Abstract
Surgical occlusion of the left atrial appendage (LAA) during cardiac surgery in patients with atrial fibrillation (AF) is known to reduce thromboembolism. However, data on the clinical significance of LAA occlusion (LAAO) in patients with mitral regurgitation (MR) are lacking. A total of 237 AF patients with chronic severe MR who underwent mitral valve (MV) surgery were retrospectively analyzed. Patients were divided into two groups according to concomitant LAAO or LAA preservation. The primary outcome was a composite of all-cause death and thromboembolic events (ischemic stroke or systemic embolism). The LAA was surgically occluded in 98 (41%) patients and preserved in 139 (59%) patients. During the follow-up period (median, 37 months), 29 primary outcomes occurred. In the Kaplan–Meyer analysis, the LAA preservation group showed a greater cumulative incidence of the primary outcome (P = 0.002) and thromboembolic events (P = 0.003) than the LAAO group. In the univariate Cox regression analysis, coronary artery disease, CHA2DS2-VASc score, a cauliflower-shaped LAA, Maze, and no LAAO were significantly associated with the primary outcome. In the multivariate Cox regression analysis, concomitant LAAO was significantly linked to the primary outcome (hazard ratio [HR]: 0.30, 95% confidence interval [CI]: 0.10–0.91, P = 0.033) and thromboembolic events (HR: 0.19, 95% CI: 0.04–0.87, P = 0.032). These benefits from LAAO were consistent, even after propensity score–matched analysis. For patients undergoing surgery for chronic MR who also have AF, concomitant surgical LAAO is associated with favorable clinical outcome.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-73400-0.
Keywords: Left atrial appendage, Occlusion, Mitral regurgitation, Atrial fibrillation, Outcomes
Subject terms: Cardiology, Diseases
Introduction
Severe mitral regurgitation (MR) causes left atrial (LA) and LA appendage (LAA) remodeling due to chronic volume overload in the LA, and many patients are accompanied by atrial fibrillation (AF) and are at risk of stroke1–4. Therefore, when performing surgery for severe MR, efforts to reduce the risk of stroke are clinically needed in addition to correcting the MR itself. Recently, a large randomized controlled trial, Left Atrial Appendage Occlusion Study (LAAOS III), demonstrated a reduced risk of ischemic stroke or systemic embolism in concurrent surgical occlusion of the LAA group compared to the no-occlusion group in patients with AF undergoing any cardiac surgery5. Based on this trial, surgical occlusion of the LAA is recommended for stroke prevention in patients with AF undergoing cardiac surgery6. However, patients with chronic severe MR should be considered separately from other cardiac conditions when assessing the benefits of LAA occlusion (LAAO). This is due to distinct LA and LAA remodeling that can develop with MR, along with significant hemodynamic changes following mitral valve (MV) surgery in severe MR7. Moreover, a sub-analysis of patients with severe MR was not conducted even in LAAOS III, thus there was still a lack of information on the potential benefits of LAAO combination in AF patients undergoing surgery for severe MR. In addition, when deciding LAAO, it is theoretically necessary to consider individualized stroke risk regarding the structure and function of LAA. In particular, as transcatheter interventions such as, mitral transcatheter edge-to-edge repair (TEER), increase in the setting of severe MR, there is growing interest in the future risk of stroke in patients who are vulnerable to blood flow stasis and thrombus formation in LA and LAA after MR correction. We hypothesized that LAAO would significantly reduce thromboembolic risk in patients undergoing surgery for severe MR. To explore this hypothesis, we aimed to conduct a study utilizing retrospective data collection from patients with AF and severe MR who underwent MV surgery. The objective of this study was to determine the association between concomitant LAAO during MR surgery and the postoperative thromboembolic risk in patients with both MR and AF.
Materials and methods
Study population
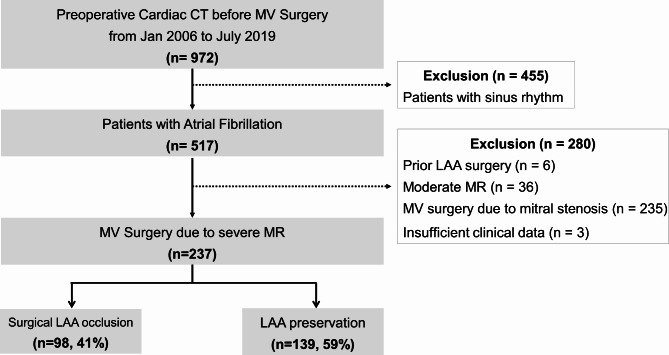
A total of 972 patients who underwent transthoracic echocardiography and cardiac computed tomography (CT) for preoperative evaluation were retrospectively identified at a single tertiary center (Severance Cardiovascular Hospital, Seoul, Republic of Korea) between January 2006 and June 2019. Patients in sinus rhythm (n = 455), those who had previously undergone LAA surgery or device occlusion (n = 6), individuals with insufficient clinical data (n = 3), patients without severe MR (n = 36), and those who had undergone MV surgery mainly due to mitral stenosis (n = 235) were excluded from the study. Finally, a total of 237 patients were analyzed (Fig. 1). This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Yonsei University Health System. Because this study used anonymized retrospective data, the requirement for informed consent was waived by the institutional review board of Yonsei University Health System. (Yonsei University Health System, Severance Hospital, Institutional Review Board, approval no. 2021-0510-003)
Fig. 1.
Flow diagram. Flow diagram for study population selection.
Echocardiography and cardiac computed tomography
Standard two-dimensional and Doppler echocardiography measurements were performed following the American Society of Echocardiography guidelines8. The LA volume index was measured using the modified Simpson’s method and indexed on the basis of body surface area. Left ventricular (LV) ejection fraction was calculated using biplane calculation. The severity of MR was assessed using a multiparametric approach according to current guidelines. Rheumatic heart disease was defined as the presence of typical morphological rheumatic valvular features, including leaflet restriction and thickening, subvalvular thickening, and commissural fusion. The cardiac CT scanning protocol, generation of 3D rendering, and data extraction were all carried out in the usual way. The LAA morphology was evaluated and classified by a single expert radiologist (Y. J. K.) blinded to the clinical data. The LAA morphologies were classified into four types (cactus, cauliflower, windsock, chicken wing), as previously described9.
Clinical data and outcomes
All demographic, anthropometric, and laboratory data were collected from patient electronic medical records. The decision to implement LAAO was made at the discretion of the surgeon during each MV surgery. There are only two methods used for LAAO: closure with an internal ligation method or amputation with a stapler device. The choice of which method to use was based on surgeon preference. The primary outcome was defined as a composite of all-cause death and thromboembolic events. Thromboembolic events were defined as a composite of ischemic stroke or systemic embolism. Analyses were performed on the association between LAAO and primary outcome, with additional analyses on thromboembolic events as a key secondary outcome. Postoperative bleeding was defined as a composite of reoperation after closure of sternotomy for the purpose of bleeding control, the transfusion of ≥ 2 U of blood products, or a chest tube output of 2 L within a 24-h period during the admission for MV surgery. Consistent AF after MV surgery was characterized by the presence of an AF rhythm documented on a 12-lead electrocardiography occurring > 3 months after the surgery. Adequate anticoagulation was defined as the use of a guided dose of a novel oral anticoagulant in the group of patients for whom novel oral anticoagulant use was recommended (MV repair or bioprosthetic MV replacement)6 or by a time in the therapeutic range (an international normalized ratio between 1.7 and 3.0) of ≥ 50% as assessed by the Rosendaal method in the warfarin group10. Patients included in the study typically underwent routine follow-up every 3–6 months, and data on all-cause mortality and thromboembolic events were collected through electronic medical record reviews. All clinical events were analyzed by two researchers independently, and the occurrence of thromboembolic events was decided based on the agreement of both researchers. Last survival dates for patients who did not develop the primary outcome were based on the date of the last outpatient visit. The data in support of the results of this study are available from the corresponding author on reasonable request.
Statistical analysis
All continuous data are presented as mean ± standard deviation values, and categorical data are expressed as numbers and percentages for each group. Baseline patients’ clinical and echocardiographic characteristics were analyzed using Student’s t test for continuous variables and the chi-squared (χ2) test for categorical variables. Kaplan–Meier survival curves were constructed based on whether concomitant LAAO was performed or not. To reduce the potential selection biases, a nearest neighbor propensity score (PS) matching method was used through variables including baseline characteristics, imaging data, and surgical data. They included age, male sex, body mass index, hypertension, diabetes mellitus, chronic kidney disease, previous stroke, coronary artery disease, CHA2DS2-VASc score (congestive heart failure, hypertension, age ≥ 75 [doubled], diabetes mellitus, prior stroke or transient ischemic attack [doubled], vascular disease, age 65–74, female), rheumatic heart disease, surgery type, concomitant surgery, Maze, LV systole/diastole diameter, LV ejection fraction, LA volume index, LAA os perimeter, cauliflower LAA shape, adequate anticoagulation, postoperative AF, and follow-up duration. For inverse probability of treatment weighting (IPTW) model, weights for patients with LAAO were the inverse of the PS, and those for patients without LAAO were the inverse of 1-PS. Stabilized weights were used to reduce variability in the IPTW model. Standardized mean differences (SMD) were estimated to assess before and after matching balance. The density plots pre- and post- IPTW were presented in Supplementary Fig. 1. The impact of risk factors on primary outcome and thromboembolic events were analyzed with univariate and multivariate Cox proportional hazard models. A multivariate Cox regression analysis was carried out to identify correlations between several variables and to examine their impact on a clinical outcome simultaneously. The hazard ratios (HR) of the primary outcome and thromboembolic events of the LAAO group compared to the LAA preservation group were exhibited using multivariate Cox regression and PS matched analysis. The variables with a p-value < 0.1 in the univariate Cox regression analysis were included in the multivariate Cox regression analysis as potential predictors of primary outcome and thromboembolic event. Subgroup analyses were performed by tests for the interaction between the two study groups and each subgroup in stratified Cox proportional hazards models without adjustment. All tests were two-sided, and statistical significance was defined as P < 0.05. All statistical analyses were performed with R statistical software (version 3.6.0; R Foundation for Statistical Computing, Vienna, Austria). We employed the following R packages for our analyses: “MatchIt” (version 4.1.0) for PS matching11, “WeightIt” (version 0.14.2) for IPTW, “survminer” (version 0.4.8) for generating Kaplan–Meier survival curves, and “survival” (version 3.2-7) for conducting the Cox proportional hazards regression model.
Results
Baseline characteristics
The mean age of participants was 62.3 ± 12 years, and the mean CHA2DS2-VASc score was 3.3 ± 1.6 points. A total of 114 (48.1%) patients were male, and 29 (12.2%) patients had a history of stroke. Additionally, among the 237 total patients, 98 (41%) underwent concomitant LAAO surgery and 139 (59%) underwent LAA preservation. Table 1 presents baseline clinical characteristics of the study population and group comparisons without or with concomitant LAAO surgery. Those who underwent LAAO were significantly younger, had lower CHA2DS2-VASc scores, and tended to have more rheumatic heart disease than patients in whom the LAA was preserved. There were no differences in surgical characteristics, such as the rate of MV repair and replacement in the two groups, with MV repair accounting for approximately 67% of the total. However, one notable difference between the two groups was that almost all patients (91.8%) in the LAAO group underwent concurrent surgical AF ablation during MV surgery (P < 0.001). Additionally, the proportion of patients undergoing coronary artery bypass grafting (CABG) during MV surgery was lower in the LAAO group (1.0% vs. 7.9%, P = 0.037). One patient in each group who had a thrombus detected in the LAA during surgery.
Table 1.
Baseline clinical characteristics of the study population before and after propensity matching.
| Before propensity score matching | After propensity score matching | |||||||
|---|---|---|---|---|---|---|---|---|
| Without LAAO (n = 139) |
With LAAO (n = 98) |
P value | SMD | Without LAAO (n = 98) |
With LAAO (n = 98) |
P value | SMD | |
| Baseline characteristics | ||||||||
| Age, years | 64.2 ± 12.3 | 59.7 ± 11.0 | 0.004 | -0.38 | 62.3 ± 13.0 | 59.7 ± 11 | 0.124 | -0.38 |
| Male sex, n | 70 (50.4%) | 44 (44.9%) | 0.486 | -0.11 | 46 (46.9%) | 44 (44.9%) | 0.774 | 0.02 |
| Body mass index, kg/m2 | 23.1 ± 3.4 | 23.5 ± 3.1 | 0.452 | 0.1 | 23.2 ± 3.3 | 23.5 ± 3.1 | 0.505 | 0.08 |
| Hypertension, n | 78 (56.1%) | 51 (52.0%) | 0.626 | -0.08 | 45 (45.9%) | 51 (52.4%) | 0.391 | -0.06 |
| Diabetes mellitus, n | 25 (18.0%) | 14 (14.3%) | 0.563 | -0.1 | 17 (17.4%) | 14 (14.3%) | 0.557 | -0.19 |
| Chronic kidney disease, n | 15 (10.8%) | 5 (5.1%) | 0.189 | -0.21 | 8 (8.2%) | 5 (5.1%) | 0.568 | -0.19 |
| Previous stroke, n | 23 (16.5%) | 6 (6.1%) | 0.027 | -0.33 | 9 (9.2%) | 6 (6.1%) | 0.420 | -0.18 |
| Coronary artery disease, n | 24 (17.3%) | 9 (9.2%) | 0.114 | -0.24 | 9 (9.2%) | 9 (9.2%) | > 0.999 | -0.09 |
| CHA2DS2-VASc score, points | 3.5 ± 1.7 | 2.9 ± 1.4 | 0.002 | -0.43 | 3.1 ± 1.5 | 2.9 ± 1.4 | 0.232 | -0.43 |
| Rheumatic heart disease, n | 24 (17.3%) | 28 (28.6%) | 0.056 | 0.27 | 19 (19.4%) | 28 (28.6%) | 0.132 | 0.35 |
| Surgery type | ||||||||
| Bioprosthetic valve, n | 15 (10.8%) | 7 (7.1%) | 0.468 | -0.13 | 8 (8.2%) | 7 (7.1%) | 0.788 | -0.17 |
| Mechanical valve, n | 34 (24.5%) | 23 (23.5%) | 0.983 | -0.02 | 25 (25.5%) | 23 (23.5%) | 0.740 | 0 |
| Mitral valve repair, n | 90 (64.7%) | 68 (69.4%) | 0.544 | 0.1 | 65 (66.3%) | 68 (69.4%) | 0.646 | 0.11 |
| Concomitant surgery | ||||||||
| Aortic valve replacement, n | 22 (15.8%) | 9 (9.2%) | 0.194 | -0.2 | 16 (16.3%) | 9 (9.2%) | 0.134 | -0.13 |
| CABG, n | 11 (7.9%) | 1 (1.0%) | 0.037 | -0.34 | 1 (1.0%) | 1 (1.0%) | > 0.999 | 0 |
| Tricuspid surgery, n | 98 (70.5%) | 69 (70.4%) | > 0.999 | 0 | 67 (68.4%) | 69 (70%) | 0.757 | 0 |
| Maze, n | 20 (14.4%) | 90 (91.8%) | < 0.001 | 2.46 | 17 (17.4%) | 90 (91.8%) | < 0.001 | 2.07 |
| Echocardiographic data | ||||||||
| LV end-systolic diameter, mm | 40.0 ± 8.0 | 39.6 ± 6.5 | 0.651 | -0.04 | 39.3 ± 7.3 | 39.7 ± 6.6 | 0.652 | 0.11 |
| LV end-diastolic diameter, mm | 58.2 ± 8.6 | 58.0 ± 7.6 | 0.874 | -0.05 | 58.0 ± 8.4 | 57.8 ± 7.8 | 0.890 | 0.07 |
| LV ejection fraction, % | 61.0 ± 11.7 | 62.0 ± 9.7 | 0.483 | 0.09 | 62.3 ± 10.2 | 62.0 ± 9.7 | 0.841 | -0.03 |
| LA volume index, mL/m2 | 117.4 ± 67.3 | 107.5 ± 50.3 | 0.198 | -0.17 | 111.9 ± 66.7 | 107.5 ± 50.3 | 0.606 | -0.12 |
| CT data | ||||||||
| LAA os perimeter, mm | 105.0 ± 24.2 | 105.8 ± 21.9 | 0.784 | -0.32 | 105.7 ± 25.4 | 105.8 ± 21.9 | 0.983 | 0.01 |
| Cauliflower LAA Shape, n | 32 (23.0%) | 11 (11.2%) | 0.032 | 0.04 | 14 (14.3%) | 11 (11.2%) | 0.521 | -0.09 |
| Adequate anticoagulation, n | 96 (69.1%) | 59 (60.2%) | 0.203 | 0.33 | 95 (96.9%) | 98 (100%) | 0.246 | 0.25 |
| Postoperative atrial fibrillation, n | 103 (74.1%) | 36 (36.7%) | < 0.001 | -0.81 | 62 (63.3%) | 36 (36.7%) | < 0.001 | -0.55 |
| Follow-up duration, months | 38.7 ± 29.5 | 42.0 ± 22.5 | 0.336 | 0.145 | 36.7 ± 27.3 | 42.0 ± 22.5 | 0.140 | -0.04 |
CABG, coronary artery bypass grafting; LA, left atrial; LAA, left atrial appendage; LAAO, left atrial appendage occlusion; LV, left ventricular; SMD, standardized mean difference
Looking at the echocardiography and CT data, the studied patients had remarkably larger LA size, but there was no statistical difference between the two groups. Moreover, there was no difference in any LAA measure evaluated by CT. Regarding LAA shape, cauliflower-shaped LAAs were less common in the LAAO group compared to the LAA preservation group (11.2% vs. 23.0%, P = 0.032). The rate of postoperative persistent AF was lower in the LAAO group owing to the high rate of Maze operation.
Clinical outcomes
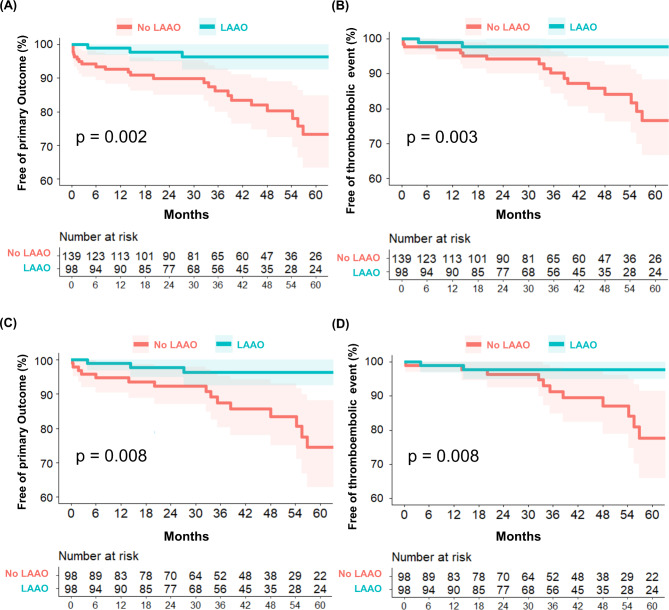
During the follow-up period (median, 37 months; interquartile range, 20–55 months), 29 primary outcomes, 8 all-cause deaths, and 21 thromboembolic events occurred (18 ischemic strokes, 1 embolic peripheral artery occlusion, and 2 intracardiac thrombi). Kaplan–Meier curves demonstrate significantly higher cumulative incidences of the primary outcome (P = 0.002) and thromboembolic events (P = 0.003) in the LAA preservation group than the LAAO group (Fig. 2A and B). In the analysis of 98 PS-matched patients per group, the primary outcomes and thromboembolic events were significantly more common in the LAA preservation group than in the LAAO group (Fig. 2C and D). These results were similar in the IPTW population (Supplementary Fig. 2).
Fig. 2.
Kaplan–Meier curves for the primary and thromboembolic outcome. Kaplan–Meier curves for the primary outcome (A) and thromboembolic outcome (B) stratified by left atrial appendage occlusion vs. preservation into two groups in original cohort. Kaplan–Meier curves for the comparison of the primary outcomes and thromboembolic outcome after propensity score matching (C and D).
In the univariate Cox regression analysis, the presence of coronary artery disease, higher CHA2DS2-VASc scores, CABG, a cauliflower-shaped LAA, Maze, and LAAO were significantly associated with the primary outcome (Table 2). Persistent AF after the MV procedure for more than 3 months after surgery was more often observed in the LAA preservation group than the LAAO group. In the multivariate Cox regression analysis, LAAO was consistently linked to the primary outcome (HR = 0.30, 95% CI = 0.10–0.91, P = 0.033) and thromboembolic events (HR = 0.19, 95% CI = 0.04–0.87, P = 0.032). HRs in the multivariate Cox regression analysis of the primary outcome and thromboembolic events estimated in the original cohort (Model 1) and PS-matched cohort (Model 2) are given in Tables 3 and 4. Patients who underwent surgical LAAO showed significantly lower HRs in both models.
Table 2.
Univariate analysis for the primary outcome and thromboembolic events.
| Primary Outcome | Thromboembolic event | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.02 (0.98–1.05) | 0.326 | 0.99 (0.96–1.04) | 0.935 |
| Male sex | 0.87 (0.42–1.80) | 0.699 | 0.52 (0.21–1.30) | 0.164 |
| Body mass index | 1.03 (0.91–1.15) | 0.686 | 1.09 (0.94–1.25) | 0.257 |
| Hypertension | 1.29 (0.61–2.74) | 0.505 | 1.05 (0.44–2.48) | 0.921 |
| Diabetes mellitus | 1.26 (0.48–3.30) | 0.643 | 0.66 (0.15–2.82) | 0.572 |
| Chronic kidney disease | 1.42 (0.43–4.70) | 0.571 | 1.35 (0.31–5.86) | 0.685 |
| Previous stroke | 1.65 (0.63–4.37) | 0.311 | 0.83 (0.19–3.58) | 0.798 |
| Coronary artery disease | 3.01 (1.37–6.57) | 0.006 | 1.19 (0.39–3.67) | 0.759 |
| CHA 2 DS 2 -VASc score | 1.28 (1.03–1.57) | 0.023 | 1.09 (0.84–1.41) | 0.507 |
| Rheumatic heart disease | 0.74 (0.28–1.93) | 0.532 | 0.84 (0.28–2.49) | 0.750 |
| Bioprosthetic valve | 0.99 (0.23–4.20) | 0.988 | 1.56 (0.36–6.82) | 0.554 |
| Mechanical valve | 0.92 (0.39–2.16) | 0.850 | 1.14 (0.44–2.94) | 0.792 |
| Mitral valve repair | 1.08 (0.49–2.37) | 0.854 | 0.78 (0.32–1.88) | 0.579 |
| Aortic valve replacement | 2.33 (0.99–5.45) | 0.052 | 2.92 (1.13–7.54) | 0.027 |
| CABG | 2.92 (1.00–8.53) | 0.049 | 0.00 (0–Inf) | 0.997 |
| Tricuspid surgery | 0.98 (0.45–2.13) | 0.958 | 0.87 (0.36–2.14) | 0.768 |
| Maze | 0.31 (0.13–0.77) | 0.011 | 0.20 (0.06–0.69) | 0.011 |
| LV end-systolic diameter | 1.03 (0.98–1.08) | 0.208 | 1.02 (0.96–1.08) | 0.585 |
| LV end-diastolic diameter | 1.02 (0.97–1.06) | 0.519 | 1.01 (0.96–1.06) | 0.705 |
| LV ejection fraction | 0.97 (0.94–1.00) | 0.061 | 0.98 (0.94–1.02) | 0.291 |
| LA volume index | 1.00 (0.99–1.01) | 0.603 | 1.00 (0.99–1.01) | 0.470 |
| LAAO | 0.22(0.08–0.63) | 0.005 | 0.14 (0.03–0.62) | 0.009 |
| LAA shape, cauliflower | 2.17 (1.00–4.67) | 0.049 | 3.13 (1.30–7.52) | 0.011 |
| LA volume | 1.00 (0.99–1.00) | 0.752 | 1.00 (0.99–1.00) | 0.378 |
| LAA volume | 1.01 (0.99–1.03) | 0.327 | 1.01 (0.99–1.03) | 0.139 |
| LAA os diameter | 1.00 (0.95–1.05) | 0.970 | 1.02 (0.96–1.07) | 0.577 |
| LAA os perimeter | 1.00 (0.99–1.02) | 0.972 | 1.01 (0.99–1.02) | 0.590 |
| LAA os area | 1.01 (0.94–1.10) | 0.756 | 1.03 (0.94–1.13) | 0.514 |
| Adequate anticoagulation | 0.78 (0.37–1.66) | 0.520 | 1.16 (0.45–2.99) | 0.761 |
| Postoperative AF | 1.97 (0.87–4.45) | 0.103 | 2.41 (0.88–6.59) | 0.086 |
CABG, coronary artery bypass grafting; LV, left ventricular; LA, left atrial; LAA, left atrial appendage; AF, atrial fibrillation; LA, left atrial; LAA, left atrial appendage; LV, left ventricular
Table 3.
Multivariate Cox proportional hazards models for the primary outcome.
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Coronary artery disease | 1.94 (0.62–6.06) | 0.255 | 2.12 (0.60–7.47) | 0.243 |
| CHAD2VASc2 score | 1.16 (0.88–1.53) | 0.283 | 1.11 (0.80–1.52) | 0.537 |
| Aortic valve replacement | 2.63 (1.06–6.56) | 0.038 | 0.82 (0.71–3.90) | 0.804 |
| CABG | 0.98 (0.26–3.63) | 0.972 | 1.00 (0.19–5.34) | 0.996 |
| Cauliflower-shaped LAA | 1.94 (0.90–4.21) | 0.093 | 1.48 (0.86–7.01) | 0.422 |
| LAAO | 0.30 (0.10–0.91) | 0.033 | 0.32 (0.09–0.85) | 0.050 |
CABG, coronary artery bypass grafting; LAA, left atrial appendage; LVEF, left ventricular ejection fraction; LAAO, left atrial appendage occlusion
Model 1: Original cohort
Model 2: Propensity score–matched cohort
Table 4.
Multivariate Cox proportional hazards models for thromboembolic events.
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Aortic valve replacement | 2.67 (1.01–7.11) | 0.048 | 0.91 (0.20–4.18) | 0.908 |
| Cauliflower-shaped LAA | 2.45 (1.02–5.87) | 0.045 | 1.77 (0.60–5.21) | 0.297 |
| Postoperative AF | 1.24 (0.42–3.66) | 0.694 | 1.92 (0.59–6.28) | 0.279 |
| LAAO | 0.19 (0.04–0.87) | 0.032 | 0.21 (0.05–0.99) | 0.049 |
AF, atrial fibrillation; LAA, left atrial appendage; LAAO, left atrial appendage occlusion
Model 1: Original cohort
Model 2: Propensity score–matched cohort
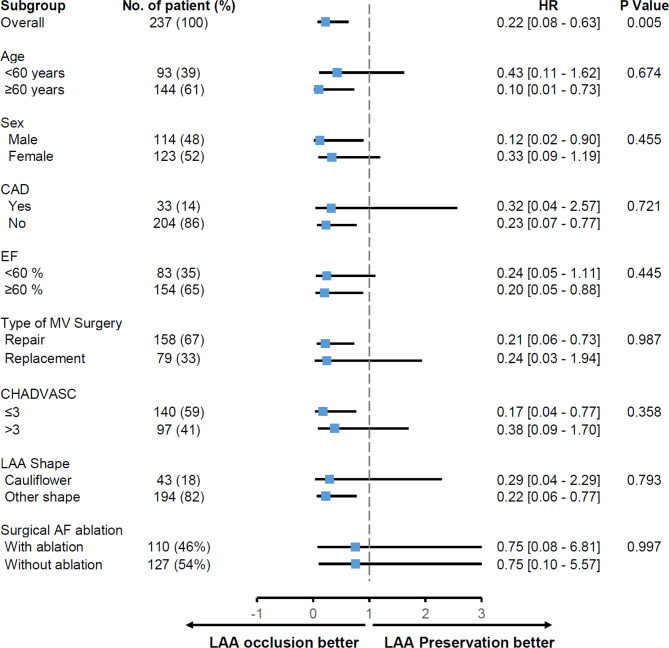
Results for the primary outcome were generally similar in most subgroups. The beneficial effects of LAAO were consistent across demographic characteristics, baseline echocardiographic and CT features, CHA2DS2-VASc score, surgical AF ablation, and type of MV surgery (Fig. 3). In patients who had undergone MV repair, LAAO was also associated with a lower risk of the primary outcome compared to LAA preservation (Supplementary Fig. 3). Postoperative bleeding occurred in two (2.1%) patients in the LAAO group and six (4.3%) patients in the LAA preservation group (P = 0.571) and was not related to LAAO/LAA preservation or the primary outcome.
Fig. 3.
Subgroup analysis. Risk of primary outcomes associated with left atrial appendage occlusion or preservation in the subgroups. CAD, coronary artery disease; EF, ejection fraction; MV, mitral valve; LAA, left atrial appendage; AF, atrial fibrillation.
Discussion
The principal findings of the study are as follows: first, concomitant LAAO in patients with AF undergoing MV surgery due to chronic mitral regurgitation is associated with a reduced risk of thromboembolic events. The benefits from concomitant LAAO during MV surgery were consistent even after multivariate adjustment analysis, PS matching, and IPTW analysis. Second, in real clinical practice, concomitant LAA surgery in patients with mitral regurgitation is primarily performed in conjunction with the Maze operation, particularly in young patients who are not undergoing additional combined procedures such as CABG. Third, in AF patients undergoing MV surgery, future thromboembolic events were associated with clinical risk factors, a cauliflower-shaped LAA, and the absence of LAAO. These results suggest that concomitant LAAO or exclusion is helpful in patients who underwent MV surgery with MR, and the decision about surgery needs to be made especially by considering the structural and functional features of the LAA.
An early study of LAA obliteration in patients who underwent MV replacement reported that LAA ligation during MV surgery is associated with a reduction in the risk of late embolism12. However, results of subsequent studies have been controversial13,14. One retrospective study of patients with rheumatic MV disease documented a trend suggesting that LAAO may reduce death and thromboembolism when surgical AF ablation was not performed, but this trend was attenuated when surgical AF ablation was performed15. The LAAOS III was the first large-scale, randomized controlled trial to evaluate the efficacy and safety of concomitant LAAO in patients with AF who underwent cardiac surgery. In this trial, patients who underwent MV surgery totaled about 36%. Although the study did not conduct a comparison of the primary endpoint (ischemic stroke and systemic embolism) in the MV procedure group, the LAAO group had a statistically significant benefit over the non-LAAO group in the subgroup of patients who underwent all kinds of valve surgery11.
Compared to previous studies, the study population of this study shows different characteristics. This study targeted only patients with AF among those who underwent surgery for chronic MR. Although our subjects were younger than participants in the LAAOS III trial, they showed a high rate of prior ischemic stroke. Looking at the LA and LAA remodeling as characteristic information of this study, both LA volume indices measured by echocardiography and CT were significantly larger than normal values16,17, and LAAs were also remarkably enlarged. This may partly explain the greater rate of thromboembolic events (8.9%) in our study compared to rates in previous retrospective studies and its similarity to that of the LAAOS III trial; the higher rate of these events may also have contributed to a statistically significant benefit of concomitant LAAO.
Significant MR is commonly associated with left atrial (LA) remodeling and AF; however, MR has been thought to have a protective effect on thrombotic risk18,19. This is because the directed MR jet reduces the flow stasis in the LA and LAA with a washout effect. Therefore, despite a markedly dilated LA and LAA in patients with severe MR, the profoundly impaired LAA filling with flow stasis seen in patients with mitral stenosis is uncommon20. Theoretically, if MV surgery was performed in patients with severe MR and preoperative AF, the protective effect against blood flow stasis in LA may be abolished, and the thromboembolic risk would probably persist or even increase because relevant risk factors are still present. Furthermore, after mitral repair or replacement, the MV pressure gradient may be elevated in some patients, which may exacerbate the LA flow stasis and thromboembolic risk. This is more pronounced in patients who have undergone TEER for severe MR, which is now widely performed. It is reported that more than one-third of patients develop new spontaneous echo contrast (SEC) after TEER21. These newly developed SECs are known to be associated with a successful reduction of severe MR. Although the relationship between new SECs and thromboembolic risk is unclear, there have been recent reports of simultaneous percutaneous LAAO and mitral TEER in patients at high embolic risk22–24. Our subgroup analysis for MV repair also supported this treatment strategy, as the benefit of LAAO in the primary outcome was maintained in patients who had undergone MV repair. In our analysis, the traditional risk factors of thromboembolism, including old age, previous ischemic stroke, higher CHA2DS2-VASc score, and postoperative AF rhythm, were not significantly related to the primary event. These findings are similar to those of previous studies15,25. Consequently, it can be inferred that the traditional risk score represented by the CHA2DS2-VASc score may not suffice to predict thromboembolic risk in patients undergoing cardiac surgery.
This study has several limitations due to it being a single-center retrospective study. First, because of the retrospective nature of this study, it is subject to selection bias. To address this issue, PS matching and IPTW were utilized; however, complete balance across all variables was not achieved, leaving inherent limitations. Nevertheless, we believe our study novel as we explored the efficacy of LAAO in a distinctive patient cohort characterized by chronic severe MR and significant cardiac remodeling. Second, in our study, patients who had undergone LAA preservation had unexpectedly higher CHA2DS2-VASc scores, more often had a history of stroke, and more often had cauliflower-shaped LAAs. This means that the LAAO surgery was performed based on the surgeon’s preference without considering the thromboembolic risk. To overcome this bias in baseline characteristics, we performed PS matching and IPTW analyses and demonstrated that LAAO was associated with a lower thromboembolic risk in these analyses as well. Third, 92% of the LAAO group underwent the Maze operation concomitantly, while only 14% of the LAA preservation group underwent the Maze operation. This makes it impossible to analyze whether the reduction in thromboembolic risk is related to Maze or LAAO because there was multicollinearity between the Maze operation and LAAO. To address this, we tried to reduce the imbalance between the two groups regarding the Maze by incorporating it into both PS matching and IPTW. In addition, we performed a survival analysis by including the postoperative AF, the primary target of the Maze procedure, as a variable. Consequently, LAAO showed a significant association with the primary outcome, whereas postoperative AF did not. Therefore, it can be speculated that LAAO may be more relevant to the primary outcome than the reduction in posterior AF with Maze. Further studies are needed to confirm this. Fourth, due to the limitations of a retrospective study, data on family history, smoking status or alcohol consumption, which may have influenced the primary outcome, were not collected and could not be included in the analyses. Finally, while all LAAO cases were confirmed successful via intraoperative transesophageal echocardiography, they have not been reassessed during the follow-up period.
For patients undergoing surgery for MR who also have AF, concomitant LAAO is advantageous in preventing thromboembolic events following MV surgery. Further large-scale prospective studies are needed to determine the independent beneficial effect of LAAO in patients who underwent MV surgery.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Author contributions
J.S. and H.-J.L.: study design, data acquisition, data analysis, data interpretation, and manuscript drafting and review; I.C., Y.J.S., S.-H.L., S.L., G.-R.H., J.-W.H.: data acquisition, interpretation, and manuscript review; Y.J.K. and C.Y.S.: study concept and design, data analysis, data interpretation, manuscript review and revision, and study supervision. All authors reviewed the manuscript.
Funding
This study was supported by a faculty research grant of Yonsei University College of Medicine (6-2021‐0096) and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (grant number HI22C0154).
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at Yonsei University College of Medicine.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiwon Seo and Hee-Jung Lee have contributed equally.
Contributor Information
Young Jin Kim, Email: dryj@yuhs.ac.
Chi Young Shim, Email: cysprs@yuhs.ac.
References
- 1.Essayagh, B. et al. Prognostic implications of left atrial enlargement in degenerative mitral regurgitation. J. Am. Coll. Cardiol.74(7), 858–870 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Thomas, K. L. et al. Prevalence, characteristics, and outcomes of Valvular Heart Disease in patients with Atrial Fibrillation: Insights from the ORBIT-AF (outcomes Registry for Better Informed Treatment for Atrial Fibrillation). J. Am. Heart Assoc.6(12). (2017). [DOI] [PMC free article] [PubMed]
- 3.Melduni, R. et al. Risk of left atrial appendage thrombus and stroke in patients with atrial fibrillation and mitral regurgitation. Heart. 108(1), 29–36 (2022). [DOI] [PubMed] [Google Scholar]
- 4.Kim, H. D. et al. Left atrial dysfunction, fibrosis and the risk of thromboembolism in patients with paroxysmal and persistent Atrial Fibrillation. Int. J. Heart Fail.4(1), 42–53 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitlock, R. P. et al. Left atrial appendage occlusion during cardiac surgery to prevent stroke. N Engl. J. Med.384(22), 2081–2091 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Joglar, J. A. et al. 2023 ACC/AHA/ACCP/HRS Guideline for the diagnosis and management of Atrial Fibrillation: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice guidelines. Circulation. 149(1), e1–e156 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inciardi, R. M. et al. Mitral regurgitation, left atrial structural and functional remodelling and the effect on pulmonary haemodynamics. Eur. J. Heart Fail.22(3), 499–506 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Mitchell, C. et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 32(1), 1–64 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Di Biase, L. et al. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J. Am. Coll. Cardiol.60(6), 531–538 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Rosendaal, F. R., Cannegieter, S. C., van der Meer, F. J. & Briet, E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb. Haemost. 69(3), 236–239 (1993). [PubMed] [Google Scholar]
- 11.Zhang, Z. Propensity score method: A non-parametric technique to reduce model dependence. Ann. Transl Med.5(1), 7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Fernandez, M. A. et al. Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: A transesophageal echocardiographic study. J. Am. Coll. Cardiol.42(7), 1253–1258 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Min, X. P., Zhu, T. Y., Han, J., Li, Y. & Meng, X. Left atrial appendage obliteration in atrial fibrillation patients undergoing bioprosthetic mitral valve replacement. Herz. 41(1), 87–94 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Almahameed, S. T. et al. Left atrial appendage exclusion and the risk of thromboembolic events following mitral valve surgery. J. Cardiovasc. Electrophysiol.18(4), 364–366 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Kim, W. K. et al. Exclusion versus preservation of the left atrial appendage in rheumatic mitral valve surgery. Heart. 106(23), 1839–1846 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Singh, A. et al. Normal values of left atrial size and function and the impact of age: Results of the World Alliance societies of Echocardiography Study. J. Am. Soc. Echocardiogr. 35(2), 154–164 (2022). e3. [DOI] [PubMed] [Google Scholar]
- 17.Sun, B. J. et al. Normal reference values for left atrial strain and its determinants from a large Korean Multicenter Registry. J. Cardiovasc. Imaging. 28(3), 186–198 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Laer, S. L. et al. Effect of mitral regurgitation on thrombotic risk in patients with nonrheumatic atrial fibrillation: A New CHA(2)DS(2)-VASc score risk modifier? Am. J. Cardiol.145, 69–76 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Kranidis, A. et al. Mitral regurgitation protects from left atrial thrombogenesis in patients with mitral valve disease and atrial fibrillation. Pacing Clin. Electrophysiol.23(11 Pt 2), 1863–1866 (2000). [DOI] [PubMed] [Google Scholar]
- 20.Ha, J. W. et al. Assessment of left atrial appendage filling pattern by using intravenous administration of microbubbles: Comparison between mitral stenosis and mitral regurgitation. J. Am. Soc. Echocardiogr. 14(11), 1100–1106 (2001). [DOI] [PubMed] [Google Scholar]
- 21.Sato, H. et al. Significance of spontaneous echocardiographic contrast in transcatheter edge-to-edge repair for mitral regurgitation. J. Am. Soc. Echocardiogr. 36(1), 87–95 (2023). [DOI] [PubMed] [Google Scholar]
- 22.Belli, M. et al. Combined MitraClip and left atrial appendage occlusion: Is it still a Utopia? Front. Cardiovasc. Med.9, 940560 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuwata, S. et al. Feasibility of concomitant MitraClip and left atrial appendage occlusion. EuroIntervention. 12(16), 1940–1945 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Tichelbacker, T. et al. MitraClip(R) and amplatzer(R) cardiac plug implantation in a single procedure: A reasonable approach? Int. J. Cardiol.220, 107–111 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Song, K., Jang, W. S., Park, N., Kim, Y. S. & Kim, J. B. Is it safe to preserve left atrial appendage during maze procedure? Korean Circ. J.53(8), 566–577 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at Yonsei University College of Medicine.