Abstract
Microfluidics represent a quality sperm selection technique. Human couples fail to conceive and this is so in a significant population of animals worldwide. Defects in male counterpart lead to failure of conception so are outcomes of assisted reproduction affected by quality of sperm. Microfluidics, deals with minute volumes (μL) of liquids run in small‐scale microchannel networks in the form of laminar flow streamlines. Microfluidic sperm selection designs have been developed in chip formats, mimicking in vivo situations. Here sperms are selected and analyzed based on motility and sperm behavioral properties. Compared to conventional sperm selection methods, this selection method enables to produce high‐quality motile sperm cells possessing non‐damaged or least damaged DNA, achieve greater success of insemination in bovines, and achieve enhanced pregnancy rates and live births in assisted reproduction—in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI). Besides, the concentration of sperm available to oocyte can be controlled by regulating the flow rate in microfluidic chips. The challenges in this technology are commercialization of chips, development of fully functional species‐specific microfluidic tools, limited number of studies available in literature, and need of thorough understanding in reproductive physiology of domestic animals. In conclusion, incorporation of microfluidic system in assisted reproduction for sperm selection may promise a great success in IVF and ICSI outcomes. Future prospectives are to make this technology more superior and need to modify chip designs which is cost effective and species specific and ready for commercialization. Comprehensive studies in animal species are needed to be carried out for wider application of microfluidic sperm selection in in vitro procedures.
Keywords: chips, microfluidics, sperm selection
Sperm selection techniques: special refrence to microfluidics.

1. INTRODUCTION
About 15% of human couples show conception failure naturally and nearly 30% livestock is infertile with male factor contributing 50% to the total. 1 The success of assisted reproductive techniques (ART) and outcomes of infertility treatment massively depends on the quality of sperm used. Many conventional techniques such as swim up, density gradient centrifugation, and magnetic activated cell sorting are being used to harvest high‐quality sperm fraction. However, such methods typically involve many steps, manual screening processes, and migration mechanism through complex environments, hence prone to human error and may also cause damage to sperm DNA. 2 , 3 Although, a new nano‐selection method (using magnetic nanoparticles to segregate apoptotic and acrosome reacted sperm fractions) proves to be a better alternative to conventional methods. But, a more recent promising and less invasive addition to sperm sorting techniques proves to be microfluidics. Microfluidics is the science and technology of accurate manipulation of fluids at sub‐millimeter scale, which is typically done in microchannels with dimensions of a few hundred micrometers. 4 This technology selects sperms in a way similar to in vivo sperm selection within the micro confined areas in cervix and oviducts of female reproductive tract, when provided optimal temperature and the chemical environment. 5 , 6 , 7 Not involving washing and centrifugation step as in other conventional techniques, microfluidics is least damaging to sperm DNA and feature least production of reactive oxygen species. 7
Many live births have been obtained after artificial insemination in bovines following selection of highly motile sperm subpopulations with the help of microfluidics. 8 The fluid dynamics in microchannel geometry and design aids in the propulsion of spermatozoa, thus ensuring ultrahigh‐throughput sorting. It uses microfabrication and sperm behavioral properties in vivo which aid in quality selection and better analysis. 7 Moreover, microfluidic selection of functional sperm is characterized by sinuous trajectories, exhibiting many curves or turns during motion. Microfluidics target selection of undamaged motile spermatozoa which otherwise is a major limitation in conventional cell sorter sowing to vulnerability of physical damage. 9 While selection of fertile sperm population for assisted reproduction in animals, advanced microfluidic chips have been tested to remove dead sperms and debris. 7 , 10 In bovines, more functional and progressively motile spermatozoa with high DNA integrity have been obtained, 9 based on the ability of sperm to cross laminar flow streamlines. Highly motile spermatozoa with intact membrane integrity and mitochondrial function from frozen‐thawed bull semen have been successfully sorted using diffuser‐type microfluidic sperm sorter. 11 DMSS is now considered by many as a high‐quality sperm selection method to achieve great potential of assisted reproduction in bovines. It is a well‐established fact that during in vivo conditions, there is facilitation of sperm migration with selection of small subpopulation of healthy and motile sperm which reach to the site of fertilization. 12 It has been reported that mouse and human tract fluid allow rheotaxis‐based flow of sperm. 13 Although many of these natural aspects are not achievable artificially but principles of novel microfluidic chips ensure laminar, unidirectional, or gradient flow in a 3D physical environment. 14 These characteristics along with provision of altered chemical composition in the medium, make the procedure closer to natural processes and improve outcomes in in vitro fertilization.
While taking application part, microfluidics has become a tool to explore new possibilities within IVF technology. 15 , 16 Besides sperm selection, microfluidics aid in embryo culture, in vitro oocyte maturation, in vitro fertilization, and sperm processing, cumulus cell removal from zygotes, and in vitro microchannel insemination. 17 This technology owing to lesser handling and manipulation of the gametes, poses lower risk of cellular damage resulting in greater success in IVF procedures. Intact sperm DNA is a key to successful fertilization, proper egg activation, and embryo development. 18 Several studies indicate that the status of sperm chromatin at the time of fertilization can influence embryonic survival, thus, accounting for a significant proportion of male infertility. Besides other external sperm quality indicators, DNA integrity is now considered an important predictor of sperm fertility potential and IVF outcomes. Plethora of microfluidic based studies and reviews are of the opinion that microfluidic‐based systems facilitate noninvasive identification and quality selection of spermatozoa in terms of viability, functional capacity, morphology, and DNA integrity for in vitro fertilization. In this review, we focus more on the published literature in animals which supports microfluidics being the latest technology for quality sperm selection and analysis in humans as well as domestic and wild animals. According to reviewed research studies, the technique is so far considered novel, fast, reliable, more sensitive, and accurate even at the single sperm level. Our review also includes way forward and prospects of quality sperm selection and analysis for better outcomes in assisted reproduction. The advances in diagnostic and therapeutic evaluation of infertility based on selection of best quality sperm and sperm‐friendly semen analysis in domestic animals is also taken care.
2. HISTORICAL PERSPECTIVE IN THE DEVELOPMENT OF MICROFLUIDIC APPROACHES FOR ART
Roberts 19 after studying motion of spermatozoa in fluid streams reported geotaxis behavior of sperm. The geotaxis was later reported to be rheotaxis behavior due to the response of spermatozoa to fluid shear and gravity. 20 Discouraging production of harmful wastes in IVF media due to higher sperm oocyte ratio in human IVF (10000 sperms/oocyte) and considering the natural fluid flow that surrounds the gametes during fertilization, climbing‐over‐a‐wall (COW) method was first proposed. 21 The method in porcine IVF allowed higher monospermic penetration rate after putting a wall between sperms and oocyte. Also, there was direct correlation of sperm concentration with oocyte penetration and an inverse correlation of sperm concentration with monospermic oocyte penetration. Later, straw method was devised to reduce the sperm concentration around oocytes and obtain sperm: oocyte ratios closer in vivo. Depositing semen at the one end and keeping oocytes at the other end of a 0.25 mL × 5 cm long semen straw, used as IVF channel, allowed lesser number of high‐quality sperms available for oocyte compared to standard drop protocol. 22 Some researchers used polydimethylsiloxane (PDMS)/borosilicate microchannel for porcine in vitro fertilization and reported lower polyspermic and higher monospermic oocyte penetration using microfluidic device. 22 , 23 , 24 , 25 Some microfluidic device with dynamic media flow 26 were used for embryo development with development of blastocyst stage in a duration similar to in vivo development. Later, spermatozoa were observed to control their direction by rheotaxis, 27 chemotaxis, and boundary following behavior. 28 Rheotactic behavior of sperm (upstream motion or movement against flow) was quantified recently using three‐inlet chip and collection chamber positioned between the spermatozoa and the flow inlet. 29 The flow pattern was used in the chip to increase the total recovery rate of spermatozoa and progressively motile spermatozoa. Some successful designs of quality sperm selection microfluidic chips are those reported in porcine, 24 , 25 humans, 30 , 31 bovine, 8 and mouse. 32
3. WHY WE NEED MICROFLUIDIC BASED APPROACH?
3.1. Sperm heterogeneity
Semen samples contain a mosaic of sperm subpopulations possessing different patterns of motility and varied morphology. Some cells do have greater motility, some are more viable, some have better morphology, and others have more intact chromatin. We desire a population of sperms having all the attributes for greater outcomes in ARTs. Such populations possess qualities of highest fertilization capability and the best features for supporting embryo development. However, effective methods are lacking that lead to separation of specific high‐quality sperm subpopulation corresponding to that selected naturally in oviduct. 33 Human spermatozoa in a sample has been shown to possess different characteristics in terms of motility, morphology, hyperactive motility, and protein tyrosine phosphorylation in response to incubation under capacitating conditions in different fractions of Percoll gradient. 34 Jenkins et al. reported existence of differentially methylated regions in the DNA between high‐quality and low‐quality sperm fractions. 35 While other study by these authors 33 reported 15% of the ejaculate containing quality subpopulation migrating toward the higher temperature within a gradient had rhodopsin at a specific cellular location. Rhodopsins are G protein‐coupled receptor proteins in mammalian sperm cell which act as thermo‐sensors and trigger signaling pathways for thermotaxis. 36 , 37 A study on bull ejaculate by D'Amours et al. 38 reported that sperm within two different sperm populations separated by density gradient centrifugation (DGC) showed that in high motile population, 80 proteins were in abundance compared to low motile spermatozoa where only 31 proteins showed abundance. Comet assay involving single‐cell analysis of DNA fragmentation revealed appreciable intra‐sample heterogeneity in human spermatozoa. 39 DNA fragmentation ranged from 0 to 70% in normozoospermic men and from 0% to 65% in epididymal mouse spermatozoa. 37 Differences in telomere lengths have also been reported by Antunes et al. 40 in different sperm subpopulations. High‐ and low‐sperm chromatin compaction in different sperm subpopulations have also been observed. 41 More comprehensive studies of semen ejaculate heterogeneity involving genome, epigenome, transcriptome, proteome, and metabolome studies may be the way forward to the selection of high‐quality sperms for assisted reproduction to improve outcomes and preserve quality genomes.
3.2. Polyspermic versus monospermic fertilization
Generally, many spermatozoa are required to be exposed to ovum in vitro to achieve fertilization out of which only a fraction of population is capable of fertilizing an oocyte. Several randomized controlled trials for the treatment of male subfertility show large variations in sperm oocyte ratios for achieving fertilization and the ratios mostly fall in the range of 50,000–10 million sperm/oocyte for IVF. 42 However, latest studies show even lower limits of this ratio by selecting quality spermatozoa. A recent study in mouse achieved 60% fertilization rate using 5 motile sperm/oocyte by enhancing sperm capacitation via creatine supplementation in the human oviductal fluid (HTF) medium. Creatine in the medium increased sperm ATP production and motility. 43 The concept of monospermic fertilization used in ICSI, dates back to COW method described in the historical perspective of this review. Natural oviduct secretions in mammals have been reported to contribute to increase monospermic fertilization through zona pellucida modulation and enhancing sperm–oocyte interaction and more power of penetration. 44 Conventional methods of sperm selection hardly meet successful selection of a single sperm for use in assisted reproduction. However, more monospermic oocyte penetration was achieved in porcine oocytes using microfluidic device. 24 , 25 But comprehensive studies in this regard are lacking in literature.
4. CONVENTIONAL METHODS OF SPERM SELECTION
Conventional methods of sperm sorting include density gradient centrifugation (DGC), sperm washing, swim‐up (SU) and magnetic activated cell sorting (MACS), and MACS in conjunction with DGC. 39 , 45 , 46 All these methods use repeated centrifugation and sperm flows in sheath fluid. Other important methods of sperm selection are hyaluronic acid‐coated slide sperm‐binding assay and electrophoretic approach. Several studies in humans and bovine reveal use of Percoll density gradient centrifugation, swim‐up, washing by centrifugation, glass wool filtration 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 for removing seminal plasma, dead cells, abnormal sperm, cryoprotective agents, and other factors for preparation of quality sperms.
Centrifugation technique at 200–1800 g with colloidal silica particles, results in appearance of distinct sperm bands separating motile sperms from immotile sperm and debris. However, the technique is associated with sperm mechanical damage, production of reactive oxygen species, and loss of DNA integrity which ultimately leads to lower fertilization rates, impaired embryo progression, and decreased pregnancy rates. 55 More recent studies in human patients with mild or idiopathic male factor by Oguz et al. 56 and animal sperm selection 57 reported SU selected sperm population having less DNA fragmentation compared to its counterpart DGC. Also, the direct micro‐SU variant shows comparable fertilization percentages by ICSI to those of DGC. SU has been also associated with higher blastocyst development in vitro more pregnancy rates (42% vs. 26% in DGC) and low abortion rate (13% vs. 29% in DGC). 58 MACS or annexin V‐MACS helps in separation of apoptotic and non‐apoptotic spermatozoa. 59 It involves coating of magnetic nanoparticles with annexin V molecule 60 and externalization of phosphatidylserine in apoptotic sperm in semen sample which isolates non‐apoptotic sperms with high DNA integrity. 61 , 62 , 63 Varied results of comparison between MACS, SU, and DGC have been reported in various studies but a recent study in human sperm by Ziarati et al. 64 reports more DNA integrity and embryo survival following MACS technique. MACS studies have not been carried out yet in livestock species. Rabbit sperms selected using AV‐MACS for AI reported no enrichment in non‐apoptotic spermatozoa and no influence on reproductive outcome. 65 Hyaluronic acid (HA), a main extracellular matrix molecule surrounding the cumulus–oocyte complex (COC) 66 bears binding sites for spermatozoa during spermatogenesis and maturation. 67 , 68 Comparison of HA‐binding assay with SU and DGC have contradictory findings but two separate studies by Parmegiani et al. 69 and Huang et al. 70 reported lower and similar level of DNA fragmentation in spermatozoa selected in HA solution compared to SU, and by HA‐coated dishes compared to DGC, respectively.
Electrophoretic approach is a more rapid isolation of motile, viable, and morphologically normal spermatozoa with good DNA integrity 49 as revealed by high‐power microscopy. 71 In fact, a negatively charged plasma membrane of spermatozoa forms basis for the developed methods of separation using electric field. It ensures selection of higher quality sperms characterized by high DNA integrity. 72 , 73 , 74 , 75 The charge‐based separation, also known as Zeta method, has allowed the selection of spermatozoa with lower DNA fragmentation compared to the HA‐coated dish selection. 73 Although results by Zeta method being promising, only a single human study has been published using spermatozoa selected for ICSI 76 and no study is available so far in animals. Other available conventional powerful cell sorters using flow cytometry are not suitable for fragile spermatozoa. Such sorters use florescence proteins, antibodies, and other compounds which are costly and the sorting process leads to greater mechanical damages and a recent study by Nakao et al. 32 shows that in mouse, after flow cytometric sorting a smaller number of selected sperms obtained had tails moving up and down compared to sperm subpopulation obtained in microfluidic chip cell sorter in the same species. As reviewed in literature we found no established study regarding selection of quality sperm using flow cytometry. Methods, based on morphometric evaluation of spermatozoa 77 and direct capture‐based spermatozoa selection with a low number of vacuoles and normal nuclear morphology under a microscope equipped with a micromanipulation system and a 6300X magnification 78 have been used. But the techniques provided sperms which neither possessed high DNA integrity nor improved IVF outcomes and live births. 79
Thus, none of the discussed techniques can isolate highly motile, morphologically normal sperm with high DNA integrity from an unprocessed semen sample. Also, variable results have been published in their outcomes after use in assisted reproduction. Moreover, many such techniques are costly, time consuming, and require more skill. All the methods bypass natural barriers that sperm would experience in vivo 80 and damage sperm DNA in centrifugation steps 81 , 82 which has already been reported to have long‐term effects on viability of embryos. 83 Besides, all these methods focus only on sperm motility as sperm quality metric. Sperm morphology and membrane properties are required to be explored for quality sperm selection and investigations regarding morphology as a sperm quality metric are not documented.
5. MICROFLUIDICS AND QUALITY SPERM SORTING
5.1. Physical aspects in microfluidics
Microfluidics refers to science and technology to manipulate small amounts of fluids accurately using microchannels of a few hundred micrometers dimensions. 84 It helps in exploring different geometrical, sperm–fluid interaction, hydrodynamics of sperm, and other biochemical mechanisms in which sperm has to interact in order to attain motility to reach the site of fertilization. It is typically done in microchannels with dimensions of a few hundred micrometers. The technique uses microfabrication and sperm behavioral properties in vivo which aid in quality sperm selection. Microchannels so far successfully used include Y‐, H‐ and Radial Microarray‐shaped. These all are PDMS fabricated and coated with poly(ethylene glycol) methyl ether methacrylate (PEGMA). PEGMA is a nonlinear analog of polyethylene glycol (PEG). Moreover, each microscale microchannel is constituted by two inlet channels (for sperm and buffer medium), two outlet channels (selected sperm collection and waste collection), and a main channel.
The devices produce new insights of sperm behavioral changes enabling propulsive or progressive motility of quality sperms to be available for oocyte during in vitro production. The propulsion of sperm is attained in microscale environments through periodic but time irreversible changes in the shape of sperm's body (scallop theorem) and locomotory drag forces when viscous forces dominate inertial forces. The drag force is better explained by low Reynold's number (R e = 4.9 × 10−3) of fluid dynamics. 85 Low Reynold's number inertial forces are negligible while viscous forces dominate which causes a drag for locomotion. Drag force allows optimal laminar flow of semen and medium.
A microfluidic chip with radial microarray design of microchannels was successfully used by Nosrati et al. 30 for quality sperm selection in humans and bull (Figure 1). In this breakthrough design, the chip consisted of top layer having two inlets and an outlet with removable top seal. Buffer and semen samples injected enable streamlined flow of sperm through radial microchannel network (500 microchannels) on bottom layer. Each microchannel (400 μm long) has a funnel‐shaped opening and dividers. Out of a 1‐mL semen sample, 1,00,000 sperms with high DNA integrity were obtained in the study. Sperms were evaluated using florescent dyes.
FIGURE 1.
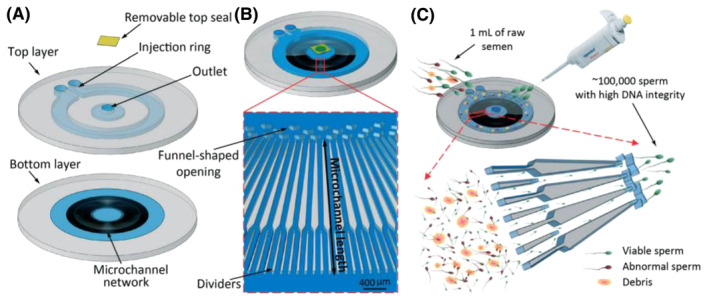
Microfluidic sperm selection device (capacity for one milliliter of raw semen). (A) Schematic view of the device: Bottom layer has a network of 500 radial microchannels with a 100 × 75 μm cross‐section, top layer (1.45 mm depth at the injection ring and 0.8 mm depth at the outlet chamber and transparent tape as the removable top seal). (B) Schematic view of the assembled device and close‐up view of the microchannel vertical walls. Each pair of consecutive dividers coalesces 5 mm before the outlet. Funnel‐shaped openings prevent sperm from re‐entering the microchannels after reaching the outlet. (C) One milliliter of raw semen is injected around the ring, live and motile sperm navigate through high viscosity fluid toward the central outlet, resulting in collection of ~100,000 sperm for ART. (Reproduced from Nosrati et al. 30 with permission.)
6. INNATE PROPERTIES OF SPERM INSPIRING NEW METHODS OF ITS SELECTION AND ANALYSIS
6.1. Regulation of sperm motion
Locomotion of mammalian spermatozoa is driven and influenced by (1) mitochondria present in midpiece of tail or flagellum, generating force for beating of principle piece, 86 (2) axoneme (9 + 2 + 2 pattern) at the central core of the flagellum, causing a wave‐like pattern through sequential sliding of the nine outer microtubule doublets via ATPactivated dynein arms over the neighboring doublet, 87 , 88 unidirectional flagellar wave owing to balance between active force of the dynein arms, passive force generated by flagellar and hydrodynamic drag force, 53 and flagellar wave interaction with surrounding medium leading to drag force acting along flagellum which in turn results in drag anisotropy. The higher ratio of normal to tangent drag compared to normal to tangent velocity forms a propulsive force in the direction normal to the motion of the filament 89 spermatozoa's helical or planar wave configurations 90 , 91 as a result of dynein motor regulatory mechanisms owing to changes in intra and extracellular conditions, ion flux changes, and share rate regulation. 92 , 93 The findings of the digital imaging studies revealed sperm's exhibiting wide range of swimming patterns starting from typical, helical, hyperactivated, hyper‐helical, and chiral ribbons. The sperm's typical swimming pattern characterized by forward progression with lateral displacement is observed in 90% population while ~5% of sperms swim along helical trajectories characterized by forward progress with a stable revolution. Swimming patterns are changed about every 10 s and ~2% of sperm exhibit a chiral ribbon swimming pattern characterized by forward progress with planar oscillation of the sperm head. Three‐dimensional shape and volume of a bovine sperm head was measured by Merola et al., 94 while using onchip holographic microscopy to quantify the volume of sperm cells. Using optical tweezers and a focused laser beam to generate an attractive force to trap and rotate the asymmetric sperm cell by regulating the laser power to record its holograms. 95 Same author in 2010 96 had reported 3D images of bovine sperm with detailed topographical information demonstrating a protuberance on the post‐acrosomal region of the sperm head.
6.2. Rheotaxis behavior of sperm and viscosity of medium
Natural in vivo mechanisms such as ciliary beating and surface secretion form viscosity gradients and flow which in turn influence sperm migration to the site of fertilization and the phenomenon is called rheotaxis. 13 , 97 Rheotaxis is an established property of sperm to swim against the flow. 98 Two important rheological variables namely, viscosity gradient and flow rate are targeted to be achieved in microfluidics for quality selection. Viscosity determines the swimming trajectory of sperm as the increase in viscosity at a fixed energy production decreases flagellar wave frequency as well as wavelength hampering progressive motility. Further a combined viscous and elastic nature of mucus potentiates swimming velocity owing to formation of highly strained fluid regions behind the tail, responsible for inhibition of backward movements of sperm and enabling forward flow. Rheotaxis is exhibited in nonuniform flow field caused by a boundary 99 and is also responsible for surface accumulation and sperm's boundary following behavior. Sperm has the capability to show rheotactic behavior in bulk fluid also (away from the surface), where weak shear flow is expected. 100 Rheotaxis, a passive process, is a result of hydrodynamics 101 and influenced by the front back asymmetry of the sperm. 99 In the presence of a shear flow, both the higher resistive force experienced by the sperm head than the tail and drag forces from the flow over the conical envelope of the flagellar wave reorient the sperm against the flow, directing movement upstream. Besides vast human sperm studies, Kantsler et al. 98 studied rheotaxis within bull sperm samples in circular crosssection microchannels. A combination of increased shear rates near a no slip boundary, conical shape of the flagellar wave envelope, and chiral flagellar beat pattern were observed to contribute to the upstream navigation of sperm with a spiral trajectory component around the circular channel circumference. Rheotaxis, in combination with boundary following behavior, has been shown to act as a long‐range guidance mechanism for sperm. Rheotaxis in bull semen has established studies in literature. A recent study by El‐sherry et al. 102 reported maximum rheotaxis behavior of bull at pH 6.4–6.9.
6.3. Chemotaxis behavior of sperm
In vivo environment of female reproductive tract provides a characteristic chemotaxis attracting sperm toward egg in response to intercellular mechanisms generating a chemical signal. 103 , 104 , 105 Such studies are available in more detail in case of sea urchin sperm. 106 , 107 The studies explain react gradient (continuous release of chemoattractant peptide by the egg thereby stimulating sperm motility, inducing intercellular alkalization and polarization), activating the sperm‐specific Ca2+ channels (CatSper), and ultimately raising intracellular Ca2+ concentration inside the cell. 108 , 109 More Ca2+ leads to increase in flagellar beating, aligning the sperm swimming trajectory and guiding sperm toward the egg. The sperm chemotactic mechanism in sea urchin predicts successful fertilization in vivo. 110 However, in mammals the chemotactic response of spermatozoa has been challenging due to unresolved chemotactic role and chemotactic intercellular mechanisms.
More advanced rapid microfluidic technologies capable of single cell analysis such as droplet based microfluidic technologies show a high‐throughput response of individual sperm to a chemical cue and promise better study of chemotaxis in a precisely controlled and isolated environment. Koyama et al. 111 and Xie et al. 15 studied the chemotactic response of mouse sperm in presence of ovarian extracts and cumulus cell masses near diffusion chamber of the microfluidic devices. The studies indicated progesterone as chemoattractant for meager sperm population (7% and 10% of sperms, respectively). However chemotactic role of progesterone was not observed in later studies of mouse like hydrogel based microfluidic chemotaxis plate form used by Chang et al. 112 did not show any chemotactic response of mouse sperms in presence of progesterone. A contradictory response in more recent microfluidic study of Zhang et al. 113 indicated progesterone acting as chemoattractant at 1 mM concentration gradient but not at 100pM gradient for human capacitated sperms. Thus, the chemotactic role of progesterone and chemotaxis behavior of sperm needs more controlled studies which can better ensure using controlled microenvironment in microfluidics.
7. MICROFLUIDIC DESIGNS
7.1. Basic microfluidic system
A basic microfluidic system consists of the main microchannel with two inlets and two outlets. Poly dimethyl silicone (PDMS) has been used as a fabricating material for construction of these devices due to it being non deleterious to sperm survival, fertilization, and early development of the embryo. 114 , 115 , 116 The device dimensions (length, width, and height) are specific to the size of sperm and species. In general, for human spermatozoa, they are 5 mm, 400 μm, and 50 μm, respectively in a K‐shaped device. The diameters of the inlet‐up are smaller (100 μm) than inlet‐down (300 μm), and outlet up and outlet down are same in size as that of inlets. Semen is injected into microchannel from the inlet up, motile sperms varying with laminar streams get pre collected inlet‐down due to its larger width than that of the inlet‐up. The height of the microchannel needs to be lesser than length of spermatozoa in order to allow its unidirectional flow, preventing z directional movements to improve sorting efficiency. Maintaining optimum viscosities of semen and media and the flow velocities of the two inlets (300 μm/s) helps in uniform distribution of concentration by simulation. Fluorescent staining as used by Nakao et al. 32 in mouse sperm and a statistical method based on flow cytometric analysis 117 are employed to characterize both live and dead sperms and to analyze the sorting efficiency of motile sperm.
7.2. Microfluidic chips
Microfluidic systems are also named as Micro‐Total Analysis Systems (μTAS) or lab‐on‐a‐chip (LOC) devices. Apparent random motion of sperm in a quiescent medium, leading to diffusion is used within space constrained microfluidic channels to separate racing sperms. Persistence length of collected sperm was increased in a similar design by addition of pillar arrays. 118 Microfluidic devices, typically fabricated by microfabrication techniques, enabling functional microscale parts for delivery and mixing of fluids 119 along with separation of fluid particles. 120 Nagata et al. 8 used a bovine microchip to select microfluidic sorted sperm for live births in AI (diffuser‐type microfluidic sperm sorter). The chip was fabricated by micromachining of the poly methyl methacrylate (PMMA) master and replica molding using poly dimethyl siloxane. 121
Some other important microfluidic chips used so far in sperm selection documented in literature include Sperm check, FertilMARQ Fertile Plus and Fertell in humans, on Chip Sort, on Chip Biotechnologies, Japan in mouse, and Fertile Bovine and Fertile Plus®, KOEK EU GmbH, Hannover, Germany for bull semen. SpermCheck, 122 a paper‐based microfluidic device, can evaluate lowest sperm count even below the WHO reference value (<20 × 106 mL−1) with 100% concurrence for diagnosis of low‐quality sperm. The commercially available test Fertell can detect semen samples with low motility. 123 FERTILE® chips are being used in IVF, IUI (intra uterine insemination), and ICSI, providing selected sperm with best DNA and physiological quality in less time. FERTILE Bovine® product mimics more to female reproductive tract system and this technology selects sperm based on natural sperm movement.
Common microfluidic chips used for sperm selection is shown in Figure 2.
FIGURE 2.

Common microfluidic chips used in semen laboratories for sperm selection.
7.3. Main functions of microfluidic devices
The devices have ability to sort sperm cells in a faster and easier way, closely mimicking the natural selection processes. Besides their role in analytical chemistry, molecular and cellular biology, microbiology, and pharmaceutical drug screening, these devices ensure more sample manipulation processes associated with cell culturing, cell separation, and DNA analysis. Moreover, the unique microscale properties of microfluidic devices have been successfully employed in assisted reproductive technologies (ART) for sperm sorting, 124 oocyte manipulation, 125 insemination, 126 embryo culturing, 127 and for assessing sperm and embryo quality. 128 The devices can select spermatozoa having greater motility, viability, morphology, and DNA integrity. 5 , 30 , 126 , 129 Besides the technology in animals reduces the content of reactive oxygen species (ROS) of sperm and the potential for DNA damage, 130 compared to conventional methods. Microfluidic sperm sorting chips show greater efficiency for bull sperm; however, investigation is still required for its ability to increase embryonic development rates and IVF outcomes in bovine. A recent study by Gonzalez‐Castro et al. 131 to select sperms on the basis of sperm motility and morphology using microfluidic chip in horses demonstrated the technology as the only sorting method to get subpopulation of sperm with greater DNA integrity. In this study, equine frozen‐thawed semen was subjected to sorting, using human microfluidic chip (FERTILE PLUS™ Sperm Sorting Chip, DxNow Inc.) with modifications in manufactures guidelines. Although microfluidic chip did not improve percentage of motile, live, morphologically normal, and swollen spermatozoa as compared to single‐layer colloidal centrifugation (SLC) and swim up (SU) sorting methods but the percentage of DNA‐fragmented sperms was significantly reduced (6% in microfluidic sorting vs. 12% in SLC and 11% SU). However, advantage of using microfluidic sorting in horses for clinical ICSI is still to be determined. Microfluidic systems help in selection of qualitatively and quantitatively sufficient sperm and reduce requirements of clinician's skill for sperm purification process. Use of mechanical conditions in micro fluidics, 127 tiny culture drops and micro well approach 132 lower possibility of dispersion of autocrine factors, which remains unidentified yet. Due to its resemblance to fallopian tube environment and providing natural boundary following navigation, rheotaxis and random motion in microchannels, it remains an advanced method of choice for sperm selection. 133
Healthy mouse off springs following IVF using high‐quality sorted sperm through microfluidic chip was reported by Nakao et al., 32 which indicates a mile stone in the microfluidic sperm sorting. The authors reported that fluoroscein‐5‐isothiocyanate (FITC)‐labeled peanut agglutinin (PNA) sperm sorted in the chip had good motility (> 50 μm/s progressive motility), fertilization, and developmental abilities (nearly 65% in highly acrosome reacted sperm and 100% for two cell‐stage embryos). Moreover, 30% live pups were obtained out of transferred embryos after IVF. Also, same study reports selection of acrosome reacted sperm having high fertilization rate following capacitation like changes in microfluidics. Such type of successes are yet awaited in other species following advanced microfluidic sperm selection procedure. Further microfluidics can be an innovative approach of sperm selection to improve IVF outcomes in mammals.
8. SPERM SELECTION BY MICROFLUIDICS
Sperm sorting by microfluidic uses microfluidic devices that isolate only motile sperm, referred as Type 1 microfluidic sperm sorting. Microfluidic devices isolating sperm cells without relying on sperm motility form Type 2 sorting, and the devices for observation and selection of individual sperm constitute Type 3 microfluidic sperm sorting. All three types have their characteristic features and separate clinical applications.
Type 1 sperm sorting devices constitute a largest group including technologies translating the process of motility screening to a microfluidic system, thus improving the swim up method. Type 1 microfluidics have ability to select enough motile sperm cells (>10 million cells) for intrauterine insemination (IUI) and pure and lesser in number (~100,000 cells) for in vitro fertilization (IVF) procedures. The sperms sorted with these systems are almost 100% motile and with acceptable morphology and DNA integrity. Type 1 sorting is a natural way to separate sperms from somatic cells and debris in semen. Moreover, dead and damaged sperm cells are also segregated. In type 1 system, motile sperm subpopulations are selected after exposure of semen sample to laminar stream‐based, surface‐modified microchannel microfluidic chips.
Type 2 microfluidics instead of using sperm motility; utilize shape, size, or other physical biomarkers of sperm as a selection criteria. Trapping of sperm in these systems are focused to retain the full fertilization capability of a subfertile semen sample by indiscriminately capturing sperm cells. Hence, the selection mechanism is not explored to sort an improved sperm subpopulation.
Type 3 microfluidic devices are employed to capture and non‐invasively investigate the characteristics of a single sperm cell without affecting its viability. Such devices use Raman spectroscopy in combination with microfluidic sperm sorting systems. Raman spectroscopy is a vibrational spectroscopy involving inelastic scattering of monochromatic light by the molecular structure of a system to determine its constituents. Individual viable sperm cells have Raman spectras with common traits, leading to identification of sperm biomarkers and measurements of sperm DNA and organelle damage. 134
Some of the technologies and their applications in semen selection are briefly summarized in Table 1.
TABLE 1.
Some remarkable microfluidic‐based technologies and their applications in sperm selection for assisted reproduction.
| Microfluidic device | Design/principle | Species | Outcome | Reference |
|---|---|---|---|---|
| H‐Filter microfluidic device | H design filter with three inlets and three outlets, only motile spermatozoa to cross the streamlines in microfluidic channel. Continuous stream instead of batchwise | Human |
|
[114] |
| Rheotaxis‐based microfluidic device | Used the tendency of spermatozoa to swim against the flow and made use of this in their device to isolate the motile ones. Only two inlets | Human |
|
[27] |
| Chemotaxis‐based device | 7 mm long Y‐channel that connected three wells. Cumulus cells placed in one well to act as chemoattractant | Humans |
|
[15] |
| Boyden chamber type Thermotaxic device | A shallow temperature gradient (0.014°C/mm) across a tube, and a stainless steel porous membrane separating two compartments. spermatozoa placed in one chamber accumulate across the membrane in the other chamber | Humans | With increasing gradient larger accumulation of sperms helped in sorting | [164] |
| Themotaxic microfluidic chip | Consisted of two collection chambers, an interfacial valve to close off, trapping different spermatozoa in their respective chambers | Humans | Thermotactic reaction was observed in 10% of the sperm populations | [165] |
| Microfluidic chip | 500 microchannels in parallel, which are connected to an inlet ring and an outlet in the center of the chip | Human | DNA fragmentation index could be lowered by a factor of 5–10 times | [30] |
| Microfluidic channel with different swimming medium | Device used controlled environment hyaluronic acid and methylcellulose as viscosity medium | Human | Hyaluronic acid was detrimental to the viability and motility of the spermatozoa when compared to methyl cellulose | [69] |
| Microfluidic chip |
In‐ and outlet with a straight channel in between Hexagonal pool of 4 mm diameter with six adjacent channels with a width and height of 700 μm by 50 μm, connected by microchannels of 5 μm by 2 μm to the main pool Chemoattractant progesterone at 100 pM and 1 mM allowed to diffuse in microchannels |
Human | Normal spermatozoa count 27.1% spermatozoa that swam toward the gradient was ~16% larger | [113] |
| Swim‐up method based transwell system | Device connected to porous membrane, allowing swim up | Human |
|
[118] |
| Thermotaxis based microfluidics | A drop of a medium containing spermatozoa connected to second drop with no cells. Under a temperature gradient. Spermatozoa respond by thermotaxis shifting toward higher and accumulate in second drop | Bull |
|
[166] |
| Rheotaxis base chip |
Designed with Serial of microchannels directed toward a well In response to the flow the spermatozoa swim toward it passing through the microchannels and accumulating in a receptive well where they can be collected for downstream applications |
Bull |
|
[8] |
| Chemotaxis based Sperm Selection Assay (SSA) device | Progesterone gradient was used for separation | Bull |
|
[167] |
| Thermotaxis based microfluidics | A drop of a medium containing spermatozoa connected to second drop with no cells, under a temperature gradient | Mice, humans |
|
[33] |
| Chip using combined thermotaxis and chemotaxis | Combined chemotaxis chip with an on‐chip heater | Humans | Almost same number of spermatozoa moved toward upstream gradients in both mechanisms | [168] |
| Microfluidic chip |
PDMS chip, 50 μm high, three 100 μm inlet channels merging into a 300 μm wide main channel and split into three outlets Sandwich structure, inner channel used to introduce the spermatozoa and the two outer channels having equal All immotile spermatozoa flow into the waste channel |
Boar, Bull | Sperm viability decreased only by 6% and in case of bull viability remained almost unaffected compare to flow cytometric analysis | [169] |
|
Microfluidic chip cell sorter On‐Chip Sort, On‐chip Biotechnologies, Japan |
Pulsed air pressure, which results in minimal damages to cells Epididymal sperm sample dissolved in calcium‐enhanced human tubal fluid (mHTF) were sorted using a signal distribution of forward scattered light (FSC) and side scattered light (SSC) Sperms gated into scatter, labeled with fluoroscein‐5‐isothiocyanate (FITC) and collected in the collection reservoir |
Mice | Selected acrosome reacted spermatozoa showed in vitro fertility and full developmental ability | [32] |
| Micro‐chamber‐based microfluidic platform | Traps nonprogressive sperm in microchambers separate, separating progressively motile sperm from | Bull |
|
[170] |
9. MICROFLUIDIC BASED SPERM ANALYSIS
Microfluidic sorted sperms are subsequently assessed for kinematic parameters, acrosome reaction, mitochondrial membrane potential, and DNA integrity. Notable parameters evaluated before use being sperm viability, motility, and morphology. But DNA integrity is now considered in latest technologies to improve in vitro production results. Semen analysis helps in quantifying sperm deficiencies like count, vitality, motility, morphology, and DNA integrity, 135 thereby aids in diagnosis and treatment of male‐factor infertility 136 , 137 , 138 , 139 , 140 , 141 and efficient sperm selection. Available clinical methods 140 , 142 in humans utilize WHO reference values for semen characteristics. 137 Conventional clinical methods include counting chambers, viability assays, Computer‐Assisted Sperm Analysis, 140 , 143 and DNA integrity assays (such as the comet assay and sperm chromatin structure assay, 144 , 145 ). However, being time consuming (counting chambers, viability assays), costly (CASA), more skill demanding, and low‐standard procedures (CASA, COMET and SCSA), 141 , 146 these methods are discouraging for improving efficiency in assisted reproduction techniques. Counting chambers method involves use of hemocytometer and Makler chambers for counting. Swelling of live sperm cells due to an influx of water to their cytoplasm from an induced osmotic pressure gradient of a hypotonic solution better distinguishes live sperm population. Although DNA integrity tests (SCSA and COMET) assist in furnishing detailed information about sperm chromatin characteristics, compaction and dis integrity, but wide applications of these techniques is also limited owing to less instrumental access, lack of standardized methods, and nonavailability of reference values for clinical evaluation. 141 This review therefore concludes that there is a gap to comprehensive regrading availability of most efficient semen analyzing techniques in order to prepare a best quality sperm to the oocyte in in vitro conditions. This way assisted reproductive outcomes might be closer to natural in vivo reproduction. Chen et al., 147 were able to calculate sperm concentration in the range of 0–252 × 106 mL based on sperm's random swimming orientation principle in microfluidic chips. De Wagenaaret al. 148 in a glass microchip with two sets of electrode gates and induced fluid flow based on electric impedance could estimate sperm abnormalities to the extent of 89%. A recent successful study by Nosrati et al. 31 reported sperm concentration in the range of 8.56–381 × 106/mL, motile sperm motility in the range of 3.73–315 × 106/mL, and sperm motility ranging from (9 to 87%), using calorimetric signal principle in a fast run (10 min) paper‐based microchip.
Compared to conventional semen analysis, processing damages to human and animal spermatozoa in microfluidic designs are least observed in several reviewed research studies. The techniques are now regarded as accurate, fast, and easy to use but need comprehensive comparative evaluation.
10. MICROFLUIDIC‐BASED SPERM RECOVERY
Isolation of nonmotile sperm from background contaminated fraction in testicles has not been studied comprehensively. Magnum et al. 149 have reviewd some studies of sperm testicular extraction but the methods prove less efficient. Microfluidics recovery of sperm from biopsy tissues, testicles, or epididymis proves faster and contamination‐free technique. This has been successfully demonstrated through the application of 3D printed microfluidic hard chips to recover sperm from mixed cell suspensions with a >96% recovery rate. 150 This helps in sperm isolation from background cells and debris without affecting its vitality, motility, morphology, or DNA fragmentation to improve ART outcomes.
11. DNA FRAGMENTATION IN MICROFLUIDICS
Selecting quality sperm needs the ejaculates to be screened better for separation of non‐DNA‐fragmented or least DNA‐fragmented sperms available for oocytes in invitro studies. DNA fragmentation in microfluidic devices is lower thereby improves fertilization, and zygote and embryo developments. Shirota et al. 5 while studying separation efficiency of a microfluidic sperm sorter reported very low DNA damage in selected sperm subpopulation. In fact, the selected spermatozoa exhibited 95% motility, and only 1% DNA fragmentation index. In a more recent study by Pérez‐Cerezales et al, 33 authors reported a range of 0%–70% DNA fragmentation of individual spermatozoa in normozoospermic men and 0%–65% in epididymal mouse spermatozoa. Gai et al., 151 while using acoustic based continuous‐flow microfluidic method for bull sperm selection, found it capable of selecting high‐quality sperm with considerably improved motility and DNA integrity compared to the initial raw bull semen. The method was reported to select sperm having improved motility, progressive motility and DNA integrity up to 50%, 60%, and 38%, respectively. Using SCSA, TUNEL, and SCD, different studies have shown lower DNA disintegrity in microfluidic chips; some recent and important findings are presented in Table 2.
TABLE 2.
DNA fragmentation in microfluidic chips versus conventional sperm sorting techniques.
| S No. | Device | DNA fragmentation (%) | Reference |
|---|---|---|---|
| 1. | Microfluidic radial 500 channels in parallel | 2.4 vs. 10.9 (control) | [30] |
| 2.. | Sperm sorter quails | 5.9 vs. 8.3 (SU) and 27 (control) | [152] |
| 3. | Sperm sorter quails | 0.8 vs. 10 (DGC‐SU) | [5] |
| 4. | Microfluidic DMSS (Bovine) | 0.37 vs. 7.08 (unsorted) | [8] |
| 5. | Fertile (Zymote) device | 0 vs. 15 (DGC‐SU) | [153] |
| 6. | Simple Periodic Array for Trapping and Isolation (SPARTAN) | 4–6 vs. 13 (SU) | [118] |
12. ROLE OF MICROFLUIDICS IN IVF
In addition to role played by microfluidics in quality sperm selection and analysis, it helps in oocyte's maturation, enucleation, transport, and cryopreservation. It also helps in development and culture of in vitro produced embryos owing to improved cleavage rates (67% vs. 49% in standard procedures). 154 Studies have indicated that oocytes maturated in microchannel had the same nuclear maturation of those maturated in standard drops. Microchannels used for maturation of individual oocytes enhance developmental potential and aids in transporting the oocytes in the microchannel up to a specific point (trap) where the fertilization is carried out. 155 . Zeringue et al. 156 first used microfluidics to remove cumulus cells of bovine oocytes and found microfluidic procedure more efficient and less stressful compared to vortexing for cumulus removal. The study reports development to more advanced stages of 2 days embryo was higher in the microfluidic device than in the control treatment (35% vs. 20%) and obtained more blastocyst (57% vs. 30%).
Yuan et al. 155 reported increased developmental potential of embryos using silicon microchannels probably due to oocyte movement with the help of pumping action of medium over the oocyte. 125 A recent study by Caliscici et al. 157 used sperm cells selected after thawing at 30°C by standard density gradients (DG) protocol (SpermFilter®, GYNEMED GmbH & Co. KG.) and a microfluidic sperm sorting (MSS) chip technique (Fertile Plus®, KOEK EU GmbH). The authors observed more embryonic development rates and outcomes in routine IVF procedure using MSS chip‐based sperm (Day 8 blastocysts rates, 33.6% vs. 22.0%, using MSS chip sorted and DG sorting sperms, respectively). Also, Caliscici et al., 2019 157 reported in IVF using microfluidic sorting chips (FERTILE PLUS), more blastocyst development rates (18.1 vs. 15.3% in MSS chip and DG, respectively) and cleavage rates (75% vs. 71.2% in MSS chip and DG, respectively). However, the extensive studies are lacking in this regard.
13. CONCLUSIONS AND FUTURE PERSPECTIVE
In spite of availability of various methods of sperm selection, conventional capillary zone electrophoresis selecting robust spermatozoa without damaging DNA 158 and microfluidics are the best selection techniques. Microfluidics is a fast technique for selection of high‐quality spermatozoa but its full potential species wise has not been fully realized. Microfluidic selection improves percentage of normal morphology, viability, and DNA integrity in quality subpopulations spermatozoa to be used in assisted reproduction. The micro environment owing to small‐scale dimensions and controlled flow of the fluid in microfluidic chips aids in selection of sperms lower in number but higher in motility, DNA integrity, and fertilizing capacity, mimicking natural selection mechanisms. The devices also aid in studying spermatozoa's different behavior types in groups as well as investigating single sperm cell characteristics. Moreover, recent development in microfluidic technology designs led to encouraging outcomes in in vitro studies. Microfluidic sperm separation can be used to conserve wildlife germplasms and the methods devised for wild animal species can have large application in other domestic animals.
Commercialization problem in such devices limits applications in humans as well as animals. High cost of PDMS for fabrication of chips is another limitation for wide application in masses. Thermoplastics based microfluidic designs have been used with varying results in published literature. Similar to sperm robotics technique of sperm selection, reviewed in Bhat et al., 159 3D printing facility in microfluidic designs, 160 with the help of thermoplastics and other polymers for rapid prototyping enables faster commercialization and overcoming limitation for microscale. 161 , 162 , 163 Majority of the studies found in literature are human side. Among animals only few bovine and porcine studies using microfluidic designs for sperm selection have been published. However, for better clinical adoption of microfluidic systems more user friendly, simple and robust designs need to be devised in humans and their modifications need to be investigated in order to apply these techniques in domestic and wild animal germplasm conservation. The sperm behavioral characteristics in vivo need to be further investigated and used as guiding physics for designing new and innovative microfluidic designs in all the species. The species‐specific microfluidic chips need to be designed for extraction of fertile spermatozoa with minimum DNA damage for use in assisted reproduction.
AUTHOR CONTRIBUTIONS
The first and second authors conceived the ideas presented in the manuscript. The first author collected literature and wrote the manuscript. The second and third authors reviewed the entire manuscript.
DISCLOSURES
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
The authors are thankful to Sher‐e‐Kashmir Institute of Agricultural Sciences and Technology of Kashmir for encouragement of scientists for research.
Bhat GR, Lone FA, Dalal J. Microfluidics—A novel technique for high‐quality sperm selection for greater ART outcomes. FASEB BioAdvances. 2024;6:406‐423. doi: 10.1096/fba.2024-00041
DATA AVAILABILITY STATEMENT
This is a review of literature. There are no new primary data presented in the manuscript, it is all cited and published in data repositories like CORD and DOI. Data sharing is not applicable.
REFERENCES
- 1. Rahman MS, Kwon WS, Pang MG. Prediction of male fertility using capacitation‐associated proteins in spermatozoa. Mol Reprod Dev. 2017;84:749‐759. [DOI] [PubMed] [Google Scholar]
- 2. Sakkas D, Ramalingam M, Garrido N, Barratt CL. Sperm selection in natural conception: what can we learn from mother nature to improve assisted reproduction outcomes? Hum Reprod Update. 2015;21(6):711‐726. doi: 10.1093/humupd/dmv042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yata VK, Yadav N, Katoch V, et al. Enrichment of motile spermatozoa from cattle semen samples by microfluidics method. Indian J Anim Sci. 2022;92(6):711‐716. doi: 10.56093/ijans.v92i6.114553 [DOI] [Google Scholar]
- 4. Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442(7101):368‐373. doi: 10.1038/nature05058 [DOI] [PubMed] [Google Scholar]
- 5. Shirota K, Yotsumoto F, Itoh H, et al. Separation efficiency of a microfluidic sperm sorter to minimize sperm DNA damage. Fertil Steril. 2016;105(2):315‐321.e1. doi: 10.1016/j.fertnstert.2015.10.023 [DOI] [PubMed] [Google Scholar]
- 6. Wu JK, Chen PC, Lin YN, Wang CW, Pan LC, Tseng FG. High‐throughput flowing upstream sperm sorting in a retarding flow field for human semen analysis. Analyst. 2017;142(6):938‐944. doi: 10.1039/C6AN02420C [DOI] [PubMed] [Google Scholar]
- 7. Nosrati R, Gong MM, San Gabriel MC, Perzada CE, Zini A, Sinton D. Paper‐based quantification of male fertility potential. Clin Chem. 2016;62:458‐465. [DOI] [PubMed] [Google Scholar]
- 8. Nagata MPB, Endo K, Ogata K, et al. Live births from artificial insemination of microfluidic‐sorted bovine spermatozoa characterized by trajectories correlated with fertility. Proc Natl Acad Sci U S A. 2018;115(14):E3087‐E3096. doi: 10.1073/pnas.1717974115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zaferani M, Palermo GD, Abbaspourrad A. Strictures of a microchannel impose fierce competition to select for highly motile sperm. Sci Adv. 2019;5(2):eaav2111. doi: 10.1126/sciadv.aav2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khodamoradi M, Rafizadeh Tafti S, Mousavi Shaegh SA, Aflatoonian B, Azimzadeh M, Khashayar P. Recent microfluidic innovations for sperm sorting. Chem. 2021;9(6):126. doi: 10.3390/chemosensors9060126 [DOI] [Google Scholar]
- 11. Ogata K, Nagata MPB, Nishizono H, et al. In vitro survival kinetics of microfluidic‐sorted bovine spermatozoa. Andrology. 2021;9(3):977‐988. doi: 10.1111/andr.12958 [DOI] [PubMed] [Google Scholar]
- 12. Coline M, MariaAM ZA, Karine R, et al. Sperm migration, selection, survival, and fertilizing ability in the mammalian oviduct. Biol Reprod. 2021;105(2):317‐331. doi: 10.1093/biolre/ioab105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miki K, Clapham DE. Rheotaxis guides mammalian sperm. Curr Biol. 2013;23(6):443‐452. doi: 10.1016/j.cub.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wheeler MB, Rubessa M. Integration of microfluidics in animal in vitro embryo production. Mol Hum Reprod. 2017;23(4):248‐256. doi: 10.1093/molehr/gaw048 [DOI] [PubMed] [Google Scholar]
- 15. Xie L, Ma R, Han C, et al. Integration of sperm motility and chemotaxis Screeningwith aMicrochannel‐based device. Clin Chem. 2010;56(8):1270‐1278. doi: 10.1373/clinchem.2010.146902 [DOI] [PubMed] [Google Scholar]
- 16. Weng L. IVF‐on‐a‐chip: recent advances in microfluidics technology for in vitro fertilization. SLAS Technol. 2019;24(4):373‐385. doi: 10.1177/2472630319851765 [DOI] [PubMed] [Google Scholar]
- 17. Zhu L, Zhang Y, Liu Y, et al. Maternal and live‐birth outcomes of pregnancies following assisted reproductive technology: a retrospective cohort study. Sci Rep. 2016;6(1):35141. doi: 10.1038/srep35141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gawecka JE, Marh J, Ortega M, Yamauchi Y, Ward MA, Ward WS. Mouse zygotes respond to severe sperm DNA damage by delaying paternal DNA replication and embryonic development. PLoS One. 2013;8(2):e56385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roberts AM. Motion of spermatozoa in fluid streams. Nature. 1970;228(5269):375‐376. doi: 10.1038/228375a0 [DOI] [PubMed] [Google Scholar]
- 20. Winet H, Bernstein GS, Head J. Observations on the response of human spermatozoa to gravity, boundaries and fluid shear. Reproduction. 1984;70(2):511‐523. doi: 10.1530/jrf.0.0700511 [DOI] [PubMed] [Google Scholar]
- 21. Funahashi H, Nagai T. Sperm selection by a climbing‐over‐a‐wall IVF method reduces the incidence of polyspermic penetration of porcine oocytes. J Reprod Dev. 2000;46(5):319‐324. doi: 10.1262/jrd.46.319 [DOI] [Google Scholar]
- 22. Funahashi H, Romar R. Reduction of the incidence of polyspermic penetration into porcine oocytes by pretreatment of fresh spermatozoa with adenosine and a transient co‐incubation of the gametes with caffeine. Reproduction. 2004;128(6):789‐800. doi: 10.1530/rep.1.00295 [DOI] [PubMed] [Google Scholar]
- 23. Clark SG, Walters EM, Beebe DJ, Wheeler MB. In vitro fertilization of porcine oocytes in polydimethylsiloxane (PDMS)‐glass microchannels. Biol Reprod. 2002;66:528. [Google Scholar]
- 24. Clark SG, Haubert K, Beebe DJ, Ferguson CE, Wheeler MB. Reduction of polyspermic penetration using biomimetic microfluidic technology during in vitro fertilization. Lab Chip. 2005;5(11):1229‐1232. doi: 10.1039/b504397m [DOI] [PubMed] [Google Scholar]
- 25. Sano H, Matsuura K, Naruse K, Funahashi H. Application of a microfluidic sperm sorter to the in‐vitro fertilization of porcine oocytes reduced the incidence of polyspermic penetration. Theriogenology. 2010;74(5):863‐870. doi: 10.1016/j.theriogenology.2010.04.011 [DOI] [PubMed] [Google Scholar]
- 26. Prapti P, KumarSK SS, Monica G, et al. Microfuidic chips: recent advances, critical strategies in design, applications and future perspectives. Microfluid Nanofluidics. 2021;25(12):99. doi: 10.1007/s10404-021-02502-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seo DB, Agca Y, Feng ZC, Critser JK. Development of sorting, aligning, and orienting motile sperm using microfluidic device operated by hydrostatic pressure. Microfluid Nanofluid. 2007;3(5):561‐570. doi: 10.1007/s10404-006-0142-3 [DOI] [Google Scholar]
- 28. Ramírez‐Gómez HV, Jimenez Sabinina V, Velázquez Pérez M, et al. Sperm chemotaxis is driven by the slope of the chemoattractant concentration field´. Elife. 2020;9:e50532. doi: 10.7554/eLife.50532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rappa K, Samargia J, Sher M, Pino JS, Rodriguez HF, Asghar W. Quantitative analysis of sperm rheotaxis using a microfluidic device. Microfluid Nanofluid. 2018;22(9):100. doi: 10.1007/s10404-018-2117-6 [DOI] [Google Scholar]
- 30. Nosrati R, Vollmer M, Eamer L, et al. Rapid selection of sperm with high DNA integrity. Lab Chip. 2014;14(6):1142‐1150. doi: 10.1039/c3lc51254a [DOI] [PubMed] [Google Scholar]
- 31. Nosrati R, Graham PJ, Liu Q, Sinton D. Predominance of sperm motion in corners. Sci Rep. 2016;6:26669. doi: 10.1038/srep26669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nakao S, Toru T, Hitomi W, Gen K, Naomi N. Successful selection of mouse sperm with high viability andfertility using microfluidics chip cell sorter. Repo RtS. 2020;10:8862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Serafín P‐C, Ricardo L‐B, de AlejandroAC C, et al. Sperm selection by thermotaxis improves ICSI outcome in mice. Sci Rep. 2018;20188(1):2902. doi: 10.1038/s41598-018-21335-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Buffone MG, Doncel GF, Marín Briggiler CI, Vazquez‐Levin MH, Calamera JC. Human sperm subpopulations: relationship between functional quality and protein tyrosine phosphorylation. Hum Reprod. 2004;19(1):139‐146. doi: 10.1093/humrep/deh040 [DOI] [PubMed] [Google Scholar]
- 35. Jenkins TG, Aston KI, Trost C, Farley J, Hotaling JM, Carrell DT. Intra‐sample heterogeneity of sperm DNA methylation. Mol Hum Reprod. 2015;21(4):313‐319. doi: 10.1093/molehr/gau115 [DOI] [PubMed] [Google Scholar]
- 36. Roy D, Levi K, Kiss V, Nevo R, Eisenbach M. Rhodopsin and melanopsin coexist in mammalian sperm cells and activate diferent signaling pathways for thermotaxis. Sci Rep. 2020;10(1):112. doi: 10.1038/s41598-019-56846-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Benko F, Urminská D, Ďuračka M, Tvrdá E. Signaling roleplay between ion channels during mammalian sperm capacitation. Biomedicine. 2023;11(9):1. doi: 10.3390/biomedicines11092519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. D'Amours O, Frenette G, Fortier M, Leclerc P, Sullivan R. Proteomic comparison of detergent‐extracted sperm proteins from bulls with different fertility indexes. Reproduction. 2010;139(3):545‐556. doi: 10.1530/REP-09-0375 [DOI] [PubMed] [Google Scholar]
- 39. Simon L, Zini A, Dyachenko A, Ciampi A, Carrell DT. A systematic review and meta‐analysis to determine the effect of sperm DNA damage oninvitrofertilization and intracytoplasmic sperm injection outcome. Asian J Androl. 2017;19(1):80‐90. doi: 10.4103/1008-682X.182822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Antunes DM, Kalmbach KH, Wang F, et al. A single‐cell assay for telomere DNA content shows increasing telomere length heterogeneity, as well as increasing mean telomere length in human spermatozoa with advancing age. J Assist Reprod Genet. 2015;32(11):1685‐1690. doi: 10.1007/s10815-015-0574-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Evenson DP. The sperm chromatin structure assay (SCSA®) and other sperm DNA fragmentation tests for evaluation of sperm nuclear DNA integrity as related to fertility. Anim Reprod Sci. 2016;169:56‐75. doi: 10.1016/j.anireprosci.2016.01.017 [DOI] [PubMed] [Google Scholar]
- 42. Tournaye H, Verheyen G, Albano C, et al. Intracytoplasmic sperm injection versus in vitro fertilization: a randomized controlled trial and a meta‐analysis of the literature. Fertil Steril. 2002;78(5):1030‐1037. doi: 10.1016/s0015-0282(02)03377-0 [DOI] [PubMed] [Google Scholar]
- 43. Umehara T, Kawai T, Goto M, Richards JS, Shimada M. Creatine enhances the durationof sperm capacitation: a novel factorfor improving in vitro fertilizationwith small numbers of sperm. Hum Reprod. 2018;33(6):1117‐1129. doi: 10.1093/humrep/dey081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mondéjar I, Martínez‐Martínez I, Avilés M, Coy P. Identification of potential oviductal factors responsible for zona pellucida hardening and monospermy during fertilization in mammals. Biol Reprod. 2013;89(3):67. doi: 10.1095/biolreprod.113.111385 [DOI] [PubMed] [Google Scholar]
- 45. Said TM, Grunewald S, Paasch U, et al. Advantage of combining magnetic cell separation with sperm preparation techniques. Reprod Biomed Online. 2005;10(6):740‐746. doi: 10.1016/s1472-6483(10)6111 [DOI] [PubMed] [Google Scholar]
- 46. Morrell JM, Rodriguez‐Martinez H. Practical applications of sperm selection techniques as a tool for improving reproductive efficiency. Vet Med Int. 2010;2011:1‐9. doi: 10.4061/2011/894767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Flesch FM, Gadella BM. Dynamics of the mammalian sperm plasma membrane in the process of fertilization. Biochim Biophys Acta. 2000;1469(3):197‐235. doi: 10.1016/s0304-4157(00)00018-6 [DOI] [PubMed] [Google Scholar]
- 48. Trentalance GM, Beorlegui NB. Sperm evaluation in cryopreserved bovine semen recovered by two selection methods. Andrologia. 2002;34(6):397‐403. doi: 10.1046/j.1439-0272.2002.00525.x [DOI] [PubMed] [Google Scholar]
- 49. Ainsworth C, Nixon B, Aitken R. Development of a Novel Electrophoretic System for Isolation of Human Spermatozoa. Human Rep. 2005;20(8):2261‐2270. [DOI] [PubMed] [Google Scholar]
- 50. Cesari A, Kaiser GG, Mucci N, et al. Integrated morphophysiological assessment of two methods for sperm selection in bovine embryo production in vitro. Theriogenology. 2006;66(5):1185‐1193. doi: 10.1016/j.theriogenology.2006.03.029 [DOI] [PubMed] [Google Scholar]
- 51. Machado GM, Carvalho JO, Filho ES, et al. Effect of Percoll volume, duration and force of centrifugation, on in vitro production and sex ratio of bovine embryos. Theriogenology. 2009;71(8):1289‐1297. doi: 10.1016/j.theriogenology.2009.01.002 [DOI] [PubMed] [Google Scholar]
- 52. Petyim S, Choavaratana R, Suksompong S, Laokirkkiat P, Makemaharn O. Outcome of sperm preparation using double‐gradients technique study in Siriraj hospital. J Med Assoc Thai. 2009;92(7):878‐884. [PubMed] [Google Scholar]
- 53. Ishibashi K, Sakakibara H, Oiwa K. Force‐generating mechanism of axonemal dynein in solo and ensemble. Int J Mol Sci. 2020;21(8):2843. doi: 10.3390/ijms21082843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Morrell JM, Rodriguez‐Martinez H. Practical applications of sperm selection techniques as a tool for improving reproductive efficiency. Vet Med Int. 2010;2011:894767. doi: 10.4061/2011/894767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mousset‐Siméon N, Rives N, Masse L, Chevallier F, Mace B. Comparison of six density gradient media for selection of cryopreserved donor spermatozoa. J Androl. 2004;25(6):881‐884. doi: 10.1002/j.1939-4640.2004.tb03157.x [DOI] [PubMed] [Google Scholar]
- 56. Oguz Y, Guler I, Erdem A, et al. The effect of swim‐up and gradient sperm preparation techniques on deoxyribonucleic acid (DNA) fragmentation in subfertile patients. J Assist Reprod Genet. 2018;35(6):1083‐1089. doi: 10.1007/s10815-018-1163-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Arias ME, Andara K, Briones E, Felmer R. Bovine sperm separation by swim‐up and density gradients (Percoll and BoviPure): effect on sperm quality, function and gene expression. Reprod Biol. 2017;17(2):126‐132. doi: 10.1016/j.repbio.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 58. Palini S, De Stefani S, Primiterra M, et al. Comparison of in vitro fertilization outcomes in ICSI cycles after human sperm preparation by density gradient centrifugation and direct micro swimup without centrifugation. J Bras Reprod Assist. 2017;21:89‐93. doi: 10.5935/1518-0557.20170022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Grunewald S, Paasch U, Glander HJ. Enrichment of non‐apoptotic human spermatozoa after cryopreservation by immunomagnetic cell sorting. Cell Tissue Bank. 2001;2(3):127‐133. doi: 10.1023/A:1020188913551 [DOI] [PubMed] [Google Scholar]
- 60. Vermes I, Haanen C, Steffens‐Nakken H, Reutellingsperger C. A novel assay for apoptosis flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J Immunol Methods. 1995;184(1):39‐51. doi: 10.1016/0022-1759(95)00072-I [DOI] [PubMed] [Google Scholar]
- 61. Lee TH, Liu CH, Shih YT, et al. Magnetic‐activated cell sorting for sperm preparation reduces spermatozoa with apoptotic markers and improves the acrosome reaction in couples with unexplained infertility. Hum Reprod. 2010;25(4):839‐846. doi: 10.1093/humrep/deq009 [DOI] [PubMed] [Google Scholar]
- 62. Gil M, Sar‐Shalom V, Melendez Sivira Y, Carreras R, Checa MA. Sperm selection using magnetic activated cell sorting (MACS) in assisted reproduction: a systematic review and meta‐analysis. J Assist Reprod Genet. 2013;30(4):479‐485. doi: 10.1007/s10815-013-9962-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bucar S, Goncalves A, Rocha E, Barros A, Sousa M, Sa R. DNA fragmentation inhuman sperm after magnetic‐activated cell sorting. J Assist Reprod Genet. 2015;32:147154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ziarati N, Tavalaee M, Bahadorani M, Nasr Esfahani MH. Clinical outcomes of magnetic activated sperm sorting in infertile men candidate for ICSI. Hum Fertil. 2019;22(2):118‐125. doi: 10.1080/14647273.2018.1424354 [DOI] [PubMed] [Google Scholar]
- 65. Vasicek J, Pivko J, Chrenek P. Reproductive performance of New Zealand white rabbits after depletion of apoptotic spermatozoa. Folia Biol. 2014;62(2):109‐117. doi: 10.3409/fb62_2.109 [DOI] [PubMed] [Google Scholar]
- 66. Dandekar P, Aggeler J, Talbot P. Structure, distribution and composition of the extracellular matrix of human oocytes and cumulus masses. Hum Reprod. 1992;7(3):391‐398. doi: 10.1093/oxfordjournals.humrep.a137656 [DOI] [PubMed] [Google Scholar]
- 67. Cayli S, Jakab A, Ovari L, et al. Biochemical markers of sperm function: male fertility and spermselection for ICSI. Reprod Biomed Online. 2003;7(4):462‐468. doi: 10.1016/s1472-6483(10)61891-3 [DOI] [PubMed] [Google Scholar]
- 68. Huszar G, Ozkavukcu S, Jakab A, Celik‐Ozenci C, Sati GL, Cayli S. Hyaluronic acid binding ability of human sperm reflects cellular maturity and fertilizing potential: selection of sperm for intracytoplasmic sperm injection. J Obstet Gynaecol. 2006;18(3):260‐267. doi: 10.1097/01.gco.0000193018.98061.2f [DOI] [PubMed] [Google Scholar]
- 69. Parmegiani L, Cognigni GE, Bernardi S, Troilo E, Ciampaglia W, Filicori M. “Physiologic ICSI”: hyaluronic acid (HA) favors selection of spermatozoa without DNA fragmentation and with normal nucleus, resulting in improvement of embryo quality. Fertil Steril. 2010;93(2):598‐604. doi: 10.1016/j.fertnstert.2009.03.033 [DOI] [PubMed] [Google Scholar]
- 70. Huang MT, Kuo‐Kuang Lee R, Lu CH, Chen YJ, Li SH, Hwu YM. The efficiency of conventional microscopic selection is comparable to the hyaluronic acid binding method in selecting spermatozoa for male infertility patients. Taiwan J Obstet Gynecol. 2015;54(1):48‐53. doi: 10.1016/j.tjog.2014.11.006 [DOI] [PubMed] [Google Scholar]
- 71. Garolla A, Fortini D, Menegazzo M, De TL NV, Moretti A, et al. High‐power microscopy for selecting spermatozoa for ICSI by physiological status. 2008. [DOI] [PubMed]
- 72. Kheirollahi‐Kouhestani M, Razavi S, Tavalaee M, et al. Selection of sperm based on combined density gradient and zeta method may improve ICSI outcome. Hum Reprod. 2009;24(10):2409‐2416. doi: 10.1093/humrep/dep088 [DOI] [PubMed] [Google Scholar]
- 73. Razavi SH, Nasr‐Esfahani MH, Deemeh MR, Shayesteh M, Tavalaee M. Evaluation of zeta and HA‐binding methods for selection of spermatozoa with normal morphology, protamine content and DNA integrity. Andrologia. 2010;42(1):13‐19. doi: 10.1111/j.1439-0272.2009.00948.x [DOI] [PubMed] [Google Scholar]
- 74. Zarei‐Kheirabadi M, Shayegan Nia E, Tavalaee M, et al. Evaluation of ubiquitin and annexin V in sperm population selected based on density gradient centrifugation and zeta potential (DGC‐zeta). J Assist Reprod Genet. 2012;29(4):365‐371. doi: 10.1007/s10815-011-9689-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zahedi A, Tavalaee M, Deemeh MR, Azadi L, Fazilati M, Nasr‐Esfahani MH. Zeta potential vs apoptotic marker: which is more suitable for ICSI sperm selection? J Assist Reprod Genet. 2013;30(9):1181‐1186. doi: 10.1007/s10815-013-0022-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nasr Esfahani MH, Deemeh MR, Tavalaee M, Sekhavati MH, Gourabi H. Zeta sperm selection improves pregnancy rate and alters sex ratio in male factor infertility patients: A double‐blind, randomized clinical trial. Int J Fertil Steril. 2016;10(2):253‐260. doi: 10.22074/ijfs.2016.4917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yániz JL, Soler C, Santolaria P. Computer assisted sperm morphometry in mammals: a review. Anim Reprod Sci. 2015;156:1‐12. doi: 10.1016/j.anireprosci.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 78. Bartoov B, Berkovitz A, Eltes F, Kogosowski A, Menezo Y, Barak Y. Real‐time fine morphology of motile human sperm cells is associated with IVF‐ICSI outcome. J Androl. 2002;23(1):1‐8. doi: 10.1002/j.1939-4640.2002.tb02595.x [DOI] [PubMed] [Google Scholar]
- 79. Fortunato A, Boni R, Leo R, et al. Vacuoles in sperm head are not associated with head morphology, DNA damage and reproductive success. Reprod Biomed Online. 2016;32(2):154‐161. doi: 10.1016/j.rbmo.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 80. Belva F, Roelants M, De Schepper J, Van Steirteghem A, Tournaye H, Bonduelle M. Reproductive hormones of ICSI‐conceived young adult men: the first results. Hum Reprod. 2017;32:439‐446. doi: 10.1093/humrep/dew324 [DOI] [PubMed] [Google Scholar]
- 81. Nabi A, Khalili MA, Halvaei I, Roodbari F. Prolonged incubation of processed human spermatozoa will increase DNA fragmentation. Andrologia. 2014;46(4):374‐379. doi: 10.1111/and.12088 [DOI] [PubMed] [Google Scholar]
- 82. Zhao F, Yang Q, Shi S, Luo X, Sun Y. Semen preparation methods and sperm telomere length: density gradient centrifugation versus the swim up procedure. Sci Rep. 2016;6(1):39051. doi: 10.1038/srep39051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Matsuura R, Takeuchi T, Yoshida A. Preparation and incubation conditions affect the DNA integrity of ejaculated human spermatozoa. Asian J Androl. 2010;12(5):753‐759. doi: 10.1038/aja.2010.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442(7101):368‐373. [DOI] [PubMed] [Google Scholar]
- 85. Phiphattanaphiphop C, Leksakul K, Phatthanakun R, Khamlor T. A novel microfluidic chip‐based sperm‐sorting device constructed using design of experiment method. Sci Rep. 2020;10(1):17143. doi: 10.1038/s41598-020-73841-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Inaba K. Sperm flagella: comparative and phylogenetic perspectives of protein components. Mol Hum Reprod. 2011;17(8):524‐538. doi: 10.1093/molehr/gar034 [DOI] [PubMed] [Google Scholar]
- 87. Riedel‐Kruse IH, Hilfinger A, Howard J, Jülicher F. How molecular motors shape the flagellar beat. HFSP J. 2007;1(3):192‐208. doi: 10.2976/1.2773861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lin J, Okada K, Raytchev M, Smith MC, Nicastro D. Structural mechanism of the dynein power stroke. Nat Cell Biol. 2014;16(5):479‐485. doi: 10.1038/ncb2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lauga E, Powers TR. The hydrodynamics of swimming microorganisms. Rep Prog Phys. 2009;72(9):96601. doi: 10.1088/0034-4885/72/9/096601 [DOI] [Google Scholar]
- 90. Ishijima S, Sekiguchi K, Hiramoto Y. Comparative study of the beat patterns of American and Asian horseshoe crab sperm: evidence for a role of the central pair complex in forming planar waveforms in flagella. Cytoskeleton. 1988;9(3):264‐270. [Google Scholar]
- 91. Woolley DM, Vernon GG. A study of helical and planar waves on sea urchin sperm flagella, with a theory of how they are generated. J Exp Biol. 2001;204(7):1333‐1345. doi: 10.1242/jeb.204.7.1333 [DOI] [PubMed] [Google Scholar]
- 92. Gibbons IR, Shingyoji C, Murakami A, Takahashi K. Spontaneous recovery after experimental manipulation of the plane of beat in sperm flagella. Nature. 1987;325(6102):351‐352. doi: 10.1038/325351a0 [DOI] [PubMed] [Google Scholar]
- 93. Woolley DM. Flagellar oscillation: a commentary on proposed mechanisms. Biol Rev Camb Philos Soc. 2010;85(3):453‐470. doi: 10.1111/j.1469-185X.2009.00110.x [DOI] [PubMed] [Google Scholar]
- 94. Merola F, Miccio L, Memmolo P, et al. Digital holography as a method for 3D imaging and estimating the biovolume of motile cells. Lab Chip. 2013;13(23):4512‐4516. doi: 10.1039/c3lc50515d [DOI] [PubMed] [Google Scholar]
- 95. Di Caprio GD, Ferrara MA, Miccio L, et al. Holographic imaging of unlabelled sperm cells for semen analysis: a review. J Biophotonics. 2015;8(10):779‐789. doi: 10.1002/jbio.201400093 [DOI] [PubMed] [Google Scholar]
- 96. CaprioG DC, Gioffre MA, Saffioti N, et al. Quantitative label‐free animal sperm imaging by means of digital holographic microscopy. IEEE J Sel Top Quantum Electron. 2010;2010(4):833‐840. [Google Scholar]
- 97. Lai D, Takayama S, Smith GD. Recentmicrofluidic devices for studying gamete and embryo biomechanics. J Biomech. 2015;48(9):1671‐1678. doi: 10.1016/j.jbiomech.2015.02.039 [DOI] [PubMed] [Google Scholar]
- 98. Kantsler V, Dunkel J, Blayney M, Goldstein RE. Rheotaxis facilitates upstream navigation of mammalian sperm cells. eLife. 2014;3:e02403. doi: 10.7554/eLife.02403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tung CK, Ardon F, Roy A, Koch DL, Suarez SS, Wu M. Emergence of upstream swimming via a hydrodynamic transition. Phys Rev Lett. 2015;114(10):108102. doi: 10.1103/PhysRevLett.114.108102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ishimoto K, Gaffney EA. Fluid flow and sperm guidance: a simulation study of hydrodynamic sperm rheotaxis. J R Soc Interface. 2015;12(106):20150172. doi: 10.1098/rsif.2015.0172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zhang Z, Liu J, Meriano J, et al. Human sperm rheotaxis: a passive physical process. Sci Rep. 2016;6(1):23553. doi: 10.1038/srep23553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Eel‐Sherry TM, Abdel‐Ghani MA, Abou‐Khalil NS, Elsayed M, Abdelgawad M. Effect of pH on rheotaxis of bull sperm using microfluidics. Reprod Domest Anim. 2019;202:1‐9. [DOI] [PubMed] [Google Scholar]
- 103. Eisenbach M, Giojalas LC. Sperm guidance in mammals – an unpaved road to the egg. Nat Rev Mol Cell Biol. 2006;7(4):276‐285. doi: 10.1038/nrm1893 [DOI] [PubMed] [Google Scholar]
- 104. Alvarez L, Friedrich BM, Gompper G, Kaupp UB. The computational sperm cell. Trends Cell Biol. 2014;24(3):198‐207. doi: 10.1016/j.tcb.2013.10.004 [DOI] [PubMed] [Google Scholar]
- 105. Bukatin A, Kukhtevich I, Stoop N, Dunkel J, Kantsler V. Bimodal rheotactic behavior reflects flagellar beat asymmetry in human sperm cells. Proc Natl Acad Sci U S A. 2015;112(52):15904‐15909. doi: 10.1073/pnas.1515159112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kaupp UB, Solzin J, Hildebrand E, et al. The signal flow and motor response controling chemotaxis of sea urchin sperm. Nat Cell Biol. 2003;5(2):109‐117. [DOI] [PubMed] [Google Scholar]
- 107. Kirkman‐Brown JC, Sutton KA, Florman HM. How to attract a sperm. Nat Cell Biol. 2003;5(2):93‐96. doi: 10.1038/ncb0203-93 [DOI] [PubMed] [Google Scholar]
- 108. Jikeli JF, Alvarez L, Friedrich BM, et al. Sperm navigation along helical paths in 3D chemoattractant landscapes. Nat Commun. 2015;6:7985. doi: 10.1038/ncomms8985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Friedrich BM, Jülicher F. Chemotaxis of sperm cells. Proc Natl Acad Sci U S A. 2007;104(33):13256‐13261. doi: 10.1073/pnas.0703530104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hussain YH, Guasto JS, Zimmer RK, Stocker R, Riffell JA. Sperm chemotaxis promotes individual fertilization success in sea urchins. J Exp Biol. 2016;219(10):1458‐1466. doi: 10.1242/jeb.134924 [DOI] [PubMed] [Google Scholar]
- 111. Koyama S, Amarie D, Soini HA, Novotny MV, Jacobson SC. Chemotaxis assays of mouse sperm on microfluidic devices. Anal Chem. 2006;78(10):3354‐3359. doi: 10.1021/ac052087i [DOI] [PubMed] [Google Scholar]
- 112. Chang H, Kim BJ, Kim YS, Suarez SS, Wu M. Different migration patterns of sea urchin and mouse sperm revealed by a microfluidic chemotaxis device. PLoS One. 2013;8(4):e60587. doi: 10.1371/journal.pone.0060587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zhang B, Yin TL, Yang J. A novel microfluidic device for selecting human sperm to increase the proportion of morphologically normal, motile sperm with uncompromised DNA integrity. Anal Methods. 2015;7(14):5981‐5988. doi: 10.1039/C5AY00905G [DOI] [Google Scholar]
- 114. Schuster TG, Cho B, Keller LM, Takayama S, Smith GD. Isolation of motile spermatozoa from semen samples using microfluidics. Reprod Biomed Online. 2003;7(1):75‐81. doi: 10.1016/s1472-6483(10)61732-4 [DOI] [PubMed] [Google Scholar]
- 115. Walters EM, Clark SG, Beebe DJ, Wheeler MB. Mammalian embryo culture in a microfluidic device. Methods Mol Biol. 2004;254:375‐382. doi: 10.1385/1-59259-741-6:375 [DOI] [PubMed] [Google Scholar]
- 116. Raty S, Walters EM, Davis J, et al. Embryonic development in the mouse is enhanced via microchannel culture. Lab Chip. 2004;4(3):186‐190. doi: 10.1039/b316437c [DOI] [PubMed] [Google Scholar]
- 117. Takeda K, Fujimura Y, Jimma F. Development of a new compact flow cytometer a using disposable microfluidic chip for contamination—free and biosafety measurement. Cytometry Res. 2011;21:43‐50. [Google Scholar]
- 118. Chinnasamy T, Kingsley JL, Inci F, et al. Guidance and self‐sorting of active swimmers: 3D periodic arrays increase persistence length of human sperm selecting for the fittest. Adv Sci. 2018;5(2):1700531. doi: 10.1002/advs.201700531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lee CY, Chang CL, Wang YN, Fu LM. Microfluidic mixing: a review. Int J Mol Sci. 2011;12(5):3263‐3287. doi: 10.3390/ijms12053263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Murphy TW, Zhang Q, Naler LB, Ma S, Lu C. Recent advances in the use of microfluidic technologies for single cell analysis. Analyst. 2017;143(1):60‐80. doi: 10.1039/c7an01346a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Briones MPP, Honda T, Yamaguchi Y, Miyazaki M, Nakamura H, Maeda H. A practical method for rapid microchannel fabrication in polydimethylsiloxane by replica molding without using silicon photoresist. J Chem Eng Jpn. 2006;39(10):1108‐1114. doi: 10.1252/jcej.39.1108 [DOI] [Google Scholar]
- 122. Coppola MA, Klotz KL, Kim KA, et al. SpermCheck® fertility, an immunodiagnostic home test that detects normozoospermia and severe oligozoospermia. Hum Reprod. 2010;25(4):853‐861. doi: 10.1093/humrep/dep413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Björndahl L, Kirkman‐Brown J, Hart G, Rattle S, Barratt CLR. Development of a novel home sperm test. Hum Reprod. 2006;21(1):145‐149. doi: 10.1093/humrep/dei330 [DOI] [PubMed] [Google Scholar]
- 124. Nosrati R, Graham PJ, Zhang B, et al. Microfluidics for sperm analysis and selection. Nat Rev Urol. 2017;14(12):707‐730. doi: 10.1038/nrurol.2017.175 [DOI] [PubMed] [Google Scholar]
- 125. Sadani Z, Wacogne B, Pieralli C, Roux C, Gharbi T. Microsystems and microfluidic device for single oocyte transportation and trapping: toward the automation of in vitro fertilising. Sens Actuators A. 2005;121(2):364‐372. doi: 10.1016/j.sna.2005.03.004 [DOI] [Google Scholar]
- 126. Matsuura K, Uozumi T, Furuichi T, Sugimoto I, Kodama M, Funahashi H. A microfluidic device to reduce treatment time of intracytoplasmic sperm injection. Fertil Steril. 2013;99(2):400‐407. doi: 10.1016/j.fertnstert.2012.10.022 [DOI] [PubMed] [Google Scholar]
- 127. Krisher RL, Wheeler MB. Towards the use of microfluidics for individual embryo culture. Reprod Fertil Dev. 2010;22(1):32‐39. doi: 10.1071/RD09219 [DOI] [PubMed] [Google Scholar]
- 128. Yanez LZ, Camarillo DB. Microfluidic analysis of oocyte and embryo biomechanical properties to improve outcomes in assisted reproductive technologies. Mol Hum. 2017;23:235‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Asghar W, Velasco V, Kingsley JL, et al. Selection of functional human sperm with higher DNA integrity and fewer reactive oxygen species. Adv Healthc Mater. 2014;3(10):1671‐1679. doi: 10.1002/adhm.201400058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Barroso G, Morshedi M, Oehninger S. Analysis of DNA fragmentation, plasma membrane translocation of phosphatidylserine and oxidative stress in human spermatozoa. Hum Reprod. 2000;15(6):1338‐1344. doi: 10.1093/humrep/15.6.1338 [DOI] [PubMed] [Google Scholar]
- 131. Gonzalez‐Castro R, Porflidt C, Patton T, Goins D. Lisa HerickhoffRetrospective analysis of commercial heterospermic and homospermic cooled boar semen: effect of the season, sample type and shipping temperature on sperm quality. Reprod Domest Anim. 2022;57(4):357‐367. doi: 10.1111/rda.14074 [DOI] [PubMed] [Google Scholar]
- 132. Vajta G, Parmegiani L, Machaty Z, Chen WB, Yakovenko S. Back to the future: optimised microwell culture of individual human preimplantation stage embryos. J Assist Reprod Genet. 2021;38(10):2563‐2574. doi: 10.1007/s10815-021-02167-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Denissenko P, Kantsler V, Smith DJ, Kirkman‐Brown J. Humanspermatozoa migration in microchannels reveals boundary‐following navigation. Proc Natl Acad Sci U S A. 2012;109(21):8007‐8010. doi: 10.1073/pnas.1202934109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Mallidis C, Sanchez V, Wistuba J, et al. Raman microspectroscopy: shining a new light on reproductive medicine. Hum Reprod Update. 2014;20(3):403‐414. doi: 10.1093/humupd/dmt055 [DOI] [PubMed] [Google Scholar]
- 135. Franken DR, Oehninger S. Semen analysis and sperm function testing. Asian J Androl. 2012;14(1):6‐13. doi: 10.1038/aja.2011.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Segerink LI, Sprenkels AJ, ter Braak PM, Vermes I, van den Berg A. Onchip determination of spermatozoa concentration using electrical impedance measurements. Lab Chip. 2010;10(8):1018‐1024. doi: 10.1039/b923970g [DOI] [PubMed] [Google Scholar]
- 137. Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231‐245. [DOI] [PubMed] [Google Scholar]
- 138. Mazzilli R, Rucci C, Vaiarelli A, et al. Male factor infertility and assisted reproductive technologies: indications, minimum access criteria and outcomes. J Endocrinol Invest. 2023;46:1079‐1085. doi: 10.1007/s40618-022-02000-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Segerink LI, Sprenkels AJ, Oosterhuis GJ, Vermes I, van den Berg A. Microfluidic chips for semen analysis. EJIFCC. 2012;23(3):66‐69. [PMC free article] [PubMed] [Google Scholar]
- 140. Park JY, Kricka LJ. Male infertility and microchips. Clin Chem. 2013;59(3):457‐458. doi: 10.1373/clinchem.2012.200394 [DOI] [PubMed] [Google Scholar]
- 141. Mortimer ST, Van Der Horst G, Mortimer D. The future of computer‐aided sperm analysis. Asian J Androl. 2015;17(4):545‐553. doi: 10.4103/1008-682X.154312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Murray KS, James A, McGeady JB, Reed ML, Kuang WW, Nangia AK. The effect of the new 2010 World Health Organization criteria for semen analyses on male infertility. Fertil Steril. 2012;98(6):1428‐1431. doi: 10.1016/j.fertnstert.2012.07.1130 [DOI] [PubMed] [Google Scholar]
- 143. Repping S, Van Weert JM, Mol BW, de Vries JW, van der Veen F. Use of the total motile sperm count to predict total fertilization failure in in vitro fertilization. Fertil Steril. 2002;78(1):22‐28. doi: 10.1016/s0015-0282(02)03178-3 [DOI] [PubMed] [Google Scholar]
- 144. Kime DE, Van Look KJ, McAllister BG, Huyskens G, Rurangwa E, Ollevier F. Computerassisted sperm analysis (CASA) as a tool for monitoring sperm quality in fish. Comp Biochem Physiol C Toxicol Pharmacol. 2001;130(4):425‐433. doi: 10.1016/s1532-0456(01)00270-8 [DOI] [PubMed] [Google Scholar]
- 145. Brazil C. Practical semen analysis: from A to Z. Asian J Androl. 2010;12:14‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Amann RP, Katz DF. Reflections on CASA after 25 years. J Androl. 2004;25(3):317‐325. doi: 10.1002/j.1939-4640.2004.tb02793.x [DOI] [PubMed] [Google Scholar]
- 147. Chen CY, Chiang TC, Lin CM, et al. Sperm quality assessment via separation and sedimentation in a microfluidic device. Analyst. 2013;138(17):4967‐4974. doi: 10.1039/c3an00900a [DOI] [PubMed] [Google Scholar]
- 148. De Wagenaar B, Dekker S, de Boer HL, et al. Towards microfluidic sperm refinement: impedance‐based analysis and sorting of sperm cells. Lab Chip. 2016;16(8):1514‐1522. doi: 10.1039/c6lc00256k [DOI] [PubMed] [Google Scholar]
- 149. Mangum CL, Patel DP, Jafek AR, et al. Towards a better testicular sperm extraction: novel sperm sorting technologies for non‐motile sperm extracted by microdissection TESE. Transl Androl Urol. 2020;9(2):S206‐S214. doi: 10.21037/tau.2019.0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Vasilescu SA, Khorsandi S, Ding L, et al. A microfluidic approach to rapid sperm recoveryfrom heterogeneous cell suspensions. Sci Rep. 2021;11(1):7917. doi: 10.1038/s41598-021-87046-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Gai J, Nosrati R, Neild A. High DNA integrity sperm selection using surface acoustic waves. Lab Chip. 2020;20(22):4262‐4272. doi: 10.1039/d0lc00457j [DOI] [PubMed] [Google Scholar]
- 152. Kishi K, Ogata H, Ogata S, et al. Frequency of sperm DNA fragmentation according to selection method:comparison and relevance of a microfluidic device and a swim‐up procedure. J Clin Diagn Res. 2015;9(11):QC14‐QC16. doi: 10.7860/JCDR/2015/10332.6811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Quinn MM, Jalalian L, Ribeiro S, et al. Microfluidic sorting selects sperm for clinical use with reduced. 2018. [DOI] [PubMed]
- 154. Hester PN, Roseman HM, Clark SG, Walters EM, Beebe DJ, Wheeler MB. Enhanced cleavage rates following in vitro maturation of pig oocytes within polydimethylsiloxane‐borosilicate microchannels. Theriogenology. 2002;57:723. [Google Scholar]
- 155. Yuan Y, Paczkowski M, Wheeler MB, Krisher RL. Use of a novel polydimethylsiloxane well insert to successfully mature, culture and identify single porcine oocytes and embryos. Reprod Fertil Dev. 2014;26(3):375‐384. doi: 10.1071/RD12326 [DOI] [PubMed] [Google Scholar]
- 156. Zeringue HC, Rutledge JJ, Beebe DJ. Early mammalian embryo development depends on cumulus removal technique. Lab Chip. 2005;5(1):86‐90. doi: 10.1039/b316494m [DOI] [PubMed] [Google Scholar]
- 157. Calisici O, Camacho AR, Seckin S, Bajcsy CÁ. Effect of sperm selection using microfluidic sperm sorting chip on bovine embryonic development in vitro. In: Conference: 33rd Annual Meeting of the Brazilian Embryo Technology Society (SBTE) and 35th Annual Meeting of the European Embryo Transfer Association (AETE. 2019. Lab: Csaba Á Bajcsy's Lab.
- 158. Wright SN, Huge BJ, Dovichi NJ. Capillary zone electrophoresis separation and collection of spermatozoa for the forensic analysis of sexual assault evidence. Electrophoresis. 2020;41(15):1344‐1353. doi: 10.1002/elps.201900455 [DOI] [PubMed] [Google Scholar]
- 159. Bhat GR, Lone FA, Assad NI. Spermbots: A promising futuristic innovation in assisted reproductive technology. The Indian J Anim Reprod. 2023;44(2):14‐17. doi: 10.48165/ijar.2023.44.02.3 [DOI] [Google Scholar]
- 160. Jahangiri AR, Ziarati N, Dadkhah E, et al. Microfluidics: the future of sperm selection in assisted reproduction. Andrology. 2023. doi: 10.1111/andr.13578. Epub ahead of print. PMID: 38148634. [DOI] [PubMed] [Google Scholar]
- 161. Meseguer F, Giménez Rodríguez C, Rivera Egea R, Carrión Sisternas L, Remohí JA, Meseguer M. Can microfluidics improve sperm quality? A prospective functional study. Biomedicine. 2024;12(5):1131. doi: 10.3390/biomedicines12051131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Sheibak N, Amjadi F, Shamloo A, Zarei F, Zandieh Z. Microfluidic sperm sorting selects a subpopulation of high‐quality sperm with a higher potential for fertilization. Hum Reprod. 2024;39(5):902‐911. doi: 10.1093/humrep/deae045 [DOI] [PubMed] [Google Scholar]
- 163. Bhattacharjee N, Urrios A, Kang S, Folch A. The upcoming 3D‐printing revolution in microfluidics. Lab Chip. 2016;16(10):1720‐1742. doi: 10.1039/c6lc00163g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Bahat A, Caplan SR, Eisenbach M. Thermotaxis of human sperm cells in extraordinarily shallow temperature gradients over a wide range. PLoS One. 2012;7(7):e41915. doi: 10.1371/journal.pone.0041915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Li Z, Liu W, Qiu T, et al. The construction of an interfacial valve‐based microfluidic chip for thermotaxis evaluation of human sperm. Biomicrofluidics. 2014;8(2):24102. doi: 10.1063/1.4866851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Mondal MA, Takagi Y, Baba SA, Hamano KI. Involvement of calcium channels and intracellular calcium in bull sperm thermotaxis. J Reprod Dev. 2017;63(2):143‐148. doi: 10.1262/jrd.2016-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Dominguez EM, Moreno‐Irusta A, Guidobaldi HA, Tribulo H, Giojalas LC. Improved bovine in vitro embryo production with sexed and unsexed sperm selected by chemotaxis. Theriogenology. 2018;122:1‐8. doi: 10.1016/j.theriogenology.2018.08.023 [DOI] [PubMed] [Google Scholar]
- 168. Ko YJ, Maeng JH, Hwang SY, Ahn Y. Design, fabrication, and testing of a microfluidic device for thermotaxis and chemotaxis assays of sperm. SLASTECHNOLOGY: translating life. SLAS Technol. 2018;23(6):507‐515. doi: 10.1177/2472630318783948 [DOI] [PubMed] [Google Scholar]
- 169. Mohammad Y, Morteza A. Progressive bovine sperm separation using parallelized microchamber‐based microfluidics†. Lab Chip. 2021;21:2791‐2804. [DOI] [PubMed] [Google Scholar]
- 170. Berendsen JTW, Eijkel JCT, Wetzels AM, Segerink LI. Separation of spermatozoa from erythrocytes using their tumbling mechanism in a pinch flow fractionation device. Microsyst Nanoeng. 2019;5:24. doi: 10.1038/s41378-019-0068-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a review of literature. There are no new primary data presented in the manuscript, it is all cited and published in data repositories like CORD and DOI. Data sharing is not applicable.


