Abstract
The aim was to evaluate predictors of clinical outcomes in infliximab (IFX)-treated Crohn’s disease (CD) patients in western China and provide evidence for future treatment optimization. Our retrospective study included CD patients at Chongqing General Hospital from July 2022 to July 2023. Clinical data of CD patients at baseline and the endpoint (the seventh IFX treatment, 38 weeks) were collected. Baseline variables of IFX-treated patients with regard to clinical remission [Crohn's Disease Activity Index (CDAI) < 150] at endpoint were assessed, and the correlation of serum vitamin D (Vit-D) levels before initiating IFX therapy and CDAI at week 38 was analyzed. Sixty patients with IFX-treated CD were included. The Vit-D-deficient rate was 51.7% at baseline, 81.7% of patients achieved clinical remission, and 66.7% achieved endoscopic remission at week 38 of IFX treatment. Vit-D level at baseline was an independent predictors of clinical remission after IFX treatment (P < 0.05). Receiver operating characteristic curve analysis showed that when Vit-D concentration was 15.81 ng/ml, the area under the curve was 0.711 (95% CI 0.523–0.899, P = 0.03). The sensitivity and specificity were 81.6% and 63.6%, respectively. Vit-D level in the normal BMI, non-smoking, immunosuppressant-treated subgroup had independent predictive value for CDAI at endpoint (P < 0.05). Baseline Vit-D level predicted clinical remission in CD patients after IFX treatment, especially in those with normal body mass index, who do not smoke, and who take IFX in combination with immunosuppressants.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10238-024-01483-0.
Keywords: Crohn’s disease, Vitamin D, Clinical remission, Infliximab, Predictors
Introduction
Inflammatory bowel diseases (IBDs) comprise a group of chronic inflammatory conditions of the gastrointestinal tract, which include Crohn’s disease (CD) and ulcerative colitis (UC) [1]. It is estimated that the disease affects up to about 3 million people in Europe, where the annual incidences of IBD are 24.3 cases/100,000 for UC and 12.7 cases/100,000 for CD [2–4]. China has the highest number of CD diagnoses in Asia (3.44 per 100,000 individuals) [5, 6].
Biologic agents against tumor necrosis factor-alpha (TNF-α) have dramatically improved the response and remission rates of CD and UC. Nevertheless, over time, a loss of response to anti-TNF-α therapy results in clinical relapse and disease progression. It is estimated that 30% of IBD patients lose response to the infliximab (IFX) throughout therapy [7]. Therefore, additional treatments, which could increase or maintain the efficacy of the anti-TNFα antibody treatment response, are of great importance [8].
In recent years, research has found that in IFX-treated IBD patients, vitamin D (Vit-D) levels were correlated with IFX plasma trough concentrations (TC) and both parameters were associated with remission. Serum Vit-D level may be a predictive marker in addition to drug TC in IBD patients treated with IFX [9]. However, clinical studies evaluating response to anti-TNF therapy and 25-hydroxyvitamin D(25-OHD) levels are limited and inconsistent. Most studies have found that Vit-D is associated with IFX treatment outcomes in CD patients [9–11], while two studies proposed opposite views [12, 13]. Yet, one of the two studies still put forward an association with the two at extreme Vit-D deficiency [12]. These studies have different conclusions due to different study designs, different countries and study populations.
In western China, latitude and sunshine are associated with higher levels of Vit-D deficiency. According to a study that included 2,317 healthy people in western China, the median 25-OHD level for all participants was 23.41 ng/mL (range 2.06 ng/mL-88.69 ng/mL). Of all samples, 35.5% and 38.6% were found to be Vit-D deficient and insufficient, respectively. Only 25.9% participants had sufficient Vit-D [14]. In addition, the western region of China is relatively backward in China due to economic development, the median diagnosis time of IBD, extra-intestinal manifestation, and perianal disease of patients in the western region are higher than those in the eastern region, and patients' diet, genetic susceptibility, health resources, attitudes toward disease, and the availability of treatments and other factors also have an important impact on the disease [15]. Therefore, it is valuable to carry out clinical studies on the relationship between Vit-D and IFX clinical efficacy in western China in order to bolster the evidence in this field.
As a result, we aimed to determine, firstly, the baseline variables of IFX-treated patients with regard to clinical remission and, secondly, the correlation of serum Vit-D levels before initiating IFX therapy with clinical remission at week 38.
Methods
Study design
This study adopted a retrospective investigation method and collected clinical data of patients in a Crohn's disease patient database at the Gastroenterology Department of Chongqing General Hospital from July 2022 to July 2023, according to the inclusion and exclusion criteria specified below. This study was approved by the Ethics Committee of Chongqing General Hospital (No. KY S 2022-023-01).
Inclusion criteria
1. CD patients diagnosed under the consensus on inflammatory bowel disease in China [16]; 2. over 18 years old and under 60 years old; 3. IFX was used for initial treatment; 4. patients with the records of baseline 25-hydroxyvitamin D levels (before the first IFX treatment); and 5. patients with recorded the Crohn's Disease Activity Index (CDAI) score at the endpoint of week 38 (the seventh IFX treatment). Exclusion criteria: 1. Patients without regular follow-up; 2. Vit-D and calcium supplementation; 3 months prior to the first IFX treatment; 3. history of glucocorticoid use 3 months before the first IFX treatment; and 4. patients with other infectious diseases, severe or chronic cardiovascular, respiratory, urinary, endocrine, reproductive, skeletal, muscular, neurological, or other systemic disorders.
The clinical data of CD patients were collected. At baseline(before the first IFX treatment), the collected clinical data included course of disease, clinical symptoms, intestinal and perianal surgery, combined immunosuppressant (IMM), Montreal classification, CDAI scores, simple endoscopic score for Crohn’s disease (SES-CD), inflammatory indicators, nutritional indicators, liver and kidney function, electrolytes, fasting blood glucose (FBG), blood lipids, and antinuclear antibodies (ANA). At the fourth IFX treatment, therapeutic drug monitoring (TDM) and anti-antibody concentration (ATI) were included. At the endpoint (the seventh IFX treatment, 38 weeks), CDAI scores, SES-CD, inflammatory indicators, and nutritional indicators were collected.
Clinical outcome assessment (including clinical remission, biochemical remission, endoscopic remission, clinical response, and endoscopic response) was performed at week 38. Patients were divided into remission group and non-remission group according to clinical remission criteria, and the relationship between all baseline indicators, TDM, ATI, and clinical remission was analyzed in both groups.
Therefore, 149 patients were initially included, and 14 were excluded due to Vit-D deficiency data (n = 13) and Vit-D treatment (n = 1); 135 eligible patients enrolled. Of these, 28 patients who had no follow-up data were excluded (23 lacked of efficacy, 21 lacked of Vit-D data, and 8 received Vit-D treatment), and between the 4th IFX and the 7th IFX treatment, 47 patients were excluded (24 lack of efficacy, 23 lack of Vit-D data, and 33 lack of CDAI scores). (See flowchart of participants and data in Supplementary Figure S1).
Clinical evaluation and classifications
IFX therapeutic doses are calculated from 5 to 10 mg/kg, meeting both the dose range and the entire infusion according to IFX specifications (100 mg/ea). Treatment intervals followed standard intervals: weeks 0, 2, and 6 in the induced remission period and every 8 weeks in the maintained remission period.
Peripheral blood 25-OHD was detected using chemiluminescence in the laboratory of the Chongqing General Hospital. The cutoff concentration for Vit-D deficiency was based on the Endocrine Society Clinical Practice Guidelines 2011 [17]: Patients with 25-OHD levels < 20 ng/mL were considered Vit-D deficient, those with > 30 ng/mL were classified as Vit-D sufficient, and those with 20–30 ng/ml were classified as Vit-D insufficient.
TDM of IFX was performed before the fourth IFX treatment using a fluorescence immunochromatography IFX detection kit (Suzhou Herui BioMed Co., Ltd.) at the Suzhou Herui IBD Diagnostic Technology Research Center. Concentrations ≥ 3ug/mL were considered sufficient [16]. ATI < 20 ng/mL was defined as negative [18]. The ATI detection kit (Suzhou Herui BioMed Co., Ltd.) is a quantitative fluorescence immunochromatographic assay tool.
At the endpoint (the seventh IFX treatment, 38 weeks), a CDAI score of < 150 was used as the clinical remission, a CDAI decrease of ≥ 70 (between baseline and the seventh IFX treatment) was used as the clinical response, a CRP < 5 was used as the biochemical remission, SES-CD ≤ 4 was an endoscopic remission, and SES-CD decrease ≥ 50% was an endoscopic response [19–22].
Statistical analysis
SPSS 26.0 statistical software was used for data analysis. The Shapiro–Wilk test was used to conduct normality test for each variable. Measurement data conforming to normal distribution were represented by mean ± standard deviation (SD); independent sample t test was used for inter-group comparison; non-normal data were represented by M (Q1,Q3); and nonparametric test was used for inter-group comparison. Categorical data use case (%) representation. Univariate analysis was carried out for each indicator, and the indicator with P < 0.1 was taken as a significant variable and included in logistic or linear regression analysis. P < 0.05 was considered statistically significant. Subgroup analysis was carried out to explore the heterogeneity of treatment effects and influencing factors among different patient populations. ROC curve analysis was performed between the above subgroup variables and the endpoint CDAI outcome. P-values < 0.05 in the final multivariate model were considered significant.
Results
Demographic characteristics
A total of 60 patients with CD met the inclusion and exclusion criteria. (See flowchart of participants and data in Supplementary Figure S1.) Patients with a median age of 27 years were enrolled in the study, and all received IFX therapy. The male-to-female ratio was 2.75 (44:16), the median Body Mass Index (BMI) was 19.97 kg/m2, and the median duration of disease was 3.81 years. 23.3% of the patients smoked and 60% had a bachelor's degree or above.
The rates of Vit-D deficiency and insufficiency were 51.67% and 40%, respectively. The main clinical symptoms were abdominal pain (60%) and diarrhea (45%), and 35% of patients received IMM in combination (including 85% who received azathioprine). According to the Montreal classification, the diagnosed age was mostly A2, the affected intestinal location was mostly L3, the disease behavior was mostly B1, and 60% had perianal lesions. 28.3% of the patients had undergone intestinal surgery, and 58.3% had undergone perianal surgery.
Treatment efficiency was evaluated before the seventh IFX treatment. 81.7% of patients achieved clinical remission, and 28.3% achieved biochemical remission. Endoscopic re-examination was performed in 42 patients, of whom 66.7% achieved endoscopic remission and 42.9% achieved endoscopic response (Table 1).
Table 1.
Demographic characteristics
| Variables | Total, n (%) or µ (IQR) |
|---|---|
| Number of patients | 60 |
| Age (y) | 27 (22.25–34) |
| Gender | |
| Female (%) | 16 (26.7) |
| Male (%) | 44 (73.3) |
| Height (m) (mean ± SD) | 1.67 ± 0.08 |
| Weight (Kg) (mean ± SD) | 54.8 ± 9.49 |
| BMI | 19.97 ± 2.88 |
| Underweight (%) | 22 (36.7) |
| Normal weight (%) | 35 (58.3) |
| Pre-obese (%) | 2 (3.3) |
| Obese (%) | 1 (1.7) |
| Vit-D | |
| Deficiency (%) | 31 (51.67) |
| Insufficiency (%) | 24 (40) |
| Sufficiency (%) | 5 (8.33) |
| Provinces | |
| Chongqing (%) | 52 (86.7) |
| Sichuan (%) | 5 (8.3) |
| Yunnan (%) | 3 (5) |
| Employment situation | |
| Unemployed (%) | 26 (43.3) |
| Employed (%) | 34 (56.7) |
| Occupation | |
| Indoor (%) | 58 (96.7) |
| Outdoor (%) | 2 (3.3) |
| Marital status | |
| Unmarried (%) | 32 (53.3) |
| Married (%) | 26 (43.3) |
| Divorced (%) | 2 (3.3) |
| Smoking status | |
| No (%) | 46 (76.7) |
| Yes (%) | 14 (23.3) |
| Education background | |
| Primary school, n (%) | 1 (1.7) |
| Middle school (%) | 10 (16.7) |
| Senior high school (%) | 13 (21.7) |
| University and above (%) | 36 (60) |
| Course of disease(y) (IQR) | 3.81 (2.4–6.61) |
| Clinical symptoms | |
| Abdominal | 36 (60) |
| Diarrhea | 27 (45) |
| Hematochezia | 3 (5) |
| Crissum diseases | 4 (6.7) |
| Extraintestinal manifestations | 2 (3.3) |
| Fever | 1 (1.7) |
| Intestinal surgery, n (%) | |
| No | 43 (71.7) |
| Yes | 17 (28.3) |
| Perianal surgery, n (%) | |
| No | 25 (41.7) |
| Yes | 35 (58.3) |
| IMM | |
| No | 39 (65) |
| Yes | 21 (35) |
| IMM type | |
| Azathioprine | 17 (85) |
| Methotrexate | 2 (10) |
| Thalidomide | 1 (5) |
| Diagnosis age, n (%) | |
| A1 ≤ 16 y | 0 (0) |
| A2 17–40y | 52 (86.7) |
| A3 > 40y | 8 (13.3) |
| Disease extent, n (%) | |
| L1 | 10 (16.7) |
| L2 | 10 (16.7) |
| L3 | 39 (65) |
| L3 + L4 | 0 (0) |
| L2 + L4 | 1 (1.7) |
| Behavior, n (%) | |
| B1 | 37 (61.7) |
| B2 | 19 (31.7) |
| B3 | 4 (6.7) |
| Perianal lesions | |
| No | 24 (40) |
| Yes | 36 (60) |
| Clinical remission | 49 (81.7) |
| Biochemical remission | 17 (28.3) |
| Clinical response | 7 (11.7) |
| Endoscopic remission | 28 (66.7) |
| Endoscopic response | 18 (42.9) |
y: year; m: meter; kg, kilogram; SD, standard deviation; BMI, body mass index; IQR, interquartile range; IMM, immunosuppressant
Baseline variables associated with clinical remission
Patients were divided into clinical remission (n = 49) and non-remission (n = 11) groups according to whether their CDAI score was < 150 at the endpoint. Among the two groups, the clinical non-remission group had a higher proportion of IMM than the remission group (81.8% vs. 24.5%, P = 0.001), but other demographic measures were not statistically different between the two groups (Supplementary materials).
Baseline biochemical markers (including inflammatory indicators such as C-reactive protein(CRP), erythrocyte sedimentation rate (ESR), high-sensitivity C-reaction protein (hs-CRP), blood routine indicators, liver and kidney function, electrolyte, micronutrient indicators (including Vit-D)), TDM, and ATI before the fourth IFX treatment were analyzed in both groups to identify baseline factors effect on clinical remission at week 38. The median IFX-TC was 4 μg/mL in the remission group and 2.1 μg/mL in the non-remission group(P = 0.061), indicating a higher drug concentration is associated with remission. Furthermore, the positive rate of ATI in remission group was lower than that in non-remission group (6.12% vs. 36.36%, P = 0.012), indicating neutralizing antibodies to IFX negatively correlated with successful treatment. Baseline Vit-D levels were higher in the clinical remission group than in the non-remission group (20.92 ng/ml vs. 16.43 ng/ml, P = 0.036), suggesting a protective effect of high resting Vit-D. Median CRP and hs-CRP levels were lower (12 mg/l vs.79.4 mg/l, P = 0.003; 21.71 mg/l vs. 66.55 mg/l, P = 0.016) and lower median ESR level (17 mm/h vs. 42 mm/h, P = 0.003). In addition, there were statistically significant differences in red blood cell (RBC), hemoglobin (Hb), prealbumin (PA), urea nitrogen (BUN), and blood Ca between the two groups (P < 0.05) (Table 2).
Table 2.
Analysis of differences in clinical factors between the two groups
| Analyte | Remission group (n = 49) | Non-remission group (n = 11) | OR (95% CI) | P |
|---|---|---|---|---|
| Vit-D | 20.92 ± 6.15 | 16.43 ± 5.7 | 0.861 (0.749–0.991) | 0.036 |
| CRP | 12 (5–46) | 79.4 (29.5–106.9) | 1.028 (1.01–1.047) | 0.003 |
| ESR | 17 (9–29.5) | 42 (27–63) | 1.053 (1.017–1.089) | 0.003 |
| Hs-CRP | 21.71 (2.61–54.39) | 66.55 (20.15–95.85) | 1.023 (1.004–1.041) | 0.016 |
| WBC | 6.61 (5.23–8.77) | 8.02 (6.33–8.51) | 1.187 (0.903–1.56) | 0.22 |
| RBC | 4.63 ± 0.6 | 4.9 ± 0.93 | 0.268 (0.091–0.785) | 0.016 |
| Hb | 121.52 ± 22.1 | 98.73 ± 23.59 | 0.956 (0.923–0.99) | 0.011 |
| MCV | 82.7 (76.45–89.55) | 79.9 (72.7–82.7) | 0.986 (0.933–1.042) | 0.614 |
| PLT | 349.77 ± 123.4 | 394.36 ± 107.79 | 1.003 (0.998–1.008) | 0.272 |
| PA | 170 (150–248.5) | 134 (102–152) | 0.974 (0.954–0.994) | 0.011 |
| ALB | 41.7 (37.65–45.15) | 35.7 (33.7–41) | 0.933 (0.857–1.016) | 0.11 |
| AST | 14.6 (11.4–20.55) | 12 (10.2–13.4) | 0.93 (0.828–1.045) | 0.222 |
| ALT | 11.1 (7.75–21.45) | 8.1 (4.7–8.7) | 0.923 (0.831–1.025) | 0.136 |
| ALP | 72.6 (60.45–93.55) | 75.3 (70–83.4) | 1.004 (0.98–1.03) | 0.727 |
| GGT | 20.3 (13.7–35) | 22.2 (17.6–33) | 1.006 (0.97–1.044) | 0.755 |
| TBil | 9 (7.3–13.25) | 6.9 (5.9–11.4) | 0.848 (0.677–1.063) | 0.153 |
| DBil | 2.1 (1.45–2.7) | 1.6 (1.1–2.8) | 0.612 (0.273–1.374) | 0.235 |
| TBA | 1.7 (1.08–2.6) | 1.25 (0.58–3.15) | 1.054 (0.737–1.507) | 0.773 |
| BUN | 4.24 ± 1.48 | 3.13 ± 0.92 | 0.486 (0.254–0.929) | 0.029 |
| Cr | 68.93 ± 15.1 | 60.34 ± 12.67 | 0.959 (0.913–1.007) | 0.09 |
| Uric Acid | 332.39 ± 95.28 | 307.3 ± 71.71 | 0.997 (0.989–1.004) | 0.409 |
| Cys-C | 0.78 (0.69–0.94) | 0.83 (0.7–0.86) | 0.972 (0.335–2.816) | 0.958 |
| Blood Ca | 2.27 ± 0.12 | 2.18 ± 0.13 | 0.003 (0–0.707) | 0.037 |
| Blood Mg | 0.85 ± 0.07 | 0.85 ± 0.11 | 0.994 (0–5327.857) | 0.999 |
| Fe | 7.8 (4.1–13.4) | 7.86 (6.38–10.38) | 0.981 (0.798–1.205) | 0.852 |
| Blood K | 4.08 (3.92–4.2) | 4.02 (3.56–4.27) | 0.169 (0.022–1.313) | 0.089 |
| Blood Na | 138.02 ± 2.18 | 138.02 ± 1.86 | 1 (0.732–1.367) | 0.998 |
| Blood P | 1.19 ± 0.23 | 1.22 ± 0.25 | 1.527 (0.076–30.586) | 0.782 |
| Blood glucose | 4.36 (3.96–4.65) | 4.35 (4.07–5.01) | 1.619 (0.695–3.773) | 0.264 |
| Serum Ferritin | 75.33 (42.78–358.56) | 151.78 (12.83–262.36) | 0.999 (0.995–1.003) | 0.612 |
| Folic acid | 6 (3–8) | 4.67 (3–5.5) | 0.841 (0.645–1.097) | 0.202 |
| VB12 | 443 (339–619) | 477 (393–572) | 1 (0.996–1.004) | 0.999 |
| EPO | 14.85 (11.5–22.2) | 16.2 (13.75–106.55) | 1.002 (0.999–1.006) | 0.177 |
| TC | 3.33 (2.82–4.17) | 0.92 (0.79–1.12) | 0.613 (0.233–1.614) | 0.322 |
| TG | 0.89 (0.68–1.12) | 0.92 (0.79–1.12) | 0.751 (0.254–2.22) | 0.604 |
| FBG | 4.2 (3.49–4.32) | 4.6 (3.87–5.15) | 1.723 (0.86–3.452) | 0.125 |
| ANA | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.999 |
| IFX-TC | 4 (0.6–6.3) | 2.1 (0.4–2.57) | 0.761 (0.571–1.014) | 0.061 |
| ATI, n(%) | 3 (6.12) | 4 (36.36) | 1.013 (0.997–1.029) | 0.012 |
OR, odds ratio; Vit-D: vitamin D; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; Hs-CRP, high-sensitivity C-reactive protein; WBC, white blood cell; RBC, red blood cell; Hb, hemoglobin; MCV, mean corpuscular volume; PLT, platelet; PA, prealbumin; ALB, albumin; AST, glutamic oxalacetic transaminase; ALT, glutamic pyruvic transaminase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transpeptidase; TBil, total bilirubin; DBil, direct bilirubin; TBA, total bile acid; BUN, urea; Cr, creatinine; UA, uric acid; Cys-C: cystatin C; VB12, vitamin B12; EPO, erythropoietin; TC, cholesterol; TG, triglycerides; FBG, fasting blood glucose; ANA, antinuclear antibodies; IFX, infliximab; TC, trough concentration; ATI, anti-TNFα antibody
The above indicators were incorporated into logistic regression for multivariate regression analysis, and the results showed that Vit-D level, CRP, and blood K at baseline were correlated with clinical remission(CDAI < 150) at the seventh IFX treatment (P < 0.05) (Table 3). Receiver operating characteristic (ROC) curve analysis showed that when Vit-D concentration was 15.81 ng/ml, the diagnostic value of CDAI (= 150) at endpoint was AUC 0.711 (95% CI 0.523–0.899), the sensitivity was 81.6%, and the specificity was 63.6% (P = 0.03) (Fig. 1), indicating blood Vit-D concentration can be used as an effective predictor of positive IFX treatment outcomes in Chinese adults with CD.
Table 3.
Relation between baseline factors and clinical remission at week 38
| Variables | OR(95%CI) | P |
|---|---|---|
| Univariate analysis | ||
| Vit-D | 0.861 (0.749–0.991) | 0.036 |
| CRP | 1.028 (1.01–1.047) | 0.003 |
| ESR | 1.053 (1.017–1.089) | 0.003 |
| Hs-CRP | 1.023 (1.004–1.041) | 0.016 |
| RBC | 0.268 (0.091–0.785) | 0.016 |
| Hb | 0.956 (0.923–0.99) | 0.011 |
| PA | 0.974 (0.954–0.994) | 0.011 |
| BUN | 0.486 (0.254–0.929) | 0.029 |
| Cr | 0.959 (0.913–1.007) | 0.09 |
| Blood Ca | 0.003 (0–0.707) | 0.037 |
| Blood K | 0.169 (0.022–1.313) | 0.089 |
| Multivariate analysis | ||
| Vit-D | 0.766 (0.615–0.953) | 0.017 |
| CRP | 1.091 (1.02–1.167) | 0.011 |
| Hs-CRP | 0.938 (0.877–1.005) | 0.068 |
| Blood K | 0.023 (0.001–0.501) | 0.017 |
Vit-D, vitamin D; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; Hs-CRP, high-sensitivity C-reactive protein; RBC, red blood cell; Hb, hemoglobin; PA, prealbumin; BUN, urea; Cr, creatinine
Fig. 1.
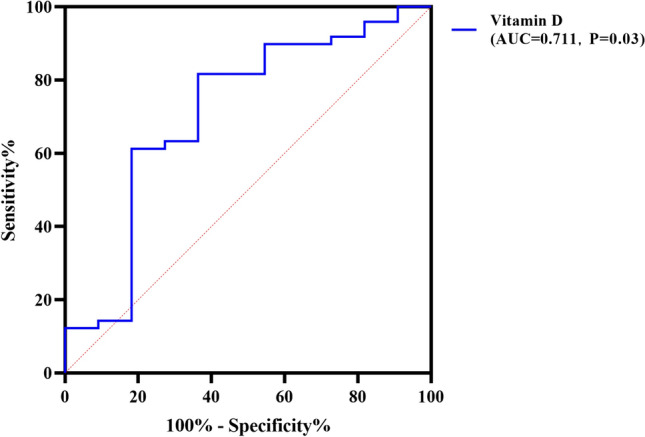
ROC curve of vitamin D level for diagnostic prediction
Subgroup association analysis of baseline variables
Baseline variables were divided into subgroups to determine the influencing factors on clinical remission in patients with CD. Due to the limited sample size, CDAI score was taken as the dependent variable, and significant factors in univariate analysis were included in linear regression for multi-factor analysis. When patients were grouped by gender, then RBC, Hs-CRP, and mean corpuscular volume (MCV) were independent predictors of CDAI score at the endpoint in the female subgroup, while Vit-D, ESR, and hs-CRP were independent predictors of CDAI in the male subgroup (P < 0.05). Patients were divided into low body weight group and normal body weight group according to BMI. It was found that no independent predictor of CDAI was found in the low body weight group, while baseline Hb, folic acid, and FBG levels in the normal body weight subgroup were independent predictors of CDAI (P < 0.05).
Dividing by smoking status, ESR and blood K levels were independent factors in the smoking group, while Vit-D, BMI, and CRP levels were independent factors in the non-smoking group (P < 0.05). Grouped according to whether or not they had undergone intestinal surgery, Vit-D, ESR, and Hs-CRP were found to be independent predictors of CDAI in patients without intestinal surgery, while RBC and BUN levels were independent predictors of CDAI in patients with intestinal surgery (P < 0.05). Grouping by perianal surgery or not, Vit-D, CRP, and course of disease were found to be independent predictors of CDAI in the non-perianal surgery group, while Vit-D, TB, blood P, and ANA levels were independent predictors in the subgroup that had perianal surgery (P < 0.05). Grouping by IMM use or not, Vit-D and WBC were found to be independent predictors of CDAI in the no combined IMM group, while ESR, RBC, Hb, DB, and BUN levels were independent predictors in the subgroup that had combined IMM (P < 0.05) (Table 4 and Supplementary materials).
Table 4.
Factors influencing clinical remission in patients with subgroups analysis
| Subgroup | Multivariate | |
|---|---|---|
| Variables | Beta (95% CI) | P |
| Gender | ||
| Male | ||
| Vit-D | − 2.942 (− 4.93 to − 0.954) | 0.005 |
| ESR | 0.95 (0.1–1.799) | 0.03 |
| Hs-CRP | − 0.618 (− 1.178 to − 0.058) | 0.032 |
| Female | ||
| RBC | − 47.136 (− 81.29 to − 12.982) | 0.011 |
| Hs-CRP | 0.886 (0.409–1.363) | 0.002 |
| MCV | − 1.612 (− 3.189 to − 0.034) | 0.046 |
| BMI | ||
| Normal | ||
| Hb | − 1.505 (− 2.66 to − 0.35) | 0.014 |
| Folic acid | − 7.674 (− 13.081 to − 2.266) | 0.009 |
| FBG | 18.203 (4.236–32.17) | 0.014 |
| Smoking | ||
| Yes | ||
| ESR | 1.102 (0.381–1.823) | 0.009 |
| K | − 62.436 (− 98.176 to − 26.697) | 0.004 |
| No | ||
| Vit-D | − 3.449 (− 5.406 to − 1.493) | 0.001 |
| BMI | − 4.214 (− 7.845 to − 0.582) | 0.024 |
| CRP | 0.282 (0.03–0.533) | 0.029 |
| Intestinal surgery | ||
| No | ||
| Vit-D | − 2.284 (− 4.193 to − 0.376) | 0.021 |
| ESR | 1.493 (0.609–2.378) | 0.002 |
| Hs-CRP | − 0.677 (− 1.23 to − 0.124) | 0.018 |
| Yes | ||
| RBC | − 38.407 (− 64.477 to − 12.338) | 0.008 |
| BUN | − 18.395 (− 31.307 to − 5.484) | 0.009 |
| Perianal surgery | ||
| No | ||
| Vit-D | − 2.991 (− 5.459 to − 0.523) | 0.022 |
| CRP | 0.35 (0.013–0.688) | 0.043 |
| Course of disease | 4.604 (0.4–8.809) | 0.035 |
| Yes | ||
| Vit-D | − 4.169 (− 7.922 to − 0.416) | 0.033 |
| TB | − 5.7 (− 9.162 to − 2.239) | 0.005 |
| Blood P | − 147.827 (− 248.852 to − 46.802) | 0.009 |
| ANA | − 82.995 (− 141.145 to − 24.845) | 0.01 |
| Combined IMM | ||
| No | ||
| Vit-D | − 2.194 (− 4.003 to − 0.385) | 0.02 |
| WBC | 5.094 (1.019–9.169) | 0.017 |
| Yes | ||
| ESR | 1.757 (0.742–2.771) | 0.002 |
| RBC | − 21.1 (− 41.65 to − 0.549) | 0.045 |
| Hb | 1.172 (0.281–2.063) | 0.014 |
| DB | − 22.948 (− 44.891 to − 1.005) | 0.042 |
| BUN | − 17.693 (− 27.591 to − 7.796) | 0.002 |
Vit-D, vitamin D; ESR, erythrocyte sedimentation rate; Hs-CRP, high-sensitivity C-reaction protein; RBC, red blood cell; MCV, mean corpuscular volume; BMI, body mass index; Hb, hemoglobin; FBG, fasting blood glucose; CRP,C-reactive protein; BUN, urea; TBil, total bilirubin; ANA, antinuclear antibodies; WBC, white blood cell; DBil, direct bilirubin
Further, ROC curve analysis was performed between the above subgroup variables and the endpoint CDAI outcomes. In the normal BMI subgroup, when Vit-D level was 16.06 ng/ml, the diagnostic value of CDAI(= 150) at endpoint was AUC (95%CI) 0.77(0.556–0.985), and the sensitivity and specificity were 0.82 and 0.71 (P = 0.029), suggesting a stronger effect in those with normal weight. In the no smoking and combined with IMM subgroup, when Vit-D level was 19.05 ng/ml and 15.81 ng/ml, respectively, the diagnostic value of CDAI(= 150) at endpoint was AUC (95% CI) 0.74 (0.54–0.94) and 0.81 (0.604–1), the sensitivity was 0.568 and 0.917, and the specificity was 0.889 (P = 0.026) and 0.667 (P = 0.019). Thus, this subgroup might benefit particularly strong from Vit-D supplementation in Crohn's disease (Table 5).
Table 5.
Diagnostic efficiency of different subgroups for endpoint CDAI outcomes by ROC curve
| Subgroup | Diagnostic value (Vit-D baseline level ng/ml) | AUC(95%CI) | Sensitivity | Specificity | P |
|---|---|---|---|---|---|
| Male | 18.95 | 0.73 (0.513–0.938) | 0.65 | 0.86 | 0.061 |
| BMI: normal weight | 16.06 | 0.77 (0.556–0.985) | 0.821 | 0.71 | 0.029 |
| No smoking | 19.05 | 0.742 (0.541–0.942) | 0.568 | 0.89 | 0.026 |
| Without intestinal surgery | 18.95 | 0.7 (0.50–0.90) | 0.6 | 0.88 | 0.081 |
| Without perianal surgery | 15.8 | 0.68 (0.43–0.94) | 0.789 | 0.67 | 0.181 |
| With perianal surgery | 18.95 | 0.71 (0.43–1.0) | 0.7 | 0.8 | 0.131 |
| Combine with IMM | 15.81 | 0.81 (0.604–1) | 0.917 | 0.67 | 0.019 |
CDAI, Crohn's Disease Activity Index; ROC, receiver operating characteristic; Vit-D, vitamin D; BMI, Body Mass Index; IMM, immunosuppressant
Discussion
Strategies to improve response rates to first-line anti-TNF therapies in CD patients are a key line of research in light of drug resistance. The current study aimed to evaluate predictors of clinical outcomes in 60 IFX-treated CD patients from the Chongqing area in western China. At baseline, over 50% of patients were vitamin D-deficient, suggesting a widespread lack of this nutrient. Despite this, 81.7% of patients achieved clinical remission of their Crohn's disease after a 38-week course of infliximab (anti-TNF) treatment.
Vit-D level at baseline was one of the key independent predictors of clinical remission in CD patients after IFX treatment (P < 0.05). ROC curve analysis showed an AUC of 0.711, indicating a high predictive power of Vit-D levels on disease outcomes. Gender, BMI, smoking status, history of intestinal or perianal surgery, and combination therapy with immunosuppressants were all statistically significant in subgroup analysis. Specifically, Vit-D level in those with normal BMI, non-smoking, and taking IMM significantly predicted better outcomes at endpoint (P < 0.05).
To the best of our knowledge, this is the first study in which an association was found between Vit-D and IFX treatment efficacy among CD patients in western China. It provides a reference for clinical optimization of IFX treatment and lays a foundation for the study of the mechanism of Vit-D in IFX-treated IBD.
These findings are consistent with most current research and mainstream opinion. The underlying mechanism between Vit-D and IFX-treated efficiency can enhance the expression of inflammatory cytokines, inhibit the expression and activity of Vit-D receptor (VDR), and lead to the enhancement of downstream inflammatory signals [23]. Some studies have found that intestinal epithelial cells are tightly connected and rely on Vit-D to maintain their integrity. In experimental mice with colitis, the Vit-D receptor gene (VDR-/-) was knocked out, and the VDR-/-mice were more prone to epithelial injury, which was typically characterized by the loss of intestinal epithelial tight junction and the destruction of epithelial integrity, increasing the chance of exogenous bacterial invasion [24–28]. In addition, Vit-D acts as an important balancing factor for adaptive immune activation, it can regulate the proliferation of T cells and B cells, and inhibit the expression of various pro-inflammatory factors [29, 30].
In terms of clinical research, many teams have reported the relationship between Vit-D level and clinical outcome of CD patients and the therapeutic effect of biologic agents [9, 31, 32]. The lack or insufficiency of Vit-D level is more common in CD patients. And patients with low Vit-D level have worse clinical remission and treatment effect of biologic agents. Vit-D can even be used as a predictor of the long-term efficacy of biologics in CD patients, and Vit-D supplementation in these patients can improve the efficacy of biologics. In this study, our finding is that Vit-D can be used as a molecular marker to predict long-term efficacy of biologics in patients with CD, which can be used as a supporting supplement to other studies. Second, our subgroup analysis looked at different clinical indicators; we found through analysis that CRP and blood K at baseline were correlated with clinical remission (CDAI < 150) at the seventh IFX treatment (weeks 38), which is consistent with other research team’s finding that the relationship between IFX exposure and maintain CRP remission [33].
In addition, we also found that the positive rate of ATI before the fourth IFX treatment was associated with clinical remission (P = 0.012). Some research have demonstrated that Vit-D levels were positively correlated with the duration of anti-TNF-α drug response, and patients with Vit-D deficiency with Vit-D deficiency are more likely to stop continuing IFX therapy because of low drug response or early onset of anti-antibodies [9, 34, 35]. What’s more, we also found that ANA levels were one of independent predictors in the subgroup that had perianal surgery. Some previous studies found that Vit-D deficiency was related to positivity for ANA (before starting anti-TNF) and anti-TNF treatment was related to ANA development. Pretreatment positivity for ANA was related to failure of the first anti-TNF [36, 37]. It suggests that further studies on these two aspects can be conducted or the combination of Vit-D can be used as a more accurate clinical predictor of IFX treatment efficacy.
This study has some defects: First, it is a single-center retrospective design of study, the sample size is small, and the causal relationship between Vit-D and clinical remission needs to be further verified in observational studies. However, retrospective single-center study is necessary for us to preliminarily establish the relationship between clinical variables, which can reflect the correlation between clinical indicators of Vit-D and clinical remission variables of patients treated with biologics, and lay the foundation for further prospective and multi-center studies. In view of the bias of single-center, retrospective and small-sample studies, we try to adopt statistics, screen out the influence of each single variable on the dependent variable after single-factor analysis, and then conduct multi-factor regression analysis to take several potentially significant variables into consideration, so as to analyze the influencing factors more comprehensively, which is more in line with the actual clinical situation. At the same time, we also use subgroup analysis to control the error deviation of the baseline level as much as possible. Second, data about dietary intake of Vit-D, calcium, and exposure to sun are insufficient. Third, in the detection of Vit-D level, high-performance liquid chromatography with mass spectrometry was superior to radioimmunoassay in the accuracy of the results. In the following research, we should collect sufficient information on the influencing factors of the research object and evaluate the advanced test technical method.
In summary, this work constitutes a major contribution of important clinical data on an understudied population (western Chinese patients with CD). Further, the results of our analysis have broad implications for patient populations around the world as IFX is currently first-line therapy for IBD in many countries. With the increasing rate of IBD incidence with industrialization and globalization, and the incidence of resistance to these biologic therapies, research and clinical data are greatly needed to understand and improve the efficacy of treatments and cures.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the Chongqing Municipal Health Commission, Chongqing Municipal Science and Technology Bureau, Chongqing Municipal Education Commission, and Children’s Hospital of Chongqing Medical University for providing funding support. The authors also thank the associate editor and reviewers for their constructive feedback that improved this paper.
Author contributions
The authors confirm contribution to the paper as follows: XQ Z and H G contributed to study conception and design; XM S, HH Z, JY S, and H W performed data collection; HH Z, XM S, and XQ Z performed interpretation of results; XM S, HH Z, XQ Z, and H G performed manuscript preparation. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by [Chongqing Municipal Education Commission, Grant Number [2021] 27-KJQN202100455] and [Science-Health Joint Medical Scientific Research Project of Chongqing, Young and Middle-aged Medical Talents Program, Grant Number 2023GDRC015].
Data availability
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author/s.
Declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hao Wang, Email: 13629755546@163.com.
Hong Guo, Email: hguo_CGH2021@163.com.
Xiaoqin Zhou, Email: zhouxiaoqin_20@126.com.
References
- 1.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–29. [DOI] [PubMed] [Google Scholar]
- 2.Ye Y, Pang Z, Chen W, Ju S, Zhou C. The epidemiology and risk factors of inflammatory bowel disease. Int J Clin Exp Med. 2015;8:22529–42. [PMC free article] [PubMed] [Google Scholar]
- 3.M’Koma AE. Inflammatory bowel disease: an expanding global health problem. Clin Med Insights Gastroenterol. 2013;6:33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burisch J, Munkholm P. The epidemiology of inflammatory bowel disease. Scand J Gastroenterol. 2015;50:942–51. [DOI] [PubMed] [Google Scholar]
- 5.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42; quiz e30. [DOI] [PubMed]
- 6.Ng SC, Tang W, Ching JY, Wong M, Chow CM, Hui AJ, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology. 2013;145:158-165.e2. [DOI] [PubMed] [Google Scholar]
- 7.Qiu Y, Chen B-L, Mao R, Zhang S-H, He Y, Zeng Z-R, et al. Systematic review with meta-analysis: loss of response and requirement of anti-TNFα dose intensification in Crohn’s disease. J Gastroenterol. 2017;52:535–54. [DOI] [PubMed] [Google Scholar]
- 8.Bendix M, Dige A, Jørgensen SP, Dahlerup JF, Bibby BM, Deleuran B, et al. Seven weeks of high-dose vitamin d treatment reduces the need for infliximab dose-escalation and decreases inflammatory markers in Crohn’s disease during one-year follow-up. Nutrients. 2021;13:1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mechie N-C, Mavropoulou E, Ellenrieder V, Kunsch S, Cameron S, Amanzada A. Distinct association of serum vitamin D concentration with disease activity and trough levels of infliximab and adalimumab during inflammatory bowel disease treatment. Digestion. 2020;101:761–70. [DOI] [PubMed] [Google Scholar]
- 10.Hizarcioglu-Gulsen H, Kaplan JL, Moran CJ, Israel EJ, Lee H, Winter H. The impact of vitamin D on response to anti-tumor necrosis factor-α therapy in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2021;72:e125–31. [DOI] [PubMed] [Google Scholar]
- 11.Mechie N-C, Mavropoulou E, Ellenrieder V, Petzold G, Kunsch S, Neesse A, et al. Serum vitamin D but not zinc levels are associated with different disease activity status in patients with inflammatory bowel disease. Medicine (Baltimore). 2019;98: e15172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santos-Antunes J, Nunes AC-R, Lopes S, Macedo G. The relevance of vitamin D and antinuclear antibodies in patients with inflammatory bowel disease under anti-TNF treatment: a prospective study. Inflamm Bowel Dis. 2016;22:1101–6. [DOI] [PubMed] [Google Scholar]
- 13.Chanchlani N, Lin S, Smith R, Roberts C, Nice R, McDonald TJ, et al. Pretreatment vitamin D concentrations do not predict therapeutic outcome to anti-TNF therapies in biologic-naïve patients with active luminal Crohn’s disease. Crohns Colitis 360. 2023;5:1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Li K, Li J, Luo Y, Cheng Y, Jian M, et al. Ethnic, geographic, and seasonal differences of vitamin D status among adults in south-west China. J Clin Lab Anal. 2020;34: e23532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wan J, Shen J, Wu X, Zhong J, Chen Y, Zhu L, et al. Geographical heterogeneity in the disease characteristics and management of patients with inflammatory bowel disease, the preliminary results of a Chinese database for IBD (CHASE-IBD). Therap Adv Gastroenterol. 2023;16:17562848231210368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inflammatory Bowel Disease Group, Chinese Society of Gastroenterology, Chinese Medical Association. Chinese consensus on diagnosis and treatment in inflammatory bowel disease (2018, Beijing). J Dig Dis. 2021;22:298–317. [DOI] [PubMed]
- 17.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. [DOI] [PubMed] [Google Scholar]
- 18.Bauman LE, Xiong Y, Mizuno T, Minar P, Fukuda T, Dong M, et al. Improved population pharmacokinetic model for predicting optimized infliximab exposure in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2020;26:429–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Satsangi J, Silverberg MS, Vermeire S, Colombel J-F. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Hoeve K, Dreesen E, Hoffman I, Van Assche G, Ferrante M, Gils A, et al. Higher infliximab trough levels are associated with better outcome in paediatric patients with inflammatory bowel disease. J Crohns Colitis. 2018;12:1316–25. [DOI] [PubMed] [Google Scholar]
- 21.Best WR, Becktel JM, Singleton JW, Kern F. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439–44. [PubMed] [Google Scholar]
- 22.Danese S, Sandborn WJ, Colombel J-F, Vermeire S, Glover SC, Rimola J, et al. Endoscopic, radiologic, and histologic healing with Vedolizumab in patients with active Crohn’s disease. Gastroenterology. 2019;157:1007-1018.e7. [DOI] [PubMed] [Google Scholar]
- 23.Szymczak-Tomczak A, Ratajczak AE, Kaczmarek-Ryś M, Hryhorowicz S, Rychter AM, Zawada A, et al. Pleiotropic effects of vitamin D in patients with inflammatory bowel diseases. J Clin Med. 2022;11:5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu S, Yoon S, Zhang Y-G, Lu R, Xia Y, Wan J, et al. Vitamin D receptor pathway is required for probiotic protection in colitis. Am J Physiol Gastrointest Liver Physiol. 2015;309:G341-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu S, Zhang Y-G, Lu R, Xia Y, Zhou D, Petrof EO, et al. Intestinal epithelial vitamin D receptor deletion leads to defective autophagy in colitis. Gut. 2015;64:1082–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdo J, Rai V, Agrawal DK. Interplay of Immunity and Vitamin D: Interactions and Implications with Current IBD Therapy. Curr Med Chem. 2017;24:852–67. [DOI] [PubMed] [Google Scholar]
- 27.Dimitrov V, White JH. Vitamin D signaling in intestinal innate immunity and homeostasis. Mol Cell Endocrinol. 2017;453:68–78. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Cui X, Li J, Wang H, Li Y, Chen Y, et al. Clinical evaluation of vitamin D status and its relationship with disease activity and changes of intestinal immune function in patients with Crohn’s disease in the Chinese population. Scand J Gastroenterol. 2021;56:20–9. [DOI] [PubMed] [Google Scholar]
- 29.Ogawa K, Matsumoto T, Esaki M, Torisu T, Iida M. Profiles of circulating cytokines in patients with Crohn’s disease under maintenance therapy with infliximab. J Crohns Colitis. 2012;6:529–35. [DOI] [PubMed] [Google Scholar]
- 30.Billiet T, Cleynen I, Ballet V, Claes K, Princen F, Singh S, et al. Evolution of cytokines and inflammatory biomarkers during infliximab induction therapy and the impact of inflammatory burden on primary response in patients with Crohn’s disease. Scand J Gastroenterol. 2017;52:1086–92. [DOI] [PubMed] [Google Scholar]
- 31.Xia S-L, Min Q-J, Shao X-X, Lin D-P, Ma G-L, Wu H, et al. Influence of vitamin D3 supplementation on infliximab effectiveness in Chinese patients with Crohn’s disease: A retrospective cohort study. Front Nutr. 2021;8: 739285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song X, Zhang H, Wang H, Li Z, Zhou X, Guo H. Correlation between treatment outcomes and serum vitamin D levels as well as infliximab trough concentration among Chinese patients with Crohn’s disease. Gastroenterol Res Pract. 2023;2023:6675401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grisic A-M, Eser A, Huisinga W, Reinisch W, Kloft C. Quantitative relationship between infliximab exposure and inhibition of C-reactive protein synthesis to support inflammatory bowel disease management. Br J Clin Pharmacol. 2021;87:2374–84. [DOI] [PubMed] [Google Scholar]
- 34.Kabbani TA, Koutroubakis IE, Schoen RE, Ramos-Rivers C, Shah N, Swoger J, et al. Association of vitamin D level with clinical status in inflammatory bowel disease: a 5-year longitudinal study. Am J Gastroenterol. 2016;111:712–9. [DOI] [PubMed] [Google Scholar]
- 35.Winter RW, Collins E, Cao B, Carrellas M, Crowell AM, Korzenik JR. Higher 25-hydroxyvitamin D levels are associated with greater odds of remission with anti-tumour necrosis factor-α medications among patients with inflammatory bowel diseases. Aliment Pharmacol Ther. 2017;45:653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pink AE, Fonia A, Allen MH, Smith CH, Barker JNWN. Antinuclear antibodies associate with loss of response to antitumour necrosis factor-alpha therapy in psoriasis: a retrospective, observational study. Br J Dermatol. 2010;162:780–5. [DOI] [PubMed] [Google Scholar]
- 37.Rodrigues S, Lopes S, Magro F, Cardoso H, Horta e Vale AM, Marques M, et al. Autoimmune hepatitis and anti-tumor necrosis factor alpha therapy: A single center report of 8 cases. World J Gastroenterol. 2015;21:7584–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author/s.


