Abstract
PCOS is one of the most common endocrine disorders among women of reproductive age. While the mechanism involved is not yet fully characterized. Our study aims to examine the pregnancy outcomes of embryo transfers in women with PCOS after pretreatment, and to explore the possible effect of high androgen levels on endometrial receptivity. Retrospective cohort study was conducted to analyze pregnancy outcomes among 2714 infertile women with tubal factor and 452 PCOS women. Endometrium samples were collected from 6 controls and 6 PCOS patients to detect the expression of endometrial receptivity marks. The implantation rate, clinical and ongoing pregnancy rates and live birth rate in women with PCOS followed fresh embryo transfers were obviously decreased even after the pretreatment. Similar pregnancy outcomes were found in frozen-thawed embryo transfer cycles between women with or without PCOS. Strikingly, serum total testosterone (TT) levels on trigger day were significantly higher in PCOS women. Women with high TT levels presented significantly lower clinical and ongoing pregnancy rates, and the expression of insulin-like growth factor binding protein 1 (IGFBP-1), and leukemia inhibitory factor (LIF) in the endometria decreased significantly as well. High doses of testosterone significantly down-regulated the expression of IGFBP-1 and LIF in Ishikawa cells. Although endocrine abnormalities had been improved before the controlled ovarian stimulation (COS) cycle started, higher serum TT levels were detected on the trigger day of the COS cycle in PCOS patients, which may contribute to the decreased fresh embryo implantation by impairing endometrial receptivity.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-74295-7.
Keywords: PCOS, Androgen, Endometrial receptivity, Embryo transfer
Subject terms: Reproductive signs and symptoms, Endocrine reproductive disorders
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder among women of reproductive age, with a prevalence rate of 5–10%1–5. The main clinical manifestations of PCOS vary greatly from person to person, including hyperandrogenism, oligomenorrhea, chronic anovulation, and hyperinsulinemia. Several studies indicated that although the ovulation rate reaches 80% when taking ovulation induction therapy, women with PCOS are more likely to encounter poor pregnancy outcomes6,7. Successful implantation requires synchrony between competent embryoes and receptive endometrial luminal epithelium during the window of implantation (WOI). A molecular dialogue is engaged within the embryo and endometrium during WOI8,9.
Though the dynamics of the transition of a non-receptive to receptive endometrium are still poorly understood, this transition involves both anatomic and functional changes to the functional layer of endometrium, which are both embodied and driven by changes in gene expression. Several gene products, known to be differentially expressed in WOI or secretory endometrium, were verified. Several proteins expressed specifically in the secretory phase of endometrium, including insulin-like growth factor-binding protein 1 (IGFBP-1), leukemia inhibitory factor (LIF), matrix metalloproteinase 9 (MMP9), etc., may play important roles in implantation and in the establishment of pregnancy10–12. Studies also show that specific growth factors, like transforming growth factor-beta (TGFβ), epidermal growth factor (EGF), and integrins, are expressed in the endometrium of WOI, and are good markers of endometrial receptivity13–15. All the above molecules are considered to be predictors for endometrial receptivity and essential for embryo implantation during WOI.
Receptive endometrium preparation is regulated by an array of endocrine, paracrine and autocrine modulators originating from the embryo and the mother. Endocrine hormones in maternal serum during the peri-implantation period are of great importance, and either directly or indirectly regulate the molecular index of endometrial receptivity. It is believed that in PCOS, low pregnancy rates and high miscarriage rates are related to high androgen levels, hyperinsulinism, and insulin resistance16,17. This metabolic disorder and endocrine abnormalities may affect endometrial metabolism and receptivity18. It is well-accepted that before the initiation of in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI)/cycles, suppressing women’s own hormone secretion improves their response to ovarian stimulation in both natural cycles and ovarian-stimulated cycles. Therefore, the practice of pretreating women with PCOS with Diane-35, an oral contraceptive (OC), for 3 months before controlled ovarian stimulation (COS) is widely adopted in a majority of reproductive centers.
However, it is still unclear if the endometrial receptivity of the COS cycle in PCOS women with normal sex hormone levels after pretreatment is comparable with that of infertile women with tubal factors. We conducted a retrospective cohort study to analyze the pregnancy outcomes of PCOS women of fresh embryo transfer after routine pretreatment and the first cycle of frozen-thawed embryo transfer. Also, we determined the expression of endometrial receptivity marks in women with PCOS during the COS cycle WOI, and explored the possible effects of endocrine hormone changes on endometrial receptivity.
Methods
Patient information
Data of women who underwent their IVF/ICSI cycles at the Reproductive Center of International Peace Maternity & Child Health Hospital affiliated with Shanghai Jiao Tong University Medical School and Reproductive Center of Taizhou Hospital were recruited from January 2010 to December 2022 in this study. The inclusion criteria for this study were as follows: (1) women aged 20–35 years; (2) BMI values within 18.5–28 kg/m219; (3) women whose retrieved oocytes were ≥ 3, with 1–2 embryos transferred. Cases of ovarian cyst or tumor, ovarian injury for operation or torsion, or premature ovarian failure, endometriosis, uterine abnormality such as a malformed uterus (uterus unicornis, septate uterus, duplex uterus or uterus bicornis), adenomyosis, submucous myoma or intrauterine adhesion were excluded. According to the inclusion and exclusion criteria, data from 452 PCOS women and 2714 women with tubal factors were enrolled and further analyzed.
The diagnosis of PCOS was made according to the Rotterdam Consensus20, based on a patient having two of these three findings or having all three findings as follows: (1) Oligo- or anovulation, which was assessed as oligomenorrhea (i.e., fewer than 7 cycles per year); (2) Biochemical or clinical hyperandrogenism, hirsutism with a modified Ferriman– Gallwey (F-G) score of more than 5 and/or clinical presence of obvious acne; (3) Polycystic ovaries (PCOs) were confirmed if there were 12 or more follicles in each ovary measuring 2–9 mm in diameter and/ or increased ovarian volume (> 10 mL) by ultrasonic examination. Women with hyperprolactinemia, hypothyroidism, androgen-secreting tumors, Cushing syndrome, congenital adrenal hyperplasia, and diabetes were excluded. PCOS patients were encouraged to control body weight, and were pretreated with OC for 3 months before IVF/ICSI treatment. Informed consent was obtained from all the patients before the IVF/ICSI protocol, and the study was approved by the local hospital’s Research and Ethics Committee.
IVF/ICSI protocol and luteal support
COS was performed using recombinant follicle-stimulating hormone (rFSH, Gonal-F; Merck-Serono, Geneva, Switzerland) or recombinant FSH with human menopausal gonadotrophin (hMG; Menopur; Ferring, Malmo, Sweden). The starting gonadotropin dose was based on patient’s antral follicles and varied from 150 to 300 IU/d for the first 5 d, initiated from day 2 or 3 of the menstrual cycle and followed by dose-adjustments according to ovarian response. The gonadotrophin-releasing hormone (GnRH) antagonist (Cetrotide® 0.25 mg/d; Merck-Serono) was applied from the day at least one follicle reached a diameter of 12 mm or day 6 of ovarian stimulation until the trigger day. When 2 leading follicles reached a mean diameter of 18 mm, recombinant hCG (Ovidrel® 250 mg; Merck-Serono) was administered for ovulation induction. Transvaginal ultrasound guide oocyte pickup (OPU) was performed 36 h later. On the third day after oocyte retrieval, fresh embryo transfer was performed and embryos of good quality was cryopreserved. Day 3 embryo of good quality was scored according to the ASEBIR consensus: day 3, 7–9 cell stage, < 10% fragmentation, and homogenous blastomere size. The number of transferred embryos varied from one to two depending on the age of the patient and embryo quality.
During frozen-thrawed embryo transfer cycles, routine hormone replacement therapy was used to prepare the endometrium. Transvaginal ultrasound scans were performed estimate the endometrial thickness and evaluted the endometrial morphology according to the clinical routine.
Luteal support was provided by vaginal administration of progesterone (Crinone® 90 mg/d, Merck Serono; Utrogestan 600 mg/d, Seid) initiated from the day of oocyte retrieval or endometrial thickness ≥ 8 mm. If pregnancy was achieved, progesterone administration was maintained up to the evidence of fetal heart activity at ultrasound.
IVF/ICSI outcomes
All pregnancies were followed up until delivery. Biochemical pregnancies were defined as those terminating after a short rise in β-hCG (≥ 5IU/L), well before the possibility of the ultrasound detection of pregnancy. Clinical pregnancy was defined as at least one gestational sac exhibiting fetal heart activity confirmed by ultrasound. Implantation rate was defined by the ratio between the number of gestational sacs with fetal heart activity and the number of embryos transferred. Spontaneous abortion was defined as a loss of a clinical pregnancy before 28 weeks′ gestation. An ongoing pregnancy was defined as the presence of at least one viable fetus 12 weeks of gestation determined by ultrasound. A live birth cycle was defined as a cycle that resulted in at least one liveborn neonate at or beyond 28 weeks of gestation.
Hormone analysis
For non-PCOS women, serum samples were collected on days 2–5 of menstruation and trigger day. For PCOS women, serum samples were collected before and after OC, on trigger day. The concentrations of serum sex hormone, including follicle stimulating hormone (FSH), luteinizing hormone (LH), estrogen (E2), total testosterone (TT), and progesterone (P), were measured at the clinical chemistry laboratory of International Peace Maternity & Child Health Hospital using chemiluminescent, two-site immunoassays on a multiparameter system (Axsym; Abbott Laboratories).
Endometrial biopsy
This study population included 6 infertile women with tubal factor (controls) and 6 PCOS women with high androgen (HA), who dropped off in vitro fertilization because of sperm retrieval failure, or selected frozen embryo transfer for personal schedule. They were recruited after written informed consent was provided. All patients had standard serum FSH, LH, E2, TT, and P level before COS started. On trigger day, there was no statistical difference in serum hormone elevations, except TT level, between these two groups. Endometrial biopsies were obtained using a biopsy catheter in all women at day 5 (hCG + 5) after hCG administration during COS cycles. Pathologic evaluations (hematoxylin and eosin staining) confirmed the endometrial secretory phase (data not shown). The rest of the endometrial tissue was stored at -80 °C for RNA and protein extraction. Ethical approval for this project was granted by the Research and Ethics Committee of the University Hospital of China.
Cell culture and quantitative real-time PCR
Ishikawa cell lines were purchased from commercial biobanks. Cells were maintained in 1:1 mixture of Dulbecco’s Modified Eagles’ Medium: Ham’s F-12 supplemented with 10% fetal bovine serum, 200 mM L-glutamine, and penicillin/streptomycin at 37° C in 5% CO2. Cells were maintained in phenol red-free DMEM/F12 medium without bovine serum for 24 h prior to hormone treatment. To mimic the endocrine environment of PCOS patients, Ishikawa cells were treated with DHT (Solarbio, China) at different concentrations of (Dmso, 10− 11, 10− 10, 10− 9, 10− 8, 10− 7mM) when they were at 70–80% confluence in 6-well plates.
Real-time polymerase chain reaction (PCR) was performed with SYBR® Green I Master Mix (TaKaRa Biotechnology, Dalian, China) after reverse transcribing 1 µg of RNA into cDNA. Primer pairs included the following: Lif:forward: CCAACGTGACGGACTTCCC, reverse: TACACGACTATGCGGTACAGC. Igfbp-1: forward: TTGGGACGCCATCAGTACCTA, reverse: TTGGCTAAACTCTCTACGACTCT.
Western blotting analysis
Western blot analysis was performed as described previously21. Endometrial samples were homogenized and IK cells were lysed before measuring the concentration of protein. In brief, the separated samples were transferred to polyvinylidene difluoride membranes and incubated overnight at 4 °C with rabbit polyclonal anti-α-IGFBP-1 (1:1000;13981-1-AP, Proteintech), anti-LIF (1:1000; 26757-1-AP, Proteintech), and human polyclonal anti-β-actin (1:0000; 66031-1-Ig Proteintech). The samples were then incubated with fluorescence-labeled anti-mouse IgG or anti-rabbit IgG antibody (1:5000, Dylight 680 or 800, KPL) for 1 h at room temperature and analyzed with an Odyssey Imager (Li-cor; Odyssey, NE, USA).
Statistical analyses
All data are presented as the mean ± SD or SEM. Independent-samples t-test, non-parametric tests, and chi-square tests were used to evaluate the statistical significance between the two groups. Univariate logistic regressions were used to estimate ORs with 95% CIs of pregnancy outcomes in relation to PCOS. Potential confounders were considered in multivariable analysis. Female age, male age, Duration of infertility, Day-3 FSH, LH, T levels were considered as continuous variables and were added to the aforementioned covariates when the estimation was done among the whole study population. Covariates methods of fertilization (IVF or ICSI), and primary or secondary infertility were included to estimate the associations between PCOS and pregnancy outcomes. SPSS (Version 19.0 for Windows) was used for the statistical analysis. P-values < 0.05 were considered statistically significant.
Results
Pregnancy outcome of PCOS patients after pretreatment
Pregnancy outcomes of fresh embryo transfer were analyzed among 200 patients with PCOS and 1562 patients with tubal factors. Clinical characteristics and IVF outcomes were shown in Table 1. Women and their partners in the PCOS group were generally younger, and the duration of fertility was longer than the controls. The ratio of primary infertility was a little higher in the PCOS group but did not reach a significant difference.
Table 1.
Clinical characteristics, basal endocrine features and pregnancy outcomes in a total cohort of 1762 infertile patients followed fresh embryo transfers.
| Tubal factor (n = 1562) | PCOS (n = 200) |
AOR (95%CI) | P-value1 | |
|---|---|---|---|---|
| Female age, years | 30.40 ± 2.662 | 29.18 ± 2.87 | -- | < 0.001 |
| Male age, years | 32.95 ± 4.37 | 31.82 ± 4.18 | -- | 0.001 |
| BMI (kg/m2) | 22.58 ± 3.68 | 22.37 ± 3.02 | -- | 0.439 |
| Duration of infertility (years) | 3.68 ± 2.31 | 3.98 ± 2.14 | -- | 0.081 |
| Primary infertility (%) | 69.14 (1080/1562) | 69.00 (138/200) | -- | 0.967 |
| Second infertility (%) | 30.86 (482/1562) | 31.00 (62/200) | -- | 0.967 |
| Day-3 FSH (IU/L) | 7.51 ± 1.80 | 6.97 ± 1.44 | -- | < 0.001 |
| Day-3 LH (IU/L) | 5.08 ± 1.59 | 5.55 ± 2.23 | -- | 0.004 |
| Day-3 E2 level (pmol/L) | 154.70 ± 60.60 | 154.71 ± 58.63 | -- | 0.998 |
| Day-3 TT level (nmol/L) | 1.34 ± 0.49 | 1.52 ± 0.66 | -- | < 0.001 |
| Implantation rate(%) |
26.81 (820/3058) |
19.13 (75/392) |
-- | 0.001 |
| Clinical pregnancy rate (%)4 | 45.07 (704/1562) | 32.00 (64/200) | 0.60 (0.43–0.87) | 0.006 |
| Miscarriage rate (%)4 | 9.52 (67/704) | 20.31 (13/64) | 2.36 (1.06–5.30) | 0.037 |
| Live birth rate (%)4 | 36.56 (571/1562) | 17.50 (35/200) | 0.36 (0.24–0.54) | < 0.001 |
| Ongoing pregnancy rate (%)4 | 37.00 (578/1562) | 21.50 (43/200) | 0.42 (0.27–0.64) | < 0.001 |
| Chemical pregnancy rate (%)4 | 5.70 (89/1562) | 10.50 (21/200) | 2.56 (1.42–4.63) | 0.002 |
| Ectopic abortion rate (%)4 | 6.96 (49/704) | 12.5 (8/64) | 2.45 (0.95–6.31) | 0.064 |
1. Data were analyzed by using Student’s t, Mann-Whitney U, and chi-square tests.
2. Mean ± SD (all such values).
3. FSH, follicle-stimulating hormone; LH, basal luteinizing hormone; TT, total testosterone; E2, estradiol.
4. Data are shown as adjusted OR (95% CI), adjusted for female age, male age, duration of infertility, primary or secondary infertility, endometrial thickness, total Gn dosage, duration of Gn, the year of patients’ treatment, and different reproductive centers.
However, clinical pregnancy rate (32.0% vs. 45.1%, Adjusted OR (AOR): 0.563, 95% CI: 0.45–0.87), ongoing pregnancy rate (21.5% vs. 37.0%, AOR:0.38, 95% CI: 0.26–0.57), and live birth rate (17.5% vs. 36. 6%, AOR:0.38, 95% CI: 0.26–0.57) in PCOS group were obviously lower compared with the control women. Meanwhile, the miscarriage rate (20.3% vs. 9.5%, AOR: 2.38, 95% CI: 1.16–4.88) and chemical pregnancy rate (10.5% vs. 5.7%, AOR: 2.27, 95% CI: 1.33–3.87) were significantly increased in women with PCOS. These results suggest that endometrial factors might significantly contribute to the poor pregnancy outcomes of the PCOS group.
Considering the heightened risk of OHSS in PCOS patients and its conceivable adverse effect on pregnancy outcomes, we segmented the study population into groups based on the occurrence of OHSS. The incidence of OHSS in the control and PCOS groups, which were 7.7% and 13.0%, respectively. Within the non-OHSS group, the clinical pregnancy rate, live birth rate, and ongoing pregnancy rate of the PCOS group were consistently lower in comparison to those of women attributed to tubal factors, while within the OHSS group, the tendency was similar (Table S1). The findings suggested that OHSS, contrary to initial assumptions, may not be a pivotal determinant affecting pregnancy outcomes in the context of PCOS.
Next, we also analyzed the clinical characteristics, basal endocrine features, and outcomes in 1152 non-PCOS and 252 PCOS women who underwent frozen-thawed cycles and first frozen embryo transfers (Table 2). No significant differences in implantation rate, clinical pregnancy rate, ongoing pregnancy rate, live birth rate, and miscarriage rate were found between PCOS and non-PCOS women. The results imply that poor pregnancy outcomes for fresh embryo transfer may be attributed to the impaired endometrial receptivity during the controlled ovarian stimulation cycle.
Table 2.
Clinical characteristics, basal endocrine features and pregnancy outcomes in women followed frozen embryo transfers.
| Tubal factor (n = 1152) | PCOS (n = 252) |
AOR (95%CI) | P-value1 | |
|---|---|---|---|---|
| Female age, years | 29.43 ± 3.012 | 28.72 ± 3.17 | -- | 0.002 |
| Male age, years | 31.81 ± 4.89 | 30.62 ± 3.74 | -- | < 0.001 |
| BMI (kg/m2) | 21.95 ± 2.57 | 23.33 ± 2.61 | -- | < 0.001 |
| Duration of infertility (years) | 3.44 ± 2.43 | 3.87 ± 2.21 | -- | 0.006 |
| Primary infertility (%) | 47.40 (546/1152) | 57.94 (146/252) | -- | 0.001 |
| Second infertility (%) | 52.60 (606/1152) | 42.06 (106/252) | -- | 0.001 |
| Implantation rate (%) | 37.04 (719/1941) | 40.01(188/469) | -- | 0.259 |
| Clinical pregnancy rate (%)3 | 53.82 (620/1152) | 57.12 (144/252) | 1.10 (0.79–1.52) | 0.583 |
| Miscarriage rate (%)3 | 14.52 (90/620) | 18.06 (26/144) | 1.30 (0.72–2.36) | 0.384 |
| Live birth rate (%)3 | 38.80 (447/1152) | 40.87(103/252) | 1.18 (0.84–1.65) | 0.351 |
| Ongoing pregnancy rate (%)3 | 44.53 (513/1152) | 46.83 (118/252) | 1.10 (0.79–1.52) | 0.585 |
| Chemical pregnancy rate (%)3 | 9.38 (108/1152) | 12.70 (32/252) | 1.33 (0.78–2.27) | 0.293 |
| Ectopic abortion rate (%)3 | 3.87 (24/620) | 2.08 (3/144) | 0.24 (0.05–1.22) | 0.086 |
1. Data were analyzed by using Student’s t, Mann-Whitney U, and chi-square tests.
2. Mean ± SD (all such values).
3. Data are shown as adjusted OR (95% CI), adjusted for female age, male age, duration of infertility, primary or secondary infertility, the year of patient’s treatment and different reproductive centers.
Endocrine features in infertile patients with PCOS during the IVF/ICSI fresh embryo transfer cycle
To illuminate the possible risk factors that may be associated with unfavorable pregnancy outcomes in women with PCOS who underwent fresh embryo transfer, we focused on hormone levels of peri-implantation, which regulate the endometrium for embryo implantation. As to IVF treatment, the total gonadotropin dose of 1673.16 IU used in the PCOS group was much lower than the dose of 2082.72 IU used in controls (P < .001). There was no significant difference in the E2 and P levels, cleavage rate, fertilization rate, available embryos rate, or high-quality embryo rate between the two groups on trigger day (Table 3). Indeed, it is noteworthy that the total T level on trigger day was significantly higher (3.1 vs. 1.8 nmol/L) and the endometrium was thinner on trigger day in women with PCOS (9.4 vs. 10.1 mm) (Table 3). The results indicate that high TT levels may impair endometrial receptivity and result in low ongoing pregnancy rate.
Table 3.
Stimulation protocol and endocrine features in infertile patients with PCOS and controls during the fresh embryo transfer cycle.
| Tubal factor (n = 1562) | PCOS (n = 200) | P-value1 | |
|---|---|---|---|
| Stimulation protocols | |||
| GnRH-a long protocol (%) | 626 (88.92) | 78 (11.08) | 0.770 |
| GnRH antagonist (%) | 936 (88.47) | 122 (11.53) | 0.770 |
| E2 on hCG day (pmol/L) | 8789.88 ± 5216.37 | 9096.44 ± 5306.87 | 0.435 |
| P on hCG day (pmol/L) | 1.97 ± 1.04 | 2.13 ± 1.22 | 0.077 |
| Serum TT level on hCG day (nmol/L)2 | 1.75 ± 0.72 3 | 3.14 ± 0.92 4 | < 0.001 |
| Endometrial thickness on hCG day (mm) | 10.10 ± 1.86 | 9.37 ± 1.62 | < 0.001 |
| Total gonadotropin dose (IU) | 2082.72 ± 726.71 | 1673.16 ± 730.55 | < 0.001 |
| Duration of gonadotropin (days) | 9.46 ± 2.06 | 9.76 ± 2.70 | 0.131 |
| Number of oocytes retrieved | 11.27 ± 5.41 | 12.34 ± 5.76 | 0.009 |
| Cleavage rate (%) | 96.94 ± 7.97 | 97.73 ± 5.85 | 0.087 |
| Fertilization rate (%) | 79.76 ± 19.11 | 80.57 ± 18.57 | 0.650 |
| Available embryos rate (%) | 71.98 ± 30.34 | 72.34 ± 24.27 | 0.571 |
| High quality embryo rate (%) | 54.60 ± 29.80 | 51.85 ± 25.98 | 0.167 |
1. Data were analyzed by using Student’s t, Mann-Whitney U, and chi-square tests.
2. 3n = 222, 4n = 70.
Expression of endometrial receptivity marks in PCOS women with HA
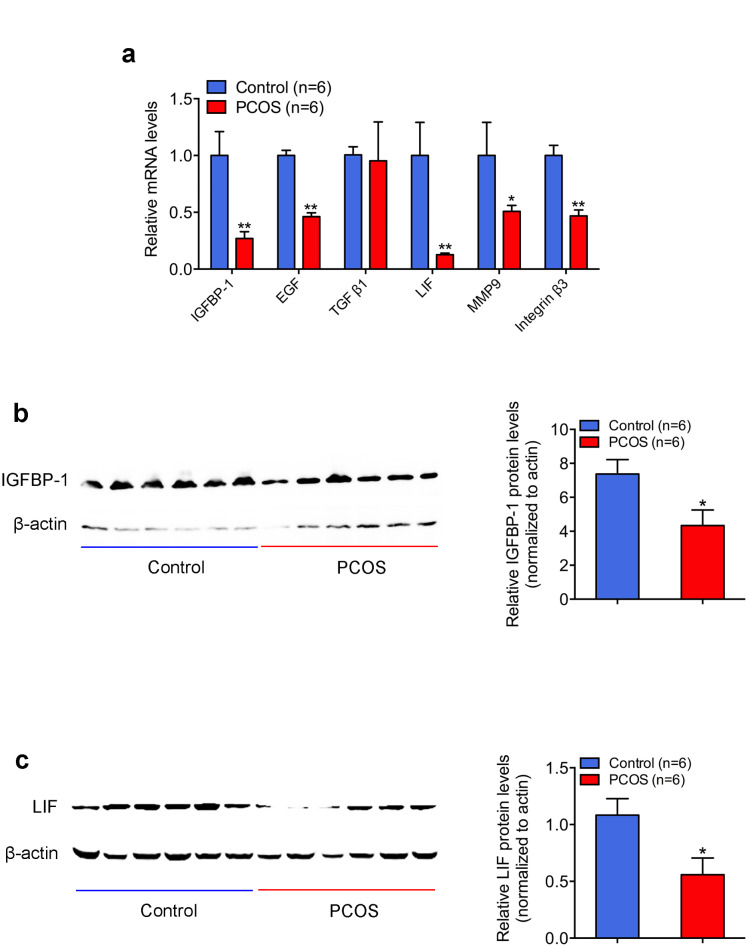
To further confirm the relationship between high androgen and lower implantation, we screened the expression levels of major factors (IGFBP-1, EGF, TGFβ1, LIF, MMP9, and integrin β3), which were known as good marks of endometrial receptivity in the human endometrium with the use of real-time reverse transcription PCR. We found that the mRNA levels of IGFBP-1, EGFR, LIF, MMP9, and integrins β3 in the endometrium of PCOS women with HA were significantly lower than in that of controls (Fig. 1a). Among them, IGFBP-1 and LIF exhibited the largest fold changes. While there was no significant difference in the EGF mRNA level between the two groups.Western blot analysis confirmed that the expression levels of IGFBP-1 and LIF in PCOS endometria were significantly lower than those in controls (Fig. 1b and c).
Fig. 1.
Expression of endometrial receptivity marks in infertile women with tubal factor and women with polycystic ovary syndrome (PCOS). (a) Messenger RNA expressions of insulin-like growth factor binding protein 1 (IGFBP-1), epidermal growth factor (EGF), transforming growth factor-beta (TGF β1), leukemia inhibitory factor (LIF), matrix metalloproteinase 9 (MMP9) and integrin β3 in human endometria. Total RNA was extracted from endometrium biopsies of infertile women with tubal factor and PCOS (n = 6 each) and then analyzed by quantitative reverse-transcription polymerase chain reaction. The values are presented as mean ± SEM. **P < .01 and *P < .05 (unpaired t-test). (b,c) IGFBP-1 and LIF protein expression. Tissue lysates (80 mg) were extracted from endometrial tissues of infertile women with tubal factor (control; n = 6) and women with PCOS (PCOS; n = 6), and then subjected to Western blot analysis with the use of anti-LIF, anti-IGFBP-1 or anti-β-actin antibody, as described in the Materials and Methods. Left figure shows one representative experiment among three separate experiments. Right histogram shows relative protein levels of IGFBP-1 and LIF normalized to β-actin (Right). The values are presented as mean ± SEM. *P < .05 (unpaired t-test).
High testosterone reduced the expression of IGFBP-1 and LIF in vitro
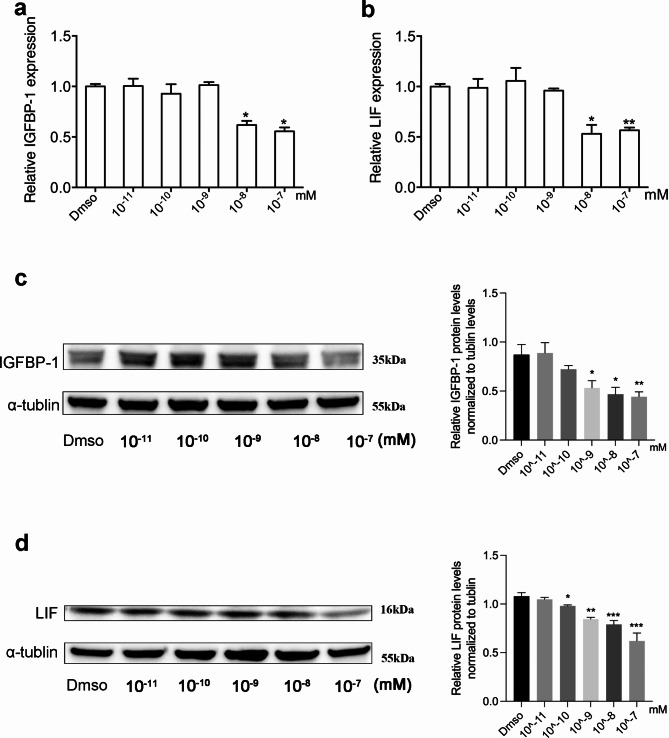
To determine whether high testosterone level would affect the expression of IGFBP-1 and LIF, we examined the mRNA and protein expression of IGFBP-1 and LIF in Ishikawa cells, which were treated with different concentrations of DHT (dihydrotestosterone). Real-time PCR and Western blot analyses showed that, compared with the control group, high doses of DHT significantly reduced the IGFBP-1 and LIF expression in both mRNA (Fig. 2a and b) and protein (Fig. 2c and d) level. This implies that high serum TT levels may result in impaired endometrial receptivity, which links to poor pregnancy outcomes in PCOS.
Fig. 2.
High testosterone levels reduced the expression of IGFBP-1 and LIF in Ishikawa cells, in vitro. (a,b) Messenger RNA expression of IGFBP-1 and LIF in Ishikawa cells treated with testosterone at different concentrations. Total RNA was extracted from Ishikawa cells (n = 6 each) and then analyzed using quantitative reverse-transcription polymerase chain reaction. The values are presented as mean ± SEM. **P < .01 and *P < .05 (unpaired t-test). (c,d) Protein expression of IGFBP-1 and LIF. Cell lysates (100 mg) were extracted from Ishikawa cells subjected to Western blot analysis with the use of anti-LIF, anti-IGFBP-1 or anti-α-tublin antibody. Left figure shows one representative experiment among three separate experiments (Left). Right histogram shows relative protein levels of IGFBP-1 and LIF normalized to α-tublin (Right).
Discussion
In this study, we found elevated serum TT levels on the trigger day of IVF in patients with PCOS, may links to impaired endometrial receptivity in fresh cycles and result in poor pregnancy outcomes.
Multiple studies had indicated poor pregnancy outcome in PCOS was due to inferior quality of eggs and impaired endometrial receptivity22–24. Patients with PCOS were also thought to be at increased risk of early pregnancy loss and higher rates of abortion when undergoing IVF treatment due to the inherent endocrine dysfunction associated with the syndrome, compared to tubal factor infertility controls7.
It was reported that pretreated with Diane-35 for three cycles could significantly reduce the body mass index (BMI), acne score, LH and TT levels, also improve acne symptoms, glucose and lipid metabolism in PCOS women25. Therefore, all patients enrolled in this study were pretreated with Diane-35 or Diane-35 for 3 cycles along with diet control. BMI < 28 and fully corrected abnormal hormone levels, including androgen, LH, and insulin, which were believed to impair both oocyte quality and quantity, were achieved before COS cycle started. As expected, comparable cleavage rate, fertility rate, available embryo rate and high-quality embryo rate were presented between controls and the PCOS group. It was supported by a recent study in which documented polycystic ovarian morphology does not have a negative impact on the quality of oocytes and embryos of IVF/ICSI cycles26. We found that though poor pregnancy outcomes of these PCOS women were restored in frozen embryo transfer cycles, the group continued to exhibit low implantation rates, clinical pregnancy rates, live birth rate risks and high miscarriage rate risks. This implied that the quality of eggs of the PCOS group in this study was similar to controls, and the lower clinical pregnancy rate might be associated with the endometrial factors. Therefore, we further compared factors on trigger day, which were believed to be involved in the pregnancy outcomes. Strikingly, a significantly higher serum TT level on trigger day was found in PCOS women, accompanied by thinner endometrial lining. Although it was reported that testosterone might exert its antiproliferative effects on the endometrium27, it was still uncertain whether other pathological factors contributed to the thinner endometrial lining in these cases.
In order to determine if high androgen on trigger day exactly links to poor pregnancy outcomes of fresh embryo transfer, strict inclusion criteria were applied to minimize confounding factors in our studies. Morbid obesity has been shown to affect ART outcomes and has been associated with higher gonadotropin requirements and lower chances of live birth for all comers, including women with PCOS18,28,29. Whether mild or moderate obesity plays a role remains undetermined. So we matched the BMI between control and PCOS patients, and, narrowed the recruited patients by BMI < 28 to minimize the confounding factors accompanied with obesity or overweight, such as altered insulin, glucose, and adipocytokines.
Next, we sampled endometria in PCOS patients with high serum TT and non-PCOS patients on trigger day. Expression of IGFBP-1 and LIF were significantly decreased in PCOS patients, which suggested poor endometrial receptivity in these patients12,30. Serum IGFBP-1 levels reflect a “receptive endometrium” and predict the likelihood of embryo implantation31. Leukemia inhibitory factor (LIF), a cytokine, is expressed in the uterine endometrial glands. It plays an important role in implantation and establishment of pregnancy. Females lacking a functional LIF gene are fertile, but their blastocysts fail to implant and do not develop32. Previous studies had reported that the concomitant increase in both serum androgens and elevations in endometrial androgen receptors (AR) might reduce endometrial receptivity as judged by the downregulation of ανβ3 integrin33. The results above suggested that elevated serum TT levels on trigger day impaired endometrial receptivity, contributing, at least in part, to the poor pregnancy outcomes in fresh embryo transfer cycle.
However, our study has some limitations. First, whether or not to apply OC before IVF/ICSI is rather conflicting in recent years. OC pretreatment usually takes place 2–3 months before the initiation of fresh embryo transfer to improve women’s reponses to hormone replacement therapy. Some clinical trials suggested that OC use in different stimulation protocols was associated with adverse pregnancy outcomes, such as lower rates of live birth and ongoing pregnancy34,35. While two meta-analyses indicated there was insufficient evidence to determine these adverse events were influenced by OC pretreatment regarding inadequate description of study methods and risks of bias36,37. Similarly, one study from a reproductive center in Zhejiang Province suggested that successive use of OC ( > = 3 months) significantly improve the rates of clinical pregnancy and small-for-gestational age infants for PCOS women38. Despite all the evidences listed above, a comprehensive understanding of the impact of OC pretreatment on pregnancy outcomes for IVF/ICSI patients is still lacking. Second, the interaction of confounding variables, such as androgens, insulin resistance and obesity, was not clarified on the poor pregnancy outcomes in PCOS. Further randomized controlled studies or well-designed experimental studies were encouraged to verify whether endometrium receptivity is impaired in PCOS women with different phenotypes. It would be beneficial for clinicians to make evidence-based clinical decisions on whether fresh or frozen-thawed embryo transfer is superior for IVF patients with PCOS. Thirdly, although the endometrium is significantly thinner in women with PCOS compared to the tubal factor group, the thickness is within the normal range and thereby the clinical importance may not be significant. Further investigation is needed to elucidate the finding.
In conclusion, our study demonstrated that PCOS women undergoing IVF/ICSI treatment with normalized sex hormone levels before ovarian stimulation, experienced lower pregnancy rates and higher miscarriage rates during the fresh embryo transfer cycle compared with that of women infertile for tubal factors. Impaired endometrial receptivity is likely attributable to elevated serum TT on trigger day.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
All authors have read and agreed with the content of the manuscript. X.H.L. designed the study. S.Y.W., J.J.C., and H.Q.W. drafted the manuscript. J.L.Z, H.Q.G, and D.D.W. conducted the experiments and data collection. X.F.J. and J.L.Z. conducted the statistical analysis. All authors commented on the drafts and approved the final draft. Written informed consent for publication was obtained from all participants.
Funding
This study was funded by the National Key Research and Development Program of China (No. 2022YFC2703801), the National Natural Science Foundation of China (No. 82071730), Research Grant from Shanghai Hospital Development Center (No. SHDC2024CRI095), and Shanghai Frontiers Science Research Base of Reproduction and Development and the Clinical Research Plan of IPMCH (IPMCH2022CR1-03).
Data availability
The data presented in this study are available on request from the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of International Peace Maternal Health Hospital (IPMCH) (GKLW2018-27 and 28 December 2018).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Si-Yi Wei, Jian-Lin Zhang and He-Qin Guan.
Contributor Information
Hui Wang, Email: wanghuilinzifu@126.com.
Dan-Dan Wu, Email: woodendenny@163.com.
Xian-Hua Lin, Email: xl_1290@126.com.
References
- 1.Rajska, A., Buszewska-Forajta, M., Rachoń, D. & Markuszewski, M. J. Metabolomic insight into polycystic ovary syndrome—an overview. Int. J. Mol. Sci. 21(14). (2020). [DOI] [PMC free article] [PubMed]
- 2.Sanchez-Garrido, M. A. & Tena-Sempere, M. Metabolic dysfunction in polycystic ovary syndrome: pathogenic role of androgen excess and potential therapeutic strategies. Mol. Metab. 35, 100937 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bozdag, G., Mumusoglu, S., Zengin, D., Karabulut, E. & Yildiz, B. O. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum. Reprod. 31(12), 2841–2855 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Li, R. et al. Prevalence of polycystic ovary syndrome in women in China: a large community-based study. Hum. Reprod. 28(9), 2562–2569 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Lizneva, D. et al. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 106(1), 6–15 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Arredondo, F. & Noble, L. S. Endocrinology of recurrent pregnancy loss. Semin. Reprod. Med. 24(1), 33–39 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Franik, S., Eltrop, S. M., Kremer, J. A., Kiesel, L. & Farquhar, C. Aromatase inhibitors (letrozole) for subfertile women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 5(5), Cd010287 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergh, P. A. & Navot, D. The impact of embryonic development and endometrial maturity on the timing of implantation. Fertil. Steril. 58(3), 537–542 (1992). [DOI] [PubMed] [Google Scholar]
- 9.Gingold, J. A. et al. Endometrial pattern, but not endometrial thickness, affects implantation rates in euploid embryo transfers. Fertil. Steril. 104(3), 620–8e5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kao, L. C. et al. Global gene profiling in human endometrium during the window of implantation. Endocrinology. 143(6), 2119–2138 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Aghajanova, L. Leukemia inhibitory factor and human embryo implantation. Ann. N. Y. Acad. Sci. 1034, 176–183 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Cheng, J., Rosario, G., Cohen, T. V., Hu, J. & Stewart, C. L. Tissue-specific ablation of the LIF receptor in the murine uterine epithelium results in implantation failure. Endocrinology. 158(6), 1916–1928 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh, M., Chaudhry, P. & Asselin, E. Bridging endometrial receptivity and implantation: network of hormones, cytokines, and growth factors. J. Endocrinol. 210(1), 5–14 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Haouzi, D. et al. Transcriptome analysis reveals dialogues between human trophectoderm and endometrial cells during the implantation period. Hum. Reprod. 26(6), 1440–1449 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Diedrich, K., Fauser, B. C., Devroey, P., Griesinger, G. & Evian Annual Reproduction Workshop, G. The role of the endometrium and embryo in human implantation. Hum. Reprod. Update. 13(4), 365–377 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Imani, B., Eijkemans, M. J., te Velde, E. R., Habbema, J. D. & Fauser, B. C. A nomogram to predict the probability of live birth after clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. Fertil. Steril. 77(1), 91–97 (2002). [DOI] [PubMed] [Google Scholar]
- 17.MacDougall, M. J., Tan, S. L., Balen, A. & Jacobs, H. S. A controlled study comparing patients with and without polycystic ovaries undergoing in-vitro fertilization. Hum. Reprod.. 8(2), 233–237 (1993). [DOI] [PubMed] [Google Scholar]
- 18.Schulte, M. M., Tsai, J. H. & Moley, K. H. Obesity and PCOS: the effect of metabolic derangements on endometrial receptivity at the time of implantation. Reprod. Sci. 22(1), 6–14 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen, C. M. Overview of obesity in Mainland China. Obes. Rev. 9(Suppl 1), 14–21 (2008). [DOI] [PubMed]
- 20.Gillman, M. W. A Life Course Approach to Chronic Disease Epidemiology, 189–217 (2004).
- 21.He, R. H. et al. Aquaporin-2 expression in human endometrium correlates with serum ovarian steroid hormones. Life Sci. 79(5), 423–429 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Palomba, S., Piltonen, T. T. & Giudice, L. C. Endometrial function in women with polycystic ovary syndrome: a comprehensive review. Hum. Reprod. Update (2020). [DOI] [PubMed]
- 23.Ludwig, M., Finas, D. F., al-Hasani, S., Diedrich, K. & Ortmann, O. Oocyte quality and treatment outcome in intracytoplasmic sperm injection cycles of polycystic ovarian syndrome patients. Hum. Reprod. 14(2), 354–358 (1999). [DOI] [PubMed] [Google Scholar]
- 24.Sengoku, K. et al. The chromosomal normality of unfertilized oocytes from patients with polycystic ovarian syndrome. Hum. Reprod. 12(3), 474–477 (1997). [DOI] [PubMed] [Google Scholar]
- 25.Wang, A., Mo, T., Li, Q., Shen, C. & Liu, M. The effectiveness of metformin, oral contraceptives, and lifestyle modification in improving the metabolism of overweight women with polycystic ovary syndrome: a network meta-analysis. Endocrine. 64(2), 220–232 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Sigala, J. et al. Is polycystic ovarian morphology related to a poor oocyte quality after controlled ovarian hyperstimulation for intracytoplasmic sperm injection? Results from a prospective, comparative study. Fertil. Steril. 103(1), 112–118 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Filho, A. M., Barbosa, I. C., Maia, H. Jr., Genes, C. C. & Coutinho, E. M. Effects of subdermal implants of estradiol and testosterone on the endometrium of postmenopausal women. Gynecol. Endocrinol. 23(9), 511–517 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Fedorcsák, P., Storeng, R., Dale, P. O., Tanbo, T. & Åbyholm, T. Obesity is a risk factor for early pregnancy loss after IVF or ICSI. Acta Obstet. Gynecol. Scand. 79(1), 43–48 (2000). [PubMed] [Google Scholar]
- 29.Veleva, Z. et al. High and low BMI increase the risk of miscarriage after IVF/ICSI and FET. Human reproduction. Hum. Reprod.. 23(4), 878–884 (2008). [DOI] [PubMed] [Google Scholar]
- 30.Giudice, L. C. Multifaceted roles for IGFBP-1 in human endometrium during implantation and pregnancy. Ann. N. Y. Acad. Sci. 828, 146–156 (1997). [DOI] [PubMed] [Google Scholar]
- 31.Douglas, N. C. et al. Differential expression of serum glycodelin and insulin-like growth factor binding protein 1 in early pregnancy. Reprod. Sci. 20(11), 1376–1381 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart, C. L. et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 359(6390), 76–79 (1992). [DOI] [PubMed] [Google Scholar]
- 33.Apparao, K. B., Lovely, L. P., Gui, Y., Lininger, R. A. & Lessey, B. A. Elevated endometrial androgen receptor expression in women with polycystic ovarian syndrome. Biol. Reprod. 66(2), 297–304 (2002). [DOI] [PubMed] [Google Scholar]
- 34.Xu, L. et al. Effects of oral contraceptive pretreatment on IVF outcomes in women following a GnRH agonist protocol. Reprod. Biomed. Online. 39(6), 924–930 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Wei, D. et al. Effect of pretreatment with oral contraceptives and progestins on IVF outcomes in women with polycystic ovary syndrome. Hum. Reprod. 32(2), 354–361 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.el Mouaaouy, A., Naruhn, M. & Becker, H. D. Diagnosis of liver metastases from malignant gastrointestinal neoplasms: results of pre- and intraoperative ultrasound examinations. Surg. Endosc. 5(4), 209–213 (1991). [DOI] [PubMed] [Google Scholar]
- 37.Farquhar, C., Rombauts, L., Kremer, J. A., Lethaby, A. & Ayeleke, R. O. Oral contraceptive pill, progestogen or oestrogen pretreatment for ovarian stimulation protocols for women undergoing assisted reproductive techniques. Cochrane Database Syst. Rev. 5(5), CD006109 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan, J. X. et al. Successive and cyclic oral contraceptive pill pretreatment improves IVF/ICSI outcomes of PCOS patients and ameliorates hyperandrogenism and antral follicle excess. Gynecol. Endocrinol. 31(4), 332–336 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.