Abstract
The human T-cell leukemia virus type 1 (HTLV-1) envelope protein is required for virus spread. This study further characterizes the role of the envelope protein in HTLV-1 immortalization. Viruses with single amino acid substitutions within the SU protein at residue 75, 81, 95, 101, 105, or 195 or with a C-terminal cytoplasmic domain truncation (CT), as well as an envelope-null (EN) virus, were generated within an infectious molecular clone, ACH. Transfection of 293T cells resulted in the release of similar amounts of virus particles from all of the mutants as determined by p19 enzyme-linked immunosorbent assay and immunoblot analysis of Gag in cell lysates and supernatants. The virus particles from all mutants except ACH-101, ACH-CT, and ACH-EN were infectious for B5 macaque cells in cell-free and cell-to-cell transmission assays and were capable of immortalizing transfected CD4+ lymphocytes. These results indicate that HTLV-1 spread is required for immortalization.
Human T-cell leukemia virus type 1 (HTLV-1) infects and immortalizes human CD4+ T cells in vitro and is associated with the development of adult T-cell leukemia/lymphoma (13, 28). The envelope glycoprotein is synthesized in infected cells as a polyprotein precursor (gp62), which is subsequently cleaved in the Golgi apparatus into two proteins, surface glycoprotein gp46 (SU) and transmembrane glycoprotein gp21 (TM) (12, 16). HTLV-1 SU is required for entry into the target cell by mediating specific attachment to an unknown cellular receptor (7). HTLV-1 TM supports fusion between viral and cellular membranes to allow entry. The 24-amino-acid cytoplasmic domain in the C terminus of TM is highly conserved in oncoretroviruses and is involved in the fusion process in a cell type-dependent manner (25). In addition, fusion between envelope-expressing cells and receptor-bearing cells leads to the formation of multinucleated cells (syncytia) (14).
Recently, viral pseudotype assays showed that SU protein plays a major role in cell-to-cell transmission of HTLV-1 (8). Furthermore, site-directed mutational analysis of SU revealed that some SU mutants exhibit severe defects in cell-to-cell transmission, despite competence for syncytium formation (5, 8). These findings suggest that the HTLV-1 envelope has multiple functions and may also be involved in postfusion steps required for full infectivity.
Several studies have indicated that envelope proteins of other retroviruses are involved in transformation, in addition to their classical role in mediating viral entry. Spleen focus-forming virus encodes a modified envelope product known as gp55, which is necessary and sufficient for virus-induced erythropoietin-independent growth of erythroid cells by mimicking the natural receptor-ligand interaction (17, 22). Expression of gp55 alone induces erythroleukemia in transgenic mice (1). Avian erythroblastosis virus S13 contains an envelope product fused to v-Sea that is capable of transforming fibroblasts and erythroblasts (4). These findings demonstrate that envelope glycoproteins may stimulate growth of infected cells and oncogenesis.
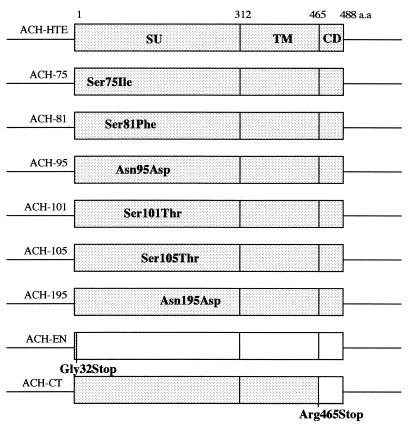
In the present study, we examined the effects of envelope mutants on HTLV-1 infectivity and immortalization. Therefore, viruses with single amino acid substitutions within the SU region at residue 75, 81, 95, 101, 105, or 195 or with a C-terminal cytoplasmic domain truncation (CT), as well as an envelope-null (EN) virus, were generated within an infectious clone, ACH (Fig. 1). Wild-type ACH-HTE and ACH point mutants contain the env genes of HTLV-1 envelope mutants from the HTE series (10, 27), provided by M. C. Dokhelar, inserted between the SphI site at position 5121 and the NsiI site at position 6565 in the ACH clone (15). It has been shown previously that these envelope expression vectors, including wild-type pHTE and pHTE-75, -81, -95, -101, -105, and -195 mutants, retained syncytium formation activity and cell-to-cell transmission ability at various levels in vitro (5, 8). For ACH-EN and ACH-CT, stop codons were inserted at an MfeI site (position 7479) and an NsiI site (position 6565) in the env open reading frame of the ACH clone, respectively, using synthetic oligonucleotides (5′-AATTGTGCTCTAGAGCAC-3′ and 5′-TCCTCTAGAGGATGCA-3′, respectively). Plasmid integrity was confirmed by automated sequencing.
FIG. 1.
Construction of ACH-envelope clones. pACH-HTE, -75, -81, -95, -101, -105, and -195 contain the env gene of pHTE mutants (5) between an SphI site and an NsiI site in the ACH molecular clone. The point mutants are designated by the wild-type residue, the position (in comparison to initiation methionine), and the mutant amino acid. ACH-EN and ACH-CT were generated by inserting linkers at an MfeI site at codon 32 and at an NsiI site at codon 465, respectively. CD, cytoplasmic domain; a.a., amino acid.
To confirm that the ACH-envelope clones were able to produce virus particles, 3 μg each of pACH-HTE and pACH-envelope clones was transfected into 293T cells (3 × 105) by a calcium phosphate precipitation method (11), and virus particles were analyzed by immunoblotting using cell lysates and supernatants obtained 48 h posttransfection. Pelleted virus particles and cell lysates were separated on sodium dodecyl sulfate (SDS)–12 or 10% polyacrylamide gels and electroblotted to polyvinylidene difluoride membranes. Immunoblot analysis was performed using an HTLV-1 patient serum or a monoclonal antibody to gp46 (1C11) (24), followed by treatment with the appropriate horseradish peroxidase-conjugated secondary antibodies, and visualization was with an ECL Western blotting detection system (Amersham, Little Chalfont, United Kingdom).
Immunoblot analysis with the patient serum revealed similar levels of Gag proteins, including p55Prgag, p24CA, and p19MA, as well as the envelope proteins, gp62Prenv, gp46SU, and gp21TM, for each pACH-envelope clone (Fig. 2A and B). An HTLV-1-infected lymphoid cell line, MT-2, was used as a positive control. Cell lysate and supernatant from parental 293T cells were used as negative controls. No envelope protein was detected in pACH-EN-transfected cells, and only uncleaved gp59 envelope precursor protein was observed in pACH-CT-transfected cells (Fig. 2A). Pelleted virus particles from supernatants of each mutant contained predominantly p24CAand p19MA, similar to MT-2 cells (Fig. 2B). The virus particles were also quantified by a p19 matrix antigen enzyme-linked immunosorbent assay (ELISA) (Cellular Products, Buffalo, N.Y.) (33). All ACH-envelope clones produced similar amounts of p19 antigen (data not shown). Immunoblot analysis with the gp46 antibody also revealed similar levels of gp62 and gp46 in pACH-101- and pACH-transfected cells in cell lysates, whereas no envelope protein was detected in pACH-EN-transfected cells and only gp59 envelope precursor protein was detected in pACH-CT-transfected cells (Fig. 2C). The virus particles of each pACH-envelope clone except pACH-EN and pACH-CT had similar levels of gp46 (Fig. 2D). However, the gp46 protein levels of ACH-81 in cell lysate and supernatants were slightly decreased compared to those of the other mutants (5). Notably, monoclonal antibody 1C11 could not recognize gp46 and gp62 in pACH-195-transfected cells in cell lysates and supernatants because the antibody reacts with an epitope of gp46 and gp62 between amino acids 190 and 209 (24), although the patient serum could detect this mutant envelope protein (Fig. 2A).
FIG. 2.
Gag and envelope protein expression of ACH-envelope clones. (A and B) Cell lysates (A) and virions released into supernatants (B) from pACH-transfected 293T cells (lanes 1 to 9, as indicated), untransfected 293T cells (lane 10), and MT-2 cells (lane 11) were subjected to SDS–12% polyacrylamide gel electrophoresis and immunoblotted with a patient serum followed by horseradish peroxidase-conjugated secondary antibodies; visualization was with an ECL system. (C and D) Cell lysates (C) and supernatants (D) from each transfectant were separated on SDS–10% polyacrylamide gels and blotted with a gp46 monoclonal antibody (1C11). * and ** indicate a p69 Env-px fusion protein in MT-2 cells (20) and a p59 protein in pACH-CT-transfected cells, respectively. The arrangement of lanes is similar to that of panel A. Specific protein bands are indicated by arrows. Numbers on the left show molecular masses in kilodaltons.
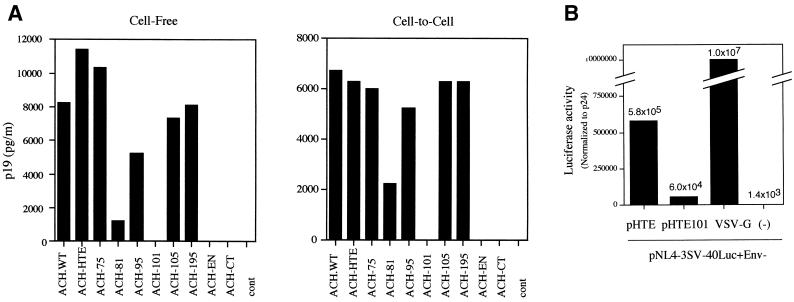
We next examined the effect of the envelope mutations on the ability of the ACH clone to infect susceptible cells by cell-free and cell-to-cell transmission assays. For this purpose, we used an HTLV-1-permissive cell line, B5, which was established from a fetal rhesus lung cell line (9). Viruses were generated by transfection of 293T cells with pACH-envelope clones as described above. Inoculation of B5 cells (5 × 103) was initiated with filtered (0.22-μm-pore-size filter) cell-free virus supernatants (approximately 0.6 ng based on p19 antigen ELISA) or by coculture with lethally irradiated (6,000 rads) transfected 293T cells (103). At 3 weeks postinfection, virus infectivity was monitored by p19 antigen ELISA of clarified supernatants. As shown in Fig. 3A, B5 cells inoculated with ACH.WT and ACH-HTE, which also encodes a wild-type envelope protein, produced similar levels of p19 antigen in cell-free and cell-to-cell transmission assays. Like the cells inoculated with ACH-HTE, the cells inoculated with ACH-envelope clones produced p19 antigen in both assays, except for ACH-101, ACH-EN, and ACH-CT, which were noninfectious. However, the levels of p19 from ACH-81 were slightly decreased compared to those of other mutants in these assays. Culture supernatants from untransfected 293T cells and irradiated cells were used as negative controls. These findings indicate that virus particles from ACH-envelope clones were infectious for the target B5 cells in cell-free and cell-to-cell transmission assays, except for ACH-101, ACH-CT, and ACH-EN, which were noninfectious. Similar results were obtained in three independent experiments. Interestingly, ACH-envelope clones were infectious for B5 cells in cell-free transmission and the pattern of cell-free infectivity was similar to that of cell-to-cell transmission, although HTLV-1 infection is thought to occur primarily by cell-to-cell contact (19, 23, 29).
FIG. 3.
(A) Effect of envelope protein mutation on HTLV-1 infectivity in cell-free and cell-to-cell transmission assays. Virus particles were generated by transfection of pACH clones into 293T cells. Inoculation of the target B5 cells was initiated with filtered cell-free virus supernatants (Cell-Free) or by coculture with lethally irradiated 293T cells transfected with pACH clones as indicated (Cell-to-Cell). At 3 weeks postinfection, virus infectivity was determined by a p19 antigen ELISA. Representative data from three independent experiments are shown. cont, control. (B) Effect of the residue 101 mutation in SU protein on virus entry. Pseudotyped viruses were generated by cotransfection of 293T cells with pNL4-3SV-40Luc+Env- and pHTE, pHTE101, or VSV-G. Inoculation of HOS cells was initiated with each cell-free pseudotyped virus particle. At 2 days after infection, virus entry was monitored by luciferase activity (normalized for HIV-1 p24 antigen levels). Representative data are shown.
We then examined whether the lack of infection with ACH-101, ACH-CT, and ACH-EN was due to defects in virus entry. Pseudotyped particles were generated using vesicular stomatitis virus glycoprotein (VSV-G), which allows entry through a different receptor for a single cycle of virus replication. Cotransfection of a VSV-G expression vector (pHCMV-G), provided by J. K. Yee (3), did not rescue virus infectivity in any case (data not shown). This is probably a result of the requirement for multiple rounds of replication in this assay for detection of p19 antigen.
We further examined the effect of the residue 101 mutation on virus entry into target cells by a pseudotype assay (34). Briefly, inoculation of human HOS cells was initiated with supernatants from 293T cells transfected with a human immunodeficiency virus type 1 (HIV-1) genome containing the luciferase gene driven by a simian virus 40 promoter in place of the env gene (pNL4-3SV-40Luc+Env-) (HIV core) and wild-type pHTE or pHTE-101. At 2 days postinfection, virus entry was monitored by luciferase assays, which were normalized for HIV-1 p24CA antigen levels. Luciferase activity in HOS cells inoculated with pseudotyped particles consisting of the HIV core and HTE-101 glycoprotein was approximately 10-fold lower than that of the HIV core with wild-type HTE. However, luciferase activity from HTE-101 pseudotyped virus particles was still higher than that of HIV core with no envelope protein. VSV-G pseudotyped virus was used as a positive control (approximately 16-fold more active than the HTLV-1 envelope, HTE) (Fig. 3B).
We then examined the effect of the envelope mutations on the ability of the ACH clone to immortalize peripheral blood mononuclear cells (PBMCs). To do this, 107 PBMCs were isolated from uninfected donors by Ficoll-Paque purification, activated for 72 h with medium containing 5 μg of phytohemagglutinin-P (Sigma, St. Louis, Mo.) per ml and 50 U of interleukin-2 (IL-2) per ml, and transfected by electroporation with 25 μg of pACH-envelope clones (31). Cell viability was monitored for 90 to 120 days posttransfection by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) conversion assays, as previously described (31).
We have shown previously that ACH.WT immortalized PBMCs and that the immortalized cells produced high levels of p19 antigen (31). Wild-type pACH-HTE- and pACH-envelope clone-transfected PBMCs continued to proliferate for at least 120 days in the presence of IL-2, whereas the cells transfected with pACH-101, pACH-EN, and pACH-CT, as well as an empty vector (pBluescript KS), grew transiently and died by 90 days after transfection. The virus particles released from the immortalized cells were quantified by the p19 antigen ELISA. All immortalized cells produced p19 antigen (greater than 4 ng per ml) in each culture supernatant at 90 days posttransfection (Table 1). We also performed virus entry complementation assays using VSV-G. Cotransfection of pHCMV-G did not immortalize PBMCs in any case.
TABLE 1.
Immortalization of transfected PBMCs by ACH-envelope clones
| Clonea | No. of immortalized culturesb/no. of electroporation expts | p19 expression in immortalized cellsc |
|---|---|---|
| ACH.WT | NTd | NT |
| ACH-HTE | 3/5 | + |
| ACH-75 | 3/4 | + |
| ACH-81 | 2/5 | + |
| ACH-95 | 4/5 | + |
| ACH-101 | 0/5 | − |
| ACH-105 | 4/5 | + |
| ACH-195 | 4/5 | + |
| ACH-EN | 0/5 | − |
| ACH-CT | 0/5 | − |
| pBluescript KS | 0/5 | − |
| ACH-101/VSV-G | 0/3 | − |
| ACH-EN/VSV-G | 0/3 | − |
| ACH-CT/VSV-G | 0/3 | − |
Clones used for this assay are shown.
Determined by MTT assays (31).
Results of p19 antigen expression in immortalized cells at 90 days posttransfection, as previously described (33). + and − p19 expression of greater than 4 ng/ml and less than 25 pg/ml, respectively.
NT, not tested.
To examine the cell surface phenotype of ACH-envelope clone-immortalized PBMCs, 106 immortalized cells were stained with anti-CD4 antibody–fluorescein isothiocyanate, anti-CD8 antibody–phycoerythrin, and isotype controls for 30 min on ice and analyzed with a FACScalibur flow cytometer (Becton Dickinson, San Jose, Calif.). As shown in Fig. 4, the majority of immortalized cells transfected with pACH-HTE, pACH-75, pACH-81, pACH-95, pACH-105, and pACH-195 expressed CD4 and lacked CD8 expression. This is consistent with previous analyses of the ACH.WT clone (32).
FIG. 4.
Cell surface phenotypes of the ACH-envelope clone-immortalized cells. Immortalized cells were stained with anti-CD4 antibody–fluorescein isothiocyanate and anti-CD8 antibody–phycoerythrin (right panels) and isotype control antibodies (left panels) and analyzed with a FACScalibur flow cytometer. FL1-H and FL2-H indicate CD4 and CD8 levels, respectively.
Finally, we confirmed by PCR-based direct sequencing that ACH-envelope mutant viruses in immortalized cells were not revertants. Genomic DNA was isolated from 107 ACH-HTE-, ACH-75-, ACH-81-, ACH-95-, ACH-105-, and ACH-195-immortalized cells with a QIAamp Blood mini Kit (Qiagen Inc., Valencia, Calif.). The env gene fragments corresponding to the mutated regions were amplified by PCR using forward primers 5′-CAGCAGATCAGGCCCTACAG-3′ and 5′-CAGCCAACTGCCTCCCACCG-3′ and reverse primers 5′-CTTCCAGTAGGGGCTGGAGA-3′ and 5′-GTAGAAGAAGAGGATGGAAC-3′, and purified DNA was subjected to direct sequencing using these forward or reverse primers. Sequence analysis indicated that all envelope mutations in the ACH clone were maintained in each immortalized cell line (data not shown). These findings indicate that the immortalization activity by ACH-envelope mutant clones did not result from a reversion of the mutations to a wild-type virus.
In this study, we analyzed effects of envelope mutations on HTLV-1 infectivity and immortalization. We demonstrated that HTLV-1-induced immortalization is strongly associated with envelope-mediated cell-free and cell-to-cell transmission. These findings suggest that HTLV-1 spread is required for immortalization.
We constructed HTLV-1 envelope mutants within an infectious molecular clone, ACH (Fig. 1). The SU protein point mutants used in this study were selected from a panel of envelope mutants as previously described, since they are all conserved among HTLV-1 and -2 isolates (5) and are essential for their fusion and infectivity (8). However, the envelope mutations are all independent of the four N-linked glycosylation sites of SU, which are also involved in fusion (26). A cytoplasmic domain truncation mutant (ACH-CT) was constructed because the cytoplasmic domain in TM contains a YXX∅ tyrosine-based motif, where X is any residue and ∅ is a hydrophobic residue, which plays a role in cell-to-cell transmission (6). For a negative control, an envelope-null virus (ACH-EN) was generated (Fig. 1).
Immunoblot analysis with an anti-gp46 antibody revealed similar levels of gp46 in wild-type ACH-HTE- and ACH-envelope mutant-transfected cells, whereas only noncleaved product, gp59, was detected in ACH-CT-transfected cells (Fig. 2). This cleavage defect is probably due to retention of the misfolded protein in the endoplasmic reticulum, which prevents transport to the Golgi compartment, where cleavage normally occurs (8).
HTLV-1 is thought to be transmitted almost exclusively via cell-to-cell contact (19, 23, 29). A quantitative single-round infection assay demonstrated that the envelope proteins play a major role in cell-to-cell transmission and that it is a principal route of transmission to the target cells, since cell-free virus particles are very poorly infectious (8). However, in our system, the virus particles from wild-type ACH-HTE and mutant ACH-envelope clones were infectious for B5 cells in both cell-to-cell and cell-free transmission assays. Our system has several differences from the single-round infection assay, which could all contribute to detection of cell-free infection: there are multiple rounds of infection, full-length HTLV-1 is used, no G418 selection is required, and virus is cultured on B5 cells.
Viral transmission assays showed that the ACH-101 mutant was noninfectious for B5 cells, although gag and envelope expression were comparable to those of wild-type virus (Fig. 2 and 3). This observation is consistent with results that the HTE-101 mutant exhibited a severe defect in cell-to-cell transmission, despite competence for processing and fusion (8), although in the case of murine leukemia virus, the envelope-mediated fusion activity was not always consistent with virus infectivity (30). These data suggest that the SU protein might be involved in postfusion events required for infectivity. To address this hypothesis, we further examined whether lack of infectivity in the residue 101 mutation is due to defects in viral entry. Pseudotype assays showed that the entry efficiency of HTE-101 pseudotyped particles was approximately 10-fold lower than that of wild-type HTE psuedotyped particles. These data suggest that residue 101 in the SU protein is involved in virus entry, and this can be one explanation for the infectivity defect.
ACH-CT was also noninfectious for B5 cells in these assays, which is presumably due to a cleavage defect (Fig. 2 and 3), as previously described (8). Alternatively, loss of the YXX∅ motif may explain the lack of infectivity, since this motif is involved in cell-to-cell transmission (6). However, little is known about the actual role of the YXX∅ motif in HTLV-1 infection (6). As expected, viral transmission was not observed with the envelope-null ACH-EN clone, suggesting that the HTLV-1 envelope is also involved in cell-free infection of this virus.
ACH-81 has slightly decreased infectivity compared to other mutants in transmission assays, which is probably due to a minor defect in processing of the envelope protein (Fig. 2C and D). This observation is consistent with previous data obtained using pHTE-81 (8).
HTLV-1 immortalizes CD4+ human lymphocytes in vitro (21, 35). Immortalization assays showed that wild-type ACH-HTE and ACH-envelope clones, except for ACH-101, ACH-EN, and ACH-CT, also immortalized CD4+ PBMCs in the culture in the presence of IL-2 (Table 1 and Fig. 4). Immortalization activity correlated with viral transmission, since only viruses capable of replication in B5 cells were able to immortalize human lymphocytes (Fig. 3 and Table 1).
The viral protein Tax is believed to be critical for immortalization of human lymphocytes based on results from overexpression of this protein using a retroviral vector in vitro (2). However, under our experimental conditions, some ACH-envelope clones could not immortalize PBMCs, although they all have wild-type Tax. These findings suggest that the HTLV-1 envelope protein, in addition to Tax, may also be directly or indirectly involved in immortalization of PBMCs. Furthermore, previous studies have shown that envelope proteins of other retroviruses stimulate cell growth of infected cells, and this may be involved in pathogenesis (4, 22). Very recently, Maeda et al. demonstrated that Jaagsiekte sheep retrovirus envelope protein directly transformed a murine cell line (18). Future analysis to determine cellular effects of expression of the HTLV-1 envelope alone in PBMCs will help us understand whether there is a direct role of the envelope protein in immortalization.
In conclusion, we demonstrated that HTLV-1 immortalization was strongly associated with envelope-mediated infectivity. These findings suggest that HTLV-1 spread is required for immortalization in our culture system, and this may help us understand more precisely the mechanism of HTLV-1 pathogenesis.
Acknowledgments
We thank M. C. Dokhelar for providing env mutants, D. Derse for providing B5 cells, F.-W. Wong and M. Robek for constructing ACH clones with env mutations, and N. Vander Heyden for preparing PBMCs.
This work was supported by Public Health Service grants.
REFERENCES
- 1.Aizawa S, Suda Y, Furuta Y, Yagi T, Takeda N, Watanabe N, Nagayoshi M, Ikawa Y. Env-derived gp55 gene of Friend spleen focus-forming virus specifically induces neoplastic proliferation of erythroid progenitor cells. EMBO J. 1990;9:2107–2116. doi: 10.1002/j.1460-2075.1990.tb07379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akagi T, Ono H, Shimotohno K. Characterization of T cells immortalized by Tax1 of human T-cell leukemia virus type 1. Blood. 1995;86:4243–4249. [PubMed] [Google Scholar]
- 3.Chen S T, Iida A, Guo L, Friedmann T, Yee J K. Generation of packaging cell lines for pseudotyped retroviral vectors of the G protein of vesicular stomatitis virus by using a modified tetracycline inducible system. Proc Natl Acad Sci USA. 1996;93:10057–10062. doi: 10.1073/pnas.93.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crowe A J, McGlade J, Pawson T, Hayman M J. Phosphorylation of the SHC proteins on tyrosine correlates with the transformation of fibroblasts and erythroblasts by the v-sea tyrosine kinase. Oncogene. 1994;9:537–544. [PubMed] [Google Scholar]
- 5.Delamarre L, Pique C, Pham D, Tursz T, Dokhelar M C. Identification of functional regions in the human T-cell leukemia virus type I SU glycoprotein. J Virol. 1994;68:3544–3549. doi: 10.1128/jvi.68.6.3544-3549.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delamarre L, Pique C, Rosenberg A R, Blot V, Grange M P, Le Blanc I, Dokhelar M C. The Y-S-L-I tyrosine-based motif in the cytoplasmic domain of the human T-cell leukemia virus type 1 envelope is essential for cell-to-cell transmission. J Virol. 1999;73:9659–9663. doi: 10.1128/jvi.73.11.9659-9663.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delamarre L, Rosenberg A R, Pique C, Pham D, Callebaut I, Dokhelar M C. The HTLV-I envelope glycoproteins: structure and functions. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13:S85–91. doi: 10.1097/00042560-199600001-00015. [DOI] [PubMed] [Google Scholar]
- 8.Delamarre L, Rosenberg A R, Pique C, Pham D, Dokhelar M C. A novel human T-leukemia virus type 1 cell-to-cell transmission assay permits definition of SU glycoprotein amino acids important for infectivity. J Virol. 1997;71:259–266. doi: 10.1128/jvi.71.1.259-266.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derse D, Mikovits J, Waters D, Brining S, Ruscetti F. Examining the molecular genetics of HTLV-I with an infectious molecular clone of the virus and permissive cell culture systems. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12:1–5. doi: 10.1097/00042560-199605010-00001. [DOI] [PubMed] [Google Scholar]
- 10.Dokhelar M C, Pickford H, Sodroski J, Haseltine W A. HTLV-I p27rex regulates gag and env protein expression. J Acquir Immune Defic Syndr. 1989;2:431–440. [PubMed] [Google Scholar]
- 11.Graham F L, van der Eb A J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 12.Hattori S, Kiyokawa T, Imagawa K, Shimizu F, Hashimura E, Seiki M, Yoshida M. Identification of gag and env gene products of human T-cell leukemia virus (HTLV) Virology. 1984;136:338–347. doi: 10.1016/0042-6822(84)90170-3. [DOI] [PubMed] [Google Scholar]
- 13.Hinuma Y, Nagata K, Hanaoka M, Nakai M, Matsumoto T, Kinoshita K I, Shirakawa S, Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci USA. 1981;78:6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoshino H, Shimoyama M, Miwa M, Sugimura T. Detection of lymphocytes producing a human retrovirus associated with adult T-cell leukemia by syncytia induction assay. Proc Natl Acad Sci USA. 1983;80:7337–7341. doi: 10.1073/pnas.80.23.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimata J T, Wong F H, Wang J J, Ratner L. Construction and characterization of infectious human T-cell leukemia virus type 1 molecular clones. Virology. 1994;204:656–664. doi: 10.1006/viro.1994.1581. [DOI] [PubMed] [Google Scholar]
- 16.Lee T H, Coligan J E, Homma T, McLane M F, Tachibana N, Essex M. Human T-cell leukemia virus-associated membrane antigens: identity of the major antigens recognized after virus infection. Proc Natl Acad Sci USA. 1984;81:3856–3860. doi: 10.1073/pnas.81.12.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J P, D'Andrea A D, Lodish H F, Baltimore D. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature. 1990;343:762–764. doi: 10.1038/343762a0. [DOI] [PubMed] [Google Scholar]
- 18.Maeda N, Palmarini M, Murgia C, Fan H. Direct transformation of rodent fibroblasts by Jaagsiekte sheep retrovirus DNA. Proc Natl Acad Sci USA. 2001;98:4449–4454. doi: 10.1073/pnas.071547598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manns A, Wilks R J, Murphy E L, Haynes G, Figueroa J P, Barnett M, Hanchard B, Blattner W A. A prospective study of transmission by transfusion of HTLV-I and risk factors associated with seroconversion. Int J Cancer. 1992;51:886–891. doi: 10.1002/ijc.2910510609. [DOI] [PubMed] [Google Scholar]
- 20.Miwa M, Shimotohno K, Hoshino H, Fujino M, Sugimura T. Detection of pX proteins in human T-cell leukemia virus (HTLV)-infected cells by using antibody against peptide deduced from sequences of X-IV DNA of HTLV-I and Xc DNA of HTLV-II proviruses. Gann. 1984;75:752–755. [PubMed] [Google Scholar]
- 21.Miyoshi I, Kubonishi I, Yoshimoto S, Akagi T, Ohtsuki Y, Shiraishi Y, Nagata K, Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981;294:770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- 22.Ney P A, D'Andrea A D. Friend erythroleukemia revisited. Blood. 2000;96:3675–3680. [PubMed] [Google Scholar]
- 23.Okochi K, Sato H, Hinuma Y. A retrospective study on transmission of adult T cell leukemia virus by blood transfusion: seroconversion in recipients. Vox Sang. 1984;46:245–253. doi: 10.1111/j.1423-0410.1984.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 24.Palker T J, Tanner M E, Scearce R M, Streilein R D, Clark M E, Haynes B F. Mapping of immunogenic regions of human T cell leukemia virus type I (HTLV-I) gp46 and gp21 envelope glycoproteins with env-encoded synthetic peptides and a monoclonal antibody to gp46. J Immunol. 1989;142:971–978. [PubMed] [Google Scholar]
- 25.Pique C, Pham D, Tursz T, Dokhelar M C. The cytoplasmic domain of the human T-cell leukemia virus type I envelope can modulate envelope functions in a cell type-dependent manner. J Virol. 1993;67:557–561. doi: 10.1128/jvi.67.1.557-561.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pique C, Pham D, Tursz T, Dokhelar M C. Human T-cell leukemia virus type I envelope protein maturation process: requirements for syncytium formation. J Virol. 1992;66:906–913. doi: 10.1128/jvi.66.2.906-913.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pique C, Tursz T, Dokhelar M C. Mutations introduced along the HTLV-I envelope gene result in a non-functional protein: a basis for envelope conservation? EMBO J. 1990;9:4243–4248. doi: 10.1002/j.1460-2075.1990.tb07872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poiesz B J, Ruscetti F W, Gazdar A F, Bunn P A, Minna J D, Gallo R C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Popovic M, Sarin P S, Robert-Gurroff M, Kalyanaraman V S, Mann D, Minowada J, Gallo R C. Isolation and transmission of human retrovirus (human T-cell leukemia virus) Science. 1983;219:856–859. doi: 10.1126/science.6600519. [DOI] [PubMed] [Google Scholar]
- 30.Rein A, Mirro J, Haynes J G, Ernst S M, Nagashima K. Function of the cytoplasmic domain of a retroviral transmembrane protein: p15E-p2E cleavage activates the membrane fusion capability of the murine leukemia virus Env protein. J Virol. 1994;68:1773–1781. doi: 10.1128/jvi.68.3.1773-1781.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robek M D, Ratner L. Immortalization of CD4+ and CD8+ T lymphocytes by human T-cell leukemia virus type 1 Tax mutants expressed in a functional molecular clone. J Virol. 1999;73:4856–4865. doi: 10.1128/jvi.73.6.4856-4865.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robek M D, Ratner L. Immortalization of T lymphocytes by human T-cell leukemia virus type 1 is independent of the Tax-CBP/p300 interaction. J Virol. 2000;74:11988–11992. doi: 10.1128/jvi.74.24.11988-11992.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robek M D, Wong F H, Ratner L. Human T-cell leukemia virus type 1 pX-I and pX-II open reading frames are dispensable for the immortalization of primary lymphocytes. J Virol. 1998;72:4458–4462. doi: 10.1128/jvi.72.5.4458-4462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trejo S R, Ratner L. The HTLV receptor is a widely expressed protein. Virology. 2000;268:41–48. doi: 10.1006/viro.2000.0143. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto N, Okada M, Koyanagi Y, Kannagi M, Hinuma Y. Transformation of human leukocytes by cocultivation with an adult T cell leukemia virus producer cell line. Science. 1982;217:737–739. doi: 10.1126/science.6980467. [DOI] [PubMed] [Google Scholar]