Abstract
Graphene-based nanomaterials have been proved to be robust sorbents for efficient removal of environmental contaminants including arsenic (As). Biobased graphene oxide (bGO-P) derived from sugarcane bagasse via pyrolysis, GO-C via chemical exfoliation, and magnetite nanoparticles (FeNPs) via green approach using Azadirachta indica leaf extract were synthesized and characterized by Ultraviolet-Visible Spectrophotometer (UV-vis.), Fourier transform infrared (FTIR) spectroscopy, X-ray diffraction (XRD), mean particle size and Scanning electron microscopy (SEM) along with Energy dispersive spectroscopy (EDX) analysis. Compared to cellulose and hemicellulose, the lignin fraction was less in the precursor material. The GOC, bGO-P and FeNPs displayed maximum absorption at 230, 236, and 374 nm, respectively. FTIR spectrum showed different functional groups (C-OH, C-O-C, COOH and O-H) modifying the surfaces of synthesized materials. Graphene based nanomaterials showed clustered dense flakes of GO-C and thin transparent flakes of bGO-P. Elemental composition by EDX analysis of GO-C (71.26% C and 27.36% O), bGO-P (74.54% C and 24.61% O) and FeNPs (55.61% Fe, 4.1% C and 35.72% O) confirmed the presence of carbon, oxygen, and iron in synthesized nanomaterials. Sorption study was conducted with soil amended with different doses of synthesized nanomaterials (10, 50 and 250 mg) and exposed to 100, 300 and 500 ppm of As. Arsenic concentrations were estimated by colorimetry and atomic absorption spectroscopy (AAS). GO-C, bGO-P, and FeNPs showed substantial As removal efficiency i.e., 81 to 99.3%, 65 to 98.8% and 73.1–89.9%, respectively. Green synthesis of bGO-P and magnetite nanoparticles removed substantial amounts of As compared to GO-C and can be effectively deployed for As removal or immobilization. Higher and medium sorbent doses (250 and 50 mg) exhibited greater As removal and data was best fitted for Freundlich isotherm evidencing favorable sorption. Nevertheless, at low sorbent doses, data was best fitted for both models. Newly synthesized nanomaterials emerged as promising materials for As removal strategy for soil nano-remediation and can be effectively deployed in As contaminated soils.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-73734-9.
Keywords: Arsenic removal, Azadirachta indica, Graphene based nanomaterials, Magnetite, Sugarcane bagasse, Pyrolysis
Subject terms: Environmental sciences, Environmental social sciences, Risk factors, Chemistry, Materials science, Nanoscience and technology, Physics
Introduction
Arsenic (As) is ubiquitous metalloid and a noxious pollutant chronically released from geogenic and anthropogenic sources and circulates in different forms in air, water, and soils before ending up in bottom sediments1–3. Major sources of arsenic are mineral dissolution/precipitation, rock erosion, industrial processes, pharmaceutical products, fossil fuel burning, agricultural operations (pesticides, fertilizers, wood preservatives), atmospheric deposition and mining operations4–6. Arsenic is considered highly toxic and hazardous posing serious health effects even at lower concentrations. Persistence and high mobility of arsenic makes it toxic. Agency for Toxic Substances and Disease Registry classified arsenic as class-1 carcinogen and ranked among 20 most hazardous substances7,8. Chronic exposure of As can result in different disorders in different organs such as liver, kidneys, skin and prostate and can impair different cellular functions9–12. Humans’ reliance on the groundwater abstracted from alluvial sediments is the foremost entry pathway of As into humans4,13. Arsenic speciation and oxidation states determine its toxicity. Major inorganic species are trivalent arsenite (AsO33–), pentavalent arsenate (AsO43–), arsine, and organic species are dimethyl arsenic acid, monomethyl arsonic acid and arsenobetaine14–17. Redox potential (Eh) and pH affecting As speciation. AsO43– predominately exists under oxidized environment with alkaline pH (> 7.5) whereas AsO33– prevails mostly under reduced environments with acidic pH. Organic species are less toxic compared to inorganic species and among inorganic species AsO33– is more toxic than AsO43–3,18.
Approximately, annual arsenic release into the environment ranges from 52,000 to 112,000 tons19. Typically, metal arsenates, arsenides and As sulphides are frequently distributed in earth crust. Different countries of the world have high levels of arsenic contaminated ground water (> 10 µg/L) and soils (> 5 mg/ kg)20,21. Nevertheless, global naturally occurring As in soils are around 5 ppm but elevated As concentrations in soils can occur because of soil nature, anthropogenic activities such as loading of As containing chemicals, pesticides and groundwater abstraction22. Unplanned substantial groundwater abstraction contaminated with As for irrigation uses can result in an increase in As concentration in surface soils that can be ultimately taken up by crop plants23,24. The European Community (EC) defined the As maximum permissible limit in agricultural soils as 20 ppm and its higher concentrations are toxic and can reduce the plant production and yields25–28. As-contaminated soil could be a primary source of As exposure to human body and potential health hazard because of translocation and magnification process. Uncontaminated soils generally have an average value of about 8.7 ppm As with a range of < 1 to 95 ppm As. Tropospheric arsenic is generally contributed by volcanic eruption, pedogensis, geothermal actions, storms of dust, and forest fires29. Contrary to different cationic contaminants, concurrent pollution of anionic arsenic with cationic metals in soils and its removal and immobilization is a challenging issue30. For example, raising soil pH to decontaminate and stabilize cationic metals might result in solubilization of anionic As31.
By considering the above scenario, there is an urgent need to design or explore efficient and sustainable approaches or methods to remove or immobilize arsenic in As contaminated soils. Different in practice approaches and methods include chemical coagulation/flocculation, precipitation, ion exchange resins, sorption, phytoremediation, phytobial and redox approaches32–36. Nonetheless, most of these methods/techniques are costly, ineffective, using more energy and producing more waste37–39. Among these physicochemical methods/techniques, sorption is considered effective, cheap, sustainable, easy handling method using less energy and producing less wastes39–42. To remove or immobilize contaminants, diverse naturally or synthetically produced sorbents such as metal oxides, slag, clay minerals (zeolite), charcoal/biochar are being used43–45. More recently, nano-sorbents are appearing most promising materials that are knocking out the conventional sorbents because of their high sorptive surface area, more functional groups, and superior chemistry46,47.
Among nanomaterials, graphene (G)-based nanomaterials are the robust sorbent materials because of their high sorptive surface area, unique surface chemistry, better physicochemical properties, ad better electrical and thermal conductivity48,49. The G family members include pristine graphene (2-D materials with stuffed C atoms and honeycomb structural arrangement), graphene oxide (GO), reduced graphene oxide (rGO), fabricated/functionalized G-based derivatives. Graphene/GO can be synthesized using different approaches by exfoliating 3-D graphite precursor50. Different members of G are unique in geometrical shapes, orientation, and surface chemistry51. Surface chemistry of G-based materials can be modified by functionalization/fabrication such as addition or removal of different functional groups (–OH, –COOH, C–O–C, C═O)49,52–55. For example, fully oxidized from of G i.e., GO is highly reactive due to high specific surface area and functionalized surface with chemically bonded O2(C = O, –COOH at layer edges and –OH, C–O–C at basal plane)34,56. The oxygen containing functional groups have electron pairs that can from metal complexation by coordinating or interacting (electrostatically) efficiently to bind metal ions33. Furthermore, oxygen atoms conferring solubility of GO in H2O by allowing H atoms to bind H2O molecules57and can enhance contaminants reactivity in soil solution phases. GO can be reduced using different methods58. Nevertheless, to synthesize oxidative or reductive forms of G such as GO and rGO via chemical exfoliation approach using Hummer’s protocol require oxidizers or strong acids resulting in emission of toxic gases, high synthesis cost, low conversion rates and laborious. To overcome these issues, it is imperative to redesign novel approaches to synthesize G-based materials which are more eco-friendly, efficient, robust, cost effective, and waste to value product59. Recently, eco-friendly approach e.g., biobased approach to synthesize these nanomaterials form carbon-based biomaterials such as agrowastes is gaining much interest because of low cost, easily available, renewable, and ecofriendly materials54,60–62. Biomass precursors (e.g. agrowastes) are becoming popular for synthesizing G based materials because of C rich structure (55 wt %), and of renewable nature63,64. Biobased graphene materials can be synthesized from different biomass by removing volatile matter from biomass polymeric chains and carbon growth by carbonization process via thermal/pyrolysis approaches. There is a dire need to use these agrowastes as a potential source for producing new generation super sorbents such as G-based materials that can be effectively used to decontaminate water and soils polluted with highly toxic inorganic metals such as arsenic. Biobased extracts contain different substances (e.g. phenols and terpenoids) that can serve as capping, reducing or stabilizing agents65,66. Plant based phytochemicals also have the potential to sorb on nanomaterials surfaces. Sugarcane bagasse is a polymer complex of lignin, cellulosic and hemicellulosic contents that can act as stabilizing/reducing agents and templates for synthesis of nanomaterials. Plant extract of Azadirachta indica contain substances like phenols, carotenoids, flavonoids, terpenoids, alkaloids, glycosides, tannin and salannin (67, 68). Recycling and using agrowastes as precursor materials for synthesis and fabrication of diverse nanomaterials also benefited in terms of circular bioeconomy. Keeping in view the above paradigmatic scenario, the present study was designed to estimate the effect of bGO-P, GO-C and FeNPS nanomaterials added in soils to estimate their efficacy for As removal/immobilization after exposure to different arsenic levels.
Materials and methods
Chemicals, solvents, and materials
Reagent grade concentrated sulphric acid (H₂SO4), hydrogen peroxide (H2O2), potassium permanganate (KMnO4), 10% hydrochloric acid (HCI) solution, graphite powder, graphene oxide, reduced graphene oxide, ferrocene, ethanol, methanol, distilled water, iron chloride hexahydrate, and sodium hexametaphosphate were used in the present study.
Synthesis of graphene-based nanomaterials and magnetite nanoparticles
Biobased materials: collection and preparation
More recently, agrowastes are emerging as precursor for synthesis of carbon-based nanomaterials. Sugarcane bagasse was collected form Tehsil Shahkot, District Nankana Sahib (31.5757° N, 73.4815° E), Punjab, Pakistan. The biomaterial (biomass) was washed well with distilled water to remove dust and attached inert material, rinsed with demineralized water, and cut into small pieces. Biomatrial was oven dried at 80 °C for 24 h in an oven. Then, material was converted into fine powered form by grinding using FOSS Cyclotec grinder.
Synthesis of graphene oxide via chemical exfoliation approach
Using graphitic route, graphene oxide (GO-C) was synthesized via chemical exfoliation approach using modified Hummer’s protocol. Steps involved in GO-C synthesis are outlined in Fig. 1a. Briefly, one gram graphite powder was taken in 500 mL beaker and 50 mL of concentrated H2SO4 was put in the beaker. After addition, it was stirred for 30 min. The color of this solution became dark after 30 min. Cooled it to below 5 °C. Then, six grams of KMnO4 was added in this mixture and stirred for two hours. Temperature was maintained at 15 °C during stirring. After stirring, 90 mL of distilled water was added slowly. The color of the mixture was dark brown at this stage. The temperature was 30 °C while stirring was carried out for two hours. To remove excess KMnO4, 280 mL of distilled water and 6 mL of H2O2 were added to stop the reaction. The mixture turns into bright yellow colour which indicated the synthesis of GO-C. Filtered reaction mixture was then rinsed with 10% HCl followed by distilled water. The final product was oven-dried for approximately eight hours at 80 °C.
Fig. 1.
(a) Synthesis of graphene oxide (GO-C) (chemical exfoliation approach) using modified Hummer’s protocol via graphitic route., (b) non-graphitic synthesis of biobased graphene oxide (bGO-P) via pyrolysis approach, and (c) Green synthesis of magnetite nanoparticles (FeNPs) using Azadirachta indica leaf extract (Sol Gel approach).
Green synthesis of biobased graphene oxide via pyrolysis approach
Using non-graphitic route, biobased graphene oxide (bGO-P) was synthesized via pyrolysis. Steps involved in bGO-P synthesis are outlined in Fig. 1b. Briefly, in a muffle furnace setting, ground biomass material (0.5 g biomaterial + 0.1 g ferrocene) was pyrolyzed at 300 °C for 10 min. With the help of motor and pestle, they were lightly mashed. After 20 min of sonication, absorption peak was estimated with UV spectrometer-1800.which showed synthesis of graphene-based materials69.
Green synthesis of magnetite nanoparticles using Azadirachta indica plant extract
Ssynthesis of magnetite iron oxide nanoparticles (FeNps) using Azadirachta indica leaf extract (Green Sol Gel approach) is outlined in Fig. 1c. Fresh leaf extract of Azadirachta indica was prepared. Then, 50 mL of Azadirachta indica leaves extract and FeCl3.6H2O solution were mixed and stirred for 30 min. The black colored solution produced was iron oxide nanoparticles. Centrifuged the nanoparticles at 4500 rpm for 15 min and washed 2–3 times with ethanol and distilled water and separated the nanoparticles. The final product was dried in a forced air oven at 75 °C for 24 h and magnetite nanoparticles were obtained after drying.
Biochemical composition of biomaterial
The biochemical composition of sugarcane bagasse was investigated using Van Soest biochemical fractionation protocol70. Cellulose, hemicellulose, and lignin fractions of biomaterial were estimated (Supplementary Table 1). Steps involved in the estimation of biochemical composition of sugarcane bagasse (Van-Soest protocol) are outlined in Fig. 2. Biomaterial was finely ground (< 1 mm) using FOSS Cyclotec and biochemical composition was estimated using Fibertec™ 1020 System M6. Among different organic pools, soluble fraction was extracted with deionized water at 20 °C, hot water-soluble fraction at 100 °C; NDS-soluble fraction by hot water extraction (100 °C) for 30 min, and then NDS fraction at 100 °C for 60 min. Similarly, ADS fraction (hemicellulose fraction), ADL fraction (cellulosic fraction) and lignin fraction (lignin-like fraction) were estimated.
Fig. 2.
Van-Soest protocol for estimation of biochemical composition of sugarcane bagasse.
Material characterization
The characterization of all the synthesized nanomaterials such UV-vis, FTIR, SEM-EDX, XRD, as well as mean particle size. UV-vis. Techniques was used to assess the optical observations of GO-C, bGO-P, and FeNPs respectively. Approximately, 0.1 g of synthesized materials were taken in falcon tubes. For proper mixing, samples were allowed to settle for ten minutes following the shaking. UV-visible spectrophotometer (UV-1800 Shimadzu, Japan) was used to obtain absorption spectrum of synthesized GO with wavelength ranging from 200 nm to 800 nm. Bruker-Alpha-II FTIR spectrometer was used for Fourier Transform Infrared (FTIR) Spectroscopy. To identify different functional groups, FTIR spectra was obtained between 400 and 4000 cm−1range and functional groups were identified by the integration of spectrum peak areas. Oxidation degree or quality can be ascertained from these peaks71. Image (morphology) and element composition analysis was performed using Scanning Electron Microscopy with Energy Dispersive X-ray (SEM-EDX) (S-3400 N), Hitachi. The mean particle size was also be calculated using the XRD patterns of all the synthesized material.
Soil sampling and physiochemical characteristics of soil
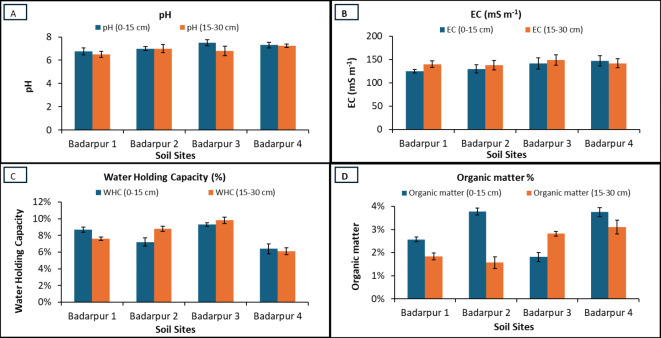
Soil samples were collected from Badarpur (31.3174° N, 74.4628° E) site of Kasuar District, Punjab, Pakistan. Soil samples were collected from 0 to 15 cm and 15–30 cm soil depth using soil cores from four sites, i.e., Badarpur 1, Badarpur 2, Badarpur 3, and Badarpur 4. These collected samples were then amended with G-based nanomaterials to estimate their arsenic sorption capacity. Because of similarity in most of physicochemical properties, soils from Badarpur 1 and 2 were mostly used in different studies. Physicochemical characteristics of soil are shown in Fig. 3A-D; Table 1. Soil texture was determined by soil hydrometer method. Approximately 50 g of soil was taken in soil dispersing cup. Distilled water was added until it was two inches from the top. Then, 5 mL of 1 M sodium hexametaphosphate was used to disperse the soil particles. After 15 min of stirring, the suspension was transferred to a sedimentation cylinder and made the volume up to 1000 mL mark with distilled water. Hydrometer was immersed after blending of the suspension. Two readings were recorded i.e., first reading after 40 s and second reading after two hours. After removing the hydrometer, temperature of the suspension was measured and recorded. For both readings, a blank cylinder containing water and sodium hexametaphosphate was also tested. Soil pH and electrical conductivity of 1: 1.25, 1: 10 and 1: 20 soils to water ratios were estimated with calibrated (buffers of pH 4 and 7) pH meter and EC meter, respectively. Soil organic matter was determined by Walkley and Black method72. One gram of dry soil was taken in 500 mL Erlenmeyer flask. 10 mL of I N potassium dichromate solution and 20 mL of a concentrated sulfuric acid solutions were added. Then, after 30 min interval, 10 m L of a concentrated phosphoric acid solution and 20 mL distilled water were added. Then, 15–20 drops of the diphenylamine indicator were added in the samples and stirred using a Teflon-coated magnetic stirring bar. Then solution was titrated against 0.5 M solution of ferrous ammonium sulphate and noted the end point when colour of the solution becomes violet to green. Similarly, two blank solutions were also tested with the same procedure. The percentage of organic matter was calculated by using these formulas73.
Fig. 3.
Soil characteristics at four sites of Badarpur (1, 2, 3, and 4) in which (A) pH at 0–15 and 15–30 cm, (B) Electrical conductivity at 0–15, and 15–30 cm, (C) water holding capacity at 0–15 and 15–30 cm, and (D) organic matter at the same depth respectively.
Table 1.
Textural analysis of soil.
| Site | Sand (%) | Silt (%) | Clay (%) | Textural Class |
|---|---|---|---|---|
| Soil depth (0–15 cm) | ||||
| Badarpur 1 | 50 | 14 | 36 | Loam |
| Badarpur 2 | 59 | 23 | 18 | Sandy clay loam |
| Badarpur 3 | 66 | 22 | 12 | Sandy clay loam |
| Badarpur 4 | 57 | 11 | 32 | Sandy loam |
| Soil depth (15–30 cm) | ||||
| Badarpur 1 | 54 | 15 | 31 | Sandy loam |
| Badarpur 2 | 51 | 14 | 35 | Loam |
| Badarpur 3 | 53 | 28 | 19 | Sandy clay loam |
| Badarpur 4 | 51 | 20 | 29 | Loam |
 |
.
 |
1 |
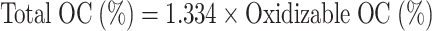 |
2 |
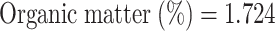 |
3 |
The water holding capacity of soils was estimated using PVC rings (3 cm in diameter) with nylon fabric perforations on one side and soil was soaked in water. The rings were reweighed. For saturation, the rings were placed in partially filled water trays for 5 to 6 h. After saturation, drying was done in an oven at 105 °C for 48 h. Weight was again noted. The water holding capacity was estimated using the following expression.
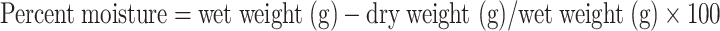 |
4 |
Sorption study
Using subsamples of soil, sorption studies were conducted to estimate the efficacy of newly synthesized GO (GO-C and bGO-P) and magnetite nanomaterials for arsenic removal in serial batch experiments. To estimate the sorption behavior of GO-C, bGO-P and FeNps to remove/immobilize As, 3 g soil of two sampling sites (Badarpur 1 and 2) were amended with three different doses of synthesized materials. The synthesized materials doses were low (10 mg), medium (50 mg) and high (250 mg). Soil samples amended with nanomaterials were exposed to different arsenic levels i.e., 100, 300 and 500 ppm of As. Solid (Soil + nanomaterials) to solution ratio was 1: 10. Three replications of each treatment were used. After 24 h of shaking on a mechanical shaker, samples were centrifuged for 5 min at 3000 rpm. After five minutes of centrifugation, samples were filtered. Then, 0.8 mL of nitric acid was added to preserve the samples and samples were refrigerated at 4 °C for further analysis. Arsenic was determined by colorimetry (Merck MQunat-117927) and atomic absorption spectroscopy.
Arsenic removal efficiency was estimated using the following expression.
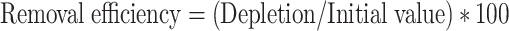 |
5 |
Sorption behaviors were estimated using Longmuir and Freundlich sorption isotherms.
The equation for Langmuir isotherm is as follows:
 |
6 |
The equation for Freundlich isotherm linear representation is as follows:
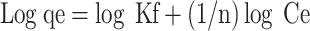 |
7 |
Statistical analysis
Data was subjected to statistical analysis according to standard procedures and means were presented with ± SD. The Origin software was used to plot spectra of FTIR and sorption isotherms.
Results and discussion
Three types of nanomaterials were synthesized in the present study, i.e., graphene oxide via chemical exfoliation approach (GO-C), biobased graphene oxide via pyrolysis approach (bGO-P), and magnetite (FeNps) nanoparticles via Green Sol Gel approach using Azadirachta indicaleaf extract. These newly synthesized nanomaterials were amended with soil and their arsenic removal capacity was evaluated after exposure to different As levels. Precursor materials for bGO-P was sugarcane bagasse and for GO-C was graphite. Agrowastes derived biomaterials like sugarcane in present study are useful material to synthesize carbon-based nanomaterials60,74,75. Azadirachta indica leaf extract was used as green approach to synthesize magnetite nanoparticles.
Biochemical composition of biomaterial
The biochemical fractions i.e., cellulose, hemicellulose, and lignin concentrations were estimated by Van-Soest protocol (Supplementary Table 1). These biochemical fractions are important components of biomaterials indicating fiber contents and structural components76. Biochemical composition provided useful information of biomaterial (sugarcane bagasse) used as precursor material for bGO-P synthesis in present study. Soluble fraction was 24.64%, more easily decomposable fraction (hemicellulose) was 21.19%, cellulose fraction was 40.65% and slow biodegradable fraction (lignin) was 16.31%. Compared to cellulose and hemicellulose, lignin fraction was less comparatively to all other constituents.
Using plants/animal-based biomass of biomaterials as a precursor material can minimize wastes and material costs77,78. Annual global production of agricultural wastes is 220 billion tons and due to renewable nature, these agrowastes (sugarcane bagasse) are a more viable option79. Dissolved substances in solvents are referred to as soluble fraction such as pectins, sugars and few kinds of hemicellulosic contents80. Their dissolution in water or other solvents is due to covalent boding. Hemicellulose contents are glycosidic linked molecules of sugars (complexed carbohydrates) contributing the plant cell wall structure and overall fiber contents. Cellulose (complex carbohydrates) is main structural component of plant cell wall and is composed of glycosidic linked molecules of glucose and insoluble in H2O due to fibrous nature. It is involved in diverse applications due to active site for OH ions and its fibrous structure makes its use possible in synthesis of nanostructures. Lignin is non-carbohydrate fraction composed of phenolic compounds. Structure and function of plants depends on these fractions.
Material synthesis and characterization
Newly synthesized materials were chemically synthesized GO via graphitic approach (GO-C), thermally oxidized (pyrolysis) GO via non-graphitic green synthesis route (bGO-P) and green synthesis of magnetite nanoparticles (FeNps) via Sol Gel approach. These synthesized nanomaterials were characterized prior to their deployment for arsenic removal.
Absorption spectrophotometric analysis of synthesized nanomaterials
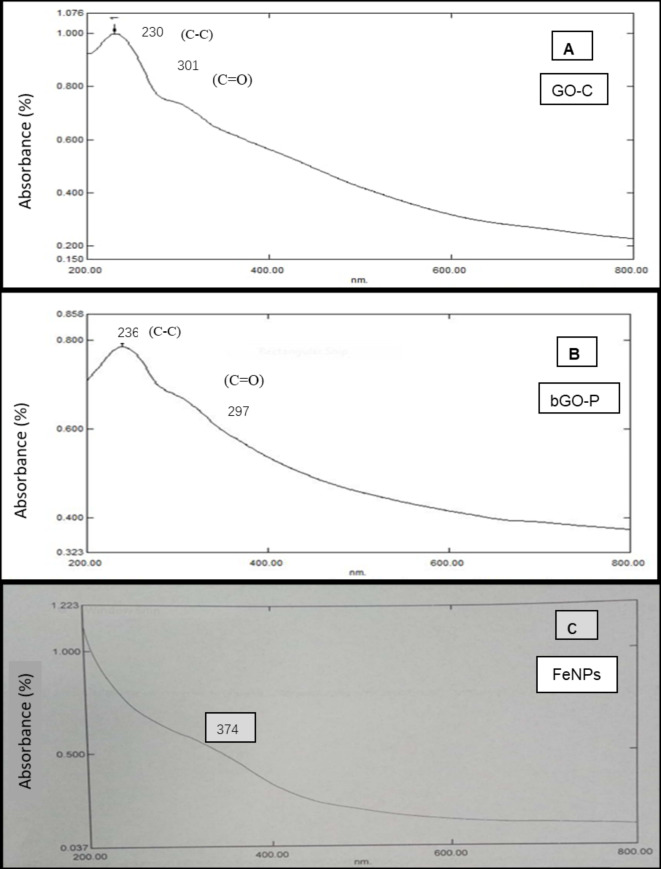
Absorption spectrophotometric analysis of newly synthesized materials was performed using UV-visible spectrophotometer (UV-1800 Shimadzu, Japan. Absorption spectra was obtained between 200 and 800 nm wavelengths of all the synthesized material (Fig. 4A, B, and C). Spectrophotometry is a useful technique to identify electronic transitional network and provide valuable information about the material structure. Absorption peak of GO is generally obtained between 225 and 240 nm81,82. In present study, absorption peaks of graphene-based materials (GO-C and bGO-P) were also obtained between 230 and 240 nm.
Fig. 4.
UV absorption spectrophotometry of (A) GO-C-graphene oxide synthesized via chemical exfoliation and (B) bGO-P-biobased graphene oxide synthesized via pyrolysis approach, and UV spectrophotometer synthesized via Sol Gel approach.
Absorption spectra of GO-C was represented in Fig. 4A. Three-dimensional graphite changed into GO-C in the environment of KMNO4, H2SO4, and H2O2 via chemical exfoliation approach (graphitic route). Absorption peak of 230 nm was obtained during spectrophotometric analysis of GO-C. UV-Vis absorption spectra of bGO-P was depicted in Fig. 4B. bGO-P was synthesized by pyrolysis using non-graphitic route. Absorption peak of 236 nm was obtained during spectrophotometric analysis of bGO-P. The absorption peaks at 230 nm (GO-C) and 236 nm (bGO-P) are attributed to carbon/oxygen and O-containing surface functional groups (epoxy and hydroxyl groups) generating localized electronic states that became active by 230 and 236 nm wavelength, respectively and absorbed these wavelengths. Resultant absorption peaks can be ascribed to π–π* transitions (covalent bonding) of remained sp2C = C bonds83. Maximum absorption (λmax) can explain the extent of remaining conjugation/oxidation in graphene oxide84. Oxidation status (degree) in GO-C and bGO-P was explained by absorption peaks of 301 and 297 nm, respectively attributed to n–π* transition of C = O bonds. These specific absorption peaks in newly synthesized nanomaterials indicating the synthesis of GO-C and bGO-P and providing information about their electro-chemical and surface properties85–87. Similarly, absorption peak of magnetite nanoparticles was 374 nm (Fig. 4C) that can be ascribed to iron atoms d orbital electronic transitions88. Electronic movement between different energy levels of d orbitals resulting these transitions corresponding to energy difference to photon absorption at 374 nm. Azadirachta indicaleaf extract was used to synthesize magnetite nanoparticles via green Sol Gel method. The results are in consonance with those reported by Zembri who synthesized nanoparticles using seaweed. No colour change was observed in the reaction mixture after addition of iron salt indicating the reduction process89.
Fourier transform infrared spectroscopy of synthesized nanomaterials
For characterization of functional groups, Fourier Transform Infrared (FTIR) spectroscopy is a powerful technique. To get FTIR spectra, samples were scanned between 400 and 4000 cm−1 range. Figure 5A represented FTIR spectra of GO-C synthesized via exfoliation approach and Fig. 5B showed FTIR spectra of bGO-P synthesized via pyrolysis approach. FTIR peaks at 3208 cm−1, 1730 cm−1, 1581 cm−1, 1017 cm−1 and 856 cm−1 in case of GO-C and FTIR peaks at 3370 cm−1, 1712 cm−1, 1602 cm−1, and 1026 cm−1 in case of bGO-P confirming the synthesis of GO-C and bGO-P, respectively. These FTIR spectra peaks evidenced the presence of different functional groups such as O-H, COOH, C-OH, and C-O. These FTIR spectra peaks are corresponding to chemical interactions/vibrations indicating properties of synthesized graphene oxide. FTIR spectra of GO-C depicted a broader peak at 3208 cm−1 (GO-C) and 3370 cm−1 (bGO-P) in high frequency domain ascribed to stretching mode of hydroxyl (O-H) bond indicating the presence of OH groups in synthesized graphene oxide nanomaterials. The stretching of O-H functional groups is generally between 2500 and 3600 cm−190. In case of bGO-P, peak obtained at 3370 cm−1 attributed to OH groups from cellulose or lignin fractions. The peaks obtained at 1730 cm−1 (GO-C) and 1712 cm−1 (bGO-P) was attributed to the carboxyl (COOH) groups. In case of bGO-P, peak obtained at 1712 cm−1can be ascribed to acetyl groups of hemicellulose showing stretching of C = O in non-conjugated organic compounds such as ketones, carbonyls, and ester groups74,91. The peaks obtained at 1581 cm−1 (GO-C) and 1602 cm−1 (bGO-P) are attributed to C = C groups. In bGO-P, FTIR spectrum peak can be attributed to vibrations of aromatic skeleton of lignin fractions. FTIR peaks obtained at 1017 cm−1 (GO-C) and 1026 cm−1 (bGO-P) assigned to the vibrations of carbonyl (C-O) groups. In bGO-P, peak obtained at 1026 cm−1 can be assigned to stretching of C–O or C–O–C in biochemical fractions (hemicellulose, cellulose, lignin). These results are in line with peaks obtained in GO-C. Likewise, FTIR peaks obtained in GO-C were attributed to stretching of C = O and C = C from non-oxidized graphite. FTIR spectra evidenced that strong oxidants like KMnO4inserted oxygen atoms into exfoliated graphite layers and made bonds with the carbon atoms there like C = O, C-H, COOH, and C-O-C90. FTIR spectra peaks of GO-C and bGO-P indicating structural nature and chemical properties of synthesized materials and confirming GO synthesis by both approaches.
Fig. 5.
The FTIR spectra of (A) GO-C-graphene oxide synthesized via chemical exfoliation and (B) bGO-P biobased graphene oxide synthesized via pyrolysis approach.
Scanning electron microscopy with energy dispersive X-ray spectroscopy
Surface morphological and topographic analysis was done with scanning electron microscopy (SEM) images of the synthesized materials. Figure 6 (A-C) represented SEM images of GO-C and bGO-P. Elemental composition was estimated with Energy dispersive X-ray spectroscopy (EDX). SEM is a useful technique to scan surface morphology and EDX analysis provided information about chemical composition of synthesized nanomaterials. SEM of GO-C showed clustered aggregated dense GO flakes with uneven spherical structural morphology (Fig. 6A) ascribed to van der Waals forces and molecular interactions92. SEM image of bGO-P is depicted in Fig. 6B. SEM image showed that thin transparent flakes of bGO-P were synthesized. EDX analysis of GO-C represented elemental composition with 71.26% carbon and 27.36% oxygen (Fig. 6A). Likewise, EDX analysis of bGO-P showed that carbon was 74.54% and oxygen was 24.61% (Fig. 6A). EDX analysis showed that the dominant element was carbon in both GO-C and bGO-P. EDX measurements indicated the formation of layered structure with oxygen containing functional groups. The presence of oxygen showed that carbon rich structure was modified by oxygen containing functional groups. These results agree with those reported in earlier literature60,93,94. Figure 6C represented SEM image and EDX analysis of magnetite nanoparticles. SEM image showed that iron nanoparticles had had a sheet above sheet-like form. EDX analysis of FeNPs showed that the percentage of iron was 55.61%, oxygen was 35.72%, and carbon was 4.1% in FeNPs and confirming the synthesis of FeNps via green Sol Gel approach.
Fig. 6.
SEM with EDX analysis of (A) GO-C-graphene oxide synthesized via chemical exfoliation and (B) bGO-P-biobased graphene oxide synthesized via pyrolysis approach, and (C) magnetite nanoparticle-green synthesis by Sol Gel approach.
X-ray diffraction analysis and mean particle size of synthesized material
The X-ray diffraction pattern and mean particle size of GO-C, bGO-P, and FeNPs NPs are shown in Fig. 7(A-C). The diffraction peak of GO-C was about 2θ = 20°, bGO-P was observed at 24° while the diffraction peak of magnetite iron oxide was about 2θ = 44° confirming the synthesis of all the synthesized procedures. The JCPDS number for metallic iron nanoparticles, like α-Fe (alpha iron), is often 06-0696, reflecting their crystal structure in X-ray diffraction. However, variations can occur based on factors like nanoparticle size, shape, and surface characteristics. Based on Debye Sherrer’s equation, the mean particle size of all synthesized materials was also be calculated, which follows the standard calculation of nanomaterials. As can be seen in Fig. 7A, GO-C NPs have the particle size of (50–70), 7 (B) bGO-P NPs are (100–120) nm, while FeNPs (7 C) have the particle size of (110–180) nm on average respectively. Several studies have examined the synthesis and properties of reduced graphene oxide (rGO) and ferric composites, such as Ma95, who presented a controllable rGO/Fe3O4 composite film. Supriya96investigated the alteration of crystal symmetry in cobalt ferrite-reduced graphene oxide nanocomposites. Studying the synthesis and properties of reduced graphene oxide (rGO) and ferric composites is significant because it can lead to the development of advanced materials with unique properties and applications. These composites have the potential to be used in various fields such as energy storage, catalysis, sensors, and biomedical applications, making them a subject of great interest in scientific research. Sagadevan97presented a chemically stabilized rGO/ZrO2 nanocomposite synthesis with improved electrical properties. Additionally, Singh98 discussed the outstanding electromagnetic interference shielding capabilities of a lightweight rGO-Fe3O4 nanoparticle composite. As a result of these studies, rGO and ferric composites have been demonstrated to be effective across a wide range of applications, including magnetoelectronic and electromagnetic shielding.
Fig. 7.

X-ray diffraction and mean particle size of (A) GO-C NPs, (B) bGO-P NPs and (C) FeNPs respectively.
Sorption study to estimate arsenic removal efficacy of synthesized nanomaterials
The arsenic removal efficacy of synthesized nanomaterials amended in soil was estimated by using different sorbent doses i.e., low (10 mg), medium (50 mg) and high (250 mg) exposed to different arsenic concentrations, i.e., 100, 300 and 500 ppm.
Arsenic estimation by colorimetric tests: Two doses (10 and 250 mg) of synthesized nanomaterials (GO-C, bGO-P and FeNPs) amended in soil were exposed to 100 and 300 ppm As concentrations. Table 2 represented arsenic removal efficiency of synthesized nanomaterials. Nanosorbents (GO-C, bGO-P and FeNPs) showed 98.5, 97.2 and 93.3% As removal efficiency, respectively when 10 mg of these nanomaterials amended with 3 g of soil were exposed to 30 mL of 100 ppm of As. Soil alone sorbed (removed) 45.5% As after exposure to 100 ppm As. Similarly, GO-C, bGO-P and FeNps depicted 96, 91 and 81.3% As removal efficiency after exposure of 250 mg of these nanomaterials to 300 ppm of As. Soil alone sorbed 38.3% As after exposure to 300 ppm As. These results showed that synthesized nanomaterials displayed substantial arsenic removal efficiency when low and high doses of sorbents were amended with soil and exposed to 100 and 300 ppm As. Nonetheless, GO-C and bGO-P removed substantial and comparable As. However, FeNps removed less As than GO-C and bGO-P when 250 mg of FeNps were exposed to 300 ppm As.
Table 2.
Arsenic removal efficiency (%) by different doses of GO-C, bGO-P and FeNPs nanomaterials amended in soil (Badarpur 1) exposed to 100 and 300 ppb arsenic.
| Sorbent | Synthesis approach | Sorbent doses (NPs + Soil) | As Removal at different levels (ppm) | As Removal (%) | |
|---|---|---|---|---|---|
| As 100 ppm | As 300 ppm | ||||
| GO-C | Chemical | 10 mg NP + 3 g soil/30mL | 1.5 ± 0.045* | 98.50 | |
| bGO-P | Pyrolysis | 10 mg NP + 3 g soil/30mL | 2.8 ± 0.123 | 97.20 | |
| FeNPs | Sol Gel | 10 mg NP + 3 g soil/30mL | 6.7 ± 0.286 | 93.30 | |
| Soil | 3 g soil/30mL | 54.5 ± 3.432 | 45.50 | ||
| GO-C | Chemical | 250 mg NP + 3 g soil/30mL | 12 ± 1.113 | 96.00 | |
| bGO-P | Pyrolysis | 250 mg NP + 3 g soil/30mL | 27 ± 1.956 | 91.00 | |
| FeNPs | Sol Gel | 250 mg NP + 3 g soil/30mL | 56 ± 3.102 | 81.33 | |
| Soil | 3 g soil/30mL | 185.2 ± 5.362 | 38.30 | ||
*Mean values with ± SD.
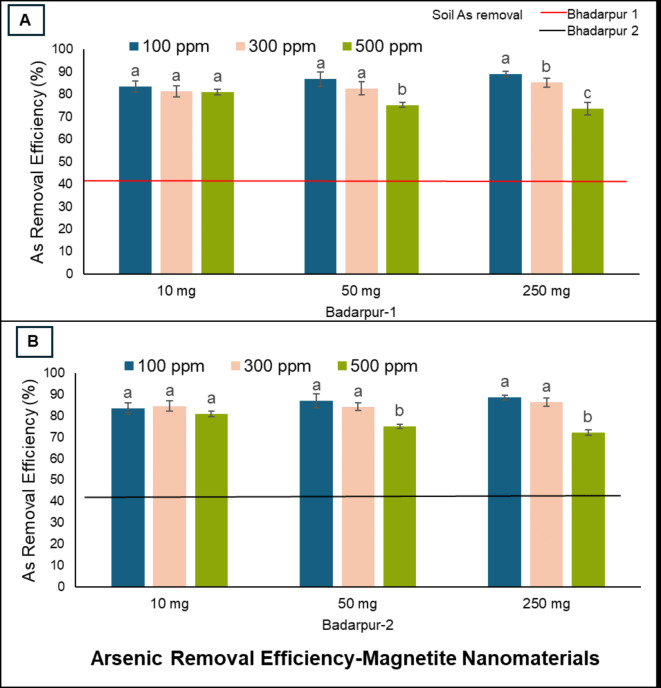
Arsenic determination by hydride generation atomic absorption spectroscopy: Figure 8(A, B) showed arsenic removal efficiency by low (10 mg), medium (50 mg) and high (250 mg) doses of nanosorbents amended in soil after exposure to 100, 300, 500 ppm of As. Low dose of GO-C amended with Badarpur 1 soil removed 87.8, 83.5 and 81% of As from 100, 300 and 500 ppm of As, respectively, and in case of Badarpur 2 soil, nanosorbents removed 88.5, 84.5 and 81.5% of As from 100, 300 and 500 ppm of As, respectively. Medium dose of GO-C amended with Badarpur 1 soil removed 97, 95.2 and 91% of As from 100, 300 and 500 ppm of As, respectively, and in case of Badarpur 2 soil, nanosorbents removed 96.7, 97.2 and 92% of As from 100, 300 and 500 ppm of As, respectively. Similarly, high dose of GO-C amended with Badarpur 1 soil removed 98.5, 97.9 and 93% of As from 100, 300 and 500 ppm of As, respectively, and in case of Badarpur 2 soil, nanosorbents removed 98.7, 99.3 and 94% of As from 100, 300 and 500 ppm of As, respectively. Likewise, low dose of bGO-P amended with Badarpur 1 soil removed 84.8, 78 and 65% of As from 100, 300 and 500 ppm of As, respectively, and in case of Badarpur 2 soil, nanosorbents removed 90.1, 82 and 78% of As from 100, 300 and 500 ppm of As, respectively. Medium dose of bGO-P amended with Badarpur 1 soil removed 93.5, 91 and 75% of As from 100, 300 and 500 ppm of As, respectively, and in case of Badarpur 2 soil, nanosorbents removed 97, 95 and 84% of As from 100, 300 and 500 ppm of As, respectively. Similarly, high dose of bGO-P amended with Badarpur 1 soil removed 98.5, 98.4 and 90% of As from 100, 300 and 500 ppm of As, respectively, and in case of Badarpur 2 soil, nanosorbents removed 98.8, 99 and 92% of As from 100, 300 and 500 ppm of As, respectively. These results indicated that both GO-C and bGO-P depicted comparable As removal efficiencies. Electrostatic interactions and surface complexations between functional groups (carbonyl, carboxyl, hydroxyl and epoxy) are the possible As sorption mechanisms and in agreement with the results reported in pertinent literature99–101. Generally, sorption occurs due to complexation (predominantly monodentate complexation and/or outer sphere complexation via H-bonding) of As oxyanions and functional groups on the surfaces of nano sorbents (85). Efficient binding between O-containing functional groups with lone electron pairs and metal/metalloid ion can result in metal complexation via electrostatic interaction and coordination102,103. In addition, the binding between O and H promotes reactivity with contaminants in soil solution phase. Hence, surface complexation is the predominant mechanism for sorption of As on surfaces of nanosorbents and in consonance of earlier studies104–106. Graphene oxide could be a promising sorbent for metal/metalloid complexation and coordination network. The obtained results are in consonance with earlier reports107,108. Nonetheless, GO-C showed slightly higher As removal efficiency compared to bGO-P. Both nanomaterials removed slightly less As when exposed to higher As level (500 ppm) compared to the other two As levels. Increased As removal at constant sorbent doses can be ascribed to increased driving force resulted by concentration gradient. Nevertheless, decreased As removal at high concentration can be attributed to saturation of active sorption sites resulting in decreased As sorption. Similar observations were reported in earlier studies106,109. Although, FeNPs added in both soils (Badarpur 1 & 2) removed substantial As, however, compared to GO-C and bGO-P, FeNps removed less As at medium and high doses (Fig. 9A, B). Like GO-C and bGO-P, FeNps removed slightly less As when exposed to high As (500 ppm) level compared to 100 and 300 ppm As levels. Like GO, magnetite nanoparticles have potential for sorption and reaction due to better surface chemistry and magnetic behavior. Results obtained in present study are in consonance with earlier studies110–113.
Fig. 8.
Arsenic removal efficiency of (A) GO-C-graphene oxide synthesized via chemical exfoliation and (B) bGO-P-biobased graphene oxide synthesized via pyrolysis approach.
Fig. 9.
Arsenic removal efficiency of magnetite nanoparticles synthesized by green approach using Sol Gel method from soil of (A). Badarpur 1 site, and (B). Badarpur 2 site.
Sorption isotherms
During sorption study, shaking was done at 300 rpm for 24 h and temperature was maintained at 25 °C. (Supplementary Fig. 1A-F) showed Langmuir isotherm analysis on the As removal by bGO-P (10, 50 and 250 mg material doses) amended in Badarpur 1 and Badarpur 2 soils and exposed to three different As levels i.e., 100, 300, and 500 ppm, respectively. (Supplementary Table 2) depicted different values of tested parameters, constants, correlation coefficients and error values. According to Langmuir model, sorbate is sorbed as a monolayer on homogenous surface without any diagonal or lateral interactions with nearby molecules.
(Supplementary Fig. 2A-F) represented Freundlich isotherm analysis on the As removal by bGO-P (10, 50 and 250 mg material doses) amended in Badarpur 1 and Badarpur 2 soils and exposed to three different As concentrations of 100, 300, and 500 ppm, respectively. (Supplementary Table 3) depicted different values of tested parameters, constants, correlation coefficients and error values. Freundlich isotherm appropriate for surfaces with heterogeneous sorbents. Langmuir and Freundlich sorption isotherm models are primarily used to study sorption characteristics of arsenic114,115. Langmuir sorption model demonstrates homogenous sorption at specific sorption sites of the sorbent indicating no further sorption at same site when sorbate adheres to the sorbent site116. This sorption behavior leads to monolayer sorption at high sorbate concentrations. Nonetheless, at low sorbate concentrations). Langmuir sorption isotherm obey Henry law and reduces to linear isotherm117. Contrarily, Freundlich sorption model demonstrates non-ideal sorption and multilayer sorption on heterogenous surfaces. (Supplementary Table 4) depicted R2 data of Langmuir and Freundlich sorption isotherms. Based on R2 values derived from both models, Freundlich isotherm showed strong correlation (R2) between the experimental and projected data at medium and high doses evidencing the favorable sorption process. Nevertheless, data was best fitted for both models at low dose (10 mg). Similarly, high dose of bGO-P (250 mg) depicted strong correlation (R2) for Langmuir sorption model.
Conclusions
Graphene-based nanomaterials (GO-C and bGO-P) were successfully synthesized by graphitic (chemical exfoliation), non-graphitic (pyrolysis using sugarcane bagasse), and magnetite nanoparticles (FeNps) via Sol-gel method using A. indica extract via green synthesis approach. Newly synthesized materials were successfully characterized using UV-Vis Spectrophotometry, Fourier Transform Infra-Red Spectroscopy and SEM with EDX.The synthesized materials (GOC, bGO-P and FeNPs) showed maximum absorption peaks at (230, 236, and 374) nm, respectively. FTIR spectrum showed different functional groups modifying material surfaces and involved in sorption. GO-C displayed clustered dense flakes, bGO-P thin transparent flakes and FeNps sheet above sheet-like pattern. EDX analysis of synthesized nanomaterials confirmed the presence of carbon, oxygen as well as iron in FeNps synthesized via green Solgel approach. GO-C and bGO-P showed substantial arsenic removal efficiency (81 to 99.3% by GO-C and 65 to 98.8% by bGO-P) exposed to different arsenic levels. Similarly, magnetite nanoparticles displayed arsenic removal efficiency ranging from 72.1 to 89.9% after exposure to different arsenic levels. The Freundlich sorption model showed a strong correlation (R2) between the experimental and projected data at medium and high sorbent doses evidencing the favorable sorption process. All synthesized materials amended in soil can be applied for effective arsenic removal or immobilization where, thesre is a problem relating toAs, however, bGO-P and FeNps synthesized via pyrolysis and Sol Gel method are green approaches that can remove comparable As. However, further research is needed to investigate the materials’ reusability, durability, and stability. In general, synthesized nanomaterials are a promising option for soil nano-remediation as they can be used in conjunction with other techniques for removal/immobilization.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors express their gratitude to the Deanship of Scientific Research, Imam Mohammad Ibn Saud Islamic University, Saudi Arabia to support this research work and the authors greatly acknowledge the financial support by HEC, Pakistan to pursue this research work.
Author contributions
Muhammad Shahbaz Akhtar: Conceptualization, Supervision, Writing– original draft, Funding acquisition, Project administration, Resources, Visualization, Data curation, Writing – review & editing; Deborah Rustam Jutt: Conceptualization, Project administration, Writing—original draft, Writing—review and editing, Sohaib Aslam: Supervision, Validation, Visualization, Writing—review and editing; Rab Nawaz: Resources, Methodology, Writing – review & editing; Muhammad Atif Irshad: Formal Analysis, Investigation, Validation; Maheer Khan: Validation, Investigation, Writing – review & editing; M. Khairy: Data Curation, Formal analysis, Writing – review & editing; Ali Irfan: Data curation, Visualization, Funding acquisition, Writing – review & editing; Sami A. Al-Hussain: Validation, Funding acquisition, Visualization, Data curation. Writing – review & editing; Magdi E. A. Zaki: Funding acquisition, Investigation, Formal analysis, Writing – review & editing.All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) and also this research is funded by Higher Education Commission of Pakistan (HEC) through NRPU Project (No. 20-16094/NRPU/R&D/HEC/2021 2021).
Data availability
All the data of this study is contained in the manuscript and further data related to this study can be obtained from corresponding author Muhammad Shahbaz Akhtar: shahbazakhtar@fccollege.edu.pk.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Muhammad Shahbaz Akhtar, Email: shahbazakhtar@fccollege.edu.pk.
Ali Irfan, Email: raialiirfan@gmail.com.
Magdi E. A. Zaki, Email: mezaki@imamu.edu.sa
References
- 1.Pang, D. et al. Superior removal of inorganic and organic arsenic pollutants from water with MIL-88A(Fe) decorated on cotton fibers. Chemosphere. 254, 126829 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Bilici, M. & HadimLioglu, S. Removal of arsenate using graphene oxide-iron modified clinoptilolite-based composites: Adsorption kinetic and column study. J. Anal. Sci. Technol.12, 22. 10.1186/s40543-021-00274-6 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Rahman, M. M., Asaduzzaman, M. & Naidu, R. Arsenic exposure from rice and water sources in the Noakhali district of Bangladesh. Water Qual. Exposure Health. 3, 1–10 (2011). [Google Scholar]
- 4.Shahid, M. et al. Arsenic level and risk assessment of groundwater in Vehari, Punjab Province, Pakistan. Exposure Health. 5, 45–57 (2017). [Google Scholar]
- 5.Wu, L. K. et al. Graphene oxide/CuFe2O4 foam as an efficient absorbent for arsenic removal from water. Chem. Eng. J.334, 1808–1819 (2018). [Google Scholar]
- 6.Raju, N. J. Arsenic in the geo-environment: A review of sources, geochemical processes, toxicity and removal technologies. Environ. Res.203, 111782 (2022). [DOI] [PubMed] [Google Scholar]
- 7.Jaggard, K. W., Qi, A. & Ober, E. S. Possible changes to arable crop yields by 2050. Philos. Trans. R. Soc. Biol. Sci.365, 2835–2851 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bretzler, A. et al. Arsenic removal with zero-valent iron filters in Burkina Faso: Field and laboratory insights. Sci. Total Environ.737 (2020|). [DOI] [PubMed]
- 9.Farooqi, A., Masuda, H. & Firdous, N. Toxic fluoride and arsenic contaminated groundwater in the Lahore and Kasur districts, Punjab, Pakistan and possible contaminant sources. Environ. Pollut.145, 839–849 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Khaja, S. M. A. et al. Arsenic and human health effects: A review. Environ. Toxicol. Pharmacol.40, 828–846 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Shakoor, M. B. et al. Unraveling health risk and speciation of arsenic from groundwater in rural areas of Punjab. Pakistan IESPR. 12, 12371–12390 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soni, R. & Shukla, D. P. Synthesis of fly ash based zeolite-reduced graphene oxide composite and its evaluation as an adsorbent for arsenic removal. Chemosphere. 219, 504–509 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Khalid, S. et al. Arsenic behaviour in soil-plant system: Biogeochemical reactions and chemical speciation influences. Enhancing Cleanup Environ. Pollutants 97–140 (2017).
- 14.Rai, A. et al. Arsenic tolerances in Rice (Oryza sativa) have a predominant role in transcriptional regulation of set of genes including sulphur assimilation pathway and antioxidant system. Chemosphere. 82, 986–995 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Luo, X. et al. Nanocomposites of graphene oxide-hydrated zirconium oxide for simultaneous removal of As(III) and As(V) from water. Chem. Eng. J.220, 98–106 (2013). [Google Scholar]
- 16.Niazi, N. K. & Burton, E. D. Arsenic sorption to nanoparticulate mackinawite (FeS): An examination of phosphate competition. Environ. Pollut.218, 111–117 (2016). [DOI] [PubMed] [Google Scholar]
- 17.LeMonte, J. J. et al. Sea level rise induced arsenic release from historically contaminated coastal soils. Environ. Sci. Technol.51, 5913–5922 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Shakoor, M. B. et al. Remediation of arsenic-contaminated water using agricultural wastes as biosorbents. Crit. Rev. Environ. Sci. Technol.46, 467–499 (2016). [Google Scholar]
- 19.Shibayama, A. Treatment of smelting residue for arsenic removal and recovery of copper using pyro-hydrometallurgical process. J. Hazard. Mater.181, 1016–1023 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Smedley, P. L. & Kinniburgh, D. G. A review of the source, behavior and distribution of arsenic in natural waters. Appl. Geochem.17, 517–568 (2002). [Google Scholar]
- 21.Farooqi, A. et al. Sources of arsenic and fluoride in highly contaminated soils causing groundwater contamination in Punjab, Pakistan. Arch. Environ. Contam. Toxicol.56, 693–706 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Mandal, B. K. & Suzuki, K. T. Arsenic around the world: A review. Talanta. 58, 201–235 (2002). [PubMed] [Google Scholar]
- 23.Meharg, A. A. & Rahman, M. M. Arsenic contamination of Bangladesh paddy field soils: Implications for rice contribution to arsenic consumption. Environ. Sci. Technol.37, 229–234 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Murphy, T. et al. Effects of arsenic, iron and fertilizers in soil on rice in Cambodia. J. Health Pollut. 8, 180910 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.FAO Arsenic contamination of irrigation water, soil and crops in Bangladesh: Risk implications for sustainable agriculture and food safety in Asia. In Regional Office for Asia and The Pacific, Rap Publication 2006/20 (ed. Heikens, A.) (Food and Agriculture Organization of the United Nation, 2006). [Google Scholar]
- 26.Kabata-Pendias, A. & Pendis, H. Trace Elements in Soils and Plants 3rd edn, 38–45 (CRC Press, 2001). [Google Scholar]
- 27.Rahman, M. A., Hasegawa, H., Rahman, M. M., Rahman, M. A. & Miah, M. A. M. Accumulation of arsenic in tissues of rice plant (Oryza sativa L.) and its distribution infractions of rice grain. Chemosphere69, 942–948 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Rahman, M. S. et al. Accumulation of arsenic and other metals in soil and human consumable foods of Meherpur district, southwestern Bangladesh, and associated health risk assessment. Environ. Sci. Eur.35, 47. 10.1186/s12302-023-00751-2 (2023). [Google Scholar]
- 29.Garelick, H., Jones, H., Dybowska, A. & Valsami-Jones, E. Arsenic pollution sources. Reviews of environmental contamination. Int. Perspect. Arsenic Polluti Remediation. 197, 17–60 (2008). [DOI] [PubMed] [Google Scholar]
- 30.Beiyuan, J. Mobility and phytoavailability of As and Pb in a contaminated soil using pine sawdust biochar under systematic change of redox conditions. Chemosphere. 178, 110–118 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Beesley, L. & Marmiroli, M. Te immobilization and retention of soluble arsenic, cadmium and zinc by biochar. Env Pollution. 159, 474–480 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Wu, Y., Ma, X., Feng, M. & Liu, M. Behavior of chromium and arsenic on activated carbon. J. Hazard. Mater.159, 380–384 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Sitko, R. Adsorption of divalent metal ions from aqueous solutions using graphene oxide. Dalton Trans.42, 5682–5689 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Bai, X., Zhao, S. & Duo, L. Impacts of carbon nanomaterials on the diversity of microarthropods in turfgrass soil. Sci. Rep.7, 1779 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung, H. Efects of graphene oxides on soil enzyme activity and microbial biomass. Sci. Total Environ.514, 307–313 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Kobya, M., Soltani, R. D. C., Omwene, P. I. & Khataee, A. A review on decontamination of arsenic-contained water by electrocoagulation: Reactor configurations and operating cost along with removal mechanisms. Environ. Technol. Innov.17, 100519 (2020). [Google Scholar]
- 37.Xu, X., Huang, H., Zhang, Y., Xu, Z. & Cao, X. Biochar as both electron donor and electron shuttle for the reduction transformation of Cr(VI) during its sorption. Environ. Pollut.244, 423–430 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Alka, S. et al. F. Arsenic removal technologies and future trends: A mini review. J. Clean. Prod.123805 (2020).
- 39.Rathi, B. S. & Kumar, P. S. A review on sources, identification and treatment strategies for the removal of toxic Arsenic from water system. J. Hazard. Mater.15, 126299 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Niazi, N. K. et al. Arsenic removal by perilla leaf biochar in aqueous solutions and groundwater: An integrated spectroscopic and microscopic examination. Environ. Pollut.232, 31–41 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Gopi, S. & Balakrishnan, P. Green Materials for Waste Water Treatment 503–528 (Elsevier eBooks, 2021).
- 42.Femina, C. C., Kamalesh, T., Senthil, K. P. & Gayathri, R. A critical review on the sustainable approaches for the removal of toxic heavy metals from water systems. Ind. Eng. Chem. Res.62, 8575–8601 (2023). [Google Scholar]
- 43.Kloster, G. A., Valiente, M., Marcovich, N. E. & Mosiewicki, M. A. Adsorption of arsenic onto films based on chitosan and chitosan/nano-iron oxide. Int. J. Biol. Macromol.165, 1286–1295 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Zheng, Q. et al. As(III) adsorption on Fe-Mn binary oxides: Are Fe and Mn oxides synergistic or antagonistic for arsenic removal? Chem. Eng. J.389, 124470 (2020). [Google Scholar]
- 45.Ochedi, F. O., Liu, Y. & Hussain, A. A review on coal fly ash-based adsorbents for mercury and arsenic removal. J. Clean. Prod.267, 122143 (2020). [Google Scholar]
- 46.Wang, S., Sun, H., Ang, H. M. & Tadé, M. O. Adsorptive remediation of environmental pollutants using novel graphene-based nanomaterials. Chem. Eng. J. (2013).
- 47.Juman, A., Naser, Z. W., Ahmed, E. & Ali, H. Nanomaterials usage as adsorbents for the pollutants removal from wastewater; A review. Mater. Today: Proc.42, 2590–2595 (2021). [Google Scholar]
- 48.Neri, G., Fazio, E., Mineo, P. G. & Scala, A. Piperno, A. SERS sensing properties of new graphene/gold nanocomposite. Nanomaterials. 9, 1236 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ali, I., Suhail, M., López, E. C., Khattab, R. A. & Albishri, H. M. Advances in graphene-based materials for the treatment of water. Arab. J. Geosci.15, 521 (2022). [Google Scholar]
- 50.Ku, S. H. & Park, C. B. Myoblast differentiation on graphene oxide. Biomater. 34, 2017–2023 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Cordaro, A., Neri, G., Sciortino, M. T., Scala, A. & Piperno, A. Graphenebased strategies in liquid biopsy and in viral diseases diagnosis. Nanomaterials. 10, 1014 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dreyer, D. R., Park, S., Bielawski, C. W. & Ruo, R. S. The chemistry of graphene oxide. Chem. Soc. Rev.39, 228 (2010). [DOI] [PubMed] [Google Scholar]
- 53.Das, T. K., Sakthivel, T. S., Jeyaranjan, A., Seal, S. & Bezbaruah, A. N. Ultra-high arsenic adsorption by graphene oxide iron nanohybrid: Removal mechanisms and potential applications. Chemosphere. 253, 126702 (2020). [DOI] [PubMed] [Google Scholar]
- 54.Sadhukhan, S. et al. Synthesis of RGO/NiO nanocomposites adopting a green approach and its photocatalytic and antibacterial properties. Mater. Chem. Phys.247, 122906 (2020). [Google Scholar]
- 55.Tariq, W. et al. Synthesis and applications of graphene and graphenebased nanocomposites: Conventional to artificial intelligence approaches. Front. Environ. Chem.3, 890408 (2022). [Google Scholar]
- 56.Stankovich, S. Graphene-based composite materials. Nature442, 282–286 (2006). [DOI] [PubMed] [Google Scholar]
- 57.Neklyudov, V. V. Solution state and complexing ability of 1, 4-bis(amidomethylsulfnyl)butane toward iron(III), copper(II), cobalt(II), nickel(II), and manganese(II). Phys. Chem.5, 17000–17008 (2017). [Google Scholar]
- 58.De Souza, R. M. et al. Occurrence, impacts and general aspects of pesticides in surface water: A review. Chem. Eng. Res. Des.135, 22–37 (2020). [Google Scholar]
- 59.Faniyi, I. O. et al. The comparative analyses of reduced graphene oxide (RGO) prepared via green, mild and chemical approaches. SN Appl. Sci.1, 1181 (2019). [Google Scholar]
- 60.Thangadurai, D. Nanomaterials from Agrowastes: Past, Present, and the future. In: (eds Kharissova, O. V., Torres-Martínez, L. M. & Kharisov, B. I.) Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications (Springer, 2021). [Google Scholar]
- 61.Pankaj, K. T. et al. Green Synthesis of Iron nanoparticles from Spinach Leaf and Banana Peel aqueous extracts and evaluation of Antibacterial potential. J. Nanomaterials11, (2021).
- 62.El-Ramady, H. et al. Nanofarming: Promising solutions for the future of the Global Agricultural Industry. Agronomy. 13, 1600 (2023). [Google Scholar]
- 63.Sufian, A., Ghosh, A., Sadiq, A. S. & Smarandache, F. A survey on deep transfer learning to edge computing for mitigating the COVID-19 pandemic. J. Syst. Architect.108, 101830 (2020). [Google Scholar]
- 64.Xie, X. & Goodell, B. Thermal degradation and conversion of plant biomass into high value carbon products. Deterioration Prot. Sustainable Biomaterials 147–158 (2014).
- 65.Li, G., Li, B., Chen, Y., Yu, B. & Chen, Z. Green synthesis of reduced graphene oxide using bagasse and its application in dye removal: A waste-to-resource supply chain. Chemosphere. 219, 148–154 (2019). [DOI] [PubMed] [Google Scholar]
- 66.Younas, M. et al. Synthesis and characterization of cerium, silver and copper oxide nanoparticles and their anticancer potential of hepatocellular carcinoma HepG2 cancer cells. J. Mol. Struct.1288, 135756 (2023). [Google Scholar]
- 67.Irshad, M. A. et al. Green synthesis and characterization of silver and copper nanoparticles and their use as an effective adsorbent for chromium removal and recovery from wastewater. Environ. Sci. Pollut. Res.30, 112575–112590 (2023). [DOI] [PubMed] [Google Scholar]
- 68.Biswas, K., Chattopadhyay, I., Banerjee, R. K. & Bandyopadhyay, U. Biological activities and medicinal properties of neem (Azadirachta indica). Curr. Sci.82, 1336–1345 (2002). [Google Scholar]
- 69.Gollavelli, G., Chang, C. C. & Ling, Y. C. Facile synthesis of smart magnetic graphene for safe drinking water: Heavy metal removal and disinfection control. ACS Sustain. Chem. Eng.1, 462–472 (2013). [Google Scholar]
- 70.Peltre, C., Dignac, M. F., Derenne, S. & Houot, S. Change of the chemical composition and biodegradability of the Van Soest soluble fraction during composting: A study using a novel extraction method. Waste Manage.30, 2448–2460 (2010). [DOI] [PubMed] [Google Scholar]
- 71.Sieradzka, M., Ślusarczyk, C., Biniaś, W. & Fryczkowski, R. The role of the oxidation and reduction parameters on the properties of the reduced graphene oxide. Coatings11, 166 (2021).
- 72.Walkley, A. & Black, I. A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci.37, 29–38 (1934). [Google Scholar]
- 73.Estefan, G., Sommer, R. & Ryan, J. Methods of soil, plant, and water analysis. A manual for the West Asia and North Africa region. 3, (2013).
- 74.Somanathan, T., Prasad, K., Ostrikov, K. K., Saravanan, A. & Krishna, V. M. Graphene oxide synthesis from agro waste. Nanomaterials. 5, 826–834 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nath, P. C. et al. Biogeneration of valuable nanomaterials from agro-wastes: A comprehensive review. Agronomy. 13, 561 (2023). [Google Scholar]
- 76.Li, T. & Takkellapati, S. The current and emerging sources of technical lignins and their applications. Biofuels Bioprod. Biorefining. 12, 756–787 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Olivares-Marín, M. et al. Cherry stones as Precursor of activated carbons for supercapacitors. Mater. Chem. Phys.114, 323–327 (2009). [Google Scholar]
- 78.Khan, T. A. et al. Hydrothermal carbonization of lignocellulosic biomass for carbon rich material preparation: A review. Biomass Bioenergy. 130, 105384 (2019). [Google Scholar]
- 79.Chandra, R., Takeuchi, H. & Hasegawa, T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev.16, 1462–1476 (2012). [Google Scholar]
- 80.Rawat, M. et al. K. A comprehensive review on nutraceutical potential of underutilized cereals and cereal-based products. J. Agric. Food Res.12, 100619 (2023). [Google Scholar]
- 81.Sangiliyandi, G., Han, J., Abdal, D., Ahmed, P. & Vasu, K. Jin-Hoi. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomed.7, 5901–5914 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Varghese, A. M. et al. Enhancing effect of UV activation of graphene oxide on carbon capture performance of metal-organic framework/graphene oxide hybrid adsorbents. Chem. Eng. J.420, 129677 (2021). [Google Scholar]
- 83.Mei, Q. et al. Highly efficient photoluminescent graphene oxide with tunable surface properties. Chem. Commun.46, 7319–7321 (2010). [DOI] [PubMed] [Google Scholar]
- 84.Marcano, D. C. Improved synthesis of graphene oxide. ACS Nano. 4, 4806–4814 (2010). [DOI] [PubMed] [Google Scholar]
- 85.Stankovich, S. et al. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon. 45, 1558–1565 (2007). [Google Scholar]
- 86.Eigler, S., Hu, Y., Ishii, Y. & Hirsch, A. Controlled functionalization of graphene oxide with sodium azide. Nanoscale. 5, 12136–12139 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li, Z. et al. Uranium (VI) adsorption on graphene oxide nanosheets from aqueous solutions. Chem. Eng. J.210, 539–546 (2012). [Google Scholar]
- 88.Fouad, D. M., El-Said, W. A. & Mohamed, M. B. Spectroscopic characterization of magnetic Fe3O4@ Au core shell nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc.140, 392–397 (2015). [DOI] [PubMed] [Google Scholar]
- 89.Zembri-Mary, G. Project Risks: Actions around Uncertainty in Urban Planning and Infrastructure Development (Wiley, 2019).
- 90.Shahriary, L. & Athawale, A. A. Graphene oxide synthesized by using modified hummers approach. Int. J. Renew. Energy Environ. Eng.2, 58–63 (2020). [Google Scholar]
- 91.Tajernia, H., Ebadi, T., Nasernejad, B. & Ghafori, M. Arsenic removal from water by sugarcane bagasse: An application of response surface methodology (RSM). Water Air Soil Pollut.225, 1–22 (2014). [Google Scholar]
- 92.Starost, K. & Njuguna, J. The influence of graphene oxide on nanoparticle emissions during drilling of graphene/epoxy carbon-fiber reinforced engineered nanomaterials. Atmosphere. 11, 573 (2020). [Google Scholar]
- 93.Aliyev, E. et al. Structural characterization of graphene oxide: Surface functional groups and fractionated oxidative debris. Nanomaterials. 9, 1180 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hashmi, A., Singh, A. K., Jain, B. & Singh, A. Muffle atmosphere promoted fabrication of graphene oxide nanoparticle by agricultural waste. Fullerenes Nanotubes Carbon Nanostruct.28, 627–636 (2020). [Google Scholar]
- 95.Ma, J., Gu, J., Wang, X., Peng, H., Wang, Q., Zhang, R., Bao, J. Effects of nano-zerovalent iron on antibiotic resistance genes during the anaerobic digestion of cattle manure. Bioresource technology, 289, 121688. (2019). [DOI] [PubMed]
- 96.Supriya, S., Kumar, S. & Kar, M. CFO-graphene nano composite for high performance electrode material. Mater. Today Proc., 4(4), 5651–5656. (2017).
- 97.Sagadevan, S., Venilla, S., Marlinda, A. R., Johan, M., Wahab, Y. A., Zakaria, R., Ahmad, N. Effect of synthesis temperature on the morphologies, optical and electrical properties of MgO nanostructures. Journal of nanoscience and nanotechnology, 20(4),2488–2494. (2020). [DOI] [PubMed]
- 98.Singh, A., Prasad, S. M. & Singh, S. Impact of nano ZnO on metabolic attributes and fluorescence kinetics of rice seedlings. Environ. Nanatechnol. Monit. Manag.9, 42–49 (2018). [Google Scholar]
- 99.Tonoy, K., Das, T. S., Sakthivel, A. J., Seal, S. & Bezbaruah, A. N. Ultra-high arsenic adsorption by graphene oxide iron nanohybrid: Removal mechanisms and potential applications. Chemosphere. 253, 126702 (2020). [DOI] [PubMed] [Google Scholar]
- 100.Zhu, J. L., Liu, Y., Fu, R., Baig, S. & Xinhua, X. Adsorption behavior and removal mechanism of arsenic on graphene modified by iron-manganese binary oxide (FeMnOx/RGO) from aqueous solutions. RSC Adv.510.1039/C5RA11601E (2015).
- 101.Foti, C. et al. Recent advances of graphene-based strategies for arsenic remediation. Front. Chem.14, 608236 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sitko, R. et al. Adsorption of divalent metal ions from aqueous solutions using graphene oxide. Dalton Trans.42, 5682–5689 (2013). [DOI] [PubMed] [Google Scholar]
- 103.Chen, W., Zhang, J., Zhang, X., Wang, W. & Li, Y. Investigation of heavy metal (Cu, Pb, Cd, and cr) stabilization in river sediment by nano-zero-valent iron/activated carbon composite. Environ. Sci. Pollut Res. Int.23, 1460–1470 (2016). [DOI] [PubMed] [Google Scholar]
- 104.Guo, L. et al. Three-dimensional Fe3O4-graphene macroscopic composites for arsenic and arsenate removal. J. Hazard. Mater.298, 28–35 (2015). [DOI] [PubMed] [Google Scholar]
- 105.Kyun, B. et al. Efficient removal of arsenic by strategically designed and layer-by-layer assembled PS @ þ rGO @ GO @ Fe 3 O 4 composites. J. Environ. Manage.201, 286–293 (2017). [DOI] [PubMed] [Google Scholar]
- 106.Sherlala, A. I. A., Raman, A. A. A. & Bello, M. M. Synthesis and characterization of magnetic graphene oxide for arsenic removal from aqueous solution. Environ. Technol.40, (). (2019). [DOI] [PubMed]
- 107.Reynosa-Martínez, A. C. et al. Effect of the degree of oxidation of graphene oxide on As(III) adsorption. J. Hazard. Mater.384, 121440 (2020). [DOI] [PubMed] [Google Scholar]
- 108.Sudip, S. Adsorption of arsenic on graphene oxide, reduced graphene oxide, and their Fe3O4 doped nanocomposites. Biointerface Res. Appl. Chem. 6196–6210 (2022).
- 109.Yoon, Y. et al. Synthesis of magnetite/non-oxidative graphene composites and their application for arsenic removal. Sep. Purif. Technol.178, 40–48 (2017). [Google Scholar]
- 110.Liu, C. H. et al. Mechanism of arsenic adsorption on magnetite nanoparticles from water: Thermodynamic and spectroscopic studies. Environ. Sci. Technol.49, 7726–7734 (2015). [DOI] [PubMed] [Google Scholar]
- 111.Yoon, Y. et al. Comparative evaluation of magnetite–graphene oxide and magnetite-reduced graphene oxide composite for As(III) and As(V) removal. J. Hazard. Mater.304, 196–204 (2016). [DOI] [PubMed] [Google Scholar]
- 112.Jain, R. Recent advances of magnetite nanomaterials to remove arsenic from water. RSC Adv.12, 32197–32209 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Almomani, F., Bhosale, R., Khraisheh, M. & Almomani, T. Heavy metal ions removal from industrial wastewater using magnetic nanoparticles (MNP). Appl. Surf. Sci.506, 144924 (2020). [Google Scholar]
- 114.Chandra, J., Park, Y., Chun, J. W., Lee, I. C. & Hwang, K. S. Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal. ACS Nano. 4, 3979–3986 (2010). [DOI] [PubMed] [Google Scholar]
- 115.Sheng, G. D. et al. Efficient removal of arsenate by versatile magnetic graphene oxide composites. RSC Adv.2, 12400–12407 (2012). [Google Scholar]
- 116.Kundu, S. & Gupta, A. K. Arsenic adsorption onto iron oxide-coated cement (IOCC): Regression analysis of equilibrium data with several isotherm models and their optimization. Chem. Eng. J.122, 93–106 (2006). [Google Scholar]
- 117.Ho, Y. S., Porter, J. F. & Mckay, G. Equilibrium isotherm studies for the sorption of divalent metal ions onto peat: Copper, nickel and lead single component systems. Water Air Soil. Poll.141, 1–33 (2002). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data of this study is contained in the manuscript and further data related to this study can be obtained from corresponding author Muhammad Shahbaz Akhtar: shahbazakhtar@fccollege.edu.pk.