Abstract
Background:
Biomarkers of eosinophilic disease activity, especially in the context of novel therapies that reduce blood eosinophil counts, are an unmet need. Absolute eosinophil count (AEC) does not accurately reflect tissue eosinophilia or eosinophil activation. Therefore, the aims of this study were to compare the reliability of plasma and urine eosinophil major basic protein 1, eosinophil cationic protein, eosinophil-derived neurotoxin (EDN), and eosinophil peroxidase measurement and to evaluate the usefulness of eosinophil granule protein (EGP) measurement for the assessment of disease activity in patients with eosinophil-associated diseases treated with mepolizumab, benralizumab, or dexpramipexole.
Methods:
Eosinophil granule protein concentrations were measured in serum, plasma, and urine from healthy volunteers and patients with hypereosinophilic syndrome (HES), eosinophilic granulomatosis with polyangiitis (EGPA), and eosinophilic asthma using a multiplex assay.
Results:
Urine EGP concentrations remained stable, whereas serum and plasma EGP concentrations increased significantly with delayed processing. Plasma (p) EDN, but not urine (u) EDN, concentration correlated with AEC and negatively correlated with prednisone dose. Both pEDN and uEDN decreased significantly following treatment of HES patients with benralizumab and EGPA patients with mepolizumab. uEDN appeared to increase with clinical relapse in both patient groups.
Conclusions:
Measurement of EGP in urine is noninvasive and unaffected by cellular lysis. Although plasma and urine EDN concentrations showed a similar pattern following benralizumab and mepolizumab treatment, the lack of correlation between AEC or prednisone dose and uEDN concentrations suggests that measurement of uEDN may provide a potential biomarker of disease activity in patients with HES and EGPA.
Keywords: benralizumab, eosinophil granule protein, eosinophilia, hypereosinophilic syndrome, mepolizumab
Graphical Abstract
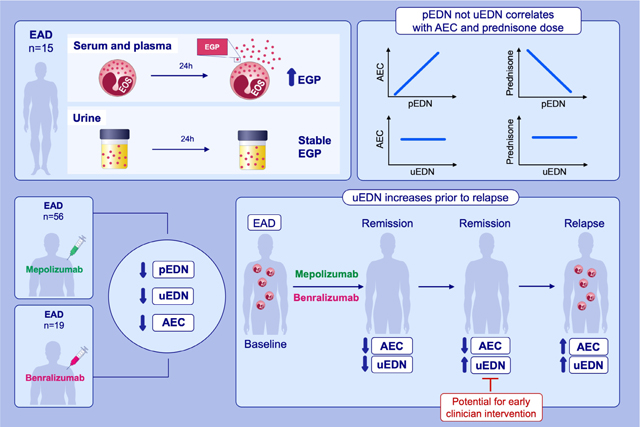
This study compares the reliability and utility of plasma and urine EGP measurements for the assessment of disease activity across a diverse group of EAD and evaluates the usefulness of EGP measurements for the assessment of disease activity in patients with EAD treated with targeted therapeutics.Urine EDN is a stable measure of eosinophilic disease activity that does not correlate with aboslule eosinophil count or prednisone dose. uEDN decreases in response to treatment with mepolizumab and benralizumab but increases prior to AEC and the onset of clinical symptoms in most EAD patients who relapse.
Abbreviations: AEC, absolute eosinophil count; EAD, eosinophil-associated diseases; EDN, eosinophil-derived neurotoxin; EGP, eosinophil granule proteins; pEDN, plasma EDN; uEDN, urine EDN
1 |. INTRODUCTION
Although peripheral blood absolute eosinophil count (AEC) has been explored as a biomarker of disease activity in eosinophil-associated diseases (EAD),1,2 the NIH Taskforce on the Research Needs of Eosinophil-Associated Diseases (TREAD) indicated in 2012, and again in 2018, that a standardized method to identify reliable noninvasive markers of eosinophilic activity remains an unmet research and clinical need.3,4 This has been especially problematic in the context of clinical trials of eosinophil-targeted therapies that reduce AEC with variable effects on tissue eosinophilia and clinical symptoms.5,6 Even in the absence of therapy, AEC may not accurately reflect organ involvement in EAD.7 For example, many patients with biopsy-documented eosinophilic esophagitis (EoE) have normal AEC despite dramatic tissue eosinophilia and clinical symptoms.8
Eosinophil granules contain many mediators, including the highly cationic eosinophil granule proteins (EGP): eosinophil major basic protein 1 (EMBP1), eosinophil cationic protein (ECP), eosinophil-derived neurotoxin (EDN), and eosinophil peroxidase (EPO).2,9–12 Whereas EPO is highly specific to eosinophils, EMBP1, ECP, and EDN can be found, albeit in lesser amounts, in other cell lineages.13–15 Since the first description of elevated serum EMBP1 concentrations in eosinophilic patients in 1981,16 EGP concentrations have been documented in blood, tissue, and a wide variety of biological fluids, including urine.17–28 Moreover, numerous studies have demonstrated an association between elevated EGP and EGP reaction product concentrations and disease activity in patients with EAD, including eosinophilic esophagitis,29–31 atopic dermatitis,32–34 eosinophilic granulomatosis with polyangiitis (EGPA),35 asthma,36,37 and hypereosinophilic syndrome (HES).38 In some cases, elevated blood and/or urine EGP concentrations have been documented in symptomatic patients with tissue eosinophilia but normal peripheral blood AEC, including patients with eosinophilic esophagitis38 and asthma.39 More recently, blood EGP concentrations have been used to support clinical trial endpoints in EAD, including HES,40 EGPA,41 and asthma,42,43 although the utility of this approach in patients with dramatic changes in AEC has not been systematically examined.
Despite these promising results, serum and plasma EGP concentrations can be falsely elevated in the setting of eosinophil lysis, particularly in patients with high AECs, and measurement of EGP in tissue typically requires invasive procedures. In this context, urine EGP measurements provide a potential noninvasive alternative. Although urine concentrations of EGP have been reported to correlate with disease severity and activity in patients with atopic dermatitis, asthma, onchocerciasis and HES,30,32– 34,44–53 urine EGP have yet to be evaluated as a biomarker of disease activity in the setting of eosinophil-targeted therapies, and studies comparing concomitant plasma and urine EGP concentrations in patients with EAD are few.54,55 The aims of the present study were (1) to compare the reliability and utility of plasma and urine EGP measurements for the assessment of disease activity across a diverse group of EAD and (2) to evaluate the usefulness of EGP measurements for the assessment of disease activity in patients with EAD treated with targeted therapeutics (mepolizumab, benralizumab, or dexpramipexole) that dramatically deplete blood and tissue eosinophils.
2 |. MATERIALS AND METHODS
2.1 |. Study populations
Eosinophilic patients in the current study were enrolled on the following Institutional Review Board (IRB)-approved trials: (1) a longitudinal study of HES (N = 45, NCT00001406); (2) the mechanistic substudy of a multicenter placebo-controlled phase 3 study of mepolizumab in patients with relapsing or refractory EGPA (N = 56, NCT02020889); (3) a single-center placebo-controlled phase 2 trial of benralizumab in patients with HES (N = 19, NCT02130882), and (4) a multicenter placebo-controlled study of dexpramipexole in patients with eosinophilic asthma (N = 99, NCT04046939). Healthy volunteers (HV) were enrolled on a protocol designed to provide clinical samples for research (N = 38, NCT00090662) (Tables S1 and S2). All participants signed written informed consent. For the mepolizumab, benralizumab, and dexpramipexole trials, plasma and/or urine samples were collected at predefined study time points for biomarker discovery (Figure S1). Samples were collected at a single visit on the HES natural history and healthy volunteer protocols. A CONSORT flow diagram included in the supplement shows the patients included and the samples analyzed for each study (Figure S2).
2.2 |. Sample collection and processing
For plasma and serum samples, blood was collected into Vacutainer K2 EDTA and SST blood collection tubes (BD, Franklin Lakes, NJ), respectively, and centrifuged (1300 × g for 10 min) at room temperature (RT) within 30 min of venipuncture unless otherwise stated. Clean catch urine samples were centrifuged (1000 × g for 10 min at 4°C) within 30 min of collection unless otherwise stated for HV and patients on the mepolizumab and benralizumab studies. For patients on the dexpramipexole study, clean catch urine samples were collected and shipped overnight at RT prior to centrifugation (1000 × g for 10 min at 4°C). All samples were stored at −80°C.
2.3 |. Multiplex assay
Plasma, serum, and urine EGP concentrations were measured simultaneously by multiplex assay as previously described.38 Briefly, samples were reduced and alkylated to prevent aggregation, as described in the supplement. Purified EGP standards were diluted in assay buffer (1× PBS, 1% BSA, and 0.05% Tween-20) to an initial concentration of 500 ng/ml each, and then serially diluted 1:3. Samples were diluted 1:220 in assay buffer for all assays except urine EMBP1, ECP, and EPO, which were diluted 1:8.8 in assay buffer. Assay results are reported as concentrations calculated from the standard curve using mean fluorescent intensity (MFI) from duplicate samples. Plasma samples with concentrations below the lower limit of detection for the assay were assigned values equivalent to the lower limit of detection: 2.2 ng/ml for EMBP1, ECP, and EDN, and 0.47 ng/ml for EPO. To normalize for the variability in urine concentration between samples, urine creatinine was measured for each sample using a creatinine (Cr) urinary detection kit (ThermoFisher, Waltham, MA). Urine samples with concentrations below the lower limit of detection for the assay were assigned values equivalent to 2.2 ng/mg Cr for EMBP1, ECP, and EDN, and 0.47 ng/mg Cr for EPO.
2.4 |. Statistical analysis
The Mann– Whitney test was used for comparison of two groups (Figures 1, 3, and S5) and the Spearman rank test for correlations (Figures 4, S3, and S6). Repeated measures were analyzed using the Friedman test with Dunn’s adjustment for comparing each day to baseline (Figure 5) or using a Wilcoxon signed rank test when only two time points and two groups were being compared (Figure 1). The Kruskal–Wallis test with Dunn’s adjustment for all pairwise multiple comparisons was used for comparing more than two groups (Figures 2 and S4). Geometric means summarize central tendencies, appropriate for the log scale or ratio scale axes. A p-value ≤ 0.05 was considered statistically significant for all tests. Methods for statistical modeling are described in the supplement.
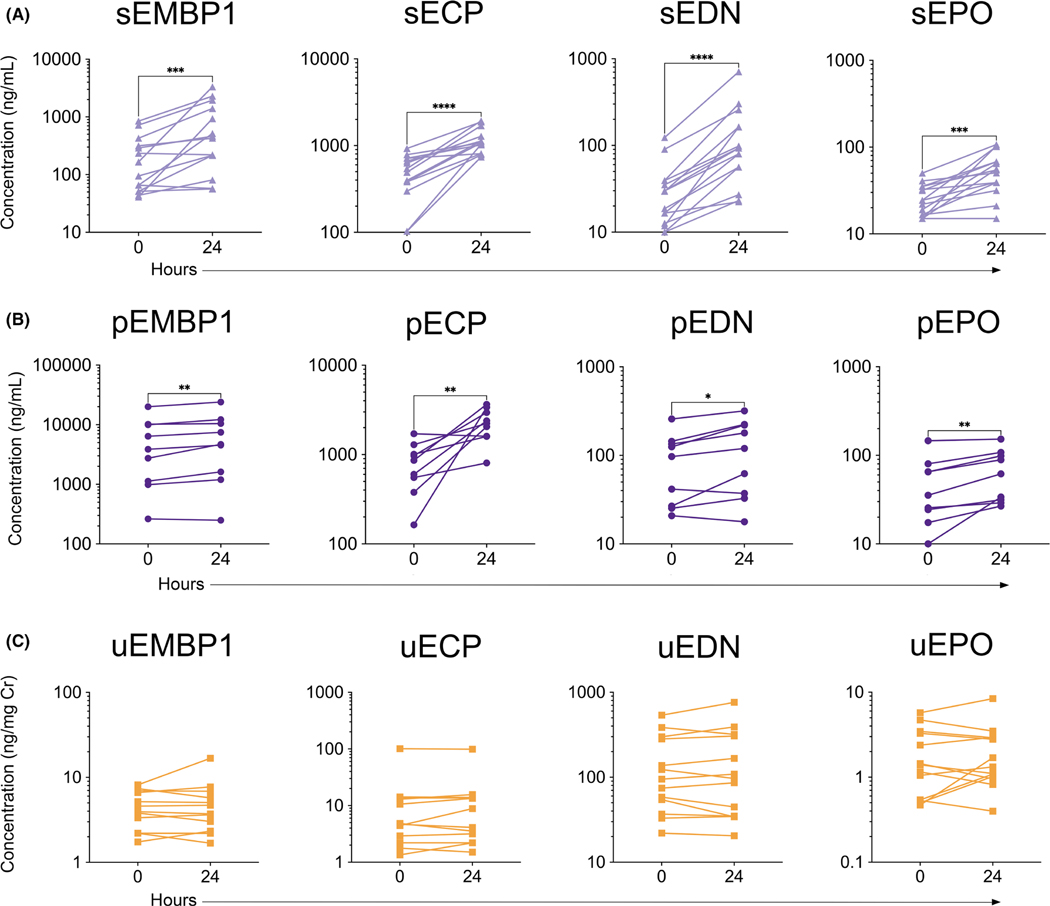
FIGURE 1.
Serum and plasma concentrations of EGP significantly increase, and urine concentrations of EGP remain stable after delayed processing. EMBP1, ECP, EDN, and EPO levels were assessed by multiplex from (A) serum (N = 15, ***p < 0.001, ****p < 0.0001), (B) plasma (N = 9, *p < 0.05, **p < 0.01), and (C) urine (N = 13) from HES patients that were processed within 30 min of collection (0 h) and after resting at room temperature for 24 h
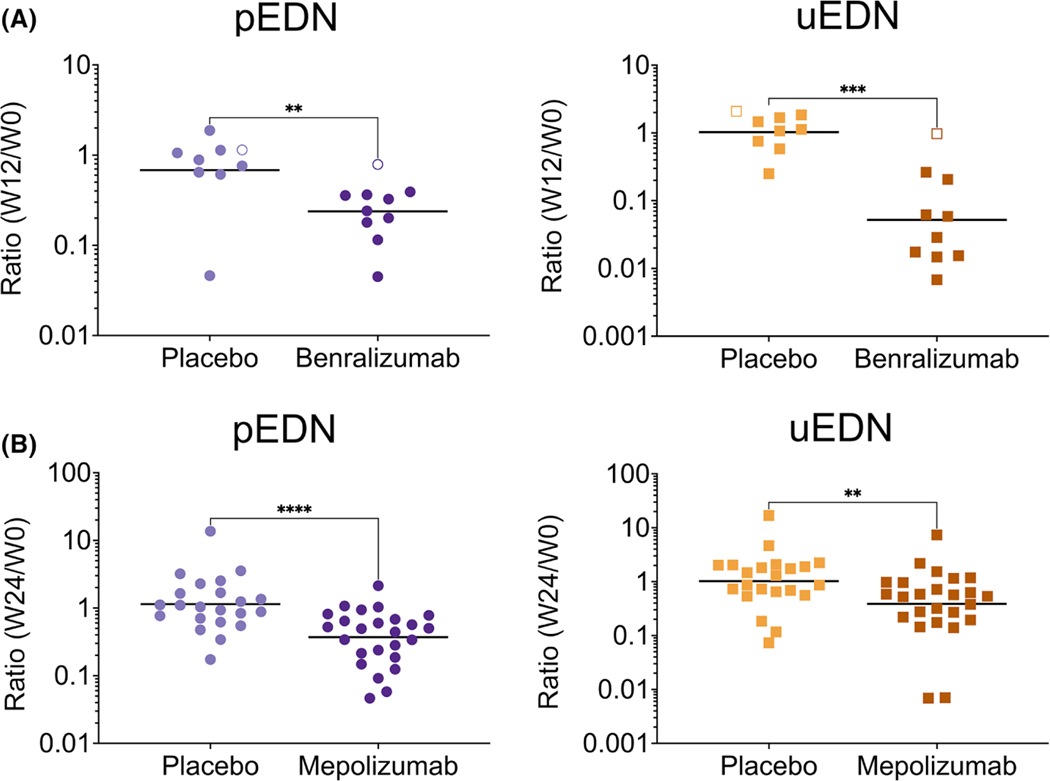
FIGURE 3.
The plasma ratio and uEDN ratio significantly decrease in response to benralizumab and mepolizumab compared to placebo. The EDN ratio was assessed from plasma (**p < 0.01, ****p < 0.0001) and urine (**p < 0.01, ***p < 0.001) collected from (A) PDGFRA-negative HES patients at baseline and week 12 receiving placebo (N = 9), or benralizumab (N = 10), the open symbols represent nonresponders, and (B) EGPA patients at baseline and week 24 receiving placebo (N = 22) or mepolizumab (N = 25)
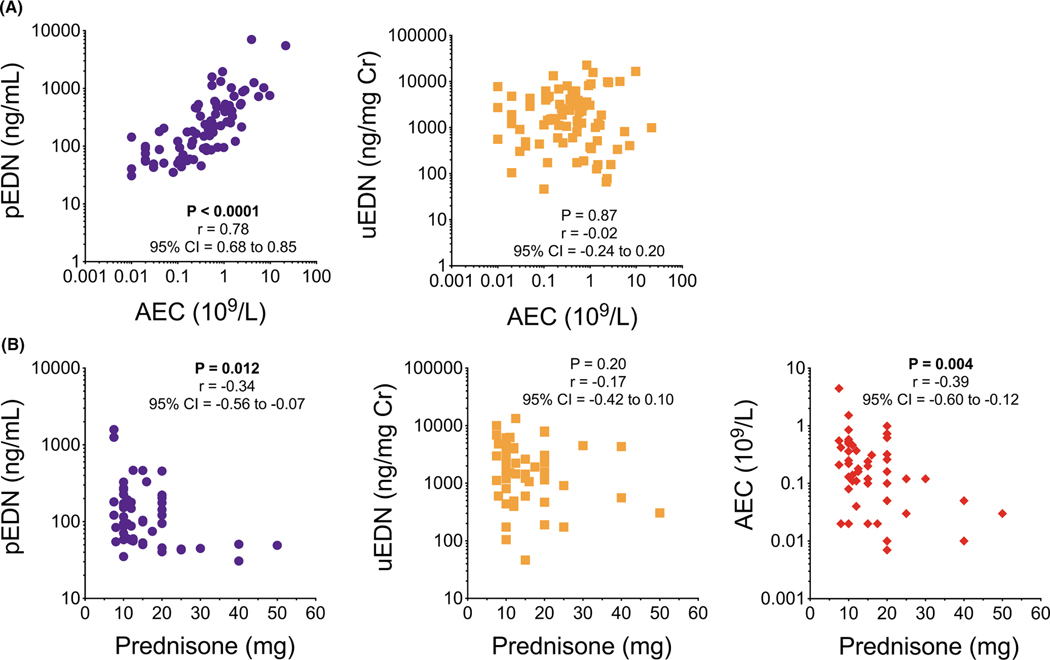
FIGURE 4.
Plasma, but not urine, EDN concentrations correlate with AEC and prednisone dose. (A) Correlation of pEDN or uEDN with AEC collected from EAD patients at baseline (N = 83). (B) Correlation of pEDN, uEDN, or AEC with prednisone dose collected from EGPA patients at baseline (N = 56)
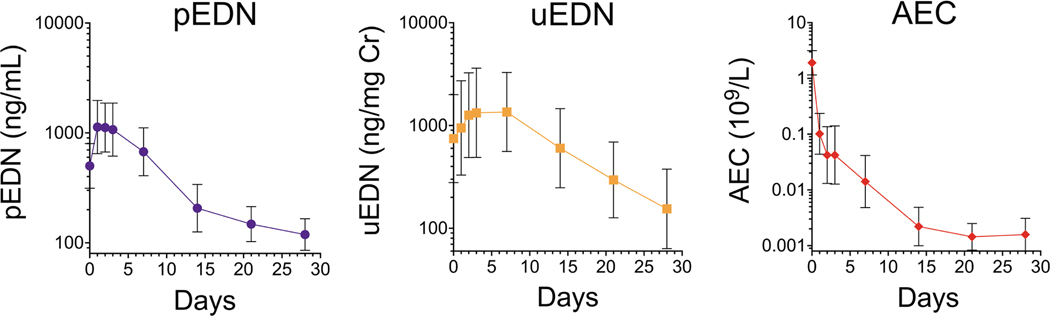
FIGURE 5.
Plasma EDN concentrations rise to a peak after single dose of benralizumab that is followed by a reduction significantly below baseline. The same pattern is seen for urine EDN levels but delayed. AEC decreases steadily after one dose of benralizumab. pEDN levels, uEDN levels and AEC were compared between each time point, with adjustment for multiple comparisons, after a single dose of benralizumab indicated by the geometric mean of patients with PDGFRA-negative HES (N = 17, nonresponders excluded), with error bars specifying the 95% confidence interval (CI)
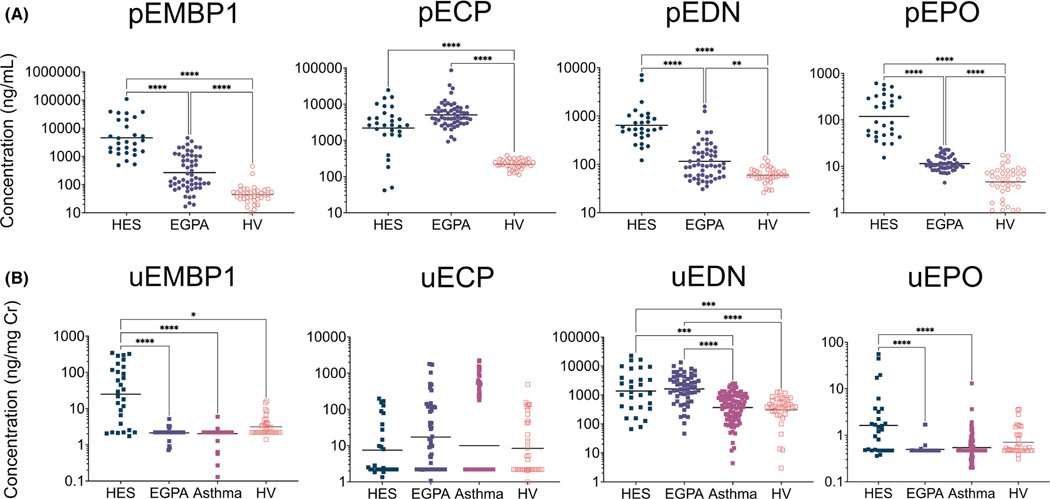
FIGURE 2.
Patients with EAD have elevated concentrations of plasma and urine EGP. (A) Plasma EGP concentrations (**p < 0.01, ***p < 0.001, ****p < 0.0001) were compared between patients with HES (N = 29), EGPA (N = 56), and HV (N = 38), with adjustment for multiple comparisons. (B) Urine EGP concentrations (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001) were compared between patients with HES (N = 29), EGPA (N = 56), eosinophilic asthma (N = 99), and HV (N = 38), with adjustment for multiple comparisons
3 |. RESULTS
3.1 |. EGP measurement stability
To assess the effect of delayed sample processing on EGP concentration, blood was drawn from patients with HES and processed within 30 min of collection (0 h) or allowed to sit at RT for 24 h before processing. Serum and plasma EGP concentrations were significantly increased for all four EGP in samples processed at 24 h compared with those processed within 30 min (Figure 1A,B). In contrast, urine EGP concentrations remained stable irrespective of processing time (Figure 1C). If the increased EGP concentrations in the samples processed after 24 h were a function of eosinophil lysis and degranulation in the blood prior to measurement, then the difference between EGP measured at 0 and 24 h would be expected to correlate with the initial AEC. The AEC measured at 0 h correlated with changes in sEMBP1 (r = 0.58, p < 0.05), sEDN (r = 0.83, p < 0.001), and sEPO (r = 0.59, p < 0.05) (Figure S3).
3.2 |. Both pEDN and uEDN concentrations decrease with benralizumab and mepolizumab treatment
The geometric mean (GM) plasma concentrations of all four EGP were increased in patients with HES (n = 29) compared with those in HV (n = 38) (4564 vs. 44 ng/mL for pEMBP1, 2190 vs. 219 ng/ml for pECP, 642 vs. 60 ng/ml for pEDN, and 118 vs. 5 ng/ml for pEPO; p < 0.001 for all comparisons) (Figure 2A). Similarly, GM plasma concentrations of all four EGP were increased in EGPA patients (n = 56) compared with HV (265 vs. 44 ng/ml for pEMBP1, p < 0.0001; 5061 vs. 219 ng/ml for pECP, p < 0.0001; 116 vs. 60 ng/ml for pEDN, p < 0.01; and 11 vs. 5 ng/ml for pEPO p < 0.0001). Notably, GM plasma concentrations of EMBP1, EDN, and EPO were significantly higher in HES patients than in EGPA patients (4564 vs. 265 ng/ml for pEMBP1, 642 vs. 116 ng/ml for pEDN, and 118 vs. 11 ng/ml for pEPO; p < 0.0001 for all comparisons).
Overall, EMBP1 and ECP were detected in higher concentrations in plasma than the more eosinophil-specific EDN and EPO. In contrast, EDN was the predominant EGP measurable in urine with measurable concentrations detected in all study participants and substantially higher GM concentrations than those for uEMBP1, uECP, and uEPO (Figure 2B). Concentrations of uEDN were similar in patients with HES and EGPA and significantly higher in both groups than in patients with eosinophilic asthma and HV (1370 ng/mg Cr vs. 370 ng/mg Cr, p < 0.001; and 1370 ng/mg Cr vs. 305 ng/mg Cr, p < 0.001, respectively, for HES and 1618 ng/mg Cr vs. 370 ng/mg Cr, p < 0.0001; and 1618 ng/mg Cr vs. 305 ng/mg Cr, p < 0.0001, respectively, for EGPA). Of note, GM uEMBP1 and uEPO concentrations were significantly increased in HES patients compared with patients with EGPA or eosinophilic asthma. HES patients had significantly higher AEC than eosinophilic asthma and EGPA patients and HV (p < 0.0001 for all comparisons) (Figure S4). Although eosinophilic asthma patients had elevated AEC compared with EGPA patients and HV (p < 0.0001 for both comparisons), none of the eosinophilic asthma patients had hypereosinophilia (AEC ≥ 1.5 × 109/L).
To determine the effect of eosinophil-targeted therapy on plasma and urine EDN concentrations, urine and plasma samples were collected at baseline and at the primary endpoint visit from patients enrolled on placebo-controlled clinical trials of benralizumab (n = 19), mepolizumab (n = 56) and dexpramipexole (n = 99). Nine of the 10 patients with HES who received benralizumab had a significant reduction in AEC (>50%) at week (W)12 compared to three of nine evaluable patients who received placebo.56 Patients receiving benralizumab experienced a significant reduction in the GM pEDN and uEDN ratios (W12/W0) compared with patients receiving placebo (0.24 vs. 0.68, p < 0.01 and 0.05 vs. 1.03, p < 0.001, respectively; Figure 3A and Table S3). Similarly, in the mechanistic substudy of the mepolizumab trial, patients with EGPA randomized to mepolizumab had a significant decrease in the GM AEC, pEDN and uEDN ratios (W24/W0) compared with patients who received placebo (AEC: 0.13 vs. 1.41, p < 0.0001, Figure S5; pEDN: 0.37 vs. 1.14, p < 0.0001 and uEDN: 0.39 vs. 1.02, p < 0.01; Figure 3B and Table S3). Dexpramipexole did not cause a change in GM uEDN concentrations from week 0 to week 12 (Table S4), and the AEC ratio (W12/W0) did not correlate with the uEDN ratio (W12/W0) for any arm of the study (placebo and dexpramipexole doses of 37.5 mg twice daily [BID], 75 mg BID, and 150 mg BID; Figure S6).
3.3 |. pEDN, but not uEDN, correlates with AEC and prednisone dose
To determine whether the observed decrease in plasma and urine EDN in patients treated with benralizumab and mepolizumab could be accounted for solely by the decrease in AEC, baseline pEDN and uEDN concentrations for the 83 participants were examined in the context of the concomitant AEC. Plasma, but not urine, concentrations of EDN correlated with AEC (r = 0.78, p < 0.0001 and r = −0.02, p = 0.870, respectively; Figure 4A). Since glucocorticoid therapy can affect eosinophil activation, migration, and survival, and all 56 of the patients with EGPA enrolled on the mepolizumab trial were receiving 7.5–50 mg prednisone/prednisolone at baseline, pEDN and uEDN concentrations were also assessed as a function of glucocorticoid dose. Glucocorticoid dose was negatively correlated with pEDN (r = −0.34, p = 0.012) and AEC (r = −0.39, p = 0.004), but not with uEDN (r = −0.17, p = 0.202) (Figure 4B).
3.4 |. Rise in pEDN precedes a peak rise in uEDN after one dose of benralizumab
To understand the kinetic effect of benralizumab on EDN concentrations in the blood and urine, samples were analyzed at days 0, 1, 2, 3, 7, 14, 21, and 28 after a single dose of benralizumab. After an initial increase in GM pEDN from 501 ng/ml at baseline to 1131 ng/ml at day 1 (p < 0.05), GM pEDN concentrations stabilized and subsequently decreased significantly below baseline concentrations by day 28 (119 ng/ml; p < 0.05). GM uEDN concentration followed a similar pattern increasing from 746 ng/mg Cr to a peak of 1353 ng/mg Cr at day 7 (p < 0.05) before decreasing (Figure 5). A GEE linear model was fit to predict the uEDN concentration. The prior day’s pEDN significantly improved the prediction of the current day’s uEDN, after controlling for the effect of day and the prior day’s uEDN concentration (p = 0.036).
3.5 |. A rise in uEDN concentration precedes relapse in benralizumab and mepolizumab treatment
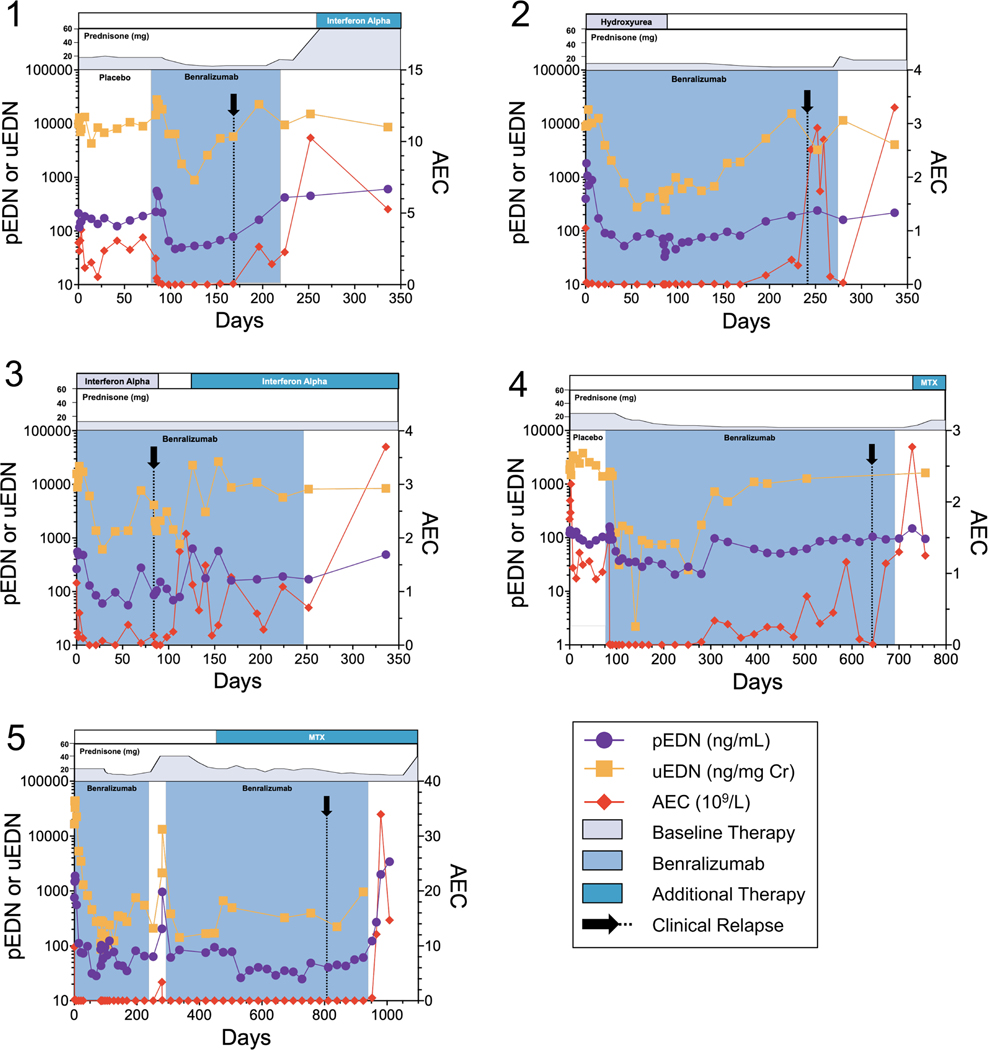
Six patients on the benralizumab trial relapsed (increasing AEC and recurrent HES symptoms) while on the open-label extension, resulting in discontinuation of benralizumab. Urine and plasma samples were collected through the point of relapse from five patients. The complete kinetics of EDN concentrations and AEC annotated with concomitant medication administration for each of the five patients is provided in Figure 6. Despite considerable variability in the timing between patients, pEDN and uEDN concentrations began to rise prior to the increase in AEC and reappearance of symptoms in all five patients.
FIGURE 6.
pEDN and uEDN concentrations increase prior to relapse on benralizumab. pEDN concentrations (purple circles, left y-axis) and uEDN concentrations (orange squares, left y-axis) prior to development of clinical symptoms related to HES disease relapse (black arrow) and/or prior to a rise in AEC (red diamonds, right y-axis)
Twenty-four patients who experienced a relapse of EGPA during the mepolizumab trial had AEC, urine and plasma samples collected at the time of the relapse ±10 days. Data from this time point were compared with those from the nearest preceding urine and plasma collection time point for each patient. (Figure S7). uEDN concentrations increased (ratio > 1) in 20 of 24 patients (83%) around the time of relapse. The corresponding AEC and pEDN concentrations increased in only 14 of 23 (61%) and 12 of 24 (50%) patients, respectively.
4 |. DISCUSSION
Using a multiplex assay, EMBP1, ECP, EDN, and EPO concentrations were simultaneously measurable in serum, plasma, and urine from a large cohort of patients with EAD. Unlike serum and plasma concentrations, urine concentrations of EGP were unaffected by delayed processing. Moreover, urine EDN concentrations, but not plasma EDN concentrations or AEC, were measurable as a variable apparently independent of prednisone dose. Both plasma and urine EDN concentrations decreased significantly upon treatment of HES patients with benralizumab and EGPA patients with mepolizumab, and urine EDN appeared to increase prior to clinical relapse in both groups. These data suggest that urine EDN concentrations may provide a better measure of disease activity and treatment response than pEDN concentrations or AEC in patients with HES or EGPA.
Eosinophil lysis in blood resting at room temperature could inflate EGP concentrations in serum or plasma, especially in patients with high AEC. Although a recent study in study healthy volunteers demonstrated that pEDN was stable for up to 7 days at RT,57 our data suggest that this is not the case in eosinophilic patients. This was especially evident in serum samples from SST tubes from HES patients and to a lesser extent in plasma samples from K2 EDTA tubes. Thus, the ability to collect urine noninvasively and the stability of urine EGP over time suggest that urine EGP concentrations may be preferable to serum or plasma concentrations as a biomarker of eosinophil activity, especially in clinical trials where patient visits are limited and samples cannot be reliably processed within 30 min.
As expected, concentrations of all four EGP measured in plasma were elevated in patients with HES and EGPA compared with HV. Although all four EGP were detectable in urine, uEDN was detected at the highest concentrations and was measurable in all but one HV. This relative abundance of uEDN compared with other uEGP has been reported51,52,58,59 and is likely multifactorial. In the case of EMBP1, which forms aggregates and complexes with other proteins in the blood, filtration through the kidney may be reduced.60–62 High concentrations of uECP have been reported only in urinary schistosomiasis,51,52 where the source (granulomas in the bladder wall) is downstream of kidney filtration. Whereas EDN and ECP are ribonucleases of similar size (ECP = 16 kDa, EDN = 18.9 kDa), EDN has a much lower isoelectric point (pI = 8.9 vs. 10.8)63–65 and its polypeptide sequence is identical to that of human urinary ribonuclease, which may account for its selective filtration through the kidney.66,67 Finally, there are no published data on uEPO, and concentrations in this study were generally low.
pEDN and uEDN concentrations decreased in response to eosinophil-lowering therapy with mepolizumab or benralizumab. To ensure that the elevated concentrations of uEDN in HES patients were not due to eosinophilic renal involvement, eGFR levels were measured and found to be comparable between the HES and eosinophilic asthma patients and were not correlated with uEDN concentration (data not shown). Unlike pEDN, uEDN concentrations showed no correlation with AEC or prednisone dose, another potential advantage in monitoring patients with EADs, who are frequently on glucocorticoid or AEC-lowering therapy. Despite evidence of active disease (based on ACQ-7 ≥ 1.5 at baseline) and higher geometric mean AECs at baseline compared with the EGPA cohort, uEDN was not increased in the asthma patients compared to HV and did not change with dexpramipexole therapy. The reasons for this are unclear since published studies of both pEDN42,68–71 and uEDN68 have demonstrated elevated EGP concentrations in patients with asthma that decline following successful treatment. The increased uEMBP1 and uEPO concentrations in patients with HES compared to patients with EGPA and eosinophilic asthma are intriguing in this regard and suggest the possibility that selective secretion of EGP may occur in EADs of differing pathogenesis.
Prospective collection of urine and plasma samples at multiple time points during a placebo-controlled trial of benralizumab in patients with HES56 provided a unique opportunity to study EGP kinetics following a single dose of an eosinophil-depleting drug. Urine and plasma were collected simultaneously and consistently in the morning for all patients to control for circadian variation in uEDN concentrations.57,72,73 The pattern of uEDN concentrations post-benralizumab treatment mirrored the pattern of pEDN concentrations, but with a delay. After an initial transient increase in pEDN followed by uEDN concentrations, presumably due to transient eosinophil degranulation in the setting of benralizumab-induced cytotoxicity,56,74,75 pEDN and uEDN concentrations decreased dramatically and remained low for the duration of the study in patients whose symptoms and eosinophilia were controlled.
Most (24 of 28; 86%) EGPA patients who received mepolizumab therapy on the mechanistic sub-study experienced at least one relapse requiring an increase in prednisone dose during the trial, and six of 17 (35%) HES patients who demonstrated a clinical and hematologic response to benralizumab experienced a relapse resulting in discontinuation of benralizumab therapy. Since clinical relapse, with the potential for end organ damage, frequently precedes a significant rise in AEC, better biomarkers are clearly needed. Increases in pEDN and uEDN concentrations appeared to precede the development of clinical symptoms and rise of AEC in patients who relapsed on benralizumab. Similarly, even though urine was only collected at three specified time points in the mepolizumab trial, an increase in uEDN within 10 days of relapse was detected in >80% of patients for whom samples were available. Corresponding increases in AEC and pEDN were observed in only 61% and 50% of patients, respectively. While preliminary, these data suggest that uEDN may be a useful early marker of relapse in patients with EGPA and HES treated with eosinophil-lowering biologics. Larger trials with more frequent urine collections are necessary to validate these findings.
This study is the largest to date directly comparing plasma and urine EGP concentrations in patients with EADs over time and in response to eosinophil-targeted therapies. Limitations included (1) the study population, which largely excluded patients with glucocorticoid-refractory eosinophilic disease, and no patients with eosinophilic cystitis or nephritis, who may have eosinophiluria, were included, (2) the infrequent prespecified time points in the mepolizumab and dexpramipexole cohorts, (3) the multiplex assay itself, which, unlike a recently described ELISA,76 does not discriminate between the precursor (proEMBP1) and mature forms of EMBP1, and (4) the fact that other inflammatory mediators, such as cytokines, chemokines, oxylipins, reactive oxygen species, and reaction products potentially contributing to disease activity, were not measured in this study. Despite these limitations, the data suggest that uEDN has several advantages over pEDN and AEC as a measure of disease activity in HES and EGPA. Placebo-controlled prospective studies with clinical disease activity endpoints are needed to confirm the utility of uEDN as a biomarker of relapse in these disorders.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank the NIH Clinical Center staff and research patients without whom the studies described would not have occurred.
FUNDING INFORMATION
This study was funded in part by the Division of Intramural Research, NIAID, NIH (AK) and in part by NIAID Grant Number 1 U01 AI097073 (MW).
Division of Intramural Research, National Institute of Allergy and Infectious Diseases; 1U01 AI097073; NIH Clinical Center
C.P. is an employee of Knopp Biosciences and owns stock or stock options in Knopp Biosciences. M.E.W. has received consulting, advisory, or speaking honoraria from the following: Amgen, AstraZeneca, Avalo Therapeutics, Boehringer Ingelheim, Cerecor, Cohero Health, Cytoreason, Eli Lilly, Equillium, Glaxosmithkline, Incyte, Kinaset, Novartis, Phylaxis, Qilu Puget Sound Biotherapeutics, Pulmatrix, Rapt Therapeutics, Regeneron, Restorbio, Roche/Genentech, Sanofi/Genzyme, Sentien, Sound Biologics, Teva, and Upstream Bio. P.A.M. reports receiving funds for the following activities in the past 2 years: (1) Consulting: AbbVie, AstraZeneca, Boeringher-Ingelheim, Bristol-Myers Squibb, ChemoCentryx, CSL Behring, Dynacure, EMDSerono, Forbius, Genentech/Roche, Genzyme/Sanofi, GlaxoSmithKline, Immagene, InflaRx, Jannsen, Kiniksa, Kyverna, Magenta, MiroBio, Mitsubishi, Neutrolis, Novartis, NS Pharma, Pfizer, Regeneron, Sparrow, Takeda, and Talaris. (2) Research Support: AbbVie, AstraZeneca, Boeringher-Ingelheim, Bristol-Myers Squibb, ChemoCentryx, Eicos, Electra, Forbius, Genentech/Roche, Genzyme/Sanofi, GlaxoSmithKline, InflaRx, Sanofi, and Takeda. (3) Stock options: Kyverna. (4) Royalties: UpToDate. P.A. declares research support and consulting fees from GlaxoSmithKline, AstraZeneca, and Sanofi, and research support from Regeneron. B.S.B. is supported in part by National Institute of Allergy and Infectious Diseases grants U19 AI136443 and R21 AI159586. He receives publication-related royalty payments from Elsevier and UpToDate®/Wolters Kluwer, remuneration for consulting services (Third Harmonic Bio, Acelyrin Inc. and Lupagen) and for serving on the scientific advisory board of Allakos Inc. He also owns stock in Allakos. He is a co-inventor on existing Siglec-8-related patents and thus receives royalty payments from Johns Hopkins University during development and potential sales of Siglec-8 antibody products. P.F.W. has acted as a consultant for GSK and has served on Data and Safety Monitoring Boards for AstraZeneca.
Abbreviations:
- AEC
absolute eosinophil count
- ANC
absolute neutrophil count
- BID
twice daily
- CEP
chronic eosinophilic pneumonia
- CI
confidence interval
- Cr
creatinine
- EAD
eosinophil-associated disorders
- EAE
episodic angioedema with eosinophilia
- ECP
eosinophil cationic protein
- EDN
eosinophil-derived neurotoxin
- EF
eosinophilic fasciitis
- EGID
eosinophilic gastrointestinal disease
- EGP
eosinophil granule proteins
- EGPA
eosinophilic granulomatosis with polyangiitis
- EoE
eosinophilic esophagitis
- EPO
eosinophil peroxidase
- FHES
familial hypereosinophilic syndrome
- GC
glucocorticoid
- GM
geometric mean
- HES
hypereosinophilic syndrome
- HV
healthy volunteers
- IRB
Institutional Review Board
- mAb
monoclonal antibody
- EMBP1
eosinophil major basic protein 1
- MFI
mean fluorescence intensity
- OCS
oral glucocorticoids
- p
plasma
- pI
isoelectric point
- RT
room temperature
- s
serum
- sc
subcutaneous
- TREAD
Taskforce on the Research Needs of Eosinophil-Associated Diseases
- u
urine
- W
week
Footnotes
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
CONFLICT OF INTEREST
All other authors declare that they have no conflict of interest.
REFERENCES
- 1.Grayson PC, Monach PA, Pagnoux C, et al. Value of commonly measured laboratory tests as biomarkers of disease activity and predictors of relapse in eosinophilic granulomatosis with polyangiitis. Rheumatology (Oxford). 2015;54:1351–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khoury P, Makiya M, Klion AD. Clinical and biological markers in hypereosinophilic syndromes. Front Med (Lausanne). 2017;4:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bochner BS, Book W, Busse WW, et al. Workshop report from the National Institutes of Health Taskforce on the Research Needs of Eosinophil-Associated Diseases (TREAD). J Allergy Clin Immunol. 2012;130:587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khoury P, Akuthota P, Ackerman SJ, et al. Revisiting the NIH taskforce on the research needs of eosinophil-associated diseases (RE-TREAD). J Leukoc Biol. 2018;104:69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly EA, Esnault S, Liu LY, et al. Mepolizumab attenuates airway eosinophil numbers, but not their functional phenotype, in asthma. Am J Respir Crit Care Med. 2017;196:1385–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuang FL, De Melo MS, Makiya M, et al. Benralizumab completely depletes gastrointestinal tissue eosinophils and improves symptoms in eosinophilic gastrointestinal disease. J Allergy Clin Immunol Pract. 2022;10:1598–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyabe Y, Kobayashi Y, Fukuchi M, et al. Eosinophil-mediated inflammation in the absence of eosinophilia. Asia Pac Allergy. 2021;11:e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J, Dierkhising R, Wu T-T, Alexander J, Weiler C. Eosinophilic gastrointestinal disorders (EGID) with peripheral eosinophilia: a retrospective review at Mayo Clinic. Dig Dis Sci. 2011;56:3254–3261. [DOI] [PubMed] [Google Scholar]
- 9.Gleich GJ, Loegering DA, Mann KG, Maldonado JE. Comparative properties of the Charcot-Leyden crystal protein and the major basic protein from human eosinophils. J Clin Invest. 1976;57:633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olsson I, Venge P. Cationic proteins of human granulocytes. II. Separation of the cationic proteins of the granules of leukemic myeloid cells. Blood. 1974;44:235–246. [PubMed] [Google Scholar]
- 11.Peterson CG, Venge P. Purification and characterization of a new cationic protein–eosinophil protein-X (EPX)–from granules of human eosinophils. Immunology. 1983;50:19–26. [PMC free article] [PubMed] [Google Scholar]
- 12.Carlson MG, Peterson CG, Venge P. Human eosinophil peroxidase: purification and characterization. J Immunol. 1985;134:1875–1879. [PubMed] [Google Scholar]
- 13.Abu-Ghazaleh RI, Dunnette SL, Loegering DA, et al. Eosinophil granule proteins in peripheral blood granulocytes. J Leukoc Biol. 1992;52:611–618. [DOI] [PubMed] [Google Scholar]
- 14.Sur S, Glitz DG, Kita H, et al. Localization of eosinophil-derived neurotoxin and eosinophil cationic protein in neutrophilic leukocytes. J Leukoc Biol. 1998;63:715–722. [DOI] [PubMed] [Google Scholar]
- 15.Butterfield JH, Weiler D, Peterson EA, Gleich GJ, Leiferman KM. Sequestration of eosinophil major basic protein in human mast cells. Lab Invest. 1990;62:77–86. [PubMed] [Google Scholar]
- 16.Wassom DL, Loegering DA, Solley GO, et al. Elevated serum levels of the eosinophil granule major basic protein in patients with eosinophilia. J Clin Invest. 1981;67:651–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morioka J, Kurosawa M, Inamura H, et al. Development of a novel enzyme-linked immunosorbent assay for blood and urinary eosinophil-derived neurotoxin: a preliminary study in patients with bronchial asthma. Int Arch Allergy Immunol. 2000;122:49–57. [DOI] [PubMed] [Google Scholar]
- 18.Armengot M, Garín L, Carda C. Eosinophil degranulation patterns in nasal polyposis: an ultrastructural study. Am J Rhinol Allergy. 2009;23:466–470. [DOI] [PubMed] [Google Scholar]
- 19.Wright BL, Leiferman KM, Gleich GJ. Eosinophil granule protein localization in eosinophilic endomyocardial disease. N Engl J Med. 2011;365:187–188. [DOI] [PubMed] [Google Scholar]
- 20.Hällgren R, Terent A, Venge P. Eosinophil cationic protein (ECP) in the cerebrospinal fluid. J Neurol Sci. 1983;58:57–71. [DOI] [PubMed] [Google Scholar]
- 21.Peterson CGB, Eklund E, Taha Y, Raab Y, Carlson M. A new method for the quantification of neutrophil and eosinophil cationic proteins in feces: establishment of normal levels and clinical application in patients with inflammatory bowel disease. Am J Gastroenterol. 2002;97:1755–1762. [DOI] [PubMed] [Google Scholar]
- 22.Modig J, Samuelsson T, Hällgren R. The predictive and discriminative value of biologically active products of eosinophils, neutrophils and complement in bronchoalveolar lavage and blood in patients with adult respiratory distress syndrome. Resuscitation. 1986;14:121–134. [DOI] [PubMed] [Google Scholar]
- 23.Colocho Zelaya EA, Orvell C, Strannegård O. Eosinophil cationic protein in nasopharyngeal secretions and serum of infants infected with respiratory syncytial virus. Pediatr Allergy Immunol. 1994;5:100–106. [DOI] [PubMed] [Google Scholar]
- 24.Reimert CM, Minuva U, Kharazmi A, Bendtzen K. Eosinophil protein X/eosinophil derived neurotoxin (EPX/EDN). Detection by enzyme-linked immunosorbent assay and purification from normal human urine. J Immunol Methods. 1991;141:97–104. [DOI] [PubMed] [Google Scholar]
- 25.Tai PC, Ackerman SJ, Spry CJ, Dunnette S, Olsen EG, Gleich GJ. Deposits of eosinophil granule proteins in cardiac tissues of patients with eosinophilic endomyocardial disease. Lancet. 1987;1:643–647. [DOI] [PubMed] [Google Scholar]
- 26.Saffari H, Hoffman LH, Peterson KA, et al. Electron microscopy elucidates eosinophil degranulation patterns in patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2014;133:1728–34.e1. [DOI] [PubMed] [Google Scholar]
- 27.Furuta GT, Kagalwalla AF, Lee JJ, et al. The oesophageal string test: a novel, minimally invasive method measures mucosal inflammation in eosinophilic oesophagitis. Gut. 2013;62:1395–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ochkur SI, Kim JD, Protheroe CA, et al. A sensitive high throughput ELISA for human eosinophil peroxidase: a specific assay to quantify eosinophil degranulation from patient-derived sources. J Immunol Methods. 2012;384:10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dellon ES, Speck O, Woodward K, et al. Markers of eosinophilic inflammation for diagnosis of eosinophilic esophagitis and proton pump inhibitor-responsive esophageal eosinophilia: a prospective study. Clin Gastroenterol Hepatol. 2014;12:2015–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wechsler JB, Ackerman SJ, Chehade M, et al. Noninvasive biomarkers identify eosinophilic esophagitis: a prospective longitudinal study in children. Allergy. 2021;76:3755–3 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peterson KA, Gleich GJ, Limaye NS, et al. Eosinophil granule major basic protein 1 deposition in eosinophilic esophagitis correlates with symptoms independent of eosinophil counts. Dis Esophagus. 2019;32:1–9. [DOI] [PubMed] [Google Scholar]
- 32.Kim HS, Kim JH, Seo YM, et al. Eosinophil-derived neurotoxin as a biomarker for disease severity and relapse in recalcitrant atopic dermatitis. Ann Allergy Asthma Immunol. 2017;119:441–445. [DOI] [PubMed] [Google Scholar]
- 33.Goto T, Morioka J, Inamura H, et al. Urinary eosinophil-derived neurotoxin concentrations in patients with atopic dermatitis: a useful clinical marker for disease activity. Allergol Int. 2007;56:433–438. [DOI] [PubMed] [Google Scholar]
- 34.Pucci N, Lombardi E, Novembre E, et al. Urinary eosinophil protein X and serum eosinophil cationic protein in infants and young children with atopic dermatitis: correlation with disease activity. J Allergy Clin Immunol. 2000;105:353–3 57. [DOI] [PubMed] [Google Scholar]
- 35.Fukuchi M, Kamide Y, Ueki S, et al. Eosinophil ETosis-mediated release of galectin-10 in eosinophilic granulomatosis with polyangiitis. Arthritis Rheumatol. 2021;73:1683–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Virchow JC, Hölscher U, Virchow C. Sputum ECP levels correlate with parameters of airflow obstruction. Am Rev Respir Dis. 1992;146:604–606. [DOI] [PubMed] [Google Scholar]
- 37.van Dalen CJ, Aldridge RE, Cha, et al. Bromotyrosines in sputum proteins and treatment effects of terbutaline and budesonide in asthma. Ann Allergy Asthma Immunol. 2009;103:348–353. [DOI] [PubMed] [Google Scholar]
- 38.Makiya MA, Herrick JA, Khoury P, Prussin CP, Nutman TB, Klion AD. Development of a suspension array assay in multiplex for the simultaneous measurement of serum levels of four eosinophil granule proteins. J Immunol Methods. 2014;411:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zimmerman B, Lanner A, Enander I, Zimmerman RS, Peterson CG, Ahlstedt S. Total blood eosinophils, serum eosinophil cationic protein and eosinophil protein X in childhood asthma: relation to disease status and therapy. Clin Exp Allergy. 1993;23:564–570. [DOI] [PubMed] [Google Scholar]
- 40.Rothenberg ME, Klion AD, Roufosse FE, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med. 2008;358:1215–1228. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura Y, Fukutomi Y, Sekiya K, et al. Low-dose mepolizumab is effective as an add-on therapy for treating long-lasting peripheral neuropathy in patients with eosinophilic granulomatosis with polyangiitis. Mod Rheumatol. 2021;32:387–395. [DOI] [PubMed] [Google Scholar]
- 42.Pham T-H, Damera G, Newbold P, Ranade K. Reductions in eosinophil biomarkers by benralizumab in patients with asthma. Respir Med. 2016;111:21–29. [DOI] [PubMed] [Google Scholar]
- 43.Büttner C, Lun A, Splettstoesser T, Kunkel G, Renz H. Monoclonal anti-interleukin-5 treatment suppresses eosinophil but not T-cell functions. Eur Respir J. 2003;21:799–803. [DOI] [PubMed] [Google Scholar]
- 44.Øymar K. High levels of urinary eosinophil protein X in young asthmatic children predict persistent atopic asthma. Pediatr Allergy Immunol. 2001;12:312–317. [DOI] [PubMed] [Google Scholar]
- 45.Oymar K, Bjerknes R. Urinary eosinophil protein X in children with atopic dermatitis: relation to atopy and disease activity. Allergy. 2000;55:964–968. [DOI] [PubMed] [Google Scholar]
- 46.Breuer K, Kapp A, Werfel T. Urine eosinophil protein X (EPX) is an in vitro parameter of inflammation in atopic dermatitis of the adult age. Allergy. 2001;56:780–784. [DOI] [PubMed] [Google Scholar]
- 47.Khalil Kalaajieh W, Hoilat R. Asthma attack severity and urinary concentration of eosinophil X protein in children. Allergol Immunopathol (Madr). 2002;30:225–231. [DOI] [PubMed] [Google Scholar]
- 48.Kristjánsson S, Strannegård IL, Strannegård O, Peterson C, Enander I, Wennergren G. Urinary eosinophil protein X in children with atopic asthma: a useful marker of antiinflammatory treatment. J Allergy Clin Immunol. 1996;97:1179–1187. [DOI] [PubMed] [Google Scholar]
- 49.Lugosi E, Halmerbauer G, Frischer T, Koller DY. Urinary eosinophil protein X in relation to disease activity in childhood asthma. Allergy. 1997;52:584–588. [DOI] [PubMed] [Google Scholar]
- 50.Tauber E, Halmerbauer G, Frischer T, et al. Urinary eosinophil protein X in children: the relationship to asthma and atopy and normal values. Allergy. 2000;55:647–652. [DOI] [PubMed] [Google Scholar]
- 51.Tischendorf FW, Brattig NW, Burchard GD, Kubica T, Kreuzpaintner G, Lintzel M. Eosinophils, eosinophil cationic protein and eosinophil-derived neurotoxin in serum and urine of patients with onchocerciasis coinfected with intestinal nematodes and in urinary schistosomiasis. Acta Trop. 1999;72:157–173. [DOI] [PubMed] [Google Scholar]
- 52.Tischendorf FW, Brattig NW, Lintzel M, et al. Eosinophil granule proteins in serum and urine of patients with helminth infections and atopic dermatitis. Trop Med Int Health. 2000;5:898–905. [DOI] [PubMed] [Google Scholar]
- 53.Cottin V, Deviller P, Tardy F, Cordier JF. Urinary eosinophil-derived neurotoxin/protein X: a simple method for assessing eosinophil degranulation in vivo. J Allergy Clin Immunol. 1998;101:116–123. [DOI] [PubMed] [Google Scholar]
- 54.Hertzman PA, Blevins WL, Mayer J, Greenfield B, Ting M, Gleich GJ. Association of the eosinophilia-myalgia syndrome with the ingestion of tryptophan. N Engl J Med. 1990;322:869–873. [DOI] [PubMed] [Google Scholar]
- 55.Wolthers OD. Eosinophil granule proteins in the assessment of airway inflammation in pediatric bronchial asthma. Pediatr Allergy Immunol. 2003;14:248–254. [DOI] [PubMed] [Google Scholar]
- 56.Kuang FL, Legrand F, Makiya M, et al. Benralizumab for PDGFRA-Negative Hypereosinophilic Syndrome. N Engl J Med. 2019;380:1336–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rutten B, Young S, Rhedin M, et al. Eosinophil-derived neurotoxin: a biologically and analytically attractive asthma biomarker. PLoS One. 2021;16:e0246627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramarokoto CE, Kildemoes AO, Randrianasolo BS, et al. Eosinophil granule proteins ECP and EPX as markers for a potential early-stage inflammatory lesion in female genital schistosomiasis (FGS). PLoS Negl Trop Dis. 2014;8:e2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lynes WL, Flynn SD, Shortliffe LD, et al. Mast cell involvement in interstitial cystitis. J Urol. 1987;138:746–752. [DOI] [PubMed] [Google Scholar]
- 60.Overgaard MT, Sorensen ES, Stachowiak D, et al. Complex of pregnancy-associated plasma protein-A and the proform of eosinophil major basic protein. Disulfide structure and carbohydrate attachment. J Biol Chem. 2003;278:2106–2117. [DOI] [PubMed] [Google Scholar]
- 61.Swaminathan GJ, Myszka DG, Katsamba PS, Ohnuki LE, Gleich GJ, Acharya KR. Eosinophil-granule major basic protein, a C-type lectin, binds heparin. Biochemistry. 2005;44:14152–14158. [DOI] [PubMed] [Google Scholar]
- 62.Soragni A, Yousefi S, Stoeckle C, et al. Toxicity of eosinophil MBP is repressed by intracellular crystallization and promoted by extracellular aggregation. Mol Cell. 2015;57:1011–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gleich GJ, Loegering DA, Bell MP, Checkel JL, Ackerman SJ, McKean DJ. Biochemical and functional similarities between human eosinophil-derived neurotoxin and eosinophil cationic protein: homology with ribonuclease. Proc Natl Acad Sci U S A. 1986;83:3146–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ackerman SJ, Loegering DA, Venge P, et al. Distinctive cationic proteins of the human eosinophil granule: major basic protein, eosinophil cationic protein, and eosinophil-derived neurotoxin. J Immunol. 1983;131:2977–2982. [PubMed] [Google Scholar]
- 65.Barker RL, Loegering DA, Ten RM, Hamann KJ, Pease LR, Gleich GJ. Eosinophil cationic protein cDNA. Comparison with other toxic cationic proteins and ribonucleases. J Immunol. 1989;143:952–955. [PubMed] [Google Scholar]
- 66.Rosenberg HF, Tenen DG, Ackerman SJ. Molecular cloning of the human eosinophil-derived neurotoxin: a member of the ribonuclease gene family. Proc Natl Acad Sci U S A. 1989;86:4460–4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beintem J, Schülle C, Iri M, Carsan A. Molecular evolution of the ribonuclease superfamily. Prog Biophys Mol Biol. 1988;51:165–192. [DOI] [PubMed] [Google Scholar]
- 68.Lee Y, Lee JH, Yang EM, et al. Serum levels of eosinophil-derived neurotoxin: a biomarker for asthma severity in adult asthmatics. Allergy Asthma Immunol Res. 2019;11:394–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Granger V, Zerimech F, Arab J, et al. Blood eosinophil cationic protein and eosinophil-derived neurotoxin are associated with different asthma expression and evolution in adults. Thorax. 2021;77:552–562. [DOI] [PubMed] [Google Scholar]
- 70.An J, Lee J-H, Sim JH, et al. Serum eosinophil-derived neurotoxin better reflect asthma control status than blood eosinophil counts. J Allergy Clin Immunol Pract. 2020;8:2681–2688.e1. [DOI] [PubMed] [Google Scholar]
- 71.Howarth P, Quirce S, Papi A, et al. Eosinophil-derived neurotoxin and clinical outcomes with mepolizumab in severe eosinophilic asthma. Allergy. 2020;75:2085–2088. doi: 10.1111/all.14266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wolthers OD, Heuck C. Circadian variations in serum eosinophil cationic protein, and serum and urine eosinophil protein X. Pediatr Allergy Immunol. 2003;14(2):130–133. [DOI] [PubMed] [Google Scholar]
- 73.Storm van’s Gravesande K, Mattes J, Gruntjens T, et al. Circadian variation of urinary eosinophil protein X in asthmatic and healthy children. Clin Exp Allergy. 1999;29(11):1497–1501. [DOI] [PubMed] [Google Scholar]
- 74.Spencer LA, Bonjour K, Melo RCN, Weller P. Eosinophil secretion of granule-derived cytokines. Front Immunol. 2014;5:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125(6):1344–1353. [DOI] [PubMed] [Google Scholar]
- 76.Squillace DL, Checkel JL, Tefferi A, Kita H, Gleich GJ. Development and application of novel immunoassays for eosinophil granule major basic proteins to evaluate eosinophilia and myeloproliferative disorders. J Immunol Methods. 2021;493:113015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.