Abstract
Abstract
Mammalian cells are suitable hosts for producing recombinant therapeutic proteins, with Chinese hamster ovary (CHO) and human embryonic kidney 293 (HEK293) cells being the most commonly used cell lines. Mammalian cell expression system includes stable and transient gene expression (TGE) system, with the TGE system having the advantages of short cycles and simple operation. By optimizing the TGE system, the expression of recombinant proteins has been significantly improved. Here, the TGE system and the detailed and up-to-date improvement strategies of mammalian cells, including cell line, expression vector, culture media, culture processes, transfection conditions, and co-expression of helper genes, are reviewed.
Key points
• Detailed improvement strategies of transient gene expression system of mammalian cells are reviewed
• The composition of transient expression system of mammalian cell are summarized
• Proposed optimization prospects for transient gene expression systems
Keywords: Recombinant protein, Transient gene expression, CHO cell, HEK293 cell, Optimization
Introduction
The proportion of biopharmaceuticals in the global pharmaceutical industry is continuously expanding with a steadily growing market scale. By 2024, the global biopharmaceutical market value will reach $389 million (O’Flaherty et al. 2020). The production of recombinant therapeutic proteins (including antibodies) is an important aspect of biopharmaceuticals, and their expression systems contain bacteria, yeast, mammalian cell lines, and plants. Chinese hamster ovary (CHO), mouse myeloma (NS0), mouse myeloma (Sp2/0), and human embryonic kidney 293 (HEK293) cells are commonly used mammalian cell systems. Out of the 107 products obtained from mammalian systems, 95 (89%) of them were produced using the CHO cells, making it the most commonly used system (Walsh and Walsh 2022). HEK293 and CHO cell lines are suitable hosts for therapeutic protein production because of their post-translational modifications (PTMs) similar to human cells, particularly glycosylation (Delafosse et al. 2016). CHO cells can produce proteins with complex structures, can be cultured on a large scale, and are less susceptible to viral infection (Zhang et al. 2013; Ward and Ober 2018; Wang et al. 2019). HEK293 cells grow easily and reproduce rapidly in serum-free suspension culture, exhibit high transfection efficiency using the most common transfection reagents, and are commonly used as models for drug discovery and toxicity testing (Hu et al. 2018).
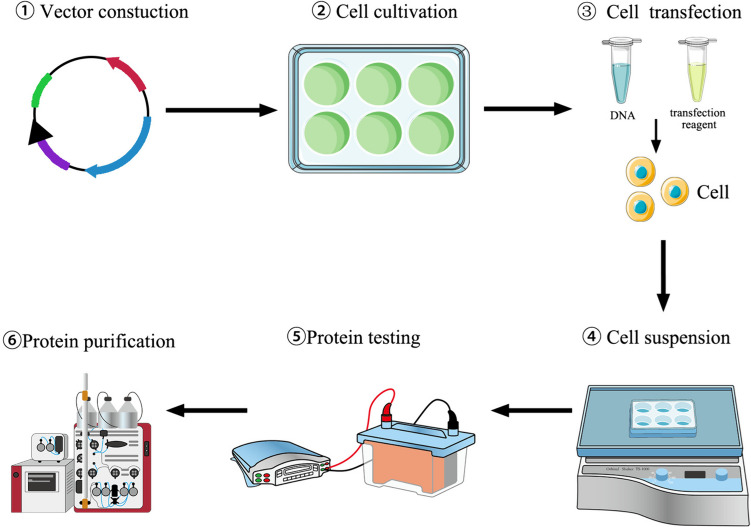
Mammalian cell expression system includes stable and transient gene expression (TGE) system. TGE systems do not require complex and extensive screening steps; the gene of interest (GOI) is transferred directly into the host cells to drive the production of recombinant proteins (Fig. 1) and can produce recombinant proteins ranging from milligrams to grams within 2–4 weeks from gene cloning to protein expression (Durocher et al. 2002; da Silva Junior 2022). The TGE system is also useful for studying the regulation of gene expression in a short time (Hacker et al. 2009), which only involves the processes of establishing cell banks and transfecting cells, eliminating cell line development, and assessing cell line stability in stable gene expression (SGE), including stable pool selection, clonal selection, clone characterization in cell growth, and product expression.
Fig. 1.
Schematic workflow of transient expression process in mammalian cells
At present, the yields from TGE systems were lower than those of the SGE system, which is the main bottleneck in large-scale TGE systems. Improving the performance of TGE mainly focuses on optimizing the processes involved in the system, including expression vector, transfection methods, transfection conditions, culture processes, media, and cell lines. Here, TGE systems and the detailed and up-to-date improvement strategies of mammalian cell are reviewed.
Composition of transient expression system
Rapid production of recombinant proteins via TGE in these mammalian cells is an important tool in the early stages of biopharmaceutical development. The TGE system mainly consists of expression vector, cell line, and culture media. The most commonly used host cell lines in TGE systems are HEK293 and CHO cells. CHO cells are derived from the ovarian tissue of female Chinese hamsters (Cricetulus griseus). With the increase in the use of CHO cells, different subtypes of CHO cell lines including CHO-S, CHO DXB11, CHO DG44, CHO-M, and GS knockout CHO cells have been isolated and developed.
HEK293 cells are immortalized cells derived from primary human embryonic kidney cells, including 293E, 293 T, and 293F cells. HEK293 is the preferred host for TGE systems because of its many industrially relevant features, such as ease of genetic manipulation, growth at high cell densities in suspension culture, and adaptation to serum-free culture conditions (Cervera et al. 2013). The TGE system showed high transfection efficiency to achieve higher product yield in HEK293 cells. However, HEK293 cell–based TGE systems have a few limitations, including different N-glycan structures of protein products, insufficient clinical use, and susceptibility to human viral contamination.
Expression vectors are important components of the TGE system including regulatory elements such as promoter, enhancer, terminator, and selection markers. The combinations of regulatory elements showed different effects on the expression of the recombinant protein (Nakamura et al. 2022; Li et al. 2022; Wang and Guo 2020). CHO cell lines expressing the Epstein-Barr virus nuclear antigen-1 (EBNA-1) protein or large T antigen showed higher TGE (Daramola et al. 2014). The overexpression of unfolded protein response factors or deletion of apoptosis-related genes significantly increased TGE in CHO cell(Johari et al. 2015).
Cell medium is the basis for the efficient expression of recombinant proteins. The safe and effective culture medium can improve the yield of recombinant proteins and simplify protein purification. In vitro culture of most cells requires the addition of 10–20% serum to the basal medium to maintain cell growth (Yao and Asayama 2017; Li et al. 2021); however, the differences in batches, susceptibility to mycoplasma contamination, and large amounts of complex protein components in the serum pose certain difficulties for downstream separation and purification. Serum-free medium (SFM) is composed of serum substitute components that are completely or partially clear in addition to the basic culture medium. SFM eliminates the influence of serum, simplifies the downstream separation and purification processes, and reduces production costs.
Improving transient gene expression strategies
Improving TGE strategies includes cell line modification, expression vector engineering, culture medium and process optimization, transfection condition screening, and co-expression of helper genes (Fig. 2, Table 1).
Fig. 2.

Strategies to improve transient expression in mammalian cells
Table 1.
Optimization strategies to improve recombinant protein transient expression in mammalian cells
| Strategy | Cell lines | VCD (fold) | Yield (fold) | qP (fold) | References | |
|---|---|---|---|---|---|---|
| Cell line modification | ||||||
| Knockout Bax and Bak gene | CHO-K1 | 0.93–1.6 | 3–4 | - | Macaraeg et al. (2013) | |
| Overexpression Bcl-xL gene | CHO-DG44 | 1.15–3 | 0.7–2.7 | 1.5–4.8 | Majors et et al. (2008) | |
| Using Expi CHO-S™ | CHO-S | – | 2.5–6 | 0.99–2.25 | Zhong et al. (2019) | |
| Using HEK293T and HEK 293-6E | HEK 293 | – | 3 –4 | – | Jäger et al. (2013) | |
| Overexpression XBP-1S and ERO1-La | CHO-S, CHO-K1 | – | 5.3–6.2 | – | Cain et al. (2013) | |
| Vector engineering | ||||||
| Promoter | CMV | CHO-K1 | – | 1.4–1.9 | 2.59–4.89 | Bayat et al. (2018) |
| CMV | CHO-S | 1.4–2 | 1.05 | – | Gupta et al. (2017) | |
| CR5, CMV5 | CHOBRI/rcTA | – | 3–4 | – | Poulain et al. (2017) | |
| CMV (NF-κB, CRE,YY1) |
CHO-S CHO-K1 |
– | 1.5 | – | Brown et al. (2015) | |
| Cis-acting element | UCOE | CHO-DG44 | – | 1.5 | – | Nematpour et al. (2017) |
| TM, WPRE, Intron A, SP163 | CHO-K1, HEK293 | – | 1.7–10.5 | – | Mariati et al. (2012) | |
| Signal peptides | human albumin and azurocidin | CHO-K1, CHO-DG44 | – | 1.5–2 | 0.15–1.7 | Kober et al. (2013) |
| Culture medium optimization | ||||||
| Peptone | CHO-DG44 | – | 1.37 | – | Davami et al. (2014) | |
| Wheat peptone | CHO-K1 | 1.3 | 1.6 | – | Burteau et al. (2003) | |
| DMSO + LiAc + fed-batch | CHO-K1 | – | 2–3 | – | Ye et al. (2009) | |
| 10% DMSO, 5 min | HEK293 | – | ~ 1.6 | – | Lynch et al. (2021) | |
| Culture process optimization | ||||||
| Mild hypothermia (32 °C) + sodium butyrate | CHO-S,CHO-K1 | – | 5.3–6.2 | – | Cain et al. (2013) | |
| Mild hypothermia (32 °C) | CHO-T | 2.23–3.45 | – | 1.76 | Sou et al. (2018) | |
| Mild hypothermia (33 °C) | HEK-293 S | – | ~ 1.5 | – | Lin et al. (2015) | |
| Light-activated | CHO-DG44 | – | 4 | – | Minami and Shah (2021) | |
| Transfection conditions screening | ||||||
| Transfection reagent (25 kDa linear PEI) | CHO-S, CHO-K1 | – | 2 | – | Daramola et al. (2014) | |
| Transfection reagent (PEI, PEIpro™) | HEK293-6E, CHO-3E7 | – | 1.1–2.3 | – | Delafosse et al. (2016) | |
| Flow electroporation | HEK293, CHO, NSO | – | 0.9–6.8 | – | Parham et al. (1998) | |
| Transfection ratio (PEI to DNA = 5:1) | Bax Bak DKO CHO-K1 | 0.93–1.6 | 3–4 | – | Macaraeg et al. (2013) | |
| Transfection ratio (3:1) + cell densities (5 × 105) | CHO-DG44 | – | – | 1.37 | Davami et al. (2014) | |
| Co-expressed help gene | ||||||
| DMA + XBP-1S | CHO-k1 | – | 1.5–2.5 | – | Rajendra et al. (2015) | |
| miR-17 + miR-92a | CHO | – | 3 | 2 | Jadhav et al. (2014) | |
| Bcl-xL | CHO-S | – | ~ 2 | – | Zustiak et al. (2014) | |
| NDPK-A + EBNA-1 | CHO-K1 | ~ 1.75–2.5 | ~ 1.5–3.5 | Budge et al. (2021) | ||
| EBNA-1 + PyLT | CHO-DG44 | – | – | 22.9 | Lee et al. (2018) | |
| EBNA-1 + GS | CHO | – | 2 | – | Daramola et al. (2014) | |
| eIF3i | CHO-K1, HEK293,NIH3T3 | – | ~ 1.1– ~ 1.4 | – | Roobol et al. (2020) | |
| Sp35 Fc to CypB = 5:1 | CHO-S | – | 6 | – | Johari et al. (2015) | |
VCD viable cell density; qP specific production; XBP-1S X-box binding protein 1 s; ERO1-La endoplasmic oxidoreductin 1 like protein; UCOE ubiquitous chromatin opening element; CR5 cumate gene switch promoter; YY1 yin yang; NF-κB nuclear factor kappa B; CRE cyclic adenosine; CHOBRI/rcTA cell line: stably expresses the cymene repressor (CymR) and the cumate reverse transactivator; SP163 163-bp long splice variant; TM triple leader sequence; WPRE woodchuck hepatitis virus post-transcriptional regulation element; EBNA Epstein-Barr virus nuclear antigen-1; DMSO dimethyl sulfoxide; LiAc lithium acetate; PEI polyethylenimine; DMA N, N-dimethyl acetamide; NDPK-A nucleoside diphosphate kinase; PyLT polyoma virus large T antigen; eIF3 eukaryotic initiation factor 3; CypB cyclophilin B
Cell line modification
Recombinant protein yields from TGE systems can be improved by modifying cell lines. Macaraeg et al. knocked out two apoptosis-inducing genes, Bax and Bak, in CHO-K1 cells to construct double-knockout (DKO) cells, and the antibody titer expressed by the DKO cells was 3–4 times higher than that in the control (Macaraeg et al. 2013). The Bcl-xL cell line constructed by Major et al. showed 70–270% increase in fusion protein production after 14 days of batch culture, maintained vitality above 90%, and exhibited reduced apoptosis (Majors et al. 2008). Zhong et al. demonstrated that in ExpiCHO-S™ TGE system, high titer of recombinant antibodies can be achieved (Zhong et al. 2019). Compared to other 293 cell TGE systems, the advantages of the Expi293F cell line include high-density growth in suspension culture, ease of transfection, amplification, and protein expression with the required PTMs. And this system allows for efficient transfection and high levels of expression (Krasnoselska et al. 2021). In the HEK293T expression system, the total length of human 17β-hydroxysteroid dehydrogenase 1 can be expressed at high levels, and a maximum expression level of 17 mg/L occurs at 72 h after transfection (Chen et al. 2017). Jäger et al. optimized the TGE of single-chain variable-region antibodies (scFv Fc) in HEK293 cells, achieving 10–20 mg/L yield in adherent HEK293T cells. Using HEK293-6E suspension cells with a truncated variant of EBNA-1, the volume production of scFv Fc antibodies increased by 10 times, reaching 140 mg/L (Jäger et al. 2013).
Expression vector engineering
Expression vector engineering is one of the main strategies used to improve the expression of recombinant proteins, including promoter optimization, signal peptide optimization, and the use of regulatory elements.
Promoters are DNA regulatory sequences that can be specifically recognized and bound by the RNA polymerase to initiate transcription effectively. The human cytomegalovirus (hCMV) major immediate-early promoter is a commonly used promoter for the production of recombinant proteins in mammalian cells. Jostock et al. (2004) demonstrated that the average IgG transient expression driven by CMV promoter, IRES-mediated bicistronic vector was above 10 μg/mL in HEK293T cells. However, in another study, under the same human CMV promoter, the dual-promoter vector system showed the highest titer expression of antibodies, with 1.4- and 1.9-fold increasing compared to the bicistronic and double vector systems, respectively (Bayat et al. 2018). Similarly, Yang et al. constructed a bicistronic, a monocistronic, and a dual-promoter vector, and the TGE of the dual-promoter vector had the highest yield about 18 ± 4 μg/mL in CHO cells (Yang et al. 2022). Therefore, the dual-promoter strategy may have more benefits in terms of recombinant antibody yield; the mechanism can be attributed to the fact that the HC and LC genes are transcribed by different promoters and are more flexible. In addition, Poulain et al. demonstrated that the recombinant protein volume yield under the cumate gene switch promoter (CR5) was three- to fourfold higher than that of the CMV or hybrid EF-1α-HTLV constitutive promoter (Poulain et al. 2017). Brown et al. demonstrated that inhibition of YY1-mediated transrepression can increase secreted alkaline phosphatase (SEAP) production by 1.5-fold, indicating that optimizing and regulating CMV promoter-mediated TGE in CHO cells can increase transient recombinant protein production (Brown et al. 2015). Gupta et al. transfected cells with codon-optimized yeast cytosolic pyruvate carboxylase (PYC 2) and a strong fusion promoter for the optimal expression of the PYC 2 enzyme to increase mAb titers by 5%, mannosylation by twofold, and galactosylation by 2.5-fold, respectively (Gupta et al. 2017).
The use of cis-acting regulatory elements such as the locus control region (LCR), nuclear matrix attachment region (MAR), and ubiquitous chromatin opening element (UCOE) significantly increased TGE levels. UCOE is an unmethylated CpG island located within the promoter of housekeeping genes and provides high levels of transgene expression by inhibiting DNA silencing via methylation in transient transfections (Doan et al. 2022), Nematpour et al. found that all UCOE cell pools had higher antibody yields than non-UCOE cells, and the use of this element with the heavy chain provided more stable cell lines with higher mAb production (Nematpour et al. 2017). MAR act as epigenetic regulators that can specifically associate with the nuclear matrix and are implicated in gene expression (Jia et al. 2019). CHO cells transfected with plasmid containing MAR showed a higher recombination protein expression compared with the control vector (Li et al. 2019; Zhao et al. 2017; Jing et al. 2020). LCR is the most vital cis-element in regulating transgene expression (Yan et al. 2008), and LCR could enhance the titer and gene transfer by lentiviral vectors (Morgan et al. 2020). Mariati et al. investigated the effect of five post-transcriptional regulatory elements (PTREs) on the TGE of recombinant proteins in CHO cells, heat shock protein 70 (Hsp70), 163 bp long splice variant (SP163), the tripartite leader sequence of human adenovirus mRNA linked with a major late promoter enhancer (TM), intron A, and woodchuck hepatitis virus post-transcriptional regulation element (WPRE), of which TM provided the highest gene expression levels, with an increase by 3.6- to 7.6-fold. The remaining regulatory elements increased the expression of one or more proteins by 1.7- to 3.2-fold. The combination of multiple PTREs increased recombinant protein expression by 10.5-fold, and the combination of WPRE with intron A, SP163, or TM had an additive effect on gene expression (Mariati et al. 2010, 2012).
The optimization of signal peptides can improve the secretion efficiency of recombinant proteins. Kober et al. compared the effects of 16 different signaling peptides and found that those extracted from human albumin and azurocidin were the most effective. These results were further confirmed by fed-batch analysis with stably transfected pools, cell-specific yields of up to 90 pg/cell/day, and titers of up to 4 g/L with albumin signal peptide (Kober et al. 2013). The choice of the most appropriate secretion signal may vary depending on the protein. Haryadi et al. selected two kappa LC signal peptides (L1 and L2) and eight HC signal peptides (H1–H8) to produce five different antibodies. The results showed that the signal peptides for different antibodies were not consistent; the best signal peptide combinations for the production of Avastin, Herceptin, Rituxan, Remicade, and Humira were H7/L1, H5/L1,H7/L2, H7/L2, and H7/L1, respectively (Haryadi et al. 2015). O’Neill et al. synthesized signal peptides by computer simulation technology to evaluate three different recombination proteins expression (one ScFv fusion protein, one easily expressed (ETE) mAb, and one difficult to express (DTE) mAb), and the results showed that optimal signal peptide solutions were highly protein-specific, and the signal peptide with the best performance increased the yield of ETE LC, DTE LC, and ScFv fusion products by 1.8 times, 2.5 times, and 2.7 times, respectively(O’Neill et al. 2023).
Culture medium and culture process optimization
Optimization of culture medium and process enhances the expression of recombinant proteins in mammalian cells. Peptone is a water-soluble protein hydrolysate containing peptides, amino acids, and inorganic salts. Davami et al. added peptone to SFM, which significantly increased TGE productivity in HEK293-EBNA cells and volumetric productivity of CHO cells by 37% (Davami et al. 2014). Burteau et al. found that supplementing with wheat peptone can increase cell growth by 30%, while IFN-γ increased production by 60%. These results indicated that plant peptones can improve mammalian cell cultivation and enhanced biological safety (Burteau et al. 2003). IgG production was increased in transiently transfected CHO cells by supplementing them with dimethyl sulfoxide (DMSO) and lithium acetate (LiAc) in batch culture, and the titer reached 80 mg/L (Ye et al. 2009). Similarly, Lynch et al. added 10% DMSO to HEK293 cell medium at 4 h after transfection, resulting in an approximately 1.6-fold increase in recombinant protein expression without significant cytotoxicity (Lynch et al. 2021). In the process of recombinant protein production, chaperones can assist protein folding and prevent protein aggregation. DOSO can provide chaperone-like functions in the protein folding pathway, reduce aggregation, and enhance production (Ye et al. 2009; Hwang et al. 2011; Xu et al. 2020).
In industrial production, mild hypothermia could enhance the yields of recombinant proteins in mammalian cells, and the change of culture temperature was usually the initial cultivation under standard physiological conditions (37 ℃), followed by the growth inhibited phase (31 ℃ or 33 ℃) (Torres et al. 2018; Torres and Dickson 2022; Yang et al. 2022). Lin et al. found that mild hypothermia (33 ℃) reduced the growth rate of HEK293S cells, while increasing green fluorescent protein (GFP) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor by approximately 1.5 times (Lin et al. 2015). The mechanism of recombinant protein yield can be enhanced by mild hypothermia through arresting cell cycle in G1/G0 phase and inhibiting cell apoptosis, promote cell viability (Avello et al. 2022), and also could enhance the expression of genes encoding unfolded protein response-specific transcriptional activators (ddit3, atfx, and bp1s5) and endoplasmic reticulum–resident proteins (grp78, trib3, grp94, and ero1α) that are related to protein folding and processing within the ER (Torres et al. 2021).
Sou et al. showed that TGE at mild hypothermia (32 °C) resulted in a 76% increase in qP compared to culturing cells at 36.5 °C. This increase is accompanied by increased consumption of amino acids and nutrients, along with increased production of nucleotides sugar species, and a higher rate of galactosylation of recombinant monoclonal antibody (Sou et al. 2018). The strategy employed by Zhang et al. in mass production of therapeutic proteins in mammalian cells is to reduce the culture temperature mildly (37 °C to 33 °C) on day 3 of suspension culture (Zhang et al. 2024).
In addition, Barron et al. (2011) showed that mild hypothermia could regulate miRNA expression, and they found that six miRNAs were significantly upregulated (mir-219, mir-518 d, mir-126, mir-30e, mir-489, and mir-345) and four were downregulated (mir-7, mir-320, mir-101, and mir-199) under mild hypothermia, indicating that the mechanism of low temperature enhances recombinant protein production may be related to the upregulation or downregulation of miRNA expression. Fischer et al. (2014) found that overexpression of the miR-30e promoted the production of SEAP in CHO cells by approximately twofold. In the CEVEC’s amniocyte production cell line, overexpression of miR-136, miR-3074, and miR-219 could significantly increase final mAb concentration (Weis et al. 2018). Sanchez et al. found that inhibition of miR-7 would almost double the production of SEAP in CHO cells (Sanchez et al. 2014).
Minami et al. achieved light-induced expression of eGFP through TGE of the CRISPR-dCas 9 effector (LACE) system activated by light, resulting in a significant increase in the TGE of eGFP (Minami and Shah 2021). Muller et al. expanded a culture of CHO cells with polyethylenimine (PEI) transfection for TGE in a new stirred culture vessel (50-mL centrifuge tube and 1-L square glass flask) with 3-L and 150-L bioreactors, resulting in a maximum recombinant antibody yield of 22 mg/L (Muller et al. 2007).
Optimizing the TGE system can not only improve the yield of recombinant proteins but also ensure their quality. Schmitt et al. found that novel “smart polymers” (SmP) could not only remove the formation of antibody aggregates in a concentration-dependent manner but also effectively reduced host cell protein levels at each step of mAb purification and significantly reduced endotoxin levels (Schmitt et al. 2017).
Transfection condition optimization
Each step of transfection has different effects; therefore, it is necessary to optimize the transfection conditions and obtain a transfection protocol with simple operation, low cost, short process duration, and high efficiency.
Daramola et al. transiently transfected CHO-S cells using cationic lipid–based reagents to obtain 60 mg/L recombinant IgG in a 1-L shake flask batch process (Daramola et al. 2014). Kadlecova et al. investigated a novel transfection agent and found that IgG yields obtained using hyperbranched polylysine (HBPL) transfection were comparable to or even higher than those obtained using PEI or Fugene 1 (Kadlecova et al. 2012). HBPL-mediated transfection does not require complex pre-incubation, works well in serum-containing media, and is biodegradable, thus preventing the accumulation of cytotoxicity and facilitating downstream processing. Delafosse et al. found that the use of deacylated PEI “Max” and PEIpro™ resulted in a significant increase in recombinant protein expression compared to that of PEI (Delafosse et al. 2016). These findings demonstrate the importance of selecting the most appropriate polymer for optimal TGE. de Los Milagros Bassani Molinas et al. developed a completely serum-free transient transfection method using a 1:1 mixture of calcium-free DMEM and FreeStyle™ 293 expression medium. Maximal transfection capability was achieved by adjusting the ratio of DNA and PEI (1:3), and a maximum transfection rate of 70–96% was achieved at 72 h post-transfection and yielded a recombinant human erythropoietin concentration of 1.6 μg/mL (de Los Milagros Bassani Molinas et al. 2014). Johari et al. designed a liposome-mediated biphasic SP35Fc-specific TGE process that utilized cyclophilin B (CYPB) co-expression to maximize cell-specific productivity and eliminate SP35Fc aggregation at an optimal SP35Fc to CYPB ratio of 5:1. The optimized manufacturing process significantly increased transient SP35FC production by sixfold without the formation of disulfide aggregates (Johari et al. 2015). In addition, a combination of pretreatment with aphidicolin (a DNA polymerase inhibitor) followed by electroporation after butyrate (a histone deacetylase inhibitor) treatment increased the fluorescence intensity, with a 4.8-fold increase in CHO cells, a 2.8-fold increase in HEK293 cells, and a 1.9-fold increase in HEK293-EBNA cells (Parham et al. 1998).
The transfection efficiency and expression of recombinant proteins can be improved by optimizing the ratio of DNA (N) to PEI (P) (Macaraeg et al. 2013). Transfection efficiency was found to be highest when the DNA/PEI (w/w) ratio was 1:3, ranging from approximately 60 to 66.93% (Ding K et al. 2017; Davami et al. 2014; Rosser et al. 2005). In addition, Rajendra et al. analyzed the use of nonspecific (filler) DNA for the transfection of CHO and HEK293E cells. When the amount of encoded DNA decreased by 67%, the production of recombinant proteins was comparable to that of the control. Filler DNA does not affect the cellular uptake or intracellular stability of encoded DNA but increases the percentage of transfected cells and stability of transgene mRNA (Rajendra et al. 2012).
Moreover, the optimization of transient transfection requires the consideration of the medium type (suitable for low or high cell density transfection), suspension cell density, pDNA (coding DNA), and PEI dosage. Haldankar et al. optimized the transfection parameters, including the transfection reagent, cell density, plasmid DNA, and transfection reagent concentration and found that the expression level of the recombinant protein was as high as 9.4 mg/L (Haldankar et al. 2006). Liu et al. used the cationic lipid transfection reagent FreeStyle™ MAX to transfect HEK293 and CHO cells by optimizing the transfection parameters and obtained approximately 70% eGFP-positive cells and 50–80 mg/L secretion of IgG antibodies. The transfection system was enlarged to 1 L to produce similar transfection efficiency and protein yield. The expression of human erythropoietin and coagulation factor IX was also higher (Liu et al. 2008). Furthermore, in the HEK293T cell line, heat treatment of plasmid DNA at 72 °C for 30 min could improve transfection efficiency (Milani et al. 2021). González-Domínguez et al. adjusted the synergistic effects between different parameters through experimental design (DoE), provided the highest transfection yield with an optimal NaCl concentration of 125 mM and an incubation time of 11 min (González-Domínguez et al. 2022).
Co-expression of helper genes
The co-expression of helper genes plays a positive role in cell growth and recombinant protein expression. Rajendra et al. showed that overexpression of X-box binding protein (XBP-1S) resulted in a 15–85% increase in the titers of several therapeutic proteins in transfected CHO cells (Rajendra et al. 2015). An engineered CHO-S cell line overexpressing XBP-1S and endoplasmic reticulum oxidoreductase (ERO1-La) produced 5.3- to 6.2-fold higher antibody yields than control (Cain et al. 2013). Overexpression of some miRNAs can improve recombinant protein production. Jadhav et al. used an established transient miRNA screening protocol to investigate the effects of miR-17, miR-92 a, and miR-17–92 a on CHO cell growth and recombinant protein productivity and found that miR-17 had a positive effect on cell growth (Jadhav et al. 2014). Ohsfeldt et al. transfected a plasmid with epidermal growth factor receptor or fibroblast growth factor receptor 3 into Bcl-xL-overexpressing CHO cells, and the CHO-Bcl-xL cells showed increased expression of both the recombinant proteins compared to wild-type CHO cells, demonstrating that co-expression of Bcl-xL could increase recombinant protein production (Ohsfeldt et al. 2012). Similarly, Zustiak et al. found that CHO cells co-transfected with Bcl-xL exhibited low cell apoptosis, high specific productivity, and approximately 100% increase in yield (Zustiak et al. 2014). Budge et al. found that the co-expression of nucleoside diphosphate kinase A (NDPK-A) resulted in increased transfection efficiency in CHO cells, which may be due to the enhanced transport of plasmid DNA into the nucleus via nucleoporin complexes. Moreover, when NDPK-A nuclear introduction and EBNA-1 were combined, the TGE of the recombinant protein significantly increased (Budge et al. 2021). In CHO cell-based TGE systems, the recombinant protein yield decreases towards the end of the culture period due to plasmid copy reduction during cell division. To solve this problem, Lee et al. established EBNA-1/polyoma virus large T antigen (PyLT)–co-expressing recombinant CHO cells. The amount of transiently expressed Fc-fusion protein was significantly higher, and the qP of EBNA-1/PyLT-rCHO cells was approximately 22.9-fold higher than that of control CHO-DG44 cells (Lee et al. 2018). In addition, Daramola et al. found that the co-expression of EBNA-1 and GS enhanced the TGE of recombinant antibodies compared to the transfection of CHO cells only with EBNA-1 (Daramola et al. 2014). The eukaryotic initiation factor 3 (eIF3) complex can be viewed as a scaffold that helps bring together the key protein machinery components required to form the 43 S pre-initiation complex in an appropriate orientation. Transient overexpression of eIF 3i in CHOK 1 cells resulted in an approximately 10% increase in the overall protein synthesis rate (Roobol et al. 2020). Vink et al. described an optimized mammalian TGE system based on the rapidly growing suspension cell line, FreeStyle™ 293-F (HEK293F) (Vink et al. 2014). Secreted antibodies of up to 400 mg/L can be obtained in less than a week by co-transfection with expression-enhancing plasmids.
Summary and prospects
The TGE system of mammalian cells has a short cycle, simple operation, and can express recombinant proteins in a short period of time. It has become an important platform for recombinant protein production, especially for preliminary studies. TGE systems include cell lines, expression vectors, and media. Through cell line modification, vector engineering, transfection condition screening, culture medium and process optimization, and co-expressed helper genes, the expression level and quality of recombinant proteins generated by TGE systems have been greatly improved. However, due to many factors affecting TGE, there are batch differences in the expression of recombinant proteins, and the expression level needs to be further improved. With the development of omics and data analysis combined with artificial intelligence, more efficient mammalian TGE systems can be used for industrialized recombinant protein production.
Acknowledgements
The authors thank Yan-fang Wang and Meng-ying Ji for the manuscript revision.
Author contribution
Y.S.F, T.Y.W, and X.Y.W designed and revised the work; Y.S.F and X.Y.W drafted the manuscript; Z.M.H, W.T.C, and S.C.N drafted the figures; T.Y.W funded the manuscript. All authors read and approved the final manuscript.
Funding
This work was funded by the Natural Science Foundation of China (U23A20270) and Natural Science Foundation of Henan Province (232300421115).
Data Availability
All data included in this study are available upon request by contact with the corresponding author.
Declarations
Conflict of interest
The authors declare no competing interests.
Human and animal rights and informed consent
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tianyun Wang, Email: wtianyuncn@126.com.
Xiaoyin Wang, Email: wxyinwxyin@163.com.
References
- Avello V, Torres M, Vergara M, Berrios J, Valdez-Cruz NA, Acevedo C, Molina Sampayo M, Dickson AJ, Altamirano C (2022) Enhanced recombinant protein production in CHO cell continuous cultures under growth-inhibiting conditions is associated with an arrested cell cycle in G1/G0 phase. PLoS ONE 17(11):e0277620. 10.1371/journal.pone.0277620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron N, Kumar N, Sanchez N, Doolan P, Clarke C, Meleady P, O’Sullivan F, Clynes M (2011) Engineering CHO cell growth and recombinant protein productivity by overexpression of miR-7. J Biotechnol 151(2):204–211. 10.1016/j.jbiotec.2010.12.005 [DOI] [PubMed] [Google Scholar]
- Bayat H, Hossienzadeh S, Pourmaleki E, Ahani R, Rahimpour A (2018) Evaluation of different vector design strategies for the expression of recombinant monoclonal antibody in CHO cells. Prep Biochem Biotechnol 48(2):160–164. 10.1080/10826068.2017 [DOI] [PubMed] [Google Scholar]
- Brown AJ, Sweeney B, Mainwaring DO, James DC (2015) NF-κB, CRE and YY1 elements are key functional regulators of CMV promoter-driven transient gene expression in CHO cells. Biotechnol J 10(7):1019–1028. 10.1002/biot.201400744 [DOI] [PubMed] [Google Scholar]
- Budge JD, Young RJ, Smales CM (2021) Engineering of Chinese hamster ovary cells with NDPK-A to enhance DNA nuclear delivery combined with EBNA1 plasmid maintenance gives improved exogenous transient reporter, mAb and SARS-CoV-2 spike protein expression. Front Bioeng Biotechnol 9:679448. 10.3389/fbioe.2021.679448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burteau CC, Verhoeye FR, Mols JF, Ballez JS, Agathos SN, Schneider YJ (2003) Fortification of a protein-free cell culture medium with plant peptones improves cultivation and productivity of an interferon-gamma-producing CHO cell line. In Vitro Cell Dev Biol Anim 39(7):291–296. 10.1290/1543-706X(2003)039%3c0291:FOAPCC%3e2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cain K, Peters S, Hailu H, Sweeney B, Stephens P, Heads J, Sarkar K, Ventom A, Page C, Dickson A (2013) A CHO cell line engineered to express XBP1 and ERO1-Lα has increased levels of transient protein expression. Biotechnol Prog 29:697–706. 10.1002/btpr.1693 [DOI] [PubMed] [Google Scholar]
- Cervera L, Gutiérrez-Granados S, Martínez M, Blanco J, Gòdia F, Segura MM (2013) Generation of HIV-1 Gag VLPs by transient transfection of HEK 293 suspension cell cultures using an optimized animal-derived component free medium. J Biotechnol 166:152–165. 10.1016/j.jbiotec.2013.05.001 [DOI] [PubMed] [Google Scholar]
- Chen J, Feng W, Zhao Y (2017) Secretory expression, purification and functional characterization of 17β-hydroxysteroid dehydrogenase type 1 from mammalian HEK293T cells. Protein Expr Purif 137:52–57. 10.1016/j.pep.2017.06.015 [DOI] [PubMed] [Google Scholar]
- da Silva Junior HC (2022) Transient gene expression in human Expi293 cells. Methods Mol Biol (Clifton, N.J.) 2406:319–325. 10.1007/978-1-0716-1859-2_18 [DOI] [PubMed] [Google Scholar]
- Daramola O, Stevenson J, Dean G, Hatton D, Pettman G, Holmes W, Field R (2014) A high-yielding CHO transient system: coexpression of genes encoding EBNA-1 and GS enhances transient protein expression. Biotechnol Prog 30:132–141. 10.1002/btpr.1809 [DOI] [PubMed] [Google Scholar]
- Davami F, Eghbalpour F, Barkhordari F, Mahboudi F (2014) Effect of peptone feeding on transient gene expression process in CHO DG44. Avicenna J Med Biotechnol 6(3):147–155 [PMC free article] [PubMed] [Google Scholar]
- de Los Milagros Bassani Molinas M, Beer C, Hesse F, Wirth M, Wagner R (2014) Optimizing the transient transfection process of HEK-293 suspension cells for protein production by nucleotide ratio monitoring. Cytotechnology 66:493–514.10.1007/s10616-013-9601-3 [DOI] [PMC free article] [PubMed]
- Delafosse L, Xu P, Durocher Y (2016) Comparative study of polyethylenimines for transient gene expression in mammalian HEK293 and CHO cells. J Biotechnol 227:103–111. 10.1016/j.jbiotec.2016.04.028 [DOI] [PubMed] [Google Scholar]
- Ding K, Han L, Zong H, Chen J, Zhang B, Zhu J (2017) Production process reproducibility and product quality consistency of transient gene expression in HEK293 cells with anti-PD1 antibody as the model protein. Appl Microbiol Biotechnol 101(5):1889–1898. 10.1007/s00253-016-7973-y [DOI] [PubMed] [Google Scholar]
- Doan CC, Ho NQC, Nguyen TT, Nguyen TPT, Do DG, Hoang NS, Le TL (2022) Enhancement of anti-TNFα monoclonal antibody production in CHO cells through the use of UCOE and DHFR elements in vector construction and the optimization of cell culture media. Prep Biochem Biotechnol 52:452–470. 10.1080/10826068.2021.1963981 [DOI] [PubMed] [Google Scholar]
- Durocher Y, Perret S, Kamen A (2002) High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acids Res 30:E9. 10.1093/nar/30.2.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, Buck T, Wagner A, Ehrhart C, Giancaterino J, Mang S, Schad M, Mathias S, Aschrafi A, Handrick R, Otte K (2014) A functional high-content miRNA screen identifies miR-30 family to boost recombinant protein production in CHO cells. Biotechnol J 9(10):1279–1292. 10.1002/biot.201400306 [DOI] [PubMed] [Google Scholar]
- González-Domínguez I, Puente-Massaguer E, Lavado-García J, Cervera L, Gòdia F (2022) Micrometric DNA/PEI polyplexes correlate with higher transient gene expression yields in HEK 293 cells. N Biotechnol 68:87–96. 10.1016/j.nbt.2022.02.002 [DOI] [PubMed] [Google Scholar]
- Gupta SK, Sharma A, Kushwaha H, Shukla P (2017) Over-expression of a codon optimized yeast cytosolic pyruvate carboxylase (PYC2) in CHO cells for an augmented lactate metabolism. Front Pharmacol 8:463. 10.3389/fphar.2017.00463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker DL, De Jesus M, Wurm FM (2009) 25 years of recombinant proteins from reactor-grown cells-where do we go from here? Biotechnol Adv 27:1023–1027. 10.1016/j.biotechadv.2009.05.008 [DOI] [PubMed] [Google Scholar]
- Haldankar R, Li D, Saremi Z, Baikalov C, Deshpande R (2006) Serum-free suspension large scale transient transfection of CHO cells in WAVE bioreactors. Mol Biotechnol 34:191–199. 10.1385/mb:34:2:191 [DOI] [PubMed] [Google Scholar]
- Haryadi R, Ho S, Kok YJ, Pu HX, Zheng L, Pereira NA, Li B, Bi X, Goh LT, Yang Y, Song Z (2015) Optimization of heavy chain and light chain signal peptides for high level expression of therapeutic antibodies in CHO cells. PLoS ONE 10(2):e0116878. 10.1371/journal.pone.0116878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Han J, Li H, Zhang X, Liu LL, Chen F, Zeng B (2018) Human embryonic kidney 293 cells: a vehicle for biopharmaceutical manufacturing, structural biology, and electrophysiology. Cells Tissues Organs 205(1):1–8. 10.1159/000485501 [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Jeon CJ, Cho SM, Lee GM, Yoon SK (2011) Effect of chemical chaperone addition on production and aggregation of recombinant flag-tagged COMP-angiopoietin 1 in Chinese hamster ovary cells. Biotechnol Prog 27(2):587–591. 10.1002/bit.22265 [DOI] [PubMed] [Google Scholar]
- Jadhav V, Hackl M, Klanert G, Hernandez Bort JA, Kunert R, Grillari J, Borth N (2014) Stable overexpression of miR-17 enhances recombinant protein production of CHO cells. J Biotechnol 175:38–44. 10.1016/j.jbiotec.2014.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäger V, Büssow K, Wagner A, Weber S, Hust M, Frenzel A, Schirrmann T (2013) High level transient production of recombinant antibodies and antibody fusion proteins in HEK293 cells. BMC Biotechnol 13:52. 10.1186/1472-6750-13-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia YL, Guo X, Ni TJ, Lu JT, Wang XY, Wang TY (2019) Novel short synthetic matrix attachment region for enhancing transgenic expression in recombinant Chinese hamster ovary cells. J Cell Biochem 120(10):18478–18486. 10.1002/jcb.29165 [DOI] [PubMed] [Google Scholar]
- Jing CQ, Guo ML, Wang C, Ni TJ, Guo X, Wang TY (2020) Fusion with matrix attachment regions enhances expression of recombinant protein in human HT-1080 cells. J Biosci Bioeng 130(5):533–538. 10.1016/j.jbiosc.2020.07.007 [DOI] [PubMed] [Google Scholar]
- Johari YB, Estes SD, Alves CS, Sinacore MS, James DC (2015) Integrated cell and process engineering for improved transient production of a “difficult-to-express” fusion protein by CHO cells. Biotechnol Bioeng 112:2527–2542. 10.1002/bit.25687 [DOI] [PubMed] [Google Scholar]
- Jostock T, Vanhove M, Brepoels E, Van Gool R, Daukandt M, Wehnert A, Van Hegelsom R, Dransfield D, Sexton D, Devlin M, Ley A, Hoogenboom H, Müllberg J (2004) Rapid generation of functional human IgG antibodies derived from Fab-on-phage display libraries. J Immunol Methods 289:65–80. 10.1016/j.jim.2004.03.014 [DOI] [PubMed] [Google Scholar]
- Kadlecova Z, Rajendra Y, Matasci M, Hacker D, Baldi L, Wurm FM, Klok HA (2012) Hyperbranched polylysine: a versatile, biodegradable transfection agent for the productionof recombinant proteins by transient gene expression and the transfection of primary cells. Macromol Biosci 12:794–804. 10.1002/mabi.201100519 [DOI] [PubMed] [Google Scholar]
- Kober L, Zehe C, Bode J (2013) Optimized signal peptides for the development of high expressing CHO cell lines. Biotechnol Bioeng 110:1164–1173. 10.1002/bit.24776 [DOI] [PubMed] [Google Scholar]
- Krasnoselska GO, Dumoux M, Gamage N, Cheruvara H, Birch J, Quigley A, Owens RJ (2021) Transient transfection and expression of eukaryotic membrane proteins in Expi293F cells and their screening on a small scale: application for structural studies. Methods Mol Biol 2305:105–128. 10.1007/978-1-0716-1406-8_5 [DOI] [PubMed] [Google Scholar]
- Lee JH, Park JH, Park SH, Kim SH, Kim JY, Min JK, Lee GM, Kim YG (2018) Co-amplification of EBNA-1 and PyLT through dhfr-mediated gene amplification for improving foreign protein production in transient gene expression in CHO cells. Appl Microbiol Biotechnol 102:4729–4739. 10.1007/s00253-018-8977-6 [DOI] [PubMed] [Google Scholar]
- Li Q, Zhao CP, Lin Y, Song C, Wang F, Wang TY (2019) Two human MARs effectively increase transgene expression in transfected CHO cells. J Cell Mol Med 23(2):1613–1616. 10.1111/jcmm.14018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Fan Z, Lin Y, Wang TY (2021) Serum-free medium for recombinant protein expression in Chinese hamster ovary cells. Front Bioeng Biotechnol 9:646363. 10.3389/fbioe.2021.646363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZM, Fan ZL, Wang XY, Wang TY (2022) Factors affecting the expression of recombinant protein and improvement strategies in Chinese hamster ovary cells. Front Bioeng Biotechnol 10:880155. 10.3389/fbioe.2022.880155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Huang Z, Wen W, Wu A, Wang C, Niu L (2015) Enhancing protein expression in HEK-293 cells by lowering culture temperature. PLoS ONE 10:e0123562. 10.1371/journal.pone.0123562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Dalby B, Chen W, Kilzer JM, Chiou HC (2008) Transient transfection factors for high-level recombinant protein production in suspension cultured mammalian cells. Mol Biotechnol 39:141–153. 10.1007/s12033-008-9051-x [DOI] [PubMed] [Google Scholar]
- Lynch J, Chung J, Huang Z, Pierce V, Saunders NS, Niu L (2021) Enhancing transient protein expression in HEK-293 cells by briefly exposing the culture to DMSO. J Neurosci Methods 350:109058. 10.1016/j.jneumeth.2020.109058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaraeg NF, Reilly DE, Wong AW (2013) Use of an anti-apoptotic CHO cell line for transient gene expression. Biotechnol Prog 29:1050–1058. 10.1002/btpr.1763 [DOI] [PubMed] [Google Scholar]
- Majors BS, Betenbaugh MJ, Pederson NE, Chiang GG (2008) Enhancement of transient gene expression and culture viability using Chinese hamster ovary cells overexpressing Bcl-x(L). Biotechnol Bioeng 101:567–578. 10.1002/bit.21917 [DOI] [PubMed] [Google Scholar]
- Mariati HSC, Yap MG, Yang Y (2010) Evaluating post-transcriptional regulatory elements for enhancing transient gene expression levels in CHO K1 and HEK293 cells. Protein Expr Purif 69:9–15. 10.1016/j.pep.2009.08.010 [DOI] [PubMed] [Google Scholar]
- Mariati HSC, Yap MG, Yang Y (2012) Post-transcriptional regulatory elements for enhancing transient gene expression levels in mammalian cells. Methods Mol Biol 801:125–135. 10.1007/978-1-61779-352-3_9 [DOI] [PubMed] [Google Scholar]
- Milani A, Bolhassani A, Rouhollah F, Naseroleslami M (2021) Which one of the thermal approaches (heating DNA or cells) enhances the gene expression in mammalian cells? Biotechnol Lett 43:1955–1966. 10.1007/s10529-021-03176-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami SA, Shah PS (2021) Transient light-activated gene expression in Chinese hamster ovary cells. BMC Biotechnol 21:13. 10.1186/s12896-021-00670-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Unti MJ, Aleshe B, Brown D, Osborne KS, Koziol C, Ayoub PG, Smith OB, O’Brien R, Tam C, Miyahira E, Ruiz M, Quintos JP, Senadheera S, Hollis RP, Kohn DB (2020) Improved titer and gene transfer by lentiviral vectors using novel, small β-globin locus control region elements. Mol the 28(1):328–340. 10.1016/j.ymthe.2019.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller N, Derouazi M, Van Tilborgh F, Wulhfard S, Hacker DL, Jordan M, Wurm FM (2007) Scalable transient gene expression in Chinese hamster ovary cells in instrumented and non-instrumented cultivation systems. Biotechnol Lett 29:703–711. 10.1007/s10529-006-9298-x [DOI] [PubMed] [Google Scholar]
- Nakamura M, Kikuta H, Misumi Y, Suzuki A, Hoshida H, Akada R (2022) Triple gene expressions in yeast, Escherichia coli, and mammalian cells by transferring DNA fragments amplified from a mother yeast expression plasmid. J Biosci Bioeng 133:587–595. 10.1016/j.jbiosc.2022.03.002 [DOI] [PubMed] [Google Scholar]
- Nematpour F, Mahboudi F, Vaziri B, Khalaj V, Ahmadi S, Ahmadi M, Ebadat S, Davami F (2017) Evaluating the expression profile and stability of different UCOE containing vector combinations in mAb-producing CHO cells. BMC Biotechnol 17:18. 10.1186/s12896-017-0330-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Flaherty R, Bergin A, Flampouri E, Mota LM, Obaidi I, Quigley A, Xie Y, Butler M (2020) Mammalian cell culture for production of recombinant proteins: a review of the critical steps in their biomanufacturing. Biotechnol Adv 43:107552. 10.1016/j.biotechadv.2020.107552 [DOI] [PubMed] [Google Scholar]
- O’Neill P, Mistry RK, Brown AJ, James DC (2023) Protein-specific signal peptides for mammalian vector engineering. ACS Synth Bio 12(8):2339–2352. 10.1021/acssynbio.3c00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsfeldt E, Huang SH, Baycin-Hizal D, Kristoffersen L, Le TM, Li E, Hristova K, Betenbaugh MJ (2012) Increased expression of the integral membrane proteins EGFR andFGFR3 in anti-apoptotic Chinese hamster ovary cell lines. Biotechnol Appl Biochem 59:155–162. 10.1002/bab.1000 [DOI] [PubMed] [Google Scholar]
- Parham JH, Iannone MA, Overton LK, Hutchins JT (1998) Optimization of transient gene expression in mammalian cells and potential for scale-up using flow electroporation. Cytotechnology 28:147–155. 10.1023/A:1008046101653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain A, Perret S, Malenfant F, Mullick A, Massie B, Durocher Y (2017) Rapid protein production from stable CHO cell pools using plasmid vector and the cumate gene- switch. J Biotechnol 255:16–27. 10.1016/j.jbiotec.2017.06.009 [DOI] [PubMed] [Google Scholar]
- Rajendra Y, Kiseljak D, Manoli S, Baldi L, Hacker DL, Wurm FM (2012) Role of non- specific DNA in reducing coding DNA requirement for transient gene expression with CHO and HEK-293E cells. Biotechnol Bioeng 109:2271–2278. 10.1002/bit.24494 [DOI] [PubMed] [Google Scholar]
- Rajendra Y, Hougland MD, Schmitt MG, Barnard GC (2015) Transcriptional and post- transcriptional targeting for enhanced transient gene expression in CHO cells. Biotechnol Lett 37:2379–2386. 10.1007/s10529-015-1938-6 [DOI] [PubMed] [Google Scholar]
- Roobol A, Roobol J, Smith ME, Carden MJ, Hershey JWB, Willis AE, Smales CM (2020) Engineered transient and stable overexpression of translation factors eIF3i and eIF3c in CHOK1 and HEK293 cells gives enhanced cell growth associated with increased c-Myc expression and increased recombinant protein synthesis. Metab Eng 59:98–105. 10.1016/j.ymben.2020.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosser MP, Xia W, Hartsell S, McCaman M, Zhu Y, Wang S, Harvey S, Bringmann P, Cobb RR (2005) Transient transfection of CHO-K1-S using serum-free medium in suspension: a rapid mammalian protein expression system. Protein Expr Purif 40(2):237–243. 10.1016/j.pep.2004.07.015 [DOI] [PubMed]
- Sanchez N, Kelly P, Gallagher C, Lao NT, Clarke C, Clynes M, Barron N (2014) CHO cell culture longevity and recombinant protein yield are enhanced by depletion of miR-7 activity via sponge decoy vectors. Biotechnol J 9(3):396–404. 10.1002/biot.201300325 [DOI] [PubMed] [Google Scholar]
- Schmitt MG, Rajendra Y, Hougland MD, Boyles JS, Barnard GC (2017) Polymer-mediated flocculation of transient CHO cultures as a simple, high throughput method to facilitate antibody discovery. Biotechnol Prog 33:1393–1400. 10.1002/btpr.2527 [DOI] [PubMed] [Google Scholar]
- Sou SN, Lee K, Nayyar K, Polizzi KM, Sellick C, Kontoravdi C (2018) Exploring cellular behavior under transient gene expression and its impact on mAb productivity and Fc-glycosylation. Biotechnol Bioeng 115:512–518. 10.1002/bit.26456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M, Dickson AJ (2022) Combined gene and environmental engineering offers a synergetic strategy to enhance r-protein production in Chinese hamster ovary cells. Biotechnol Bioeng 119:550–565. 10.1002/bit.28000 [DOI] [PubMed] [Google Scholar]
- Torres M, Zúñiga R, Gutierrez M, Vergara M, Collazo N, Reyes J, Berrios J, Aguillon JC, Molina MC, Altamirano C (2018) Mild hypothermia upregulates myc and xbp1s expression and improves anti-TNFα production in CHO cells. PLoS ONE 13:e0194510. 10.1371/journal.pone.0194510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M, Akhtar S, McKenzie EA, Dickson AJ (2021) Temperature down-shift modifies expression of UPR-/ERAD-related genes and enhances production of a chimeric fusion protein in CHO cells. Biotechnol J 16(2):e2000081. 10.1002/biot.202000081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink T, Oudshoorn-Dickmann M, Roza M, Reitsma JJ, de Jong RN (2014) A simple, robust and highly efficient transient expression system for producing antibodies. Methods 65:5–10. 10.1016/j.ymeth.2013.07.018 [DOI] [PubMed] [Google Scholar]
- Walsh G, Walsh E (2022) Biopharmaceutical benchmarks. Nat Biotechnol 40(12):1722–1760. 10.1038/s41587-022-01582-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TY, Guo X (2020) Expression vector cassette engineering for recombinant therapeutic production in mammalian cell systems. Appl Microbiol Biotechnol 104:5673–5688. 10.1007/s00253-020-10640-w [DOI] [PubMed] [Google Scholar]
- Wang Q, Chung CY, Yang W, Yang G, Chough S, Chen Y, Yin B, Bhattacharya R, Hu Y, Saeui CT, Yarema KJ, Betenbaugh MJ, Zhang H (2019) Combining butyrated ManNAc with glycoengineered CHO cells improves EPO glycan quality and production. Biotechnol J 14:e1800186. 10.1002/biot.201800186 [DOI] [PubMed] [Google Scholar]
- Ward ES, Ober RJ (2018) Targeting FcRn to generate antibody-based therapeutics. Trends Pharmacol Sci 39:892–904. 10.1016/j.tips.2018.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis BL, Guth N, Fischer S, Wissing S, Fradin S, Holzmann KH, Handrick R, Otte K (2018) Stable miRNA overexpression in human CAP cells: engineering alternative production systems for advanced manufacturing of biologics using miR-136 and miR-3074. Biotechnol Bioeng 115(8):2027–2038. 10.1002/bit.26715 [DOI] [PubMed] [Google Scholar]
- Xu P, Xu S, He C, Khetan A (2020) Applications of small molecules in modulating productivity and product quality of recombinant proteins produced using cell cultures. Biotechnol Adv 43:107577. 10.1016/j.biotechadv.2020.107577 [DOI] [PubMed] [Google Scholar]
- Yan J, Xiao Y, Wang S, Gong Z, Huang S, Zeng Y (2008) Expression of green fluorescent protein under the regulation of human locus control region elements HS2 and HS3 in transgenic mice. Int J Hematol 88(1):36–42. 10.1007/s12185-008-0089-0 [DOI] [PubMed] [Google Scholar]
- Yang Y, Li Z, Li Q, Ma K, Lin Y, Feng H, Wang T (2022) Increase recombinant antibody yields through optimizing vector design and production process in CHO cells. Appl Microbiol Biotechnol 106:4963–4975. 10.1007/s00253-022-12051-5 [DOI] [PubMed] [Google Scholar]
- Yao T, Asayama Y (2017) Animal-cell culture media: history, characteristics, and current issues. Reprod Med Biol 16:99–117. 10.1002/rmb2.12024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Kober V, Tellers M, Naji Z, Salmon P, Markusen JF (2009) High-level protein expression in scalable CHO transient transfection. Biotechnol Bioeng 103:542–551. 10.1002/bit.22265 [DOI] [PubMed] [Google Scholar]
- Zhang H, Wang H, Liu M, Zhang T, Zhang J, Wang X, Xiang W (2013) Rational development of a serum-free medium and fed-batch process for a GS-CHO cell line expressing recombinant antibody. Cytotechnology 65:363–378. 10.1007/s10616-012-9488-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang Y, Yi D, Zhang C, Ning B, Fu Y, Jia Y, Wang T, Wang X (2024) Synergistic promotion of transient transgene expression in CHO cells by PDI/XBP-1s co-transfection and mild hypothermia. Bioprocess Biosyst Eng 47(4):557–565. 10.1007/s00449-024-02987-5 [DOI] [PubMed]
- Zhao CP, Guo X, Chen SJ, Li CZ, Yang Y, Zhang JH, Chen SN, Jia YL, Wang TY (2017) Matrix attachment region combinations increase transgene expression in transfected Chinese hamster ovary cells. Sci Rep 7:42805. 10.1038/srep42805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X, Ma W, Meade CL, Tam AS, Llewellyn E, Cornell R, Cote K, Scarcelli JJ, Marshall JK, Tzvetkova B, Figueroa B, DiNino D, Sievers A, Lee C, Guo J, Mahan E, Francis C, Lam K, D’Antona AM, Zollner R, Zhu HL, Kriz R, Somers W, Lin L (2019) Transient CHO expression platform for robust antibody production and its enhanced N-glycan sialylation on therapeutic glycoproteins. Biotechnol Prog 35:e2724. 10.1002/btpr.2724 [DOI] [PubMed] [Google Scholar]
- Zustiak MP, Jose L, Xie Y, Zhu J (2014) Enhanced transient recombinant protein production in CHO cells through the co-transfection of the product gene with Bcl-xL. Biotechnol J 9:1164–1174. 10.1002/biot.201300468 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data included in this study are available upon request by contact with the corresponding author.