Abstract
Background
For patients with clinically node-positive (cN+) breast cancer undergoing neoadjuvant chemotherapy (NAC), retrieving previously clipped, biopsy-proven positive lymph nodes during sentinel lymph node biopsy [i.e., targeted axillary dissection (TAD)] may reduce false negative rates. However, the overall utilization and impact of clipping positive nodes remains uncertain.
Patients and Methods
We retrospectively analyzed cN+ ISPY-2 patients (2011–2022) undergoing axillary surgery after NAC. We evaluated trends in node clipping and associations with type of axillary surgery [sentinel lymph node (SLN) only, SLN and axillary lymph node dissection (ALND), or ALND only] and event-free survival (EFS) in patients that were cN+ on a NAC trial.
Results
Among 801 cN+ patients, 161 (20.1%) had pre-NAC clip placement in the positive node. The proportion of patients that were cN+ undergoing clip placement increased from 2.4 to 36.2% between 2011 and 2021. Multivariable logistic regression showed nodal clipping was independently associated with higher odds of SLN-only surgery [odds ratio (OR) 4.3, 95% confidence interval (CI) 2.8–6.8, p < 0.001]. This was also true among patients with residual pathologically node-positive (pN+) disease. Completion ALND rate did not differ based on clip retrieval success. No significant differences in EFS were observed in those with or without clip placement, both with or without successful clip retrieval [hazard ratio (HR) 0.85, 95% CI 0.4–1.7, p = 0.7; HR 1.8, 95% CI 0.5–6.0, p = 0.3, respectively].
Conclusion
Clip placement in the positive lymph node before NAC is increasingly common. The significant association between clip placement and omission of axillary dissection, even among patients with pN+ disease, suggests a paradigm shift toward TAD as a definitive surgical management strategy in patients with pN+ disease after NAC.
Keywords: Neoadjuvant chemotherapy, Breast cancer, Clipped node, Sentinel lymph node surgery, Targeted axillary dissection
Breast cancer management typically requires an assessment of axillary lymph node status to inform prognosis and guide therapeutic decision making. Historically, axillary lymph node dissection (ALND) was the standard of care for axillary staging but is associated with significant morbidity, including a 6–30% risk of lymphedema.1–3 Over the last two decades, several prospective, randomized clinical trials have demonstrated the reliability of using sentinel lymph node (SLN) surgery as a less morbid means for nodal staging in patients with clinically node negative (cN0) disease without impacting recurrence or survival when compared with ALND.4–7 There has consequently been a notable reduction in the use of ALND, with SLN surgery being utilized for axillary staging in patients with cN0 disease in both in the setting of upfront surgery and after neoadjuvant chemotherapy (NAC).4,7–11
However, for patients with cN+ disease who proceed to upfront surgery and for those who have residual nodal disease after NAC, current guidelines recommend ALND.8 Since advances in systemic therapy have led to higher rates of complete pathologic nodal response after NAC, there is increasing interest in accurately identifying patients with cN+ disease who have eradication of nodal disease (i.e., convert to pN0) and can be spared ALND. While several studies have shown that traditional SLN surgery in patients with cN+ disease after NAC results in false negative rates (FNR) exceeding 10%, several techniques have been identified that may mitigate this problem.12–14 Specifically, the ACOSOG Z1071 trial showed that a FNR < 10% can be achieved with the use of dual tracer and excision of ≥ 3 SLNs.12
An additional technique for reducing the FNR of SLN surgery after NAC in patients with cN+ disease includes placement of a clip in the biopsy-proven positive lymph node prior to NAC and subsequent resection at the time of SLN surgery.15 This combination of SLN surgery and removal of the previously clipped node has been termed targeted axillary dissection (TAD) and has been shown to be associated with FNRs as low as 2.4%.16 Findings from the recent, prospective SenTa study suggest that TAD without ALND confers similar survival and recurrence outcomes compared with TAD with ALND17, and further data from prospective trials are awaited regarding the optimal management of pathologic node positive disease after NAC. The implementation of lymph node clipping and subsequent localization and resection of the clipped node is inconsistent, however, and its utility has been debated.18,19
In this study, we analyzed data from the ISPY-2 trial, a prospective, randomized, multicenter NAC trial to understand the prevalence of nodal clipping and associated axillary surgical procedures. While the ISPY-2 trial protocol includes recommendations for axillary management, the specific surgical approach is not mandated, making these data a unique representation of nodal clipping practices across 24 medical centers in the USA, in the context of a trial that includes careful preoperative imaging (with serial breast magnetic resonance imaging) and thorough pathologic assessment of surgical specimens.
Patients and Methods
Study Population and Data Collection
ISPY-2 is a multicenter, randomized neoadjuvant chemotherapy trial for patients with molecularly high-risk breast cancer (NCT01042379). Per protocol, patients are randomized to neoadjuvant novel systemic therapy agents followed by surgical resection, with pathologic complete response (pCR) rates being the primary study endpoint. While type of breast and axillary surgery is not dictated by the trial, recommended standards for axillary management within the trial have been published and include the following: axillary ultrasound and percutaneous needle biopsy of the most abnormal node, if abnormal nodes are present, is required. For patients with cN+ disease, SLN surgery is permitted but requires use of dual tracer, with resection of all sentinel nodes and removal of at least two nodes required if no clip was placed. Placement of a clip in the positive axillary node in patients with node positive disease and preoperative localization of the clipped node is strongly recommended but not required; for those patients with a clipped node, resection of the clipped node should be performed. In instances of pathological positive node(s), additional axillary surgery is not obligated and is left to the discretion of the treating surgeon in both cN0 and cN+ groups.10,20 Of note, in rare instances where axillary management guidelines are not met, patients are still evaluated on trial; however, in the unusual occurrence of patients undergoing no axillary surgery at all, the primary efficacy endpoint is administratively considered a non-pCR, since pCR status cannot be ascertained.
We retrospectively analyzed all patients with cN+ breast cancer who were enrolled in ISPY-2 from 01/2011 to 12/2021. Clinical node positivity was determined by percutaneous needle biopsy (fine needle aspiration or core needle biopsy) of an abnormal axillary node prior to NAC. All included patients received NAC followed by axillary surgery, categorized as either SLN-only surgery, SLN and axillary dissection (ALND), or ALND-only, based on procedures defined in operative reports. We collected baseline clinicopathologic features, including age, self-reported race/ethnicity, tumor receptor subtype, clinical T (cT) category at diagnosis, clinical N (cN) category at diagnosis, pathologic T (pT) category, pathologic N (pN) category, residual cancer burden (RCB) class, year and type of axillary surgery, nodal clip placement, and number of nodes removed. Patients were categorized into one of two groups: those with clip placement in the biopsy-proven axillary lymph node prior to NAC and those without clip placement in an axillary lymph node prior to NAC. Clip localization method was determined by review of operative, pathology, and/or imaging reports. Successful clip retrieval was determined by confirmation of the clip and/or clip localization device being removed at the time of surgery within any operative reports, pathology reports, and/or postoperative hospital visit records. For cases in which there was no mention of clip or localization device removal at the time of surgery, these cases were categorized as “No mention of clip removal” and were excluded from analyses of clip retrieval rates. For cases in which there was confirmation of a localization device being removed but no mention of whether or not the clip was retrieved (n = 18), we assumed these cases to have successful clip retrieval. Event-free survival (EFS) was defined as patient survival without local or distant breast cancer recurrence or death; patients without a recurrence event or death were censored at the date of last follow-up.
We specifically investigated (1) what proportion of cN+ patients had a clip placed in an axillary lymph node prior to NAC and whether this has changed over time, (2) whether patients with a clip placed in a lymph node underwent different axillary surgery procedures compared with those without a clip placed, and (3) whether clipping a node is associated with higher rates of completion axillary dissection, particularly in those for whom the clip is not retrieved. Finally, we investigated EFS in those with and without nodal clip placement, as well as in those with and without successful clip retrieval.
Statistical Analyses
Demographic information and clinicopathologic features were compared between patients with and without a clipped node using Pearson’s chi-squared test, Fisher’s exact test, and Wilcoxon rank-sum test. A multivariable logistic regression model was developed to assess the influence of lymph node clipping on use of SLN only, adjusted for surgery year and cN category. Event-free survival was evaluated using the log-rank test and Kaplan–Meier survival analysis. Univariable and multivariable Cox proportional hazards models were used to assess hazard ratios (HRs) with 95% confidence intervals (CIs). Statistical analyses were performed using R version 4.3.1. Two-tailed p values < 0.05 were considered statistically significant.
Results
Clinicodemographic Information and Trends in Lymph Node Clipping
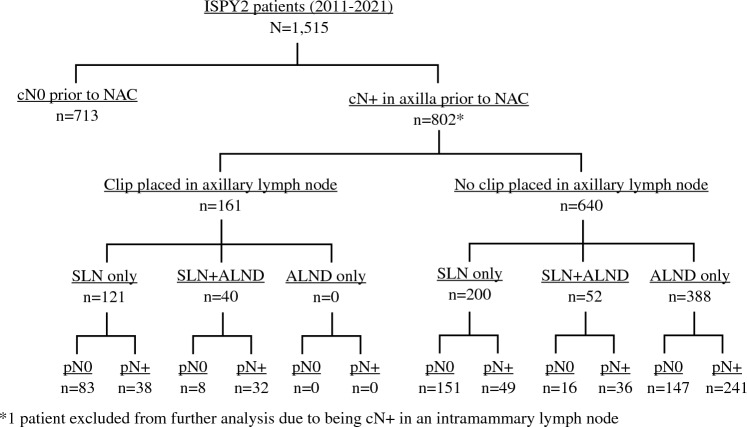
Between 2011 and 2021, there were 1515 patients enrolled in ISPY-2 who completed NAC and surgery, with 802 (52.9%) identified as cN+. One cN+ case was excluded from our analyses due to being cN+ in an intramammary node but missing axillary nodal status. After this exclusion, our final cohort consisted of 801 patients with clinical node positivity in the axilla (Fig. 1). Average age ± standard deviation (SD) of the 801 patients with cN+ disease was 48.1 ± 11.3 years. Tumor receptor subtype was hormone receptor (HR) positive and HER2 negative in 50.0% of cases (401 patients), HER2 positive in 22.6% (181 patients), and HR negative and HER2 negative in 27.3% (219 patients). The majority of patients with cN+ disease had cN1 disease (80.8%), 8.2% had cN2 disease, and 11% had cN3 disease at presentation.
Fig. 1.
Patient distribution by cN status, use of clip placement in an axillary node, and type of axillary surgery. cN0 clinically node negative, cN+ clinically node positive, NAC neoadjuvant chemotherapy, SLN sentinel lymph node, ALND axillary lymph node dissection, pN0 pathologically node negative, pN+ pathologically node positive
Among these 801 cN+ patients, 161 (20.1%) had a clip placed in a biopsy-proven positive axillary node prior to NAC, while 640 (79.9%) did not. The proportion of patients with cN+ disease undergoing clip placement increased over time from 2.4% in 2011 to 36.2% in 2021 (Table 1). Compared with those without clipped nodes, the clipped node cohort had a higher proportion of patients with cT1 (3.1% versus 0.5%) and cT2 disease (66% versus 58%), and a higher proportion with residual cancer burden (RCB) class 0 (42% versus 30%) (Table 1). There was no difference in age, tumor receptor subtype, or type of breast surgery performed between the clipped and nonclipped cohorts.
Table 1.
Characteristics and clinicopathologic features of cN+ patients with and without clipped nodes
| Use of nodal clipping | ||||
|---|---|---|---|---|
| Characteristic | Overall, N = 801a |
Clipped node, N = 161a | No clipped node, N = 640a |
p valueb |
| Age | 48.09 (11.28) | 46.94 (11.45) | 48.38 (11.22) | 0.2 |
| Surgery year | < 0.001 | |||
| 2011 | 41/801 (5.1%) | 1/41 (2.4%) | 40/41 (98%) | |
| 2012 | 59/801 (7.4%) | 1/59 (1.7%) | 58/59 (98%) | |
| 2013 | 70/801 (8.7%) | 6/70 (8.6%) | 64/70 (91%) | |
| 2014 | 74/801 (9.2%) | 6/74 (8.1%) | 68/74 (92%) | |
| 2015 | 78/801 (9.7%) | 10/78 (13%) | 68/78 (87%) | |
| 2016 | 62/801 (7.7%) | 11/62 (18%) | 51/62 (82%) | |
| 2017 | 78/801 (9.7%) | 15/78 (19%) | 63/78 (81%) | |
| 2018 | 76/801 (9.5%) | 16/76 (21%) | 60/76 (79%) | |
| 2019 | 114/801 (14%) | 35/114 (31%) | 79/114 (69%) | |
| 2020 | 80/801 (10.0%) | 35/80 (44%) | 45/80 (56%) | |
| 2021 | 69/801 (8.6%) | 25/69 (36%) | 44/69 (64%) | |
| Race | 0.069 | |||
| Asian | 53/801 (6.6%) | 14/161 (8.7%) | 39/640 (6.1%) | |
| Black | 103/801 (13%) | 12/161 (7.5%) | 91/640 (14%) | |
| Other | 15/801 (1.9%) | 2/161 (1.2%) | 13/640 (2.0%) | |
| White | 630/801 (79%) | 133/161 (83%) | 497/640 (78%) | |
| Ethnicity | 0.6 | |||
| Hispanic or Latino | 98/801 (12%) | 17/161 (11%) | 81/640 (13%) | |
| Not Hispanic or Latino | 699/801 (87%) | 144/161 (89%) | 555/640 (87%) | |
| Unknown | 4/801 (0.5%) | 0/161 (0%) | 4/640 (0.6%) | |
| Tumor subtype | 0.056 | |||
| HR+HER2− | 401/801 (50%) | 88/161 (55%) | 313/640 (49%) | |
| HR−HER2− | 219/801 (27%) | 48/161 (30%) | 171/640 (27%) | |
| HER2+ | 181/801 (23%) | 25/161 (16%) | 156/640 (24%) | |
| Clinical T category at diagnosis | 0.001 | |||
| T1 | 8/801 (1.0%) | 5/161 (3.1%) | 3/640 (0.5%) | |
| T2 | 477/801 (60%) | 106/161 (66%) | 371/640 (58%) | |
| T3 | 272/801 (34%) | 47/161 (29%) | 225/640 (35%) | |
| T4 | 44/801 (5.5%) | 3/161 (1.9%) | 41/640 (6.4%) | |
| Clinical N category at diagnosis | 0.082 | |||
| N0 | 0/801 (0%) | 0/161 (0%) | 0/640 (0%) | |
| N1 | 647/801 (81%) | 140/161 (87%) | 507/640 (79%) | |
| N2 | 66/801 (8.2%) | 8/161 (5.0%) | 58/640 (9.1%) | |
| N3 | 88/801 (11%) | 13/161 (8.1%) | 75/640 (12%) | |
| Pathologic T category | 0.036 | |||
| T0 | 236 /798 (30%) | 62/160 (39%) | 174/638 (27%) | |
| T1 | 240/798 (30%) | 45/160 (28%) | 195/638 (31%) | |
| T2 | 153/798 (19%) | 29/160 (18%) | 124/638 (19%) | |
| T3 | 93/798 (12%) | 13/160 (8.1%) | 80/638 (13%) | |
| T4 | 14/798 (1.8%) | 0/160 (0%) | 14/638 (2.2%) | |
| Tis | 62/798 (7.8%) | 11/160 (6.9%) | 51/638 (8.0%) | |
| Unknown | 3 | 1 | 2 | |
| Pathologic N category | 0.008 | |||
| N0 | 405/801 (51%) | 91/161 (57%) | 314/640 (49%) | |
| N1 | 253/801 (32%) | 56/161 (35%) | 197/640 (31%) | |
| N2 | 99/801 (12%) | 11/161 (6.8%) | 88/640 (14%) | |
| N3 | 44/801 (5.5%) | 3/161 (1.9%) | 41/640 (6.4%) | |
| RCB class | <0.001 | |||
| 0 | 260/801 (32%) | 68/161 (42%) | 192/640 (30%) | |
| 1 | 100/801 (12%) | 17/161 (11%) | 83/640 (13%) | |
| 2 | 273/801 (34%) | 60/161 (37%) | 213/640 (33%) | |
| 3 | 168/801 (21%) | 16/161 (9.9%) | 152/640 (24%) | |
| Type of breast surgery | 0.12 | |||
| Partial mastectomy | 281/734 (38%) | 54/139 (39%) | 227/595 (38%) | |
| Nipple-sparing mastectomy | 107/734 (15%) | 25/139 (18%) | 82/595 (14%) | |
| Skin-sparing mastectomy | 111/734 (15%) | 26/139 (19%) | 85/595 (14%) | |
| Total mastectomy | 235/734 (32%) | 34/139 (24%) | 201/595 (34%) | |
| Unknown | 67 | 22 | 45 | |
| Type of axillary surgery | <0.001 | |||
| SLN Only | 321/801 (40%) | 121/161 (75%) | 200/640 (31%) | |
| ALND and SLN | 92/801 (11%) | 40/161 (25%) | 52/640 (8.1%) | |
| ALND only | 388/801 (48%) | 0/161 (0%) | 388/640 (61%) | |
| Average total number of nodes removed at surgery | 11.37 (8.81) | 6.89 (6.57) | 12.50 (8.95) | < 0.001 |
| SLN only subset |
N = 321 4.15 (2.91) |
N = 121 4.21 (3.14) |
N = 200 4.11 (2.77) |
0.9 |
| ALND and SLN subset |
N = 92 15.52 (8.27) |
N = 40 14.98 (7.59) |
N = 52 15.94 (8.81) |
0.8 |
| ALND only subset |
N = 388 16.37 (8.07) |
N = 0 0 (0) |
N = 388 16.37 (8.07) |
– |
| Total positive nodes removed at surgery | 2.12 (4.08) | 1.27 (2.44) | 2.34 (4.37) | 0.005 |
| Number of positive sentinel nodes removed | 0.51 (1.69) | 0.76 (1.09) | 0.45 (1.80) | < 0.001 |
Data expressed as mean (SD) or n/N (%).
HR hormone receptor, HER2 human epidermal growth factor receptor 2, RCB residual cancer burden, SLN sentinel lymph node, ALND axillary lymph node dissection
aMean (SD); n/N (%)
bWilcoxon rank-sum test; Pearson’s chi-squared test; Fisher’s exact test
Associations between Clip Placement and Type of Axillary Surgery
Overall, axillary surgery performed was SLN only in 40.1%, SLN and ALND in 11.5%, and ALND only in 48.4% (Table 1). On univariate analysis, the clipped node cohort had a significantly higher proportion of those who underwent SLN-only surgery (75.2% versus 31.2%, p < 0.001) and a concomitant lower proportion undergoing ALND-only surgery (0% versus 60.6%, p < 0.001) compared with the nonclipped node cohort (Table 1). Clip placement remained associated with a significantly higher rate of SLN-only surgery compared with no clip placement within each cN category (75% versus 34% for cN1, p < 0.001; 63% versus 21% for cN2, p = 0.023; 85% versus 23% for cN3, p < 0.001) (Table 2). When adjusted for both year of surgery and cN category, multivariable logistic regression showed that nodal clipping was independently associated with higher odds of SLN-only surgery [odds ratio (OR) 4.3, 95% CI 2.8–6.8, p < 0.001].
Table 2.
Type of axillary surgery by clip placement and by cN category
| Type of axillary surgery | ||||
|---|---|---|---|---|
| Variable | N | All other axillary surgery, N = 371a | SLN surgery only, N = 276a | p valueb |
| Patients with cN1 | ||||
| Nodal clip placement | 647 | < 0.001 | ||
| No clip placed | 336/507 (66%) | 171/507 (34%) | ||
| Clip placed | 35/140 (25%) | 105/140 (75%) | ||
| Patients with cN2 | ||||
| Nodal clip placement | 66 | 0.023 | ||
| No clip placed | 46/58 (79%) | 12/58 (21%) | ||
| Clip placed | 3/8 (38%) | 5/8 (63%) | ||
| Patients with cN3 | ||||
| Nodal clip placement | 88 | < 0.001 | ||
| No clip placed | 58/75 (77%) | 17/75 (23%) | ||
| Clip placed | 2/13 (15%) | 11/13 (85%) | ||
an/N (%)
bPearson’s chi-squared test
Clip placement remained associated with SLN-only surgery even among patients who remained pathologically node positive (pN+). Among the 161 patients in the clipped node cohort, pN+ disease was found in 70 (43.5%). Of those 70 patients with pN+ disease, axillary surgical management was SLN only in 54.3%, SLN and ALND in 45.7%, and ALND only in none. Among the 640 patients in the nonclipped node cohort, pN+ disease was found in 326 (50.9%). Of those 326 patients with pN+ disease, surgical management of the axilla was SLN only in 15%, SLN and ALND in 11%, and ALND only in 74%. Patients with pN+ in the clipped node group were significantly more likely to undergo SLN-only surgery compared with patients with pN+ in the nonclipped node cohort (54.3% versus 15.0%, p < 0.001).
Clip Localization and Retrieval Rates
Clip localization method and retrieval status were available in 147 of 161 clipped node cases (91.3%). The overall clip retrieval rate was 85.7% (126/147) and the preoperative clip localization rate was 73.5% (108/147). Use of clip localization was associated with a higher rate of successful retrieval of the clipped node compared with procedures done without localization (96% versus 56%, p < 0.001). When stratified by type of localization, successful retrieval of the clipped node did not differ by localization method (MagSeed: 25/25, 100%; radioactive seed: 29/30, 97%; Savi Scout 32/33, 97%; wire 18/20, 90%; p = 0.356). The rate of completion axillary dissection did not differ between cases with successful clip retrieval (n = 126) compared with those without successful clip retrieval (n = 21) (completion axillary dissection rate of 24.6% versus 23.8%, respectively, p > 0.9). Of the clipped node patients who underwent completion axillary dissection, 16.1% in the clip-retrieval group were pN0, and 40% of those without clip retrieval were pN0. In the overall subset of patients with clip placement who converted to pN0 status (n = 83), there was no difference in the rate of completion axillary dissection among those with clip retrieval versus no clip retrieval (7.2% versus 14.3%, p = 0.3).
Number of Nodes Removed in SLN-Only Cohort
Among the 321 patients who underwent SLN-only surgery, the average number of nodes excised did not differ between clipped node (n = 121) and nonclipped node (n = 200) patients (mean 4.2 versus 4.1 nodes, p = 0.9). However, within the clipped node cohort, the use of clip localization (i.e., TAD) was associated with greater number of lymph nodes removed compared with those undergoing SLN-only surgery without clip localization (mean 4.6 versus 3.4 nodes, p = 0.011).
Event-Free Survival in cN+ Cohort
With mean follow-up time of 3.5 years (standard deviation 1.9), there were 146 patients with events, including 49 local recurrences (of which 12 cases involved axillary recurrence), 109 distant recurrences, and 80 deaths. Of those 12 patients who experienced axillary recurrence, 33.3% had undergone SLN only, 6.7% had undergone SLN and ALND, and 50.0% had undergone ALND. By pathologic nodal response, the axillary recurrence rate was 0.7% in patients with ypN0 disease and 2.3% in patients with ypN+ disease (p = 0.074).
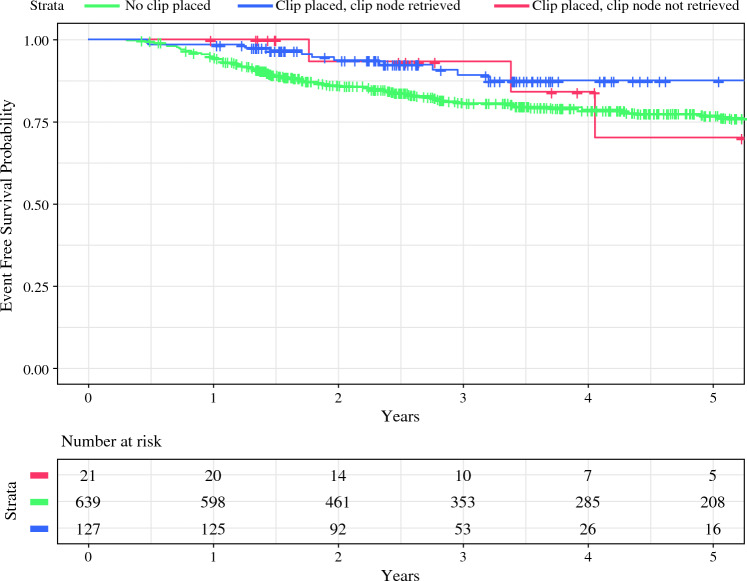
In unadjusted evaluation of EFS at 5 years by log-rank test, the cumulative estimated EFS was 77% for those without clip placement, 87% for those with a clip placed and successfully retrieved, and 70% for those with a clip placed but not retrieved (p = 0.053) (Fig. 2). In a multivariable Cox proportional hazards model adjusted for age, race/ethnicity, tumor receptor subtype, cT category, cN category, residual cancer burden (RCB) class, year of surgery, type of breast and axillary surgery, and number of nodes removed, there was no significant difference in EFS between those without clip placement compared with those with (HR 0.7, 95% CI 0.4–1.7, p = 0.7) or without (HR 1.8, 95% CI 0.5–6.0, p = 0.3) successful clip retrieval (Table 3).
Fig. 2.
Kaplan-Meier survival analysis for event-free survival (EFS) based on clip placement and clip retrieval status
Table 3.
Univariable and multivariable Cox proportional hazards model for event-free survival (EFS) in cN+ patients with and without a clipped node
| EFS univariate analysis | EFS multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | N | Event N | HRa | 95% CIa | p valueb | HRa | 95% CIa | p valueb |
| Clip placement | ||||||||
| No clip placed | 639 | 128 | – | – | – | – | ||
| Clip placed and clipped node retrieved | 127 | 11 | 0.48 | 0.26, 0.89 | 0.019 | 0.85 | 0.42, 1.74 | 0.7 |
| Clip placed, but clipped node not retrieved | 21 | 3 | 0.76 | 0.24, 2.38 | 0.6 | 1.80 | 0.54, 6.01 | 0.3 |
| Age at screening | 801 | 146 | 1.00 | 0.98, 1.01 | 0.5 | 1.00 | 0.98, 1.01 | 0.8 |
| Surgery year | ||||||||
| 2011 | 41 | 13 | – | – | – | – | ||
| 2012 | 59 | 12 | 0.65 | 0.30, 1.43 | 0.3 | 0.66 | 0.29, 1.50 | 0.3 |
| 2013 | 70 | 17 | 0.80 | 0.39, 1.65 | 0.5 | 1.05 | 0.50, 2.19 | 0.9 |
| 2014 | 74 | 16 | 0.71 | 0.34, 1.47 | 0.4 | 0.56 | 0.26, 1.19 | 0.13 |
| 2015 | 78 | 17 | 0.73 | 0.36, 1.51 | 0.4 | 0.69 | 0.33, 1.46 | 0.3 |
| 2016 | 62 | 12 | 0.68 | 0.31, 1.49 | 0.3 | 0.59 | 0.26, 1.37 | 0.2 |
| 2017 | 78 | 17 | 0.80 | 0.39, 1.66 | 0.6 | 0.78 | 0.36, 1.68 | 0.5 |
| 2018 | 76 | 13 | 0.72 | 0.33, 1.56 | 0.4 | 0.74 | 0.33, 1.66 | 0.5 |
| 2019 | 114 | 14 | 0.53 | 0.25, 1.13 | 0.10 | 0.72 | 0.32, 1.63 | 0.4 |
| 2020 | 80 | 7 | 0.46 | 0.18, 1.17 | 0.10 | 0.62 | 0.22, 1.71 | 0.4 |
| 2021 | 69 | 8 | 0.86 | 0.35, 2.13 | 0.8 | 0.00 | 0.00, inf. | > 0.9 |
| Race | ||||||||
| White | 630 | 113 | – | – | – | – | ||
| Asian | 53 | 8 | 0.81 | 0.40, 1.67 | 0.6 | 0.62 | 0.29, 1.33 | 0.2 |
| Black | 103 | 24 | 1.35 | 0.87, 2.10 | 0.2 | 1.24 | 0.75, 2.04 | 0.4 |
| Other | 15 | 1 | 0.41 | 0.06, 2.94 | 0.4 | 1.27 | 0.17, 9.52 | 0.8 |
| Ethnicity | ||||||||
| Not Hispanic or Latino | 699 | 130 | – | – | – | – | ||
| Hispanic or Latino | 98 | 16 | 0.92 | 0.55, 1.55 | 0.8 | 1.22 | 0.69, 2.16 | 0.5 |
| Unknown | 4 | 0 | 0.00 | 0.00, Inf | > 0.9 | 0.00 | 0.00, inf. | > 0.9 |
| Clinical T Category at diagnosis | ||||||||
| T1/T2 | 485 | 67 | – | – | – | – | ||
| T3/T4 | 316 | 79 | 2.04 | 1.47, 2.82 | < 0.001 | 1.52 | 1.05, 2.21 | 0.026 |
| Clinical N category at diagnosis | ||||||||
| N1 | 647 | 102 | – | – | – | – | ||
| N2 | 66 | 25 | 2.78 | 1.80, 4.31 | <0.001 | 2.77 | 1.72, 4.47 | < 0.001 |
| N3 | 88 | 19 | 1.49 | 0.91, 2.43 | 0.11 | 2.08 | 1.20, 3.60 | 0.009 |
| Tumor receptor subtype | ||||||||
| HR+ HER2– | 401 | 68 | – | – | – | – | ||
| HER2+ | 181 | 20 | 0.59 | 0.36, 0.97 | 0.037 | 1.11 | 0.64, 1.91 | 0.7 |
| HR– HER2– | 219 | 58 | 1.70 | 1.20, 2.42 | 0.003 | 2.72 | 1.83, 4.05 | < 0.001 |
| RCB class | ||||||||
| 0 | 260 | 14 | – | – | – | – | ||
| 1 | 100 | 15 | 2.89 | 1.40, 5.99 | 0.004 | 2.29 | 1.02, 5.15 | 0.045 |
| 2 | 273 | 60 | 4.52 | 2.53, 8.09 | <0.001 | 5.89 | 3.11, 11.2 | < 0.001 |
| 3 | 168 | 57 | 7.55 | 4.21, 13.6 | <0.001 | 8.55 | 4.39, 16.6 | < 0.001 |
| Type of axillary surgery | ||||||||
| All other axillary surgery | 480 | 112 | – | – | – | – | ||
| SLN surgery only | 321 | 34 | 0.48 | 0.33, 0.71 | <0.001 | 0.67 | 0.38, 1.18 | 0.2 |
| Type of breast surgery | ||||||||
| Partial mastectomy | 281 | 39 | – | – | – | – | ||
| Nipple sparing mastectomy | 107 | 17 | 1.21 | 0.69, 2.15 | 0.5 | 1.14 | 0.62, 2.08 | 0.7 |
| Skin sparing mastectomy | 111 | 19 | 1.30 | 0.75, 2.25 | 0.3 | 0.98 | 0.53, 1.82 | > 0.9 |
| Total mastectomy | 235 | 62 | 1.97 | 1.32, 2.94 | < 0.001 | 1.35 | 0.85, 2.14 | 0.2 |
| Total Nodes Removed | 801 | 146 | 1.02 | 1.01, 1.04 | 0.009 | 0.99 | 0.96, 1.01 | 0.3 |
HR hazard ratio, CI confidence interval, HR hormone receptor, HER2 human epidermal growth factor receptor 2, RCB residual cancer burden, SLN sentinel lymph node
aHR hazard ratio, CI confidence interval
bWald test
Discussion
In this analysis evaluating patients with cN+ disease treated with NAC on the ISPY-2 trial, we found that rates of nodal clipping have increased significantly over the last decade and this practice is associated with increased use of SLN surgery, independent of surgery year and clinical nodal burden. Importantly, the clipped node cohort had significantly lower rates of axillary dissection than the nonclipped node cohort, even in the setting of residual nodal disease. Additionally, patients with clipped nodes had a comparable number of nodes removed at SLN surgery/TAD compared with those without a clip placed, and there was no difference in EFS between clipped and nonclipped node patients. Overall, these findings reflect a paradigm shift in the management of the axilla, suggesting that surgeons are increasingly utilizing TAD as definitive surgical management of the axilla rather than as a staging procedure in the post-NAC setting.
TAD was initially conceived as a staging procedure to address limitations in the accuracy of SLN biopsy alone for patients with cN+ disease after NAC. Since 2013, several prospective trials have evaluated the accuracy of SLN surgery after NAC in patients with cN+. The initial key trials demonstrated FNRs ranging from 8.4 to 14.2%.12–14 In recent years, the use of clip placement prior to NAC to facilitate identification and resection of the biopsy proven lymph node at time of surgery has been proposed as a means of reducing the FNR of SLN surgery after NAC. Specifically, the term TAD has been coined to describe cases where the index positive node is clipped during biopsy and subsequently localized preoperatively in addition to SLN surgery. However, use of TAD has been inconsistent.15,16,21
Our study showed that both clip placement and SLN surgery have significantly increased over time in patients with cN+ disease and that clip placement was independently associated with SLN-only surgery overall and within each cN category. Additionally, we found that among patients with pN+ disease after NAC, axillary dissection was omitted in 54% of the clipped node group, compared with only 15% of the nonclipped node group. These findings may reflect the fact that surgeons who adopted the practice of node clipping also adopted SLN surgery after NAC; however, the high rates of omitting axillary dissection in patients with pN+ disease who underwent TAD suggest that clip placement may have been pursued with the intention of limiting axillary surgery regardless of nodal response. While not yet the standard of care, such approaches are increasingly employed in the effort to minimize the morbidity of axillary management and reduce the risk of lymphedema, particularly in the setting of data suggesting that tumor biology and response are more strongly related to recurrence risk than extent of surgery.22 This approach has been described as “tailored axillary surgery,” with the goal of selectively removing positive nodes to achieve residual disease burden that can be controlled with radiotherapy.23 The ongoing prospective, multicenter, international TAXIS trial is currently testing whether tailored axillary surgery with nodal radiation is noninferior to ALND with nodal radiation in patients with nodal disease in both the adjuvant and post-neoadjuvant settings.23,24
In our analysis we note that clips were more likely to be placed in patients who had lower cT category disease at diagnosis. This observation suggests that the decision to place clips may be influenced by pre-NAC staging factors. Additionally, patients with a greater response to NAC, evidenced by lower RCB, were also more likely to have clips placed at diagnosis. However, it is important to acknowledge that the decision to place clips is typically made prior to the onset of NAC. Therefore, while these associations may suggest a selective use of clip placement in patients who were anticipated to potentially avoid ALND after NAC, we cannot conclude causality.
Reassuringly, we found no difference in the number of nodes removed during SLN surgery for clipped versus nonclipped patients. While the ISPY-2 trial guidelines recommend localization of clipped nodes prior to surgery, the specific localization method is not mandated and not all patients with clipped nodes had preoperative localization performed. We found that the method of localization was not associated with differential rates of successful clip retrieval. When clip localization was used, the clipped node was more likely to be retrieved, at the cost of a slightly but significantly higher number of total nodes removed (mean of 4.6 versus 3.4 nodes, p = 0.011). One hypothesis for this difference in number of lymph nodes removed is that when localization is used, surgeons are more likely to continue to search for additional nodes to ensure specific removal of the localizer and the clipped node. Alternatively, to avoid missing the localized node, surgeons may be resecting a larger specimen that includes the marked lymph node and additional adjacent nodes leading to a higher number of nodes resected in patients with clip localization. However, it is worth noting that this variance may lack clinical significance, thereby supporting the value of localization use for identifying the clipped node. Our findings differ from those in the OMA study, which showed fewer nodes removed with TAD compared with SLN surgery without clip localization and removal (mean of 3 versus 4 nodes, respectively).25 However, the OMA study only included patients who converted to pN0 status, while ours included a high proportion of patients who remained pN+. Initial analyses from the ongoing TAXIS study showed that tailored axillary surgery in those with residual nodal disease excised a median of 4 lymph nodes (with interquartile range 3–5), which appears somewhat in line with our findings.26
Finally, we found no differences in EFS for those with or without a clip, or for those without successful clip retrieval. However, we acknowledge that our cohort of patients with clips placed had a lower burden of disease, potentially confounding our results. Yet, our finding is still consistent with recent studies demonstrating overall low nodal recurrence rates for patients with cN+ disease,27,28 a trend that remains true regardless of nodal clipping.18,19
Overall, our findings show that surgeons are increasingly utilizing nodal clipping in patients with cN+ disease prior to NAC, and this practice is associated with potential benefit of deescalation of axillary surgery without demonstrated harm thus far. Nonetheless, placing a clip in the node at time of diagnosis is potentially an additional procedure with associated financial costs, as is the localization procedure which is performed after NAC. If clip placements are performed at the time of needle biopsy, it likely presents minimal risk and a small amount of extra time for patients and radiologists/surgeons. The use of ultrasound-visible clips can facilitate localization with intraoperative ultrasound, but other factors such as visibility and ease of identification must be considered.29 The impact of various clips on magnetic resonance imaging (MRI) interpretation is also a consideration, particularly in the I-SPY2 trial, which employs serial MRI to assess response while on NAC. Advances in localization techniques will be essential as the use of TAD and tailored axillary surgical approaches increases.
These data from our multicenter study across the United States suggest that clip placement in patients with cN+ disease is increasingly common, possibly both for the purposes of improving the sensitivity of SLN surgery after NAC, and also to facilitate definitive axillary management even in patients with pN+ disease, consistent with tailored axillary surgery. While our study benefits from the multicenter nature and rigorous data collection in the setting of a prospective trial, there are numerous limitations to these analyses that must be acknowledged. First, the ISPY-2 trial was not designed to test surgical interventions. Accordingly, surgical procedures performed are subject to selection bias, and some information, including the use of clip placement, was collected retrospectively. Although we identified a strong association between clip placement and avoidance of ALND, even when adjusted for surgical year and cN category, we cannot conclude causation. As we do not know the decision-making process underlying management decisions, we can only postulate based on our observations. Future work will include surveying surgeons regarding axillary management decisions, particularly the omission of ALND for patients with pN+ disease despite the lack of prospective, randomized data supporting this approach. Importantly, data on the use of radiotherapy was not available for this analysis, which is a critical consideration when evaluating recurrence outcomes. Lastly, we were also limited by our mean follow-up time of 3.5 years, although all patients had molecularly high-risk breast cancer, which has a higher risk of earlier recurrence.
Conclusion
Analysis of 801 patients with cN+ disease from a prospective NAC trial showed that clip placement in a positive axillary lymph node prior to NAC is increasingly common and is independently associated with SLN-only and facilitates avoidance of ALND with no negative impact on EFS. Clip placement in the node at the time of diagnosing cN+ disease may help tailor the surgical approach to the axilla.
Funding
The ISPY-2 clinical trial is funded by Quantum Leap.
Disclosure
J.C.B. reports institutional research grant from Eli Lilly and SimBioSys; royalties from UpToDate; honoraria from EndoMag, PER, PeerView, and OncLive; received travel support from EndoMag and Alliance for Clinical Trials in Oncology; is a member of DSMC for Cairns Surgical; serves on board of directors for ASBS; and is an unpaid working group Chair at Society of Surgical Oncology Breast Disease Site. C.Y. reports institutional research grant from NCI/NIH; salary and travel support from Quantum Leap Healthcare Collaborative (QLHC); University of California Inventor Share; and patent “Breast cancer response prediction subtypes” (no. 18/174,491). M.G. reports funding from the Breast Cancer Research Foundation. M.P. reports institutional research funding and travel support from Intuitive Surgical; honoraria from Kubtec Medical Imaging and OncLive; participation on DSMB at Mayo Clinic; and co-development contract with Optiscan. M.H.M. reports roles on the SSO Development Committee and ASBrS Scientific Committee. M.C.L. reports institutional research grant and honoraria from Elucent Medical; participation on the Provepharm Advisory Board; and owns stock in J&J. S.F. reports consulting fees from Stryker and institutional equipment support from Montefiore. I.G. reports consulting fees from Abbvie and honoraria from OncLive/MJH Life Sciences. K.H. reports publishing royalties from UpToDate and participation in the QLHC DSMB. C.S. reports participation on the Executive Committee for AJCC. L.J.E. reports institutional research funding from Moderna for DCIS trial; publishing royalties from UpToDate; and travel support from and participation on Medical Advisory Board at Blue Cross Blue Shield. R.M. reports research support and honoraria from GE Healthcare and NCI; institutional research funding from UCSF; and is an unpaid member of the Scientific Advisory Board for the Lobular Breast Cancer Alliance. C.R. reports previous consultant for Elucent Medical (localizing device), terminated association 12/2022, and consultant for agenda, terminated 12/2022. J.T. reports institutional primary investigator on clinical trial for Intuitive Surgical.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bland KI, Scott-Conner CE, Menck H, Winchester DP. Axillary dissection in breast-conserving surgery for stage I and II breast cancer: a National Cancer Data Base study of patterns of omission and implications for survival. J Am Coll Surg. 1999;188(6):586–95. 10.1016/s1072-7515(99)00056-3. [DOI] [PubMed] [Google Scholar]
- 2.Khafagy M, Mostafa A, Fakhr I. Distribution of axillary lymph node metastases in different levels and groups in breast cancer, a pathological study. J Egyptian Natl Cancer Instit. 2011;23(1):25–30. 10.1016/j.jnci.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Lucci A, McCall LM, Beitsch PD, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25(24):3657–63. 10.1200/jco.2006.07.4062. [DOI] [PubMed] [Google Scholar]
- 4.Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11(10):927–33. 10.1016/S1470-2045(10)70207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349(6):546–53. 10.1056/NEJMoa012782. [DOI] [PubMed] [Google Scholar]
- 6.Veronesi U, Paganelli G, Viale G, et al. Sentinel-lymph-node biopsy as a staging procedure in breast cancer: update of a randomised controlled study. Lancet Oncol. 2006;7(12):983–90. 10.1016/s1470-2045(06)70947-0. [DOI] [PubMed] [Google Scholar]
- 7.Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: The American College of Surgeons Oncology Group Z0011 Randomized Trial. Ann Surg. 2010;252(3):426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gradishar WJ, Moran MS, Abraham J, et al. Breast Cancer, Version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2022;20(6):691–722. 10.6004/jnccn.2022.0030. [DOI] [PubMed] [Google Scholar]
- 9.Hunt KK, Yi M, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy is accurate and reduces the need for axillary dissection in breast cancer patients. Ann Surg. 2009;250(4):558–66. 10.1097/SLA.0b013e3181b8fd5e. [DOI] [PubMed] [Google Scholar]
- 10.Boughey JC, Yu H, Dugan CL, et al. Changes in surgical management of the axilla over 11 years—report on more than 1500 breast cancer patients treated with neoadjuvant chemotherapy on the prospective I-SPY2 trial. Ann Surg Oncol. 2023;30(11):6401–10. 10.1245/s10434-023-13759-y. [DOI] [PubMed] [Google Scholar]
- 11.Geng C, Chen X, Pan X, Li J. The feasibility and accuracy of sentinel lymph node biopsy in initially clinically node-negative breast cancer after neoadjuvant chemotherapy: a systematic review and meta-analysis. PLoS One. 2016;11(9):e0162605. 10.1371/journal.pone.0162605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310(14):1455–61. 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33(3):258–64. 10.1200/jco.2014.55.7827. [DOI] [PubMed] [Google Scholar]
- 14.Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14(7):609–18. 10.1016/s1470-2045(13)70166-9. [DOI] [PubMed] [Google Scholar]
- 15.Boughey JC, Ballman KV, Le-Petross HT, et al. Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0–T4, N1–N2) who receive neoadjuvant chemotherapy: results from ACOSOG Z1071 (Alliance). Ann Surg. 2016;263(4):802–7. 10.1097/sla.0000000000001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caudle AS, Yang WT, Krishnamurthy S, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016;34(10):1072–8. 10.1200/jco.2015.64.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuemmel S, Heil J, Bruzas S, et al. Safety of targeted axillary dissection after neoadjuvant therapy in patients with node-positive breast cancer. JAMA Surg. 2023;158(8):807–15. 10.1001/jamasurg.2023.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montagna G, Lee MK, Sevilimedu V, Barrio AV, Morrow M. Is nodal clipping beneficial for node-positive breast cancer patients receiving neoadjuvant chemotherapy? Ann Surg Oncol. 2022;29(10):6133–9. 10.1245/s10434-022-12240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrio AV, Montagna G, Mamtani A, et al. Nodal recurrence in patients with node-positive breast cancer treated with sentinel node biopsy alone after neoadjuvant chemotherapy—a rare event. JAMA Oncol. 2021;7(12):1851–5. 10.1001/jamaoncol.2021.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boughey JC, Alvarado MD, Lancaster RB, et al. Surgical standards for management of the axilla in breast cancer clinical trials with pathological complete response endpoint. NPJ Breast Cancer. 2018;4:26. 10.1038/s41523-018-0074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siso C, de Torres J, Esgueva-Colmenarejo A, et al. Intraoperative ultrasound-guided excision of axillary clip in patients with node-positive breast cancer treated with neoadjuvant therapy (ILINA Trial): A new tool to guide the excision of the clipped node after neoadjuvant treatment. Ann Surg Oncol. 2018;25(3):784–91. 10.1245/s10434-017-6270-z. [DOI] [PubMed] [Google Scholar]
- 22.Mukhtar RA, Chau H, Woriax H, et al. Breast conservation surgery and mastectomy have similar locoregional recurrence after neoadjuvant chemotherapy: results from 1462 patients on the prospective, randomized I-SPY2 trial. Ann Surg. 2023;278(3):320–7. 10.1097/sla.0000000000005968. [DOI] [PubMed] [Google Scholar]
- 23.Heidinger M, Knauer M, Tausch C, Weber WP. Tailored axillary surgery—A novel concept for clinically node positive breast cancer. Breast. 2023;69:281–9. 10.1016/j.breast.2023.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henke G, Knauer M, Ribi K, et al. Tailored axillary surgery with or without axillary lymph node dissection followed by radiotherapy in patients with clinically node-positive breast cancer (TAXIS): study protocol for a multicenter, randomized phase-III trial. Trials. 2018;19(1):667. 10.1186/s13063-018-3021-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montagna G, Mrdutt MM, Sun SX, et al. Omission of axillary dissection following nodal downstaging with neoadjuvant chemotherapy. JAMA Oncol. 2024. 10.1001/jamaoncol.2024.0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber WP, Matrai Z, Hayoz S, et al. Tailored axillary surgery in patients with clinically node-positive breast cancer: Pre-planned feasibility substudy of TAXIS (OPBC-03, SAKK 23/16, IBCSG 57–18, ABCSG-53, GBG 101). Breast. 2021;60:98–110. 10.1016/j.breast.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piltin MA, Hoskin TL, Day CN, Davis J Jr, Boughey JC. Oncologic outcomes of sentinel lymph node surgery after neoadjuvant chemotherapy for node-positive breast cancer. Ann Surg Oncol. 2020;27(12):4795–801. 10.1245/s10434-020-08900-0. [DOI] [PubMed] [Google Scholar]
- 28.Kahler-Ribeiro-Fontana S, Pagan E, Magnoni F, et al. Long-term standard sentinel node biopsy after neoadjuvant treatment in breast cancer: a single institution ten-year follow-up. Eur J Surg Oncol. 2021;47(4):804–12. 10.1016/j.ejso.2020.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Guergan S, Hoopmann U, Roehm C, et al. Evaluation of sonographic detectability of different markers within an in vitro simulation model of the axilla. Arch Gynecol Obstet. 2021;304(3):839–48. 10.1007/s00404-021-06085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]