Abstract
Background
Thiol/disulphide homeostasis (TDH) has a critical role in many cellular activities such as antioxidant protection. Alterations of oxidative stress in the transition period play an important role in development of some diseases and disorders in dairy cows.
Objectives
The purpose of this study is to assess the total antioxidant status (TAS), total oxidant status (TOS), oxidative stress index (OSI), total thiol, native thiol, disulphide and lipid profile in Simmental and Montofon dairy cows (aged 2–3) before and after calving.
Methods
Blood samples were collected 233–280 days of pregnancy and the 30 days of post‐partum. Serum total thiol, native thiol and disulphide levels were determined as well as TAS, TOS and paraoxonase‐1 (PON‐1) levels were measured using colorimetric assays. Triglycerides (TG), total cholesterol, HDL cholesterol and LDL cholesterol levels were measured with an automatic analyser.
Results
Total thiol (p = 0.038) and disulphide (p = 0.015) levels were higher after calving compare to pregnancy in Montofon. TAS was found lower (p < 0.001), and OSI was higher in both breeds (Montofon p = 0.012, Simmental p = 0.028) after calving than in pregnancy. When compared between pregnancy and after calving levels in the same breed, HDL was found to be higher after calving (p < 0.001) and TG was lower after calving (p = 0.020) in Montofon. PON (p = 0.090), HDL (p < 0.001) and cholesterol levels were found higher (p < 0.001) and TG level was lower (p < 0.001) after calving in Simmental.
Conclusions
According to our results, we observed different responses between two breeds before and after calving. There are few studies about TDH in animal research, and this is the first study in the literature that evaluates the TDH along with oxidative stress and lipid profiles in dairy cows in the periparturient and post‐partum period.
Keywords: antioxidant status, dairy cows, lipid profiles, periparturient period, post‐partum period, thiol‐disulphide homeostasis
Very few studies have investigated thiol/disulphide homeostasis in veterinary medicine and this is the first study that evaluates the thiol‐disulphide homeostasis along with oxidative stress and lipid profiles in dairy cows in the periparturient and post‐partum period.

1. INTRODUCTION
The transition period, which covers the period between 3 weeks before and 3 weeks after the birth in dairy cattle, is one of the most critical physiological stages in which most metabolic and infectious diseases occur (Drackley, 1999; Van Saun, 2016). Understanding the causes and consequences of metabolic changes that occur during this period is essential for postnatal health management. Hence, it often causes great economic losses to dairy farmers due to the fact that the poor transition period in dairy cattle compromises on production and reproduction (Wankhade et al., 2017). Alterations of oxidative stress in the transition period play an important role in development of some diseases and disorders in dairy cows. Besides, emergence of health problems in the transition period is an important risk factor for subsequent productive and reproductive performance (Ferguson, 2005).
Reactive oxygen species (ROS) comprise approximately 1%–2% of the total metabolits and play an important role in many metabolic activities such as protein phosphorylation, cellular immunity, ovulation, implantation, blastocyst formation, luteolysis, acrosome reaction, fertilization and luteal protection during pregnancy. However high ROS levels cause lipid, protein and DNA damage and lead to loss of cell function (Celi, 2011; Celi et al., 2012). Normally, there is a balance between ROS and the amount of antioxidants. Oxidative stress occurs when the amount of ROS increases and the balance between prooxidants (free radical) and antioxidants is disturbed. ROS is a double‐edged sword; they have important roles in physiological processes, and they also have a role in pathological processes in the female reproductive system (Agarwal et al., 2005). ROS and antioxidants play an important role in the regulation of reproductive processes in both animals and humans. Imbalances between pro‐oxidants and antioxidants can lead to some reproductive diseases in females (Lu et al., 2018).
Harmful effects of ROS are balanced by enzymatic and non‐enzymatic antioxidant systems. Enzymatic scavengers such as superoxide dismutase (SOD), catalase, glutathione peroxidase (GPX), thioredoxins, peroxiredoxin and glutathione transferase play important role in antioxidant defence (Birben et al., 2012). The non‐enzymatic antioxidants are compounds that have sulfhydryl groups (–SH), called thiols (R–SH), and these compounds are particularly important (Karoui et al., 1996). Disulphide bonding structures are changed in thiol groups due to reaction with oxidants as a consequence of their oxidation. In this manner, thiol and disulphide balance are maintained. Besides, structural and functional changes occur in proteins due to the loss of thiol groups (Erel & Neselioglu, 2014; Pasaoglu et al., 2004). Thiol/disulphide homeostasis (TDH) has an important role in many cellular activities including antioxidant system, detoxification, cell growth and programmed cell death (Erel & Neselioglu, 2014; Jones & Liang, 2009).
Dairy cows are particularly susceptible to health disorders during pregnancy. Blood biochemical parameters in dairy cows are very important in determining and monitoring animal's health status and incidence of diseases. Oxygen radicals are produced more in domestic animals during certain periods. Maintaining the balance between the production and scavenging of ROS is essential for homeostasis. In recent years, there has been a growing concern for detection of free radical damage markers for metabolic status assessment in dairy cows (Abuelo et al., 2015; Tufarelli et al., 2023). But there are few studies about TDH in animal research especially in dairy cows. The significance of TDH in dairy cows becomes evident due to importance of TDH in the antioxidant system and the limited number of studies in dairy cows (Deveci & Erdal, 2022; Müller et al., 2023). To date, there is no information available concerning the total thiol‐native thiol status in periparturient dairy cows. In the present study, total thiol‐native thiol status and the possible relationships between oxidative and biochemical parameters were assessed in dairy cows.
Therefore, the purpose of the study was to investigate the dynamic TDH, total antioxidant status (TAS), total oxidant status (TOS), oxidative stress index (OSI), paraoxonase 1 (PON1) activity and triglyceride (TG), HDL, LDL and total cholesterol (TC) levels in pregnancy and post‐partum period of dairy cows. The results of this study will enable us to thiol/disulphide status and relationship between oxidative and biochemical parameters in dairy cows in periparturient and post‐partum period.
2. MATERIALS AND METHODS
2.1. Animal selection
The trial was carried out in a commercial dairy herd in Burdur. The animals were kept at the same farm with the same housing conditions and same feeding diets to eliminate the influence of relevant factors. Clinical examinations were performed for all cows. None of the cows showed cases of clinical diseases or disorders. All cows were Brucella negative. The sample size of animal material was decided using G*Power software (version 3.1.9.7). The minimum number of animals was calculated as 25, using the settings of effect size = 0.30, error probability α = 0.05, power (1 − β) = 0.80, number of groups = 2, number of measurements = 2, correlation among measures = 0.5 and nonsphericity correction ε = 1. In line with the resources of the research infrastructure, the study started with 9 pregnant Montofon and 16 pregnant Simmental cattle.
2.2. Sampling and measurements
First samples collected when the animals (aged between 2 and 3) were between 233 and 280 days of pregnancy for before calving period. Second samples were collected from the same animals at 30 days of post‐partum for after calving period. Blood samples were collected from the caudal vein of animals. Serum separator tubes were used for collecting sera. Blood samples were stored immediately at 4°C, and serum samples were stored at −20°C until analysis. TG, TC, HDL cholesterol (HDL‐C) and LDL‐cholesterol levels were measured with a Relassay Selectra E automatic analyser. Sampling sizes were calculated using G‐Power 3.1 based on the values of power (1 − β) = 0.80 and α = 0.05.
2.3. Serum native thiol, total thiol and disulphide measurements
Serum native thiol, total thiol and disulphide levels were analysed using the method described by Erel and Neselioglu (2014), with Relassay diagnostic kit (Relassay). First, disulphide bonds were reduced to functional thiol groups with sodium boron hydride. Then, formaldehyde was used to remove sodium boron hydride. Finally, all thiol groups (reduced or not) were determined as a result of the reaction with 5,5′‐dithiobis‐(2‐nitrobenzoic) acid.
2.4. TAS and TOS measurements
TAS and TOS measurements were performed with Relassay commercial assay kit (Relassay). In TAS assay, ABTS radical, which is a dark blue green colour, is reduced to colourless ABTS form by antioxidants in sample. The changes of absorbance at 660 nm wavelength reflect the level of total antioxidant level in the sample. TAS measurement is calibrated using stable antioxidant standard solution which is a vitamin E analogue. In TOS assay, the ferrous ion chelator complex oxidized to ferric ion by oxidants in sample. The enhancer molecules in the reaction medium were prolonged the oxidation reaction. The ferric ions form a coloured complex with chromogen in the acidic medium. The intensity of colour is related to the total amount of oxidant molecules present in the sample. TOS measurement is calibrated using hydrogen peroxide, and the results are expressed in terms of micromolar hydrogen peroxide equivalent per litre (µmol H2O2 Equiv./L).
2.5. Calculation of oxidative stress index (OSI)
As an indicator of the degree of oxidative stress, the TOS:TAS ratio was defined as the OSI. To calculate OSI, the unit for TAS (mmol/L Trolox equivalents) was converted to mmol/L Trolox equivalents, and the OSI value was calculated as
2.6. Paraoxonase‐1 (PON1) measurement
PON measurements were performed with Relassay commercial assay kit (Relassay). Serum PON1 activity measurements were performed in the absence of basal activity. Paraoxon hydrolysis (diethyl‐p‐nitrophenylphosphate) rate was measured spectrophotometrically by monitoring the increase in absorbance at 412 nm wavelength and 37°C. The amount of generated p‐nitrophenol was calculated from the molar absorptivity coefficient at pH 8, which was 17,000 M 1 cm−1. PON1 activity was expressed as U/L serum.
2.7. Statistical analysis
The findings were presented as mean values accompanied by standard deviations. Normality assumption was checked for each group using Kolmogorov–Smirnov Test of Normality and Q–Q plots. The biochemical composition data, including Total Thiol (µmol/L), Native Thiol (µmol/L), disulphide, TAS (mmol/L), TOS (µmol/L), OSI, PON‐1 (U/L), HDL (mg/dL), LDL (mg/dL), TG (mg/dL) and Cholesterol, were assessed using repeated measures ANOVA procedure. In the statistical design, fixed effect was assigned to groups, whereas a repeated‐measure effect was assigned to pregnancy status. The Tukey multiple comparison test was employed to assess significant disparities among the average means. Statistical significance was determined when the p‐value was less than 0.05 for differences observed among mean values. The statistical analysis of the data was conducted using JAMOVİ 2.3.2 software (The Jamovi Project, 2022).
3. RESULTS
When compared between animal breeds, LDL was found to be higher in pregnant Simmental than in pregnant Montofon, and cholesterol levels were higher in pregnant Montofon. After calving, TG was measured higher in Montofon than in Simmental (Table 1). Total Thiol (p = 0.038) and disulphide (p = 0.015) values of Montofon in after calving were higher compare to pregnancy (Figure 1). TAS was found lower (p < 0.001), and OSI was higher in both breeds (Montofon p = 0.012, Simmental p = 0.028), after calving than in pregnancy (Figure 1). When compared between pregnancy and after calving values in the same breed, HDL was found to be higher (p < 0.001) and TG was lower after calving (p = 0.020) in Montofon (Figure 2). PON (p = 0.090), HDL (p < 0.001), and cholesterol were found higher (p < 0.001), and TG was lower (p < 0.001) after calving in Simmental (Figure 2).
TABLE 1.
Comparison of biochemical parameters in Montofon and Simmental breeds.
| Before calving | After calving | ||||||
|---|---|---|---|---|---|---|---|
| Measurement | Group | Mean | Standard deviation | p value | Mean | Standard deviation | p value |
| Total thiol (µmol/L) | Montofon | 513.89 | 88.85 | 0.607 | 670.67 | 220.34 | 0.886 |
| Simental | 584.31 | 155.35 | 622.25 | 116.45 | |||
| Native thiol (µmol/L) | Montofon | 155.78 | 27.98 | 0.579 | 160.33 | 61.62 | 0.991 |
| Simental | 177.63 | 46.08 | 153.19 | 56.06 | |||
| Disulphide | Montofon | 179.06 | 38.10 | 0.705 | 255.17 | 81.51 | 0.858 |
| Simental | 203.34 | 60.80 | 234.53 | 49.76 | |||
| TAS (mmol/L) | Montofon | 1.01 | 0.07 | 0.953 | 0.75 | 0.18 | 0.797 |
| Simental | 1.03 | 0.08 | 0.80 | 0.14 | |||
| TOS (µmol/L) | Montofon | 5.27 | 1.78 | 0.367 | 9.08 | 9.19 | 0.766 |
| Simental | 4.14 | 1.55 | 6.50 | 4.16 | |||
| OSI | Montofon | 0.53 | 0.19 | 0.341 | 1.12 | 0.77 | 0.519 |
| Simental | 0.41 | 0.17 | 0.78 | 0.44 | |||
| PON‐1 (U/L) | Montofon | 342.89 | 94.07 | 0.902 | 414.33 | 144.77 | 0.972 |
| Simental | 378.06 | 136.35 | 445.94 | 189.30 | |||
| HDL (mg/dL) | Montofon | 102.78 | 13.07 | 0.792 | 139.44 | 19.68 | 0.999 |
| Simental | 107.69 | 12.56 | 138.44 | 15.92 | |||
| LDL (mg/dL) | Montofon | 37.67 | 4.47 | 0.014 * | 49.89 | 18.38 | 0.510 |
| Simental | 46.50 | 9.26 | 54.94 | 17.99 | |||
| TG (mg/dL) | Montofon | 33.00 | 5.57 | 0.213 | 21.78 | 9.60 | 0.030 * |
| Simental | 39.63 | 8.90 | 13.00 | 5.11 | |||
| Cholesterol (mg/dL) | Montofon | 103.33 | 11.49 | < 0.001 * | 128.11 | 31.43 | 0.629 |
| Simental | 48.24 | 30.93 | 146.31 | 38.51 | |||
Note: Bold values were considered statistically significant where *p values < 0.05.
Abbreviations: OSI, oxidative stress index; PON‐1, paraoxonase 1; TAS, total antioxidant status; TG, Triglycerides; TOS, total oxidant status.
FIGURE 1.
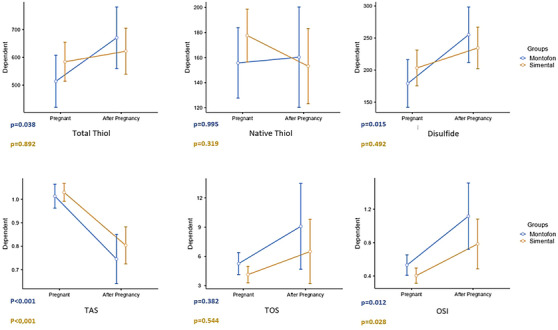
Comparison of total thiol, native thiol, disulphide, total antioxidant status (TAS), total oxidant status (TOS) and oxidative stress index (OSI) levels before and after calving.
FIGURE 2.
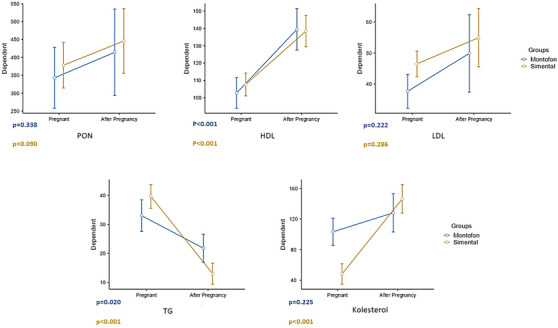
Comparison of paraoxonase (PON), HDL, LDL, triglycerides (TG) and cholesterol levels before and after calving.
4. DISCUSSION
TDH plays a key role in antioxidant defence and is associated with many cellular activities, such as antioxidant protection, cell growth, detoxification, programmed cell death and cellular signal transduction. Thus, TDH has become crucial in the evaluation of medical conditions in humans in recent years (Erdoğan et al., 2019).
Ozler et al. (2015) showed that serum native and total thiol levels were significantly decreased in preeclampsia, and they concluded that TDH has crucial in the management of preeclampsia (Ozler et al., 2015). Moreover, studies have reported that plasma and erythrocyte thiol groups (SH) were decreased after calving in dairy cows (Bernabucci et al., 2002). Deveci et al. (2022) showed that native thiol, total thiol and disulphide levels were significantly increased in dairy cattle with foot disease as well as Müller et al. (2023) reported that disulphide levels were decreased in chronically lame dairy cows and they concluded that TDH had shifted to the reductive state (Deveci & Erdal, 2022; Müller et al., 2023). In this research, we observed that total thiol (p = 0.038) and disulphide (p = 0.015) levels were significantly increased after calving in only Montofon, whereas there was no remarkable change in native thiol (µmol/L) level. To the best of our knowledge, thiol antioxidants and thiol‐disulphide redox are essential for the antioxidant system in organism, and we suggest that these results are associated with the disruption of redox balance after calving in dairy cows (Rahman, 2007). Based on our data, we considered that the change of thiol and disulphide levels leads to disruption of the balance between thiols and disulphides and caused abnormal TDH. As there is already a poor body of existing research on TDH in animal research especially in dairy cows, we suggest that these results may contribute to a metabolic status assessment in dairy cattle.
Metabolic status of dairy cattle has been extensively used in health monitoring; therefore, research on free radical damage in veterinary medicine has been a growing concern as a complementary tool in the assessment of metabolic status in dairy cattle (Castillo et al., 2005, 2006). The relationship between oxidative stress and metabolic status has been reported in many conditions, especially in diseases, such as mastitis, metritis, retention secundinarium, acidosis, ketosis and milk fever, where antioxidant levels decrease (Celi, 2011).
Bernabucci et al. (2002) investigated the oxidative status and relationships between oxidative and metabolic status in dairy cows during the transition period, and they observed that plasma and erythrocyte thiol groups (SH) and SOD levels were decreased, and reactive oxygen metabolites, thiobarbituric acid‐reactive substances and plasma GPX (GSH‐Px) levels were increased after calving in dairy cows (Bernabucci et al., 2002). Moreover, Sayiner et al. (2021) demonstrated that GPx levels were increased in early post‐partum process in dairy cows (Sayiner et al., 2021). Contrary to these findings, Sharma et al. (2011) demonstrated that blood glutathione (GSH) levels were significantly lower in early lactating cows than pregnant cows, whereas they did not observe any change in blood GPx levels (Sharma et al., 2011). In addition to that, Gong et al. (2016) also demonstrated that serum malondialdehyde (MDA) and GPX (GSH‐Px) levels increased during the early lactation period in dairy cows, whereas serum thioredoxin reductase activity and TAS decreased (Gong & Xiao, 2016). It has been shown that the OSI index is an important parameter than antioxidant and oxidant capacity for evaluating the metabolic status in dairy cows, and according to our study, TOS and OSI were increased after calving in dairy cows on both Simmental and Montofon cattles. Moreover, it was observed that antioxidant enzyme PON1 activity was increased after calving and this result consistent with to study of Bernabucci et al. (2002) that they reported increased antioxidant enzyme GSH‐Px after calving in dairy cows (Bernabucci et al., 2002). Based on our data, we considered that the increase of serum PON1 activity may be associated with an increase of TOS and OSI parameters and these findings indicated that cows are at a greater risk of oxidative stress during this period. A decreased TAS observed in both breeds was significant. These changes may be seen as a result of compensation of increase in TOS values.
Omidi et al. (2017) investigated the plasma total antioxidant capacity (TAS), total thiols and MDA levels in different stages of lactation cycle and dry period of dairy cows. They concluded that TAS could be used as a relevant indicator in measuring the cumulative effects of antioxidants, in addition to the metabolic profile tests currently used to analyse dairy cattle health (Omidi et al., 2017). In our study, we also observed significant changes in TAS. In addition to this, we concluded that OSI is more reliable indicator to access oxidative stress.
The expected values of various biochemical components are essential for the healthy functioning of various systems of the body, including the reproductive system, and it has also been suggested that alterations in various biochemical parameters can have an impact on infertility. Therefore, serum biochemical profile can be helpful of managing the metabolic status in dairy cows (Park et al., 2010). Sayiner et al. (2021) reported that serum cholesterol levels significantly decreased in post‐partum period of dairy cows and they concluded that this may be related to the energy deficiency (Sayiner et al., 2021). Our results showed that serum HDL levels were increased (p < 0.001), whereas serum TG levels decreased (p = 0.020) in Montofon cattles after calving. Moreover, serum cholesterol (p < 0.001) and HDL (p < 0.001) levels were increased, and serum TG (p < 0.001) levels were decreased in Simmental cattles in the same period. We found that LDL levels were higher in pregnant Simmental than in pregnant Montofon, and cholesterol levels were higher in pregnant Montofon. After calving, TG was measured higher in Montofon than in Simmental. Previous studies showed that serum cholesterol levels decreased, whereas serum TG levels were increased during the latter stages of pregnancy and we suggest that our data obtained from after calving period may be related to this change that occurs during latter stages of pregnancy according to these studies (Castillo et al., 2005; Marcos et al., 1990; Tainturier et al., 1984). We concluded that these results regarding lipid metabolism may be attributable to changes in endocrine status and to the characteristic decrease in dry matter intake during this period, influencing lipid metabolism.
Turk et al. (2005, 2004) pointed out that liver failure caused by post‐partum lipid mobilization, TG accumulation and following dysfunction; post‐partum increases of HDL‐Cs and oxidative stress; and combinations of these factors are the primary factors causing reduced PON production (Turk et al., 2005, 2004). Similar to this result, we observed increased OSI, but contrary to these findings, increased PON activity, HDL level and reduction of TG levels in post‐partum period. Senoh et al. (2019) observed no difference in pre‐ and post‐partum PON activities in subclinical ketosis group (Senoh et al., 2019). This may be the result of different adoptation response of different breeds to lipid metabolism.
In conclusion, our present study demonstrates for the first time serum total thiol, native thiol and disulphide levels in dairy cows before and after calving. Furthermore, oxidative stress and lipid parameters were determined. Our results showed different responses between two breeds in periparturient and post‐partum periods in terms of lipid metabolism and oxidative status. A limitation of this study is that two breeds were studied; therefore, the relationships of thiol/disulphide and oxidant–antioxidant status in periparturient and post‐partum period need to be investigated in future studies preferably on different breeds.
AUTHOR CONTRIBUTIONS
Burcu Menekse Balkan: Conceptualization; methodology; investigation; formal analysis; supervision; visualization; writing and review–editing. Ogunc Meral: Conceptualization; methodology; investigation; formal analysis; writing and review–editing. Bekir Cetintav: Methodology; data curation; investigation; formal analysis; visualization and review‐editing. Guzin Ozkurt: Methodology; investigation; formal analysis; writing and review–editing. Ali Burak Balkan: Methodology; investigation; formal analysis and review–editing. Tevhide Sel: Conceptualization; methodology; investigation and review–editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
FUNDING INFORMATION
None.
ETHICS STATEMENT
Present study approved by the Ethical Review Committee for the use of Animals, under the administrative control of Animal Experiments Local Ethics Committee of the University of Burdur Mehmet Akif Ersoy decision dated 16.10.2019 and numbered 579.
Balkan, B. M. , Meral, O. , Cetintav, B. , Ozkurt, G. , Balkan, A. B. , & Sel, T. (2024). Evaluation of thiol/disulphide and oxidant–antioxidant status of dairy cows in periparturient and post‐partum period. Veterinary Medicine and Science, 10, e70023. 10.1002/vms3.70023
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available from the corresponding author upon request.
REFERENCES
- Abuelo, A. , Hernández, J. , Benedito, J. L. , & Castillo, C. (2015). The importance of the oxidative status of dairy cattle in the periparturient period: Revisiting antioxidant supplementation. Journal of Animal Physiology and Animal Nutrition, 99(6), 1003–1016. 10.1111/jpn.12273 [DOI] [PubMed] [Google Scholar]
- Agarwal, A. , Gupta, S. , & Sharma, R. K. (2005). Role of oxidative stress in female reproduction. Reproductive Biology and Endocrinology [Electronic Resource]: RB&E, 3, 28. 10.1186/1477-7827-3-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabucci, U. , Ronchi, B. , Lacetera, N. , & Nardone, A. (2002). Markers of oxidative status in plasma and erythrocytes of transition dairy cows during hot season. Journal of Dairy Science, 85(9), 2173–2179. 10.3168/jds.S0022-0302(02)74296-3 [DOI] [PubMed] [Google Scholar]
- Birben, E. , Sahiner, U. M. , Sackesen, C. , Erzurum, S. , & Kalayci, O. (2012). Oxidative stress and antioxidant defense. The World Allergy Organization Journal, 5(1), 9–19. 10.1097/WOX.0b013e3182439613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo, C. , Hernandez, J. , Bravo, A. , Lopez‐Alonso, M. , Pereira, V. , & Benedito, J. L. (2005). Oxidative status during late pregnancy and early lactation in dairy cows. Veterinary Journal, 169(2), 286–292. 10.1016/j.tvjl.2004.02.001 [DOI] [PubMed] [Google Scholar]
- Castillo, C. , Hernández, J. , Valverde, I. , Pereira, V. , Sotillo, J. , Alonso, M. L. , & Benedito, J. L. (2006). Plasma malonaldehyde (MDA) and total antioxidant status (TAS) during lactation in dairy cows. Research in Veterinary Science, 80(2), 133–139. 10.1016/j.rvsc.2005.06.003 [DOI] [PubMed] [Google Scholar]
- Celi, P. (2011). Oxidative stress in ruminants. In Mandelker L. & Vajdovich P. (Eds.), Studies on veterinary medicine (Vol. 5, pp. 191–231). Humana Press. 10.1007/978-1-61779-071-3_13 [DOI] [Google Scholar]
- Celi, P. (2011). Oxidative stress in ruminants. In Mandelker L. & Vajdovich P. (Eds.), Studies on veterinary medicine (pp. 191–231). Humana Press. [Google Scholar]
- Celi, P. , Merlo, M. , Barbato, O. , & Gabai, G. (2012). Relationship between oxidative stress and the success of artificial insemination in dairy cows in a pasture‐based system. Veterinary Journal, 193(2), 498–502. 10.1016/j.tvjl.2012.02.002 [DOI] [PubMed] [Google Scholar]
- Deveci, M. Z. Y. , & Erdal, H. (2022). Determination of dynamic thiol‐disulfide levels in dairy cattle with foot disease. Veterinarski Arhiv, 92, 657–666. DOI: 10.24099/vet.arhiv.1785 [DOI] [Google Scholar]
- Drackley, J. K. (1999). ADSA foundation scholar award. Biology of dairy cows during the transition period: The final frontier? Journal of Dairy Science, 82(11), 2259–2273. 10.3168/jds.s0022-0302(99)75474-3 [DOI] [PubMed] [Google Scholar]
- Erdoğan, H. , Çamkerten, İ. , Çamkerten, G. , Ural, K. , Erdoğan, S. , Günal, İ. , & Erel, Ö. (2019). The effect of hot‐iron disbudding on thiol‐disulphide homeostasis in calves. Kafkas Universitesi Veteriner Fakultesi Dergisi, 25(3), 335–339. 10.9775/kvfd.2018.20950 [DOI] [Google Scholar]
- Erel, O. , & Neselioglu, S. (2014). A novel and automated assay for thiol/disulphide homeostasis. Clinical Biochemistry, 47(18), 326–332. 10.1016/j.clinbiochem.2014.09.026 [DOI] [PubMed] [Google Scholar]
- Ferguson, J. D. (2005). Nutrition and reproduction in dairy herds. The Veterinary Clinics of North America. Food Animal Practice, 21(2), 325–347. 10.1016/j.cvfa.2005.03.001 [DOI] [PubMed] [Google Scholar]
- Gong, J. , & Xiao, M. (2016). Selenium and antioxidant status in dairy cows at different stages of lactation. Biological Trace Element Research, 171(1), 89–93. 10.1007/s12011-015-0513-2 [DOI] [PubMed] [Google Scholar]
- Jones, D. P. , & Liang, Y. (2009). Measuring the poise of thiol/disulfide couples in vivo. Free Radical Biology & Medicine, 47(10), 1329–1338. 10.1016/j.freeradbiomed.2009.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoui, H. , Hogg, N. , Fréjaville, C. , Tordo, P. , & Kalyanaraman, B. (1996). Characterization of sulfur‐centered radical intermediates formed during the oxidation of thiols and sulfite by peroxynitrite. ESR‐spin trapping and oxygen uptake studies. Journal of Biological Chemistry, 271(11), 6000–6009. 10.1074/jbc.271.11.6000 [DOI] [PubMed] [Google Scholar]
- Lu, J. , Wang, Z. , Cao, J. , Chen, Y. , & Dong, Y. (2018). A novel and compact review on the role of oxidative stress in female reproduction. Reproductive Biology and Endocrinology [Electronic Resource]: RB&E, 16(1), 80. 10.1186/s12958-018-0391-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos, E. , Mazur, A. , Cardot, P. , & Rayssiguier, Y. (1990). The effect of pregnancy and lactation on serum lipid and apolipoprotein B and A‐I levels in dairy cows. Journal of Animal Physiology and Animal Nutrition, 64, 133–138. [Google Scholar]
- Müller, H. , Herzberg, D. , Chihuailaf, R. , Strobel, P. , Werner, M. , & Bustamante, H. (2023). Changes in dynamic thiol/disulfide homeostasis, and substance P, B‐endorphin and α‐tocopherol concentrations in the spinal cord of chronically lame dairy cows. Animals (Basel), 13(10), 1620. 10.3390/ani13101620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omidi, A. , Fathi, M. H. , & Parker, M. O. (2017). Alterations of antioxidant status markers in dairy cows during lactation and in the dry period. Journal of Dairy Research, 84(1), 49–53. 10.1017/S0022029916000753 [DOI] [PubMed] [Google Scholar]
- Ozler, S. , Erel, O. , Oztas, E. , Ersoy, A. O. , Ergin, M. , Sucak, A. , Neselioglu, S. , Uygur, D. , & Danisman, N. (2015). Serum thiol/disulphide homeostasis in preeclampsia. Hypertension in Pregnancy, 34(4), 474–485. 10.3109/10641955.2015.1077859 [DOI] [PubMed] [Google Scholar]
- Park, M. S. , Yang, Y. X. , Shinde, P. L. , Choi, J. Y. , Jo, J. K. , Kim, J. S. , Lohakare, J. D. , Yang, B. K. , Lee, J. K. , Kwon, I. K. , & Chae, B. J. (2010). Effects of dietary glucose inclusion on reproductive performance, milk compositions and blood profiles in lactating sows. Journal of Animal Physiology and Animal Nutrition, 94(5), 677–684. 10.1111/j.1439-0396.2009.00962.x [DOI] [PubMed] [Google Scholar]
- Pasaoglu, H. , Sancak, B. , & Bukan, N. (2004). Lipid peroxidation and resistance to oxidation in patients with type 2 diabetes mellitus. The Tohoku Journal of Experimental Medicine, 203(3), 211–218. 10.1620/tjem.203.211 [DOI] [PubMed] [Google Scholar]
- Rahman, K. (2007). Studies on free radicals, antioxidants, and co‐factors. Clinical Interventions in Aging, 2(2), 219–236. [PMC free article] [PubMed] [Google Scholar]
- Sayiner, S. , Darbaz, I. , Ergene, O. , & Aslan, S. (2021). Changes in antioxidant enzyme activities and metabolic parameters in dairy cows during different reproductive periods. Theriogenology, 159, 116–122. 10.1016/j.theriogenology.2020.10.024 [DOI] [PubMed] [Google Scholar]
- Senoh, T. , Oikawa, S. , Nakada, K. , Tagami, T. , & Iwasaki, T. (2019). Increased serum malondialdehyde concentration in cows with subclinical ketosis. Journal of Veterinary Medical Science, 81(6), 817–820. 10.1292/jvms.18-0777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, N. , Singh, N. K. , Pandey, V. , & Verma, K. (2011). Oxidative stress and antioxidant status during transition period in dairy cows. Asian—Australasian Journal of Animal Sciences, 24(4), 479. [Google Scholar]
- Tainturier, D. , Braun, J. P. , Rico, A. G. , & Thouvenot, J. P. (1984). Variations in blood composition in dairy cows during pregnancy and after calving. Research in Veterinary Science, 37, 129–131. [PubMed] [Google Scholar]
- The Jamovi Project . (2022). Jamovi. (Version 2.3) [Computer Software] . Jamovi. https://www.jamovi.org [Google Scholar]
- Tufarelli, V. , Colonna, M. A. , Losacco, C. , & Puvača, N. (2023). Biological health markers associated with oxidative stress in dairy cows during lactation period. Metabolites, 13(3), 405. 10.3390/metabo13030405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk, R. , Juretić, D. , Geres, D. , Turk, N. , Rekić, B. , Simeon‐Rudolf, V. , Robić, M. , & Svetina, A. (2005). Serum paraoxonase activity in dairy cows during pregnancy. Research in Veterinary Science, 79(1), 15–18. 10.1016/j.rvsc.2004.09.010 [DOI] [PubMed] [Google Scholar]
- Turk, R. , Juretic, D. , Geres, D. , Turk, N. , Rekic, B. , Simeon‐Rudolf, V. , & Svetina, A. (2004). Serum paraoxonase activity and lipid parameters in the early postpartum period of dairy cows. Research in Veterinary Science, 76(1), 57–61. 10.1016/j.rvsc.2003.08.001 [DOI] [PubMed] [Google Scholar]
- Van Saun, R. J. (2016). Indicators of dairy cow transition risks: Metabolic profiling revisited. Tierarztliche Praxis. Ausgabe G, Grosstiere Nutztiere, 44(2), 118–126. doi: 10.15653/TPG-150947 [DOI] [PubMed] [Google Scholar]
- Wankhade, P. R. , Manimaran, A. , Kumaresan, A. , Jeyakumar, S. , Ramesha, K. P. , Sejian, V. , Rajendran, D. , & Varghese, M. R. (2017). Metabolic and immunological changes in transition dairy cows: A review. Veterinary World, 10(11), 1367–1377. doi: 10.14202/vetworld.2017.1367-1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon request.


