation after the administration of a synbiotic product, with a focus on behavioral and cognitive symptoms. We enrolled patients with stable PD who met diagnostic criteria for functional constipation and/or irritable bowel syndrome with constipation according to Rome IV Criteria. Patients received a synbiotic treatment (Enterolactis Duo, containing the probiotic strain Lacticaseibacillus paracasei DG and the prebiotic fiber inulin) for 12 weeks. A neurological and a gastroenterological evaluation were collected before and after the treatment. In addition, 16S rRNA gene profiling and short chain fatty acid quantification were performed to characterize the microbial ecosystem of fecal samples collected before (n = 22) and after (n = 9) the synbiotic administration. 30 patients were consecutively enrolled. After treatment, patients performed better in MDS-UPDRS part 1 (p = 0.000), SCOPA-AUT (p = 0.001), TAS-20 (p = 0.014), HAM-D (p = 0.026), DIFt (p = 0.003), PAS-A (p = 0.048). Gastroenterological evaluations showed improvements in PAC-SYM score (p < 0.001), number of complete bowel movement (p < 0.001) and BSFS (p < 0.001). After the synbiotic administration, we observed a significant increase in the abundance of the order Oscillospirales, as well as the Oscillospiraceae family and the species Faecalibacterium prausnitzii within this order in fecal samples. Synbiotic treatment demonstrates potential efficacy in ameliorating non-motor features in PD patients.
Keywords: Parkinson’s disease, Non motor symptoms, Constipation, Movement disorders
Subject terms: Neuroscience, Gastroenterology, Neurology
Introduction
Parkinson’s disease (PD) is a complex neurodegenerative disorder characterized by a range of motor and non-motor symptoms.
Gastrointestinal dysfunction, and specifically constipation, is one of the most frequent non-motor symptoms of PD. Between 50 and 80% of patients with PD experience constipation1. Constipation is also one of the earliest features of autonomic dysfunction, often preceding the onset of motor symptoms by up to 15 years, and is associated with disease duration and severity2.
Individuals with PD exhibit alterations in the composition and diversity of their gut microbiota compared to healthy individuals. These alterations may include changes in the abundance of specific microbial species or a shift in the overall microbial profile and are related with constipation and clinical phenotypes3–5. Moreover, the role of the gut microbiota in shaping brain function is increasingly acknowledged.
The gut-brain axis consists of a bidirectional communication between the central nervous system (CNS) and the enteric nervous system (ENS)6. Recent research highlighted the significance of the intestinal microbiota in influencing these interactions via neural, endocrine, immune, and humoral pathways7, contributing to the pathogenesis and/or progression of several neurological disorders, including PD8.
Probiotics and prebiotics, aimed to balance the gut microbiota, are emerging as a potential therapeutic approach for PD patients. Probiotics are live microorganisms conferring health benefits when administered in adequate amounts9, whereas a prebiotic is defined as “a substrate that is selectively utilized by host microorganisms conferring a health benefit”10. The combined administration of probiotics and prebiotics can yield additional benefits compared to the administration of individual components, through what is now recognized as ‘complementary synbiotic products’11. Minimum criteria for the existing probiotic and prebiotic must be met for both components of a complementary synbiotic11.
In recent years, the evidence regarding the effectiveness of probiotics in balancing the gut microbiota and treating constipation in PD patients is growing12. On the other hand, several studies have explored the potential of both prebiotics and probiotics to improve stress related disorders, anxiety and depressive symptoms in healthy population13,14. Also, while research in this area is still evolving, some studies have shown promising results regarding the potential cognitive benefits of prebiotics and probiotics. For example, certain probiotic strains of bifidobacteria and lactobacilli have been associated with improvements in cognitive functions in healthy elderly15,16, but also in individuals with mild cognitive impairment (MCI), memory complaints17,18and in patients with Alzheimer Disease (AD)19–21.
In this study we aimed to explore the beneficial effects of the administration of a synbiotic dietary supplement (Enterolactis Duo) in PD patients with constipation, with a focus on behavioral and cognitive symptoms in addition to motor symptoms. In addition, the potential modification of bacterial taxa and microbial metabolites in feces were also assessed in a subgroup of patients. Enterolactis Duo contains the probiotic strain Lacticaseibacillus paracasei DG and the prebiotic fiber inulin. Indeed, L. paracaseiis known for alleviating gastrointestinal symptoms in patients suffering from chronic constipation22, while inulin is a prebiotic that can improve gut microbiota composition23.
Methods
Participants & methods
This is a single-arm, open label study. Thirty participants aged from 18 to 80 years with idiopathic PD, in stable motor conditions and fulfilling Rome IV criteria for functional constipation (FC) or irritable bowel syndrome with constipation (IBS-C)24 were consecutively recruited at the Parkinson’s Disease Center and Movement Disorder Clinic at the University of Salerno from 2019 to 2022. All the patients gave their written informed consent to the study. The protocol was approved by the Ethics Committee of ASLNAPOLI3SUD on 25/09/2018 and registered in ClinicalTrials.gov (ref. no.: NCT04293159).
Baseline demographic and clinical features of enrolled PD patients are reported in Table 1.
Table 1.
Baseline characteristics of enrolled PD patients.
| Males, n (%) | 20 (66.7%) |
|---|---|
| Age, median (IQR) | y 64.7 ± 7.1 |
| Disease duration, median (IQR) | y 8.9 ± 4.6 |
| BMI ± SD | 27.5 kg/m2 ± 3.7 |
| FC, n (%) | 28 (93.3%) |
| IBS-C, n (%) | 2 (6.7%) |
n, number; SD, standard deviation.
Changes in antiparkinsonian drugs were not allowed during the study. Participants with organic gastrointestinal disorders, history of major abdominal surgery, or concomitant systemic conditions that could cause bowel disturbances were excluded. Patients on advanced PD therapies and patients who regularly took laxatives or other therapies for constipation that could not be discontinued were not recruited.
Study protocol
Before the baseline visit, every PD patient completed a questionnaire to confirm their fulfilment of the Rome IV criteria for FC or IBS-C. All the examinations (e.g., blood biochemistry, endoscopic and radiological investigations to exclude secondary causes of constipation) were performed when indicated.
Neurological and gastroenterological evaluations were performed at baseline (T0) before starting the synbiotic treatment.
- Neurological evaluation consisted of:
- Cognitive and behavioral evaluation consisted of:
- Montreal Cognitive Assessment (MoCA), a screening test that assesses global cognitive function and consists of 10 subtests; it measures the visuospatial abilities, executive functions, attention, concentration, working memory, memory, language and orientation27
- Toronto Alexithymia Scale (TAS-20)28, a self-report questionnaire designed to measure alexithymia, a personality trait characterized by difficulty in identifying and expressing emotions, whose prevalence is about double in PD patients compared to normal population29. The TAS-20 assesses three main components of alexithymia:
- Difficulty Identifying Feelings (DIF)
- Difficulty Describing Feelings (DDF)
- Externally Oriented Thinking (EOT)
- Reading the Mind in the Eyes Test (RMET), a task that assesses the theory of mind component of social cognition and shows respondents the eye region of 36 Caucasian faces and asks them to select one of four accompanying labels that best describes the mental state of the individual pictured30.
- Parkinson Anxiety Scale (PAS), a questionnaire that consists of 12-item observer or patient-rated scale and includes three subscales assessing persistent, episodic anxiety and avoidance behavior (PAS-A, PAS-B, PAS-C, respectively)31.
- State-Trait Anxiety Inventory (STAI-Y), a self-report questionnaire to assess general anxiety; it allows a clear separation between state and trait anxiety, it is particularly suitable for detecting transitory rises of anxiety and it is less oriented towards somatic components of anxiety (e.g., pain, difficulty in breathing, sleep disorders) than other widely used scales, with lower risk of overlap between medical and psychological determinants of anxiety32
- Beck Depression Inventory-II (BDI II), a self-report questionnaire to assess the affective somatic and the cognitive elements of depression, for example sadness, pessimism, self-criticalness, agitation, suicide thoughts, irritability, loss of energy33
- Hamilton depression rating scale (HAM-D), a multiple-item questionnaire used to rate the severity of depression by probing mood, feelings of guilt, suicide ideation, insomnia, agitation or retardation, anxiety, weight loss, and somatic symptoms34.
- Gastroenterological evaluation consisted of:
- Patients Assessment of Constipation Symptoms (PAC-SYM) Questionnaires. The PAC-SYM consists of 12 items that cover three domains related to constipation symptoms: stool symptoms, rectal symptoms, abdominal symptoms. For each item, patients rate the severity and frequency of their symptoms over a specified timeframe. The severity rating ranges from 0 (none) to 4 (very severe), and the frequency rating ranges from 0 (never) to 4 (always). The scores for each item are summed to obtain domain scores and a total PAC-SYM score, providing an overall measure of constipation symptom severity35.
- Stool consistency was recorded as numerical values using the Bristol Stool Form Chart Adjectival scale for stool consistency (BSFS), using the validated Bristol stool form scale (a 7-point scale from 1 = separate hard, lumps like nuts to 7 = watery)36.
- Daily measurement of the number of bowel movements was summarized weekly37.
Patients received one sachet containing synbiotic Enterolactis Duo, four times a day, away for meals, for 12 weeks. At the end of 12 weeks (T1) we re-evaluated:
motor and non-motor symptoms of PD using MDS-UPDRS, SCOPA-AUT, MOCA, TAS-20, RMET, PAS scales STAY-Y STATE, STAY-Y TRAIT BDI II, and HAM-D;
the diagnosis of functional constipation or irritable bowel syndrome with constipation according to the Rome IV Criteria.
the “constipation severity and frequency” according to PAC-SYM as well as stool consistency and daily measurement of the number of bowel movements summarized weekly.
During the study period, the patients were allowed to take bisacodyl (max 2 tablets a day, 5 mg each) and rectal enemas (one weekly) or otilonium bromide (max 2 tablets a day, 40 mg each) as rescue therapies for constipation or abdominal pain, respectively.
Synbiotic formulation
Enterolactis Duo contains the probiotic strain Lacticaseibacillus paracasei DSM 34,154 (L. casei DG®; L. paracasei DG) and the prebiotic inulin. Each 5.0 g sachet contains: soluble inulin, fructose, human-derived L. paracasei DG (microorganism deposited at the Pasteur Institute in Paris under the code 1-1572), vitamin B6 hydrochloride, vitamin B2, thiamine mononitrate (vitamin B1). Each sachet contained not less than 8 billion colony formant units (CFUs). Product compositions per 100 g and per dose (1 sachet, 5.0 g) are listed in the following Table 2.
Table 2.
Treatment composition.
| Component | Per 100 g | Per sachet (5.0 g) |
|---|---|---|
| Proteins | Absent | Absent |
| Total carbohydrate | 18.648 g | 0.932 g |
| Fats | Absent | Absent |
| L. casei DG® (Lacticaseibacillus paracasei I1572, DSM 34,154) | ≥ 16 × 109 CFUs | ≥ 8 × 109 CFUs |
| Inulin | 80 g | 4.0 g |
Metataxonomic analysis of fecal samples
Fecal sample metataxonomics was performed through 16S rRNA gene profiling as described earlier38. In brief, after collection, fecal samples were stored in a home refrigerator and delivered to the laboratory within 24 h, where they were stored at − 80 °C until they were processed together. DNA was extracted from 150 mg of feces using the QIAsymphony PowerFecal Pro DNA Kit (Qiagen, Milan, Italy) following the manufacturer’s instructions. Subsequently, a fragment of the 16S rRNA gene encompassing the V3-V4 variable regions was amplified with primers 341F (5′-CCT ACG GGN GGC WGC AG-3′) and 805 R (5′-GAC TAC HVG GGT ATC TAA TCC-3′). Then, amplicons were sequenced using the NovaSeq 6000, 2 × 250 bp (NovaSeq 6000 SP Reagent Kit, 500 cycles) by LC Sciences (Ho TX). Sequencing reads were analyzed using the bioinformatic pipeline Quantitative Insights Into Microbial Ecology (QIIME) 2 version 2022.2, with the Greengenes database v. 13_8 for taxonomic assignment to amplicon sequence variants (ASVs) using the Divisive Amplicon Denoising Algorithm (DADA2; Callahan et al. 2016). Taxonomic diversity was analyzed through QIIME 2 using the algorithms Observed Features and Shannon index for the α-diversity, and Weighted and Unweighted UniFrac for the β-diversity.
Fecal organic acid quantification
Fecal samples were also used for the quantification of the following organic acids: acetate, butyrate, propionate, valerate, isovalerate, and succinate. The protocol adopted was based on Ultra-Performance Liquid Chromatography-High-Resolution Mass Spectrometry (UPLC-HR-MS) as previously described in detail39. In specific, we used an Acquity UPLC separation module (Waters, Milford, MA) coupled with an Exactive Orbitrap MS using a HESI-II probe for electro-spray ionization (Thermo Scientific, San Jose, CA).
Statistical analysis
Demographic and clinical characteristics of the sample were analyzed with descriptive statistics. We checked for normality distribution with the Kolmogorov–Smirnov test.
T test was used to evaluate changes in motor and non-motor parametric variables from baseline to follow up. Wilcoxon signed rank test was used to assess differences in the distribution of non-parametric data (including organic acids levels in feces) from baseline to follow-up. Statistical significance for correlations was set at p < 0.05. Commercial software “Statistical Package for the Social Sciences” (SPSS 29.0) was used for data analyses.
Statistical calculations of microbiome data were carried out using R programming language (version 3.4.2). Sequencing dead abundances underwent centered log-ratio transformation (CLR). Differently abundant taxa in feces between the groups were identified using Wilcoxon–Mann–Whitney test on CLR-transformed abundances.
Results
Neurological effects
After treatment with symbiotic, patients performed better in MDS-UPDRS part 1 (15.13 ± 7.98 vs 10.37 ± 6.05, p = 0.000), SCOPA-AUT (17.0 (7.3) vs 13.5 (9.8), p = 0.001), TAS-20 (54.00 ± 13.41 vs 49.87 ± 12.03, p = 0.014), HAM-D (12.5 (10.3) vs 9.0 (12.0), p = 0.026), DIFt (19.23 ± 7.78 vs 16.63 ± 7.41p = 0.003), PAS-A (7.30 ± 4.97 vs 6.03 ± 5.24, p = 0.048). No significant changes were observed in the average scores of MoCA test and the total motor score evaluated with the MDS-UPDRS III (p > 0.05) (Table 3).
Table 3.
Neurological and behavioral features of PD patients at T0 and T1.
| T0 N° 30 |
T1 N° 30 |
p | |
|---|---|---|---|
| MDS-UPDRS I mean ± SD | 15.13 ± 7.98 | 10.37 ± 6.05 | < 0.001 |
|
MDS-UPDRS II mean ± SD |
11.57 ± 6.69 | 10.60 ± 7.08 | 0.427 |
|
MDS-UPDRS III mean ± SD |
23.83 ± 9.66 | 23.57 ± 8.97 | 0.874 |
| MDS-UPDRS IV Median (IQR) | 2.0 (3.0) | 2.0 (3.0) | 0.720 |
|
SCOPA-AUT* Median (IQR) |
17.0 (7.3) | 13.5 (9.8) | 0.001 |
|
MoCA Median (IQR) |
25.0 (7.0) | 24.0 (5.3) | 0.651 |
|
TAS-20 mean ± SD |
54.00 ± 13.41 | 49.87 ± 12.03 | 0.014 |
|
DIFt mean ± SD |
19.23 ± 7.78 | 16.63 ± 7.41 | 0.034 |
|
DDF mean ± SD |
14.10 ± 4.96 | 12.87 ± 4.23 | 0.103 |
|
EOT mean ± SD |
19.83 ± 4.96 | 19.33 ± 5.01 | 0.538 |
|
RMET mean ± SD |
20.53 ± 5.09 | 20.87 ± 5.14 | 0.662 |
|
PAS-A mean ± SD |
7.30 ± 4.97 | 6.03 ± 5.24 | 0.048 |
|
PAS-B Median (IQR) |
2.0 (4.0) | 2.0 (4.0) | 0.200 |
|
PAS-C Median (IQR) |
2.0 (4.0) | 2.0 (3.3) | 0.363 |
|
STAI-Y TRAIT mean ± SD |
37.27 ± 11.28 | 36.50 ± 11.90 | 0.123 |
|
STAI-Y STATE Median (IQR) |
37.0 (11.5) | 33.5 (10.3) | 0.664 |
|
BDI-II Median (IQR) |
12.0 (10.3) | 9.5 (8.3) | 0.289 |
|
HAM-D mean ± SD |
12.5 (10.3) | 9.0 (12.0) | 0.026 |
We reported mean and SD for normally distributed variables. We reported median (IQR) for not normally distributed variables.
IQR, interquartile range; n, number; SD, standard deviation; MDS-UPDRS; SCOPA-AUT, scales for outcomes in PArkinson’s disease-autonomic dysfunction; MoCA, Montreal Cognitive Assessment; TAS-20, Toronto Alexithymia Scale; DIFt, difficulty identifying feelings; DDF, difficulty describing feelings; EOT, externally oriented thinking; RMET, reading the mind in the eyes test; PAS, Parkinson Anxiety Scale; STAI-Y, state-trait anxiety inventory; BDI-II, beck depression inventory-II; HAM-D, Hamilton depression rating scale.
Statistically significant differences (p < 0.05) are reported in bold.
By analyzing changes in items of the MDS-UPDRS part I, significant changes after synbiotic treatment were found in MDS-UPDRS I-1 (cognitive impairment) (1.07 ± 1.19 vs 0,57 ± 0.90, p = 0.003), MDS-UPDRS I-6 (features of dopamine dysregulation syndrome) (0.50 ± 1.11 vs 0,0.03 vs 0.19, p = 0.037), MDS-UPDRS I-11 (constipation problems) (2.15 ± 1.22 vs 1.11 ± 1.10, p = 0.001). We also analyzed changes in SCOPA-AUT sub-scores, finding significant changes at T1 in gastrointestinal sub-score (questions 1–7) (7.00 ± 2.46 vs 4.20 ± 2.32, p = 0.001).
Gastroenterological effects
Among the 30 patients who successfully completed treatment, 11 patients (36.7%) did not fulfil the Rome IV Criteria for FC/IBS-C after treatment.
As shown in Table 4 treatment with synbiotic significantly reduced the PAC-SYM score at T1 (p < 0.001), particularly for sub-scales ABDOMINAL SYMPTOMS (p = 0.002), RECTAL SYMPTOMS (p = 0.014) and STOOL SYMPTOMS (p < 0.001).
Table 4.
PACSYM score at T0 and T1.
| PAC_SYM | T0, Median(IQR) | T1, Median(IQR) | p value |
|---|---|---|---|
| Total PAC_SYM | 1.4(0.9) | 0.9(0.8) | < 0.001 |
| ABDOMINAL | 0.5(1.0) | 0.0(0.7) | 0.002 |
| Discomfort | 0.0(2.0) | 0.0(1.3) | 0.237 |
| Pain | 0.0(1.0) | 0.0(0.0) | 0.187 |
| Bloating | 1.0(3.0) | 0.0(1.3) | 0.003 |
| Stomach cramps | 0.0(0.0) | 0.0(0.0) | 0.102 |
| RECTAL | 0.6(1.3) | 0.0(0.7) | 0.014 |
| Painful bowel movement | 0.0(2.0) | 0.0(0.3) | 0.272 |
| Rectal burning during or after a bowel movement | 0.0(2.0) | 0.0(0.3) | 0.069 |
| Rectal bleeding or tearing during or after a bowel movement | 0.0(1.0) | 0.0(0.0) | 0.024 |
| STOOL | 2.4(1.0) | 1.2(1.4) | < 0.001 |
| Incomplete bowel movement | 2.0(2.0) | 1.0(2.0) | 0.002 |
| Bowel movement were too hard | 3.0(1.0) | 1.0(2.0) | 0.036 |
| Bowel movement were too small | 2.0(3.0) | 0.0(2.0) | 0.012 |
| Straining or squeezing to try and pass bowel movements | 3.0(1.0) | 2.0(2.0) | 0.001 |
| Feeling like you had to pass a bowel movement but you could not (‘false alarm’) | 2.2(2.0) | 0.0(1.0) | < 0.001 |
A footnote to explain the [bold] values has been added to table [4]. Please could you confirm the footnote? Or, confirm that we should remove the [bold] and the footnote from the table?.
Furthermore, we observed a significant increase in the number of bowel movements (p < 0.001) and a decrease in the numerical values of the Bristol Stool Form Chart Adjectival scale for stool consistency (BSFS) (p = 0.001) (Table 5).
Table 5.
Bowel function at T0 and T1.
| T0, Median(IQR) | T1, Median(IQR) | p value | |
|---|---|---|---|
| Number of bowel movements | 3.0(3.5) | 5.5(4.1) | 0.000 |
| Bristol stool types | 2.0(2.0) | 3.0(2.0) | 0.001 |
Statistically significant differences (p < 0.05) are reported in bold.
Among patients who completed the treatment, 4 patients (13.3%) required additional treatment with rescue therapy (Bisacodyl), with an average intake of 1.5 (± 0.6) tablets weekly, for 3.8 (± 2.1) weeks on average. None of patients took rectal enemas or otilonium bromide.
Fecal microbial ecosystem effects
A single fecal sample was collected from 7 patients at T0 and T1.
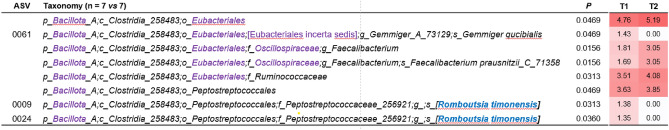
The analysis of the bacterial community structure in fecal samples before and after synbiotic intake revealed significant changes in the abundance of 8 taxa (Fig. 1). Specifically, the orders Oscillospirales and Peptostreptococcales exhibited significant increases. Within the Oscillospirales order, we observed a significant rise in the family Oscillospiraceae, the genus Faecalibacterium, and the species Faecalibacterium prausnitzii. Additionally, two ASVs ascribed to the species Romboutsia timonensis and one ASV ascribed to the species Gemmiger qucibialis showed significant decreases.
Figure 1.
Fecal bacterial taxa that exhibited significant changes after the synbiotic intake period. ASV refers to Amplicon Sequence Variant. Taxonomic lineage is provided: p, phylum; c, class; o, order; f, family; g, genus; s, species. Taxonomic names highlighted in blue were identified through manual BLASTN searches in GenBank using the corresponding read sequence. Names in square brackets indicate non-validated taxonomy. Corrections to the taxonomic names compared to those in the Greengenes database are highlighted in purple. p values were obtained from Mann–Whitney tests on CLR-transformed bacterial abundances. The numbers in the white-red heatmap indicate the median CLR-transformed abundance of the respective bacterial taxon at T0 and T1. The intensity of the red color in the heatmap corresponds to the increase in abundance.
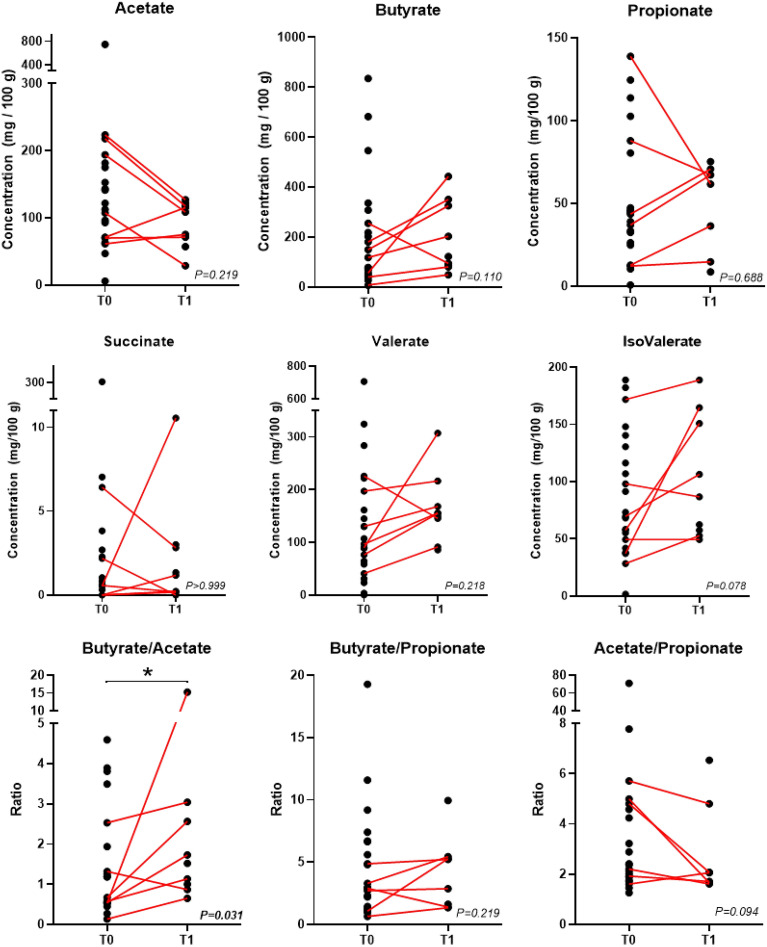
The analysis of organic acids in the fecal samples showed a reduction in acetate levels and an increase in butyrate, valerate, and isovalerate levels in most PD patients (Fig. 2). However, statistical significance was not achieved for any of these organic acids. A statistically significant change was however observed in the butyrate/acetate ratio, which was found to be increased after the synbiotic intake.
Figure 2.
Organic acids in fecal samples of PD patients. Paired data (i.e., T0 and T1 for the same patient) were available for n = 7 patients except for propionate (n = 6). p values are according to the Wilcoxon test; *, p < 0.05.
Discussion
Probiotics can regulate the composition of the gut microbiota, enhance gut barrier function, and exert anti-inflammatory and immunomodulatory effects through various mechanisms40.
Several clinical trials have indicated that probiotics positively affect gastrointestinal symptoms in PD patients, particularly those related to constipation, abdominal pain, and abdominal distension, and this is a growing area of research41–46. Also, several studies on animal models of PD indicate that different probiotics can have positive effects on dopaminergic transmission, as dopaminergic neuron preservation and prevention of neuronal damage in the substantia nigra40. Several strains of Lacticaseibacillus caseiare commonly used as probiotics in various food products and dietary supplements, and clinical trials have shown their potential health benefits, including enhancement of the immune system’s function47, anti-oxidative and anti-inflammatory effects48, maintenance of a balanced gut microbiota49and alleviation of constipation symptoms22. We found only one previous 6-week open label study investigating the effects of the administration of L. paracaseiShirota (LcS) in PD patients, demonstrating a significant improvement of constipation symptoms. However, neurological effects were not investigated44.
Inulin is a non-digestible oligosaccharide that has been linked to the growth of beneficial intestinal and probiotic bacteria, improvement of bowel regularity, enhancement of nutrient absorption, modulation of the immune response50. We did not find previous studies investigating the effects of inulin supplementation or this type of synbiotic in PD population.
Our study took a comprehensive approach, investigating both the non-motor and motor features but also the gastroenterological symptoms in PD patients.
The main finding of our study is that the consumption of a probiotic and prebiotic combination (Enterolactis Duo®) over 12 weeks significantly improved constipation but also other non-motor symptoms, such as anxiety, alexythimia, dysautonomia (with a global improvement in various body functions, including gastrointestinal, urinary, cardiovascular, thermoregulatory, pupillomotor, and sexual dysfunction) in PD patients.
There is a relationship between the gut microbiome and mental health51. Findings from clinical studies demonstrated that probiotics, in healthy subjects, significantly reduce panic anxiety and negative affect13; reduce self-reported cognitive reactivity to sad mood (indexed by the LEIDS-r)14; alleviate psychological distress (measured by the Hopkins Symptom Checklist-HSCL-90 scale, the Hospital Anxiety and Depression Scale-HADS and by the Coping Checklist—CCL)52. Several RCTs also demonstrated beneficial effects of probiotic supplementation on patients with depressive disorders: probiotic supplements are significantly associated with decrease of Beck Depression Inventory total scores53, of Zung Self-Rating Depression Scale (Z-SDS) scores54, Hamilton Depression Rating Scale-17 and Depression and Somatic Symptoms Scale55. Moreover, a treatment with the probiotic strain LcS was found to be associated to a significant decrease in anxiety symptoms as measured by Beck Anxiety Inventories among people with chronic fatigue syndrome (CFS)56.
According with previous results using different probiotics, our study shows that taking probiotics in PD has a favorable impact on depression as measured by Hamilton Depression Scale, and on anxiety, assessed by PAS scale in our study and mainly by Hamilton Anxiety Scale in previous studies57,58. Interestingly, one of these studies investigated LcS supplementation on clinical responses over 12 weeks58.
Some previous studies investigated probiotics supplementation effects on UPDRS I58,59, UPDRS III57–59 and global UPDRS60in PD patients. In one previous study, using the LcS strain, an improvement on UPDRS I score was reported58. Indeed, our study showed for the first time that synbiotic supplementation can improve non-motor symptoms assessed by the MDS-UPDRS part I and, by analyzing effects on single items of the scale, particularly cognitive impairment, dopamine dysregulation syndrome and constipation were significantly improved. On the other hand, we did not find an improvement on the MoCA score. Indeed, the cognitive item in UPDRS part I and MOCA are two different measures. It is possible that the cognitive abilities measured by MOCA are not significantly affected by synbiotic supplementation, while a subjective improvement in cognitive efficiency visible in daily life activities is perceived by the patient and/or caregiver and this may be revealed by the cognitive item in UPDRS I. Specifically, the MOCA is a neuropsychological screening test and as such is made up of tasks that have a numerical equivalent and has specific psychometric properties, designed to guarantee a reliable measurement of the global cognitive state of an individual. In order to obtain a total score of MOCA, the patients perform 10 tasks and the final score does not include the functional aspects of cognitive deficits. The UPDRS part I is a rating scale composed by questions relating to all the non-motor symptoms of PD, measured in the last week; the clinician extrapolates from the caregiver/patient’s answers a label that corresponds to an arbitrary numeric scale. In the UPDRS part I, the cognitive item includes heterogeneous and cumulative information (such as cognitive slowing, reasoning impairment, memory loss, attention deficit and orientation) and to assign the score it is necessary to understand the impact that cognitive deficits have on daily life. Moreover, cognitive slowing and reasoning impairment are not specific targets of the MOCA test.
Moreover, our study is the first to investigate synbiotic supplementation effects on alexythimia (assessed by TAS-20) and dysautonomia (assessed by SCOPA-AUT), demonstrating improvements in both non-motor symptoms.
An interesting and innovative data relating to the domain of affective disorders is the improvement of alexithymia after use of probiotics. There are no studies in the literature that have analyzed the relationship between alexithymia and the use of probiotics. The data regarding PD patients show high rates of clinically significant alexithymia29, but the neurophysiological mechanism is not clear. It is likely that alexithymia in PD represents a secondary consequence of the loss of dopaminergic input to fronto-opercular structures61. On the other hand, a previous study did not find a significant association between dopamine medication and alexithymia in PD patients62. Regardless of heterogeneous results, it would also be useful to consider the role of physiological reactivity in shaping feelings63. In fact, neural circuits involved in ‘interoception’–i.e., perception of the current state of the viscera (e.g. heart rate, perspiration, etc.)– have been suggested to play a role in emotional awareness64. Therefore, the probiotics could act on “the microbiome–gut–brain axis”, bringing advantages to the emotional brain network or amplifying the attention paid to emotional stimuli65.
The data obtained from the analysis of the fecal microbial ecosystem corroborate the clinical findings of this study. Specifically, we observed a significant increase in members of the Oscillospiraceae family, particularly F. prausnitzii, which are among the major butyrate-producing bacteria in the human colon. Numerous studies have reported a reduction in the abundance of F. prausnitziiin PD patients66. Furthermore, an increase in putative acetate-producer bacterial taxa has been suggested in PD67. Notably, acetate serves as a precursor for butyrate production in F. prausnitzii68. Accordingly, alongside the increase in this bacterium, we observed a significant rise in the ratio between butyrate and acetate levels in fecal samples following synbiotic intake. Importantly, decreased levels of bacterially produced butyrate have been associated with epigenetic changes in leukocytes and neurons from PD patients, as well as the severity of their depressive symptoms69. Therefore, we can speculate that modifications in the fecal microbiome following synbiotic intake may contribute to the improvements in behavioral and cognitive symptoms observed in the PD patients of this study.
Our data are preliminary but important because the limited differentiation of feeling states in alexithymia appears to cause patients great difficulty in regulating and resolving negative affect.
To the best of our knowledge, the current study was the first report that investigated the effect of probiotic adjuvant treatment on autonomic functions in PD patients. Autonomic dysfunction in PD includes orthostatic hypotension (OH), gastrointestinal tract dysfunction, urinary dysfunction, erectile dysfunction (ED), and diaphoresis. Gastrointestinal tract dysfunctions in PD include not only constipation but also dysphagia, drooling, nausea, gastroparesis, and incomplete bowel emptying70. The underlying causes for gastrointestinal dysfunction in PD are multifaceted: enteric synucleinopathy, dysbiosis, enteric nervous system dysfunction due to impaired dopamine signaling and central mechanisms may all contribute to their onset and clinical manifestations71.
Our study showed that the administration of a synbiotic significantly improved patients’ SCOPA-AUT scores demonstrating a global improvement of dysautonomia, and of all upper and lower gastrointestinal dysautonomia symptoms (GIDS). Our results could offer new options for helping alleviate these symptoms, ultimately improving the overall well-being of PD patients.
Our study did not demonstrate significant changes in the motor symptoms investigated with MDS-UPDRS, while some previous reports showed motor improvements after probiotics supplementations57,60. We suggest that this discrepancy may be explained by the different enrollment criteria, since in our study only patients with stable disease and without motor fluctuations were recruited.
Probiotics, by introducing beneficial bacteria to the gut, may help restore the dysbiosis of PD patients and improve communication along the gut-brain axis, possibly affecting alpha-synuclein–related neurodegeneration in the Enteric Nervous System (ENS).
Gut bacteria have been shown to produce and modulate various neurotransmitters, including glutamate, serotonin, dopamine, norepinephrine, histamine and acetylcholine and gamma-aminobutyric acid (GABA). Lactobacillus has been found to produce various neurotransmitters in vitro, with the specific neurotransmitters produced depending on the Lactobacillus species72. By influencing the production and metabolism of these neurotransmitters, probiotics may have indirect effects on non-motor symptoms in PD.
Also, chronic inflammation and immune dysregulation are thought to contribute to PD pathogenesis and progression73and specifically in non-motor symptoms such as depression, fatigue and cognitive impairment. Recent studies have shown elevated levels of serum inflammatory markers in PD patients, associated with severity of non-motor symptoms74. Probiotics can modulate the immune response and reduce inflammation by influencing the production of anti-inflammatory cytokines and promoting the integrity of the gut barrier75. Moreover, there are growing evidence of neuroprotective effects of short-chain fatty acids (SCFAs) producing bacteria such as Lactobacillus76. SCFAs can exert both systemic anti-inflammatory effects and microglia inflammation regulation, can preserve blood–brain barrier (BBB) integrity, and promote neurogenesis76. Preclinical studies in animal models have also demonstrated the potential antidepressant effects of SCFAs77. By increasing SCFA production, probiotics and prebiotics may have a positive impact on non-motor symptoms in Parkinson’s patients.
Additionally, gut microbiota is involved in drug metabolism and therefore in levodopa pharmacokinetics. Intestinal microorganisms such as Enterococcus faecalis, Helicobacter pylori, and Clostridium sporogenescan affect the metabolism and absorption of levodopa78, and dysbiosis could have implications on the absorption of levodopa from the jejunum79. Through restoration of balance in gut microbiota, synbiotic treatment could also offer a strategy to optimize levodopa metabolism and its therapeutic effects on motor and non-motor symptoms.
The main limitations of our study were the lack of a control group and the limited number of fecal samples examined. However, this open label study showed promising results on the effects of a synbiotic product on non-motor symptoms in PD., The research on probiotics in PD is still in its early stages, and further well-designed clinical trials are needed to establish the effectiveness, optimal strains, dosages, and duration of probiotic supplementation for improving non-motor symptoms in PD patients.
Author contributions
A. V.: research project conception, review and critique,of statistical analysis, writing of the first draft. C.S., B.M., F.F., F.N., E.R., D.F.M., B.G., M.M.C., S.A., S.M., G.S., B.P. : research project execution, review and critique of statistical analysis,review and critique of manuscript. I.P. and P.M.T.: research project conception and organization, statistical analysis design and execution, review and critique of manuscript All authors reviewed the manuscript.
Funding
No specific funding was received for this work.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval
All the patients gave their written informed consent to the study. The protocol was approved by the Ethics Committee of ASLNAPOLI3SUD on 25/09/2018 and registered in ClinicalTrials.gov (ref. no.: NCT04293159), and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors equally contributed to the study: P. Iovino and M. T. Pellecchia.
References
- 1.Fasano, A., Visanji, N. P., Liu, L. W., Lang, A. E. & Pfeiffer, R. F. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol.14, 625–639 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Krogh, K., Ostergaard, K., Sabroe, S. & Laurberg, S. Clinical aspects of bowel symptoms in Parkinson’s disease. Acta Neurol. Scand.117(1), 60–64 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Scheperjans, F. et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord.30(3), 350–358 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Lin, C. H. et al. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J. Neuroinflammation16(1), 129. 10.1186/s12974-019-1528-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen, T. et al. The Association between the gut microbiota and Parkinson’s Disease, a meta -analysis. Front. Aging Neurosci.13, 636545 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agirman, G., Yu, K. B. & Hsiao, E. Y. Signaling inflammation across the gut-brain axis. Science374(6571), 1087–1092. 10.1126/science.abi6087 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Stasi, C., Rosselli, M., Bellini, M., Laffi, G. & Milani, S. Altered neuro-endocrine-immune pathways in the irritable bowel syndrome: The top-down and the bottom-up model. J. Gastroenterol.47(11), 1177–1185 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Mulak, A. & Bonaz, B. Brain-gut-microbiota axis in Parkinson’s disease. World J. Gastroenterol.21(37), 10609–10620 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill, C. et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol.11, 506–514. 10.1038/nrgastro.2014.66 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Gibson, G. R. et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol.14(8), 491–502. 10.1038/nrgastro.2017.7 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Swanson, K. S. et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of symbiotics. Nat. Rev. Gastroenterol. Hepatol.17(11), 687–701. 10.1038/s41575-020-0344-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie, L., Chen, D., Zhu, X. & Cheng, C. Efficacy and safety of probiotics in Parkinson’s constipation: A systematic review and meta-analysis. Front. Pharmacol.13, 1007654. 10.3389/fphar.2022.1007654 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tran, N. et al. The gut-brain relationship: Investigating the effect of multispecies probiotics on anxiety in a randomized placebo-controlled trial of healthy young adults. J. Affect. Disord.252, 271–277. 10.1016/j.jad.2019.04.043 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Steenbergen, L., Sellaro, R., van Hemert, S., Bosch, J. A. & Colzato, L. S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun.48, 258–264. 10.1016/j.bbi.2015.04.003 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Chung, Y.-C. et al. Fermented milk of Lactobacillus helveticus IDCC3801 improves cognitive functioning during cognitive fatigue tests in healthy older adults. J. Funct. Foods10, 465–474. 10.1016/j.jff.2014.07.007 (2014). [Google Scholar]
- 16.Inoue, T. et al. Effect of combined bifidobacteria supplementation and resistance training on cognitive function, body composition and bowel habits of healthy elderly subjects. Benef. Microbes9(6), 843–853. 10.3920/BM2017.0193 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Hwang, Y. H. et al. Efficacy and safety of Lactobacillus Plantarum C29-fermented soybean (DW2009) in individuals with mild cognitive impairment: A 12-week, multi-center, randomized, double-blind, placebo-controlled clinical trial. Nutrients11(2), 305. 10.3390/nu11020305 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi, Y., Kuhara, T., Oki, M. & Xiao, J. Z. Effects of Bifidobacterium breve A1 on the cognitive function of older adults with memory complaints: A randomised, double-blind, placebo-controlled trial. Benef. Microbes10(5), 511–520. 10.3920/BM2018.0170 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Agahi, A. et al. Does severity of Alzheimer’s disease contribute to its responsiveness to modifying gut microbiota? A double blind clinical trial. Front. Neurol.9, 662. 10.3389/fneur.2018.00662 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akbari, E. et al. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: a randomized, double-blind and controlled trial. Front. Aging Neurosci.8, 256. 10.3389/fnagi.2016.00256 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamtaji, O. R. et al. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: A randomized, double-blind, controlled trial. Clin. Nutr.38(6), 2569–2575. 10.1016/j.clnu.2018.11.034 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Ou, Y. et al. Lactobacillus casei strain shirota alleviates constipation in adults by increasing the pipecolinic acid level in the gut. Front. Microbiol.21(10), 324. 10.3389/fmicb.2019.00324 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vandeputte, D. et al. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut66(11), 1968–1974. 10.1136/gutjnl-2016-313271 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.https://theromefoundation.org/rome-iv/rome-iv-criteria/ consulted on 4 July (2023).
- 25.Goetz, C. G. et al. Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord.23, 2129–2170. 10.1002/mds.22340 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Visser, M., Marinus, J., Stiggelbout, A. M. & Van Hilten, J. J. Assessment of autonomic dysfunction in Parkinson’s disease: The SCOPA-AUT. Mov. Disord.19(11), 1306–1312. 10.1002/mds.20153 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Nasreddine, Z. S. et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc.53, 695–699. 10.1111/j.1532-5415.2005.53221.x (2005). [DOI] [PubMed] [Google Scholar]
- 28.Leising, D., Grande, T. & Faber, R. The Toronto Alexithymia Scale (TAS-20): A measure of general psychological distress. J. Res. Personal.43(4), 707–710. 10.1016/j.jrp.2009.03.009 (2009). [Google Scholar]
- 29.Assogna, F. et al. Alexithymia in Parkinson’s disease: A systematic review of the literature. Parkinsonism Relat. Disord.28, 1–11. 10.1016/j.parkreldis.2016.03.021 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Baron-Cohen, S., Wheelwright, S., Hill, J., Raste, Y. & Plumb, I. The, “Reading the Mind in the Eyes” test revised version: A study with normal adults, and adults with Asperger syndrome or high-functioning autism. J. Child Psychol. Psychiatry42(2), 241–251 (2001). [PubMed] [Google Scholar]
- 31.Leentjens, A. F. et al. The Parkinson Anxiety Scale (PAS): Development and validation of a new anxiety scale. Mov. Disord.29(8), 1035–1043. 10.1002/mds.25919 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Spielberger, C., Gorsuch, R., Lushene, R., Vagg, P. R., & Jacobs, G. Manual for the State-Trait Anxiety Inventory (Form Y1 – Y2) (1983).
- 33.Beck, A. T., Steer, R. A. & Brown, G. K. Manual for the Beck Depression Inventory-II (Psychological Corporation, 1996). [Google Scholar]
- 34.Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry23(1), 56–62. 10.1136/jnnp.23.1.56 (1960). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frank, L., Kleinman, L., Farup, C., Taylor, L. & Miner, P. Jr. Psychometric validation of a constipation symptom assessment questionnaire. Scand. J. Gastroenterol.34, 870–877 (1999). [DOI] [PubMed] [Google Scholar]
- 36.Lewis, S. J. & Heaton, K. W. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol.32, 920–924 (1997). [DOI] [PubMed] [Google Scholar]
- 37.Oliviero, G. et al. Impact of COVID-19 lockdown on symptoms in patients with functional gastrointestinal disorders: Relationship with anxiety and perceived stress. Neurogastroenterol. Motil.33(5), e14092. 10.1111/nmo.14092 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gargari, G. et al. Fecal short-chain fatty acids in non-constipated irritable bowel syndrome: A potential clinically relevant stratification factor based on catabotyping analysis. Gut Microbes15(2), 2274128. 10.1080/19490976.2023.2274128 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gargari, G. et al. Fecal Clostridiales distribution and short-chain fatty acids reflect bowel habits in irritable bowel syndrome. Environ. Microbiol.20(9), 3201–3213. 10.1111/1462-2920.14271 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Mirzaei, H. et al. Probiotics and the treatment of Parkinson’s disease: An update. Cell. Mol. Neurobiol.42(8), 2449–2457. 10.1007/s10571-021-01128-w (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan, A. H. et al. Probiotics for constipation in parkinson disease: A randomized placebo-controlled study. Neurology96(5), e772–e782 (2021). [DOI] [PubMed] [Google Scholar]
- 42.Du, Y. et al. Probiotics for constipation and gut microbiota in Parkinson’s disease. Parkinsonism Relat. Disord.103, 92–97. 10.1016/j.parkreldis.2022.08.022 (2022). [DOI] [PubMed] [Google Scholar]
- 43.Ibrahim, A. et al. Multi-strain probiotics (Hexbio) containing MCP BCMC strains improved constipation and gut motility in Parkinson’s disease: A randomised controlled trial. PloS one15(12), e0244680. 10.1371/journal.pone.0244680 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cassani, E. et al. Use of probiotics for the treatment of constipation in Parkinson’s disease patients. Minerva Gastroenterol. e Dietol.57(2), 117–121 (2011). [PubMed] [Google Scholar]
- 45.Iorio, L. et al. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: An RCT. Neurology87(12), 1274–1280. 10.1212/WNL.0000000000003127 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Barichella, M. et al. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: An RCT. Neurology87(12), 1274–1280. 10.1212/WNL.0000000000003127 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Nagao, F., Nakayama, M., Muto, T. & Okumura, K. Effects of a fermented milk drink containing Lactobacillus casei strain Shirota on the immune system in healthy human subjects. Biosci. Biotechnol. Biochem.64(12), 2706–2708. 10.1271/bbb.64.2706 (2000). [DOI] [PubMed] [Google Scholar]
- 48.Zhang, Y. et al. The antioxidative effects of probiotic Lactobacillus casei Zhang on the hyperlipidemic rats. Eur. Food Res. Technol.231, 151–158. 10.1007/s00217-010-1255-1 (2010). [Google Scholar]
- 49.Matsumoto, K. et al. Effects of a probiotic fermented milk beverage containing Lactobacillus casei strain Shirota on defecation frequency, intestinal microbiota, and the intestinal environment of healthy individuals with soft stools. J. Biosci. Bioeng.110(5), 547–552. 10.1016/j.jbiosc.2010.05.016 (2010). [DOI] [PubMed] [Google Scholar]
- 50.Bărboi, O. B., Ciortescu, I., Chirilă, I., Anton, C. & Drug, V. Effect of inulin in the treatment of irritable bowel syndrome with constipation (Review). Exp. Ther. Med.20(6), 185. 10.3892/etm.2020.9315 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shoubridge, A. P. et al. The gut microbiome and mental health: Advances in research and emerging priorities. Mol. Psychiatry27, 1908–1919. 10.1038/s41380-022-01479-w (2022). [DOI] [PubMed] [Google Scholar]
- 52.Messaoudi, M. et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr.105(5), 755–764. 10.1017/S0007114510004319 (2011). [DOI] [PubMed] [Google Scholar]
- 53.Kazemi, A., Noorbala, A. A., Azam, K., Eskandari, M. H. & Djafarian, K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin. Nutr.38(2), 522–528. 10.1016/j.clnu.2018.04.010 (2019). [DOI] [PubMed] [Google Scholar]
- 54.Saccarello, A. et al. Oral administration of S-Adenosylmethionine (SAMe) and Lactobacillus Plantarum HEAL9 improves the mild-to-moderate symptoms of depression: A randomized, double-blind, placebo-controlled study. Prim. Care Companion CNS Disord.22(4), 19m02578. 10.4088/PCC.19m02578 (2020). [DOI] [PubMed] [Google Scholar]
- 55.Chen, H. M. et al. Psychophysiological effects of Lactobacillus plantarum PS128 in patients with major depressive disorder: A preliminary 8-week open trial. Nutrients13(11), 3731. 10.3390/nu13113731 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rao, A. V. et al. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog.1(1), 6. 10.1186/1757-4749-1-6 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun, H. et al. Probiotics synergized with conventional regimen in managing Parkinson’s disease. NPJ Parkinsons Dis.8(1), 62. 10.1038/s41531-022-00327-6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang, X. et al. Effect of Lacticaseibacillus Paracasei strain shirota supplementation on clinical responses, gut microbiota and faecal metabolites in patients with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Lacticaseibacillus Paracasei10.2139/ssrn.4087361 (2022). [Google Scholar]
- 59.Lu, C. S. et al. The add-on effect of Lactobacillus plantarum PS128 in patients with parkinson’s Disease: A pilot study. Front. Nutr.8, 650053. 10.3389/fnut.2021.650053 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tamtaji, O. R. et al. Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Clin. Nutr.38(3), 1031–1035. 10.1016/j.clnu.2018.05.018 (2019). [DOI] [PubMed] [Google Scholar]
- 61.Ricciardi, L. et al. Alexithymia in neurological disease: A review. J. Neuropsychiatry Clin. Neurosci.27(3), 179–187. 10.1176/appi.neuropsych.14070169 (2015). [DOI] [PubMed] [Google Scholar]
- 62.Enrici, I. et al. Emotion processing in Parkinson’s disease: A three-level study on recognition, representation and regulation. PLoS ONE10(6), e0131470 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bard, P. Emotion: I The Neuro-humoral Basis of Emotional Reactions. In A handbook of general experimental psychology 264–311 (Clark University Press, 1934). [Google Scholar]
- 64.Damasio, A., Damasio, H. & Tranel, D. Persistence of feelings and sentience after bilateral damage of the insula. Cereb. Cortex23, 833–846 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.LeDoux, J. E. & Pine, D. S. Using neuroscience to help understand fear and anxiety: A two-system framework. Am. J. Psychiatry173, 1083–1093 (2016). [DOI] [PubMed] [Google Scholar]
- 66.Nowak, J. M., Kopczyński, M., Friedman, A., Koziorowski, D. & Figura, M. Microbiota Dysbiosis in Parkinson disease-in search of a biomarker. Biomedicines10(9), 2057. 10.3390/biomedicines10092057 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu, G. et al. Serum short-chain fatty acids and its correlation with motor and non-motor symptoms in Parkinson’s disease patients. BMC Neurol.22(1), 13. 10.1186/s12883-021-02544-7 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heinken, A. et al. Functional metabolic map of Faecalibacterium prausnitzii, a beneficial human gut microbe. J. Bacteriol.196(18), 3289–3302. 10.1128/JB.01780-14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xie, A. et al. Bacterial butyrate in Parkinson’s disease is linked to epigenetic changes and depressive symptoms. Mov. Disord.37(8), 1644–1653. 10.1002/mds.29128 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alli, S. R. et al. The gut microbiome in depression and potential benefit of prebiotics, probiotics and symbiotics: A systematic review of clinical trials and observational studies. Int. J. Mol. Sci23, 4494 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fasano, A., Visanji, N. P., Liu, L. W., Lang, A. E. & Pfeiffer, R. F. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol.14(6), 625–639. 10.1016/S1474-4422(15)00007-1 (2015). [DOI] [PubMed] [Google Scholar]
- 72.Sarkar, A. et al. Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci.39(11), 763–781. 10.1016/j.tins.2016.09.002 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tansey, M. G. et al. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol.22, 657–673. 10.1038/s41577-022-00684-6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim, R. et al. Serum inflammatory markers and progression of nonmotor symptoms in early Parkinson’s disease. Mov. Disord.37(7), 1535–1541. 10.1002/mds.29056 (2022). [DOI] [PubMed] [Google Scholar]
- 75.Cristofori, F. et al. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: A door to the body. Front. Immunol.12, 578386. 10.3389/fimmu.2021.578386 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cheng, Y., Liu, J. & Ling, Z. Short-chain fatty acids-producing probiotics: A novel source of psychobiotics. Crit. Rev. Food Sci. Nutr.62(28), 7929–7959. 10.1080/10408398.2021.1920884 (2022). [DOI] [PubMed] [Google Scholar]
- 77.Tang, C. F. et al. Short-chain fatty acids ameliorate depressive-like behaviors of high fructose-fed mice by rescuing hippocampal neurogenesis decline and blood-brain barrier damage. Nutrients14(9), 1882. 10.3390/nu14091882 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nyholm, D. & Hellström, P. Effects of Helicobacter pylori on levodopa pharmacokinetics. J. Parkinsons Dis.11, 61–69. 10.3233/JPD-202298 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Kessel, S. et al. Gut bacterial deamination of residual levodopa medication for Parkinson’s disease. BMC Biol.18, 137. 10.1186/s12915-020-00876-3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.