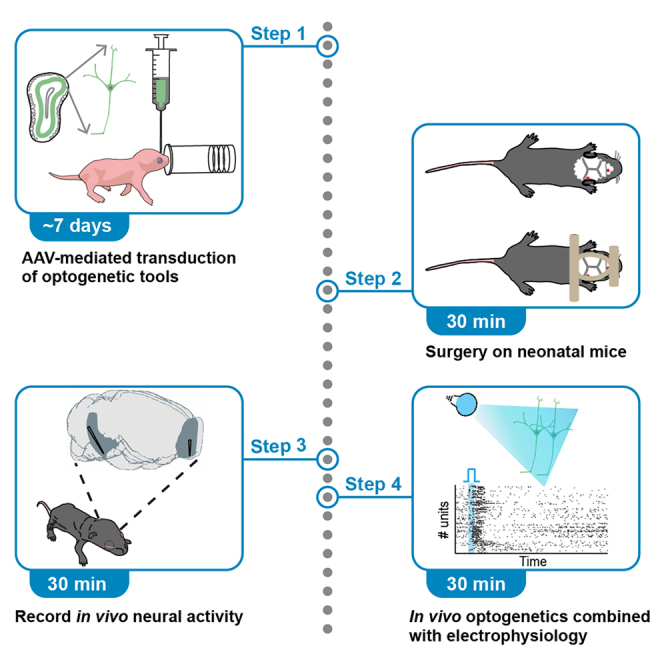
Summary
Optogenetic manipulation has proven a powerful tool for investigating the mechanisms underlying the function of neuronal networks, but implementing the technique on mammals during early development remains challenging. Here, we present a comprehensive workflow to specifically manipulate mitral/tufted cells (M/TCs), the output neurons in the olfactory circuit, mediated by adeno-associated virus (AAV) transduction and light stimulation in neonatal mice and monitor neuronal and network activity with in vivo electrophysiology. This method represents an efficient approach to elucidate functional brain development.
For complete details on the use and execution of this protocol, please refer to Chen et al.1,2,3
Subject areas: biophysics, developmental biology, neuroscience, systems biology
Graphical abstract
Highlights
-
•
Steps for combined in vivo optogenetics and electrophysiology in awake mice
-
•
Cell-type specific optogenetic manipulation mediated by AAV transduction in neonatal mice
-
•
Monitoring light-evoked neuronal and network activity of the olfactory circuits
-
•
Adaptable for other brain areas and cell types
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Optogenetic manipulation has proven a powerful tool for investigating the mechanisms underlying the function of neuronal networks, but implementing the technique on mammals during early development remains challenging. Here, we present a comprehensive workflow to specifically manipulate mitral/tufted cells (M/TCs), the output neurons in the olfactory circuit, mediated by adeno-associated virus (AAV) transduction and light stimulation in neonatal mice and monitor neuronal and network activity with in vivo electrophysiology. This method represents an efficient approach to elucidate functional brain development.
Before you begin
How sensory perception interplays with cognitive functions is a fascinating topic in the field of neuroscience. In mammals, olfaction is one of the first senses that maturates.4,5 While previous studies have identified the tight anatomical connections between olfactory system and higher-order cortical regions,6,7 their role is still largely unknown. Until recently, the impact of olfactory outputs on cognitive processing along development represented an open question, mainly due to technical challenges. For example, the neonatal stage is characterized by rapid and dynamic changes in the structure and function of the brain, therefore dissecting the developmental trajectory of the circuits linking olfaction with cognition requires complex and longitudinal experimental designs. During the last decade, optogenetics has become the tool of choice for dissecting brain circuits and identifying their function, since it allows precise manipulation of distinct neuronal populations by light.8,9 Recently, we adapted this method for developing olfactory circuit and revealed the critical role of neonatal olfactory bulb (OB) activity in the development of the entorhinal-hippocampal system accounting for cognitive abilities.1,2,3 We provide here a detailed description of how to implement in vivo optogenetics to M/TCs of neonatal mice, which can be also applied to other brain regions or cell types with simple experimental modifications.
Institutional permissions
All experiments described in this protocol were carried out on transgenic mice of both sexes which express Cre recombinase controlled by the mouse T-box 21 (also called Tbet) promoter in M/TCs of the OB. These mice together with their mothers were housed in the animal facility of the University Medical Center Hamburg-Eppendorf at a 12-h light/12-h dark cycle and the mothers were fed ad libitum. The viral vector (AAV9) used in our experiments is listed as a biosafety level 1 agent in Germany. All procedures were performed in accordance with German laws and the guidelines of the European Community for the use of animals in research and were approved by the local ethical committee (G17/015). Readers who want to adhere to this protocol for experimental practice will need to obtain similar permission and guidance from the animal care/biosafety committee at the relevant institution(s).
Mating and breeding of animals
Timing: 3 weeks
-
1.
Set up a mating cage by putting two healthy adult female mice with a single adult male in a cage with fresh beddings and nesting materials at a 12-h light/dark cycle as well as ad libitum feeding and water.
Note: We use male heterozygous Tbet-cre mice to cross with female wild-type C57BL/6J mice. According to Mendelian inheritance, 50% of the mouse descendants are Tbet-cre positive and the others are Tbet-cre negative.
-
2.
Perform vaginal plug checking and document the day, to schedule the experiments (the day of plug detection was considered E0.5 and the day of birth was considered P0).
-
3.
When the pregnancy is confirmed, house the pregnant female mouse individually and place a small shelter in the home cage.
Acquire viral vectors
Timing: depends on virus production and shipping
-
4.
Choose AAV according to the opsin, desired expression pattern, serotype, promoter, fluorophore and titer.
Note: We use AAV9 encoding cre-dependent channelrhodopsin (ChR2) and enhanced yellow fluorescent protein (EYFP) to specifically manipulate M/TCs in the OB. The expression of the virus is controlled by the Ef1a promotor and the transduction performance of AAV9 is relatively more efficient for neonatal mice compared to other AAV serotypes.10 We recommend a virus at a high titer for adequate ChR2 expression in all M/TCs (e.g., ≥ 1×1013 vg/mL from Addgene virus facility).
-
5.
Aliquot the virus on ice and store it at −80°C.
CRITICAL: To prevent the degradation of virus quality, we recommend 3–4 μL aliquots for single use and avoid repeated freezing-thawing cycles of the virus.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| AAV9-Ef1a-DIO-hChR2(E123T/T159C)-EYFP (stored at −80°C) | Addgene | Cat# 35509, RRID: Addgene_35509 |
| Chemicals, peptides, and recombinant proteins | ||
| Isoflurane (stored at 20°C–25°C) | Baxter | Cat# HDG9623 |
| Omnifill C dental cement | Omnident | Cat# 85182 |
| Phosphate-buffered saline (PBS; stored at 20°C–25°C) | Gibco | Cat# 20012027 |
| Isotonic sodium chloride (0.9%; stored at 20°C–25°C) | B. Braun | Cat# 3570310 |
| Mixture of 0.25% bupivacaine & 1% lidocaine (stored at 20°C–25°C) | University Medical Center Hamburg-Eppendorf | N/A |
| Hydrogen peroxide (3%; stored at 20°C–25°C) | Carl Roth | Cat# 1A8Y.1 |
| Ethanol (stored at 20°C–25°C) | Carl Roth | Cat# K928.3 |
| Paraformaldehyde (PFA, 4%; stored at 20°C–25°C) | Carl Roth | Cat# P087.1 |
| DiI (1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; stored at 4°C) | Molecular Probes | Cat# D282 |
| Ketamine (stored at 20°C–25°C) | Richter Pharma AG | Ketamidor |
| Xylazine (stored at 20°C–25°C) | Bayer | Rompun |
| MetriZyme detergent (stored at 20°C–25°C) | Metrex | Cat# SKU 10-4000 |
| Experimental models: Organisms/strains | ||
| Mouse: Tbet-cre (B6; CBA-Tg(Tbx21-cre) 1Dlc/J; male/female, postnatal day (P) 8–11) | Jackson Laboratory | RRID: IMSR_JAX: 024507 |
| Mouse: Tbet-cre (B6; CBA-Tg(Tbx21-cre) 1Dlc/J; male, ≥2 months) | Jackson Laboratory | RRID: IMSR_JAX: 024507 |
| Mouse: C57BL/6J (female, ≥2 months) | Jackson Laboratory | RRID: IMSR_JAX: 000664 |
| Software and algorithms | ||
| Cheetah 6 | NeuraLynx | http://neuralynx.com/ |
| OMICRON Control Center | Omicron-Laserage | v3.6.10 |
| Other | ||
| Sterican single-use hypodermic needle (27G) | B. Braun | N/A |
| Microdissection scissors | Fine Science Tools | Cat# 14084-08 |
| Dumont forceps | Fine Science Tools | Cat# 11270-20 |
| Hot bead sterilizer | Fine Science Tools | Cat# 18000-50 |
| Microliter syringe (model 701 RN, 33G) | Hamilton | Cat# 65460-05 |
| Infusion microinjector and pump controller (not substitutable) | World Precision Instruments | MICRO4 |
| Animal anesthesia system (not substitutable) | Harvard Apparatus | Cat# 75-0235 |
| Temperature controller (not substitutable) | FHC | Cat# 40-90-8D |
| Heating pad (not substitutable) | FHC | Cat# 40-90-2 |
| Stereomicroscope | Olympus | SZX10 |
| Cotton bud | DM | Cat# 724841 |
| Round plastic bar (outer diameter: 2 mm) | aero-naut Modellbau | Cat# 772831 |
| Silver wire (diameter: 200 μm; not substitutable) | Science Products | AG-8W |
| Stereotaxic apparatus | Stoelting | Cat# 51603 |
| Diode laser (473 nm; not substitutable) | Omicron-Laserage | LuxX 473-100 |
| Optic patch cable (not substitutable) | Thorlabs | Cat# FG050UGA |
| Optical power meter | Thorlabs | Cat# PM121D |
| Recording optrode (1 shank, 16 channels, 50 μm inter-site spacing) | NeuroNexus | A1x16-5mm-50-703-OA16LP |
| Recording electrode (1 shank, 16 channels, 100 μm inter-site spacing) | NeuroNexus | A1x16-5mm-100-703-A16 |
| Multichannel extracellular amplifier | NeuraLynx | Digital Lynx SX |
| Arduino Uno SMD | Arduino | A000073 |
Step-by-step method details
Virus injection for ChR2 transduction
Timing: 5 min/animal
Here we describe the procedure for microinjection of an AAV construct into the OB of Tbet-Cre mice at P1 (Figure 1A) to enable the expression of ChR2 in M/TCs. For specifically targeting other types of neurons or other brain regions, small modifications regarding mouse line, virus type, and injection volume would be needed.
-
1.Set up the injection system (Figure 1B).
-
a.Attach the microliter syringe to the infusion pump fixed on the stereotaxic apparatus.
-
b.Set the volume of withdrawal via the pump controller according to the number of pups and the amount of each injection.
-
c.Withdraw the set amount of ice-cooled virus-containing solution into the syringe.
-
a.
CRITICAL: Make sure the tip of the syringe is completely embedded in the solution during withdrawal and no air bubbles are present in the syringe.
Optional: Depending on the experimental design, dilute the virus aliquot in 0.01 M PBS to the final working concentration if necessary.
-
2.
Take half the pups from the homecage litter and make a temporary shelter for them on the heating pad at 37°C.
CRITICAL: We recommend taking half of the total number of pups for each round of injection and keeping the remaining pups with their mother. Taking all pups at once increases the chance that the mother mouse will neglect them when they are returned.
-
3.Anesthetize the animal.
-
a.Tenderly place one pup in the stereotaxic apparatus equipped with an anesthesia mask and a heating pad. Turn on the anesthesia system and adjust the vaporizer to change the isoflurane concentration (5% for initial induction for around 3 min then 3% for maintenance of anesthesia).
-
b.Gently advance the non-traumatic bars of the stereotaxic apparatus to both sides of the head to fix the pup.
-
c.Confirm the depth of anesthesia by assessing the response to a slight tail pinch with forceps.
-
a.
-
4.Microinjection into the OB.
-
a.Set the volume of infusion to 200 nL via the pump controller.
-
b.Disinfect the skin of the pup with 70% ethanol (or other local disinfectant).
-
c.Mark the location of the right OB which is in front of the blood vessel over the frontonasal suture and clearly visible through the skin (Figure 2B (i)).
-
d.Puncture the skin and skull on the marked location with the tip of a sterilized fine needle.Note: For mice aged at P0-1, no incision or sutures are needed to inject the OB.
-
e.Slowly lower the attached microliter syringe to advance the tip into the OB at a 1–1.2 mm depth (Figure 2B (ii)).
-
f.Start the microinjection under the infusion mode at a rate of 100 nL/min.
 CRITICAL: Avoid excessive injection pressure which might cause damage to brain tissue.
CRITICAL: Avoid excessive injection pressure which might cause damage to brain tissue. -
g.Following the microinjection, leave the tip of the syringe in situ for an additional 2 min to avoid leak or reflux of the fluid.
-
h.Carefully retract the syringe out of the brain.
-
i.Clean the fluid on the injection site and disinfect the skin of the pup again.Optional: Obtain tail biopsies for genotyping to identify Tbet-Cre positive animals following the PCR protocol provided by the Jackson Laboratory.11Optional: To differentiate the pups, mark them (e.g., by tattooing the paws).
-
j.Remove the pup from the apparatus and place it into a recovery box on the heating pad with beddings from the homecage.
 CRITICAL: Make sure the pup has regular vital signs after recovery from anesthesia. This takes 5–10 minutes.
CRITICAL: Make sure the pup has regular vital signs after recovery from anesthesia. This takes 5–10 minutes.
-
a.
-
5.
Repeat the anesthesia and microinjection (major steps 3–4) on the other pups.
-
6.
Return the injected pups to their homecage after full recovery.
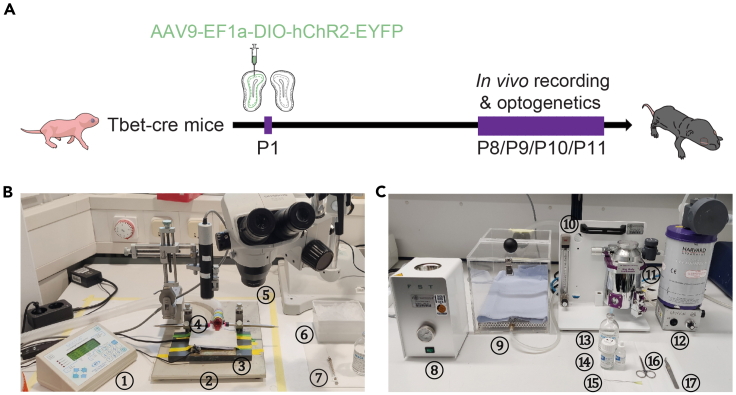
Figure 1.
Experimental design and setups
(A) Experimental timeline. At P1, transduction of light-gated ChR2 channels in M/TCs was performed by AAV injections into the right OB of Tbet-cre mice. At P8, P9, P10 or P11, acute in vivo electrophysiological recordings were performed in the right OB combined with optogenetic activation of ChR2-expressing M/TCs.
(B) The setup for microinjections on neonatal mice (devices corresponding to the numbers: ①infusion pump; ②stereotaxic apparatus; ③heating pad; ④anesthesia mask; ⑤stereomicroscope; ⑥ice box with viral construct; ⑦microliter syringe).
(C) The setup for in vivo surgery (devices corresponding to the numbers: ⑧hot bead sterilizer; ⑨anesthesia induction box; ⑩anesthesia flow meter; ⑪isoflurane vaporizer; ⑫gas evacuation unit; ⑬isotonic sodium chloride solution; ⑭dental cement powder (left) and aqueous solution (right); ⑮needle; ⑯scissors; ⑰forceps).
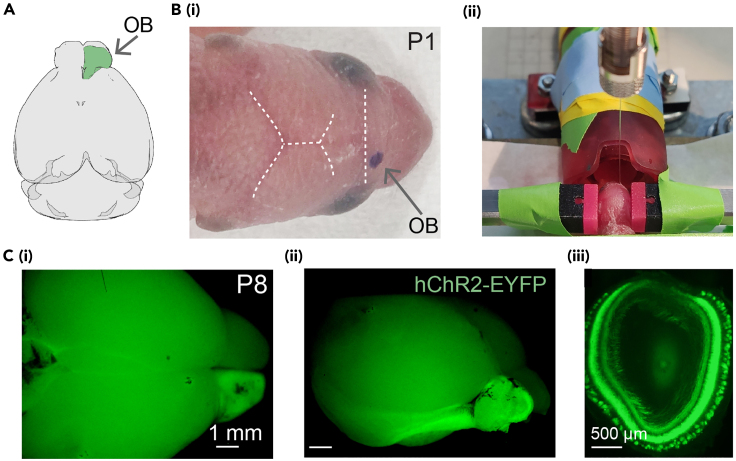
Figure 2.
Procedure for ChR2 transduction in the OB by virus injection at P1
(A) (i) 3D schematic of the OB position in the brain with the injected area in green (top view).
(B) (i) An anesthetized P1 mouse with injection location for the right OB marked. (ii) A head-fixed P1 mouse in front of the anesthesia mask and the tip of the microliter syringe positioned into the right OB for viral injection.
(C) Pictures showing hChR2-EYFP expression in the right OB (i: top view; ii: lateral view of the brain) from a P8 Tbet-cre mouse, and expression pattern across the OB layers on a coronal brain slice (iii).
Surgery for in vivo electrophysiological recordings
Timing: 30 min/animal
Here we describe the procedure for the in vivo surgery preceding electrophysiological recording simultaneously performed in both OB and lateral entorhinal cortex (LEC) of neonatal mice (P8-11) expressing ChR2 in M/TCs.
-
7.Anesthetize the animal.
-
a.Take one pup out from the homecage.
-
b.Anesthetize the pup.
-
i.Weigh the pup and put it in the induction box (Figure 1C).
-
ii.Turn on the animal anesthesia system and adjust the vaporizer to increase the isoflurane concentration to 5% for around 5 min.
-
iii.Confirm deep anesthesia by tail or toe pinch.
-
i.
-
c.Transfer the anesthetized pup to the surgery table equipped with anesthesia mask and temperature controller set at 37°C.
-
d.Adjust the isoflurane concentration to the required range and test the depth of anesthesia again.
-
a.
Note: We recommend 2.5%–3% for the maintenance of anesthesia in neonatal mice. It is necessary to adjust the concentration according to the breathing of the pup.
-
8.Surgery on the animal under anesthesia.
-
a.Disinfect the skin above the skull with 70% ethanol (or other local disinfectant).
-
b.Apply the mixture of bupivacaine (0.25%) and lidocaine (1%) by subcutaneous injection for local anesthesia and pain relief.Note: Follow your local animal care guidelines regarding pre- and postoperative analgesia.
 Pause point: Wait for 5 min for the analgesia to work before the next steps.
Pause point: Wait for 5 min for the analgesia to work before the next steps. -
c.Cut the fur and make an incision on the skin above the skull with sterilized scissors.
-
d.Remove the skin above the skull and residual tissue gently (Figure 3A (i)).
-
e.Clean the skull using 3% hydrogen peroxide-soaked cotton buds.
-
f.Dry the skull and mark the locations of the target areas and reference site on cerebellum (Figure 3A (i)).Note: Stereotaxic coordinates of OB: 0.5–0.8 mm anterior to frontonasal suture, 0.5 mm lateral to midline; LEC: 0–0.3 mm anterior to lambda, 6.5 mm lateral to the midline. The mark over cerebellum location is for drilling a hole to insert a silver wire as ground and reference for the recording.
-
g.Under a blue light (wavelength 440–514 nm) such as a fluorescent flashlight or microscope, the hChR2-EYFP expression in the OB can be easily visualized through the surface of the brain (Figure 3A (iii)).
-
h.Use dental cement to strengthen the fragile skull of neonatal mice and to fix two round plastic bars for tight head fixation during the recording (Figure 3A (ii)).
-
i.Prepare Omnifill C dental cement by appropriately mixing the powder and liquid.Note: The amount can be adjusted to change the viscosity upon preference.
-
ii.Mount the plastic bars on the nasal and occipital bones with the dental cement.
-
iii.Add more dental cement between the two bars for tighter connection.Note: For recording in other regions, adjust the cement amount and position to ensure the accessibility of the targeted regions. Also, take care that the cement does not adhere to the nose or eyelids of the pup.
-
i.
-
i.Perform craniotomies under the stereomicroscope (Figure 3A (ii)).
-
i.Drill two small holes of 0.5 mm radii on the marked locations on the skull (i.e., OB and LEC) and carefully drip 0.9% sodium chloride to reduce bleeding and heat damage to brain tissue. Also, drill a hole in the skull over the cerebellum for inserting a reference wire.
 CRITICAL: To ensure the quality of the recordings, avoid impertinent drilling to minimize the damage to brain tissues, neuronal death and neuroinflammation.
CRITICAL: To ensure the quality of the recordings, avoid impertinent drilling to minimize the damage to brain tissues, neuronal death and neuroinflammation. -
ii.Gently remove the dura mater of the holes with the tip of a sterilized fine needle.
-
i.
-
a.
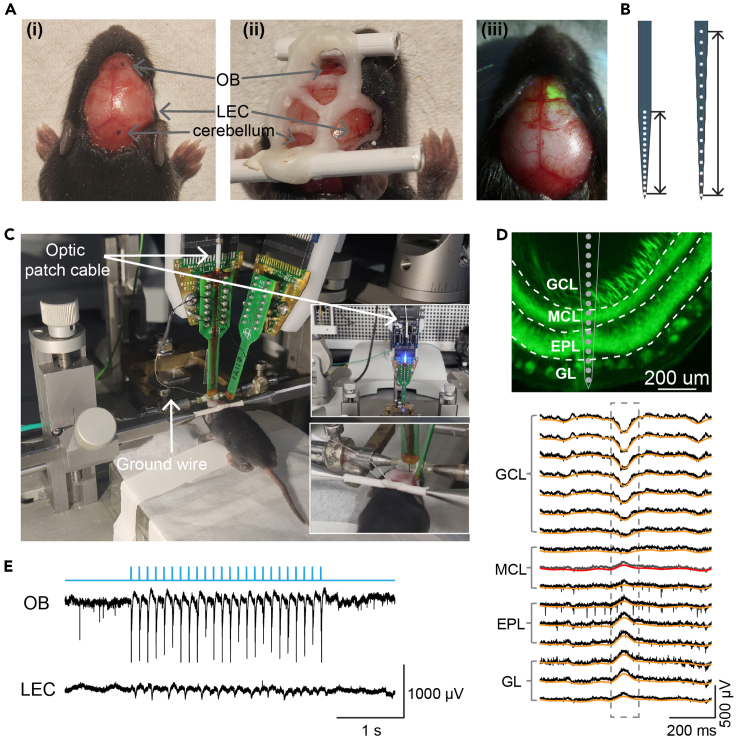
Figure 3.
Procedure for in vivo electrophysiological recording combined with optogenetics
(A) Anesthetized neonatal mouse with recording locations marked (i) and exposed (ii) for multi-site extracellular recordings. The two plastic bars were mounted on the nasal and occipital bones respectively with the dental cement for tight head fixation during the recording. (iii) Overlaid photograph (from pictures taken under 440 nm blue light and neutral white light) showing the expression of hChR2-EYFP in the OB through the surface of the brain.
(B) Schematic reconstruction of extracellular recording electrode tips with 50 μm (left) and 100 μm (right) inter-site spacing. Scale bars correspond to 750 μm (left) and 1500 μm (right) respectively.
(C) Head-fixed neonatal mouse in the stereotaxic apparatus with optrode inserted in OB and electrode inserted in LEC. The optrode is attached to the laser delivery system (upper inner photo) for optogenetic stimulation. The silver wire is positioned into the cerebellum as ground and reference (lower inner photo).
(D) Digital post-mortem photomontage reconstructing the location of the 16 recording channels of optrode in ventral OB of a P11 hChR2-EYFP expressing mouse (top; GCL: granule cell layer; MCL: mitral cell layer; EPL: external plexiform layer; GL: glomerular layer), and the laminar spontaneous activity across the corresponding OB layers (down; raw recording traces in black, 1–12 Hz band-pass filtered LFP traces in orange). Note that the 1–12 Hz LFPs reverse at the channel below the first MCL recording channel (traces in gray and red).
(E) Representative traces of simultaneous extracellular recordings in OB and LEC with both LFPs and MUA during blue light (473 nm) pulse stimulation at 8 Hz. The TTL signals indicating 3-ms light square pulses are marked in light blue.
In vivo multi-site electrophysiological recordings and optogenetic stimulation
Timing: 1 h/animal
Here we describe the procedure for in vivo acute electrophysiological recordings performed simultaneously in both OB and LEC, as well as the simultaneous light stimulation in P8-11 mice with M/TCs expressing ChR2. Detailed descriptions for post-hoc brain dissection with intact OB for verification of ChR2 expression after the recordings and stimulations are also provided here. The protocol for light stimulation is also applicable to other brain regions with the presence of the opsin.
-
9.Multi-site extracellular recordings on the awake animal.
-
a.Transfer the pup to the setup for electrophysiological recording and fix it with the plastic bars in the stereotaxic apparatus.
-
b.Mount the optrode and electrode (with 16 recording sites, Figures 3B and 3C) on the headstages of the extracellular amplifier.Note: To combine in vivo electrophysiology and light stimulation, the optrode needs to be attached with the optic patch cable. Also check the connection between the optrode, optic patch cable and diode laser before inserting the optrode into the OB.Optional: To better visualize the electrode track in post-mortem histological confirmation, use a micropipette to drop 1 μL of fluorescent dye (e.g., 1 μg/μL DiI diluted in pure ethanol) to the tip of the electrode and wait for the dye to dry before mounting it to the headstage.
-
c.Turn on the extracellular amplifier and data acquisition software.
-
d.Insert a silver wire into the hole over the cerebellum as ground and reference for the recordings.
-
e.By adjusting the manipulator arms of the stereotaxic apparatus, slowly insert then lower the mounted optrode and electrode into appropriate coordinates of OB and LEC respectively (optrode for ventral OB: 1.8–2 mm deep, 0° from the vertical plane; electrode for LEC: 2–2.4 mm deep, 15° from the vertical plane).
-
f.Confirm the position of the optrode in mitral cell layer (MCL) according to the reversal of low-frequency (1–12 Hz) oscillations which is prominent and visible in the raw recording traces of the local field potentials (LFPs) between MCL and granule cell layer (GCL) of ventral OB (Figure 3D).
 Pause point: For stable recording quality, pups should be allowed to recover for 20 min after the insertion of electrodes.
Pause point: For stable recording quality, pups should be allowed to recover for 20 min after the insertion of electrodes. -
g.Collect the digitized raw extracellular recording data of spontaneous activity with both LFPs and multi-unit activity (MUA).Note: In our experiments, the signals were sampled at 32000 Hz and band-pass (0.1–9000 Hz) filtered by the acquisition software.
-
a.
-
10.Prepare the light stimulation system.
-
a.Turn the 473 nm laser on through the control software.
-
b.Adjust the optical output to the desired power.
 CRITICAL: Avoid tangling the optic patch cable for stable optical output.
CRITICAL: Avoid tangling the optic patch cable for stable optical output. -
c.Give a short light pulse (3 ms duration, intensities ranging between 0.05–0.4 mW measured at the tip of the optrode) to test the light-induced activity and confirm the successful activation of M/TCs in the OB.
-
a.
-
11.Apply light stimulation on the awake animal.
-
a.Set the parameters to deliver the desired stimulation sequence using a pulse generator.Note: We use 3 ms square pulses at a rate of 2/4/8/16/32 Hz, with alternating epochs of 3 s On/3 s Off and repeating 30 times generated by a custom-written controlling program in Arduino.
-
b.Monitor and collect the digitized extracellular recording data as well as the transistor-to-transistor logic (TTL) pulse signals for light delivery (Figure 3E).
-
a.
-
12.Sacrifice the pup after the recording procedure for post-mortem verification of ChR2 expression and electrode location.
-
a.Carefully retract the optrode/electrode out of the brain.Note: For cleaning the optrode/electrode, soak the probe in a detergent containing proteolytic enzyme for 15 min and rinse it with a few drops of distilled water afterward.
-
b.Remove the pup from the stereotaxic apparatus.
-
c.Euthanize the pup and perform transcardiac perfusion with 0.9% sodium chloride then 4% PFA at a constant speed of ∼0.2 mL/s for 20 min.Note: We use an overdose intraperitoneal injection of 10% ketamine/2% xylazine. Please follow your local animal care guidelines regarding animal euthanasia.
-
d.Remove the brain for post-mortem verifications.
-
i.Cut off the head from the neck.
-
ii.Make an incision from the back of the head with the scissors.
-
iii.Cut along the midline of the skull to the extremity of the nasal bone then cut laterally across the lambda and the frontal bone over the OB.
-
iv.Peel back the segments of the skull to expose the underlying brain with the forceps.
 CRITICAL: The frontal bone is tougher than the other parts of the skull. Avoid too strong pressure when cutting and moving the frontal bone to prevent OB damage.
CRITICAL: The frontal bone is tougher than the other parts of the skull. Avoid too strong pressure when cutting and moving the frontal bone to prevent OB damage. -
v.Gently resect all the connective tissue and nerves anchoring the brain to the lower skull cavity with the scissors.
-
vi.Carefully lift the OBs from the connection with the remaining frontal bone.
-
vii.Carefully separate the intact brain from the cranial vault.
-
viii.Transfer the brain to the fixing solution (e.g., 4% PFA with a pH of 7.2) for 12–24 h to preserve the tissue structure and morphology before post-mortem histology.
-
i.
-
a.
Expected outcomes
Optogenetic techniques provide a spatially and temporally precise approach for studying the function of diverse brain circuits, including the sensory systems. Taking the advantages of a transgenic cre-recombinase mouse line and cre-dependent expression of an optogenetic tool, we injected an AAV9 encoding light-gated ChR2 into the right OB of P1 Tbet-cre mice to target the opsin expression specifically in M/TCs. At P8, the robust co-expression of the fluorophore EYFP with ChR2 was observed in the OB, labeling both the soma and axonal projections of M/TCs (Figure 2C). This indicates a stable expression of ChR2 and the feasibility of performing light stimulation at this developmental time point. Adequate ChR2 expression following virus injection targeting other brain regions was also found at either this time point or even earlier in our previous research.12,13 Thus, the protocol enables the manipulation of specific neuronal populations during the neonatal period.
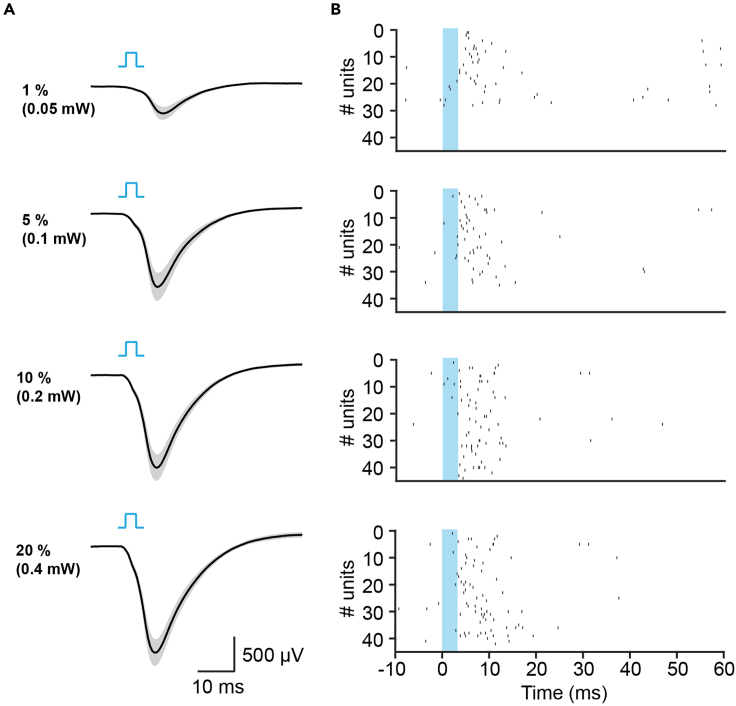
Based on the sufficient ChR2 expression at P8 and the fact that olfaction is already functional in mice at this age, we activated the ChR2-expressing M/TCs in the OB of head-fixed neonatal (P8-11) mice using blue light pulses (473 nm, 3 ms). The extracellular neural activity in OB and LEC were simultaneously recorded to investigate the influence of light-activated M/TCs on the neuronal activity within the olfactory network. In addition to adequate virus expression, the successful activation of the opsin-expressing neurons is also dependent on the appropriate stimulation parameters (Figure 4). First, we visualized the LFPs and spiking activity (extracted by spike sorting according to the waveform features beforehand) in the OB in response to the first 3-ms light pulse under different light intensities to evaluate the effects of in vivo M/TC activation. The magnitude of the light-induced averaged LFPs (Figure 4A) as well as the numbers of the responding single units (Figure 4B) grew with the increasing intensities from 0.05 mW to 0.2 mW (1%–10% of the maximal laser power), and the responses to 0.2 mW are comparable with that to 0.4 mW (20% of the maximal laser power), indicating sufficient optogenetic activation at light intensities in a range between 0.2-0.4 mW in this experimental setup.
Figure 4.
Effects of optogenetic stimulation on ChR2-expressing M/TCs with different light intensities
(A) Averaged MCL field potentials induced by the first blue light (473 nm) pulse delivered to the OB of P8-11 mice with the light intensity set as 1%, 5% 10% and 20% of the maximal intensity (1.75 mW) of laser power. The corresponding light intensities measured at the tip of the optrode are indicated following the percentage. The 3-ms light square pulses are denoted by light blue bars.
(B) Raster plots of OB neuronal firing in response to the first blue light pulse delivered in M/TCs of P8-11 mice at the corresponding intensity in (A).
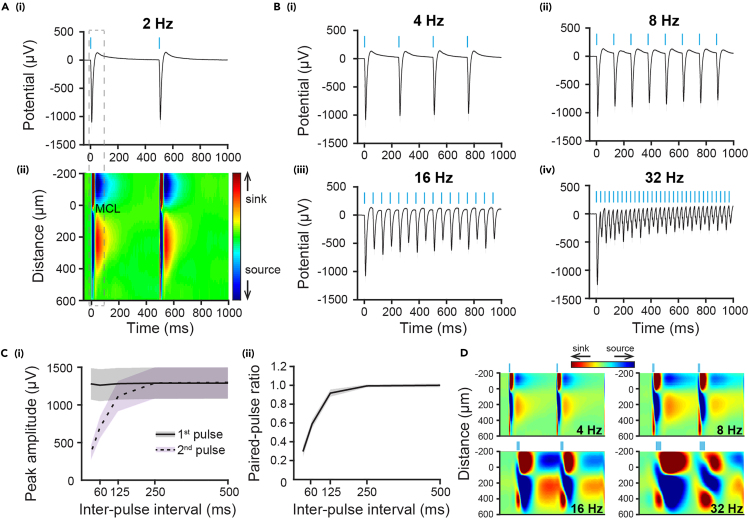
We next measured the LFP responses in the MCL in response to rhythmic light pulse sequences delivered at different frequencies. Reliable light-evoked field potentials with large amplitude were found in MCL following each single light pulse (Figures 5A and 5B), which confirms the effective repetitive activation of M/TCs. Notably, the direct activation of M/TCs causes a reversal of transmembrane currents shown by current-source density (CSD) analysis with a sink at the reversal channel of MCL and time-aligned with the peak amplitude of LFPs (Figure 5A (ii)), which can be used for the further dissection of the neuronal population involved in generating LFP dynamics in the OB. Quantification of peak amplitudes of the light-evoked LFPs revealed lower peak amplitudes for the 2nd pulses at higher frequencies (16 and 32 Hz, i.e., with 62.5 and 31.25 ms inter-pulse interval) even though the 1st pulses could trigger LFPs with comparable peak amplitudes (Figure 5C (i)). This correspondingly leads to a lower paired-pulse ratio (given by responses to 2nd pulse/responses to 1st pulse) at higher frequencies (Figure 5C (ii)), which is in line with our previous findings from in vitro patch-clamp recordings showing that the M/TC firing follows progressively less in response to the stimulation at higher frequencies.3 This indicates that the activation of the neuronal population in the OB triggered by the 2nd pulse is lower when compared with that triggered by the 1st pulse. Similarly, laminar CSD analysis for the light-evoked LFPs following the 2nd pulse in vivo showed progressively weaker magnitude of inversion, which could reflect the decay for excitatory synaptic activation of neuronal ensemble along the increasing stimulation frequencies. These results together not only rule out that the evoked activity is induced by a photovoltaic effect of light stimulation,14 but also suggest that even though ChR2 (E123T/T159C mutant) used here rapidly responds to light stimulation at the millisecond level,15 the repetitive light pulses at a high frequency might cause less efficient synaptic transmission or lower synaptic currents triggered by the light activation, which lead to a reduction of response amplitude upon the 2nd pulse. Thus, the frequency of the rhythmic light pulses is also an important parameter to consider for light stimulation design.
Figure 5.
LFP responses to light stimulation of ChR2-expressing M/TCs
(A) (i) Averaged field potentials induced by 2 Hz blue light (473 nm) pulses in the MCL of OB from P8-11 mice. The 3-ms light square pulses are denoted by light blue bars. (ii) Color map of current source density (CSD) across 16 recording channels in the OB during the same periods as (i).
(B) Averaged field potentials induced by blue light pulses delivered at 4 Hz (i)/8 Hz (ii)/16 Hz (iii)/32 Hz (iv) in the MCL of OB from P8-11 mice. Data are presented as median ± standard error of the median.
(C) (i) Averaged peak amplitude of potentials induced by the first (black solid line) and second (black dashed line) light pulses at different inter-pulse intervals. (ii) Quantified paired-pulse ratio (2nd peak amplitude/1st peak amplitude). Data are presented as median ± standard error of the median.
(D) Color maps of CSD in the OB in response to the first and second blue light pulses at 4 Hz, 8 Hz, 16 Hz and 32 Hz. The 3-ms light square pulses are denoted by light blue bars.
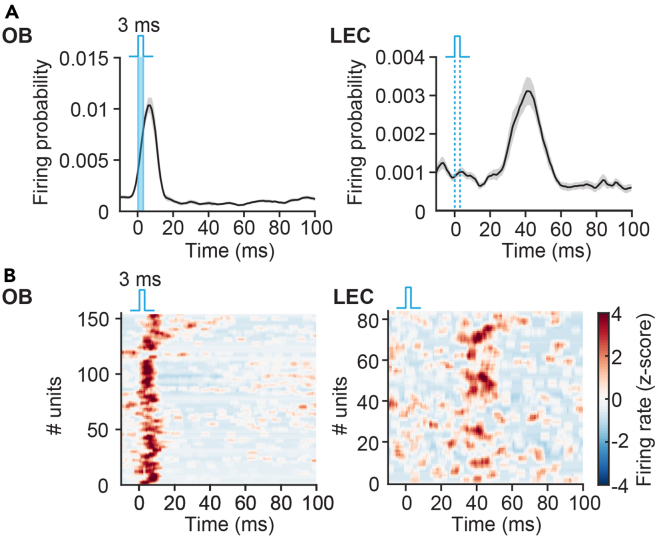
Due to its spatiotemporal precision, optogenetics represents a useful method in dissecting the function of the large-scale neural network including the olfactory circuits. For example, we simultaneously recorded the activity in the LEC, one of the target areas of M/TC axonal projections, and investigated the light-evoked activity in OB and LEC, in particular the evoked spike timing. Light stimulation of M/TCs increased neuronal firing in both regions, with the peak of evoked firing activity in downstream LEC being substantially delayed when compared to the OB (Figure 6; OB peaked at ∼8 ms after stimulus onset, LEC peaked at ∼40 ms after stimulus onset). This indicates that the direct activation of M/TCs not only elicits the firing in OB, but also induces neuronal firing in LEC through long-range functional connections already at neonatal age.
Figure 6.
Neuronal firing responses to light stimulation of ChR2-expressing M/TCs
(A) Averaged probability of neuronal firing in the OB and LEC of P8-11 mice in response to 3-ms blue light (473 nm) pulses delivered to the OB. The 3-ms light square pulses are denoted by light blue bars. Data are presented as median ± standard error of the median.
(B) Z-scored firing rates of neurons in OB and LEC of P8-11 mice in response to 3-ms blue light pulses delivered to the OB.
Limitations
We introduce here a comprehensive protocol for combining in vivo acute electrophysiology with AAV-mediated optogenetic activation of M/TCs in mice at neonatal age. Special care for the animals is mandatory for the successful application of the methods. Thus, a certain amount of time in practice should be invested before executing the entire protocol described here. Neonatal pups require regular interactions and feeding by their mother and therefore, it is almost infeasible to perform extracellular recordings chronically, given the implantation of recording devices significantly interferes with normal maternal care and restricts both the somatic and brain development of the pups.
Expression of optogenetic actuators can be achieved with different strategies, such as injecting the viral vector encoding the desired opsin into the brain region of interest,14 crossing a transgenic mouse line carrying the desired opsin with another line with cre recombinase expressed in specific neuronal populations,16 or prenatally transfecting the neuronal precursor cells by in utero electroporation (IUE).17 The cre-dependent AAV-driven ChR2 expression described here to manipulate the principal projection neurons in OB is region and cell-type specific, but it also has relatively low flexibility for the experimental timing, since it requires several days after the injection to achieve adequate expression. On the other hand, while crossing transgenic mouse lines provides a possibility for cell type-specific opsin expression without additional experimental operation, it is costly and time-consuming to generate and maintain the required mouse lines. IUE proves to successfully target cortical and sub-cortical areas for optogenetic studies shortly after birth with sufficient opsin expression among the specific neuronal population,17 but there is a potential for off-target opsin expression in other brain areas. In addition, not all cell populations can be targeted through the labeling with IUE. Furthermore, the in utero operation might be technically challenging particularly for those without expertise in embryonic manipulation techniques and can cause negative stress on the mother mice. In general, the expression strategy should be designed according to the experimental aims. Thus, experimenters have to consider, which neuronal type(s), brain areas and layers as well as which developmental time window to be targeted and manipulated.
Troubleshooting
Problem 1
Mice do not recover after the anesthesia (refer to steps 3–4 and 7–8).
Potential solution
There are several possibilities.
-
•
The temperature of the heating pad is too high or too low.
-
•
The concentration of the isoflurane is set too high.
-
•
Brain damage during microinjections or craniotomies.
We suggest checking the setup before every injection and making sure the breathing is regular under isoflurane anesthesia. Careful and attentive injection and drilling are also crucial.
Problem 2
The injected region does not express the virus or has inadequate virus expression (refer to step 4). This is the most common possibility accounting for failed stimulation.
Potential solution
-
•
Optimize the injection site according to the post-mortem histology.
-
•
Avoid using a virus after multiple freeze/thaw cycles.
-
•
Make sure the needle is not clogged (e.g., confirming the outflow of virus from the needle before and after every injection through the stereoscope, or using a micro-pressure injection system coupled with a glass micropipette).
-
•
After the injection, leave the needle inserted for a longer time and raise it as slowly as possible.
-
•
Allow sufficient time for expression before the next step experiment(s).
-
•
Check the appropriate virus-genotype pairing.
Problem 3
The mother of the injected pups does not take care of the pups (refer to step 6).
Potential solution
-
•
Clean up and disinfect the possible bleeding on the injection sites.
-
•
Return the pups only after they fully recover (i.e., breathing normally, adjusting body posture autonomously and showing healthy pink coloring and body temperature).
-
•
Keep some beddings with the pups when returning them to the homecage to make them have sufficient homecage smell.
-
•
Make sure the homecage has appropriate nesting materials for better maternal care.
Problem 4
The signal-to-noise ratio of the recording is low (refer to step 9).
Potential solution
-
•
Shield the recording table and all noisy power sources (if not possible to remove them).
-
•
Make sure the ground silver wire is fully embedded inside the reference site and securely pinned to the head stage.
-
•
Confirm the tight head fixation of the pup.
-
•
Clean the recording electrodes after every recording.
-
•
If necessary, use a new electrode.
Problem 5
Low or inadequate effect of the optogenetic stimulation (refer to step 11).
Potential solution
-
•
Make sure the stimulated region has sufficient opsin expression.
-
•
Make sure the connection of the devices for stimulation is correct.
-
•
Adjust the power and frequency of light for reliable firing activity.
-
•
Test the light transition through the optic fiber and optrode to confirm the sufficient light intensity before every recording.
-
•
Perform careful histological verification for both opsin expression and optrode insertion following the failed stimulation.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Ileana L. Hanganu-Opatz (hangop@zmnh.uni-hamburg.de).
Technical contact
Technical questions on executing this protocol should be directed to and will be answered by the technical contact, Yu-Nan Chen (yunan.chen@zmnh.uni-hamburg.de).
Materials availability
The study did not generate new unique reagents.
Data and code availability
-
•
The original datasets and analysis code for the current study are available from the corresponding authors upon request.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the corresponding authors upon request.
Acknowledgments
We thank Dr. Sabine Gretenkord for her contribution to the establishment of the methodology, as well as Peggy Putthoff, Achim Dahlmann, and Annette Marquardt for their excellent technical assistance. This work was funded by grants from the German Research Foundation (Ha4466/11-1, Ha4466/20-1, Ha4466/22-1, and SFB 936 178316478 to I.L.H.-O.), European Research Council (ERC-2015-CoG 681577 to I.L.H.-O.), Horizon 2020 DEEPER (101016787 to I.L.H.-O.), MSCA-ITN (860563 to I.L.H.-O.), and Landesforschungsförderung Hamburg (LFF73 and LFF76 to I.L.H.-O.).
Author contributions
Investigation, Y.-N.C. and J.K.K.; analysis and visualization, Y.-N.C.; writing – original draft, Y.-N.C.; writing – review and editing, Y.-N.C., J.K.K., S.H.B., and I.L.H.-O.; funding acquisition, I.L.H.-O.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Yu-Nan Chen, Email: yunan.chen@zmnh.uni-hamburg.de.
Ileana L. Hanganu-Opatz, Email: hangop@zmnh.uni-hamburg.de.
References
- 1.Chen Y.-N., Kostka J.K., Bitzenhofer S.H., Hanganu-Opatz I.L. Olfactory bulb activity shapes the development of entorhinal-hippocampal coupling and associated cognitive abilities. Curr. Biol. 2023;33:4353–4366.e5. doi: 10.1016/j.cub.2023.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gretenkord S., Kostka J.K., Hartung H., Watznauer K., Fleck D., Minier-Toribio A., Spehr M., Hanganu-Opatz I.L. Coordinated electrical activity in the olfactory bulb gates the oscillatory entrainment of entorhinal networks in neonatal mice. PLoS Biol. 2019;17 doi: 10.1371/journal.pbio.2006994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kostka J.K., Hanganu-Opatz I.L. Olfactory-driven beta band entrainment of limbic circuitry during neonatal development. J. Physiol. 2023;601:3605–3630. doi: 10.1113/JP284401. [DOI] [PubMed] [Google Scholar]
- 4.Schaal B., Saxton T.K., Loos H., Soussignan R., Durand K. Olfaction scaffolds the developing human from neonate to adolescent and beyond. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020;375 doi: 10.1098/rstb.2019.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller S.S., Spear N.E. Olfactory learning in the rat neonate soon after birth. Dev. Psychobiol. 2008;50:554–565. doi: 10.1002/dev.20318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burwell R.D. The Parahippocampal Region: Corticocortical Connectivity. Ann. N. Y. Acad. Sci. 2000;911:25–42. doi: 10.1111/j.1749-6632.2000.tb06717.x. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd G.M. Perspectives on olfactory processing, conscious perception, and orbitofrontal cortex. Ann. N. Y. Acad. Sci. 2007;1121:87–101. doi: 10.1196/annals.1401.032. [DOI] [PubMed] [Google Scholar]
- 8.Deisseroth K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci. 2015;18:1213–1225. doi: 10.1038/nn.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deisseroth K., Hegemann P. The form and function of channelrhodopsin. Science. 2017;357 doi: 10.1126/science.aan5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gombash S.E., Cowley C.J., Fitzgerald J.A., Hall J.C.E., Mueller C., Christofi F.L., Foust K.D. Intravenous AAV9 efficiently transduces myenteric neurons in neonate and juvenile mice. Front. Mol. Neurosci. 2014;7 doi: 10.3389/fnmol.2014.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Protocol 27165 - Tg(Tbx21-cre)1Dlc. https://www.jax.org/Protocol?stockNumber=024507&protocolID=27165.
- 12.Xu X., Song L., Kringel R., Hanganu-Opatz I.L. Developmental decrease of entorhinal-hippocampal communication in immune-challenged DISC1 knockdown mice. Nat. Commun. 2021;12:6810–6817. doi: 10.1038/s41467-021-27114-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chini M., Pfeffer T., Hanganu-Opatz I. An increase of inhibition drives the developmental decorrelation of neural activity. Elife. 2022;11 doi: 10.7554/eLife.78811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cardin J.A., Carlén M., Meletis K., Knoblich U., Zhang F., Deisseroth K., Tsai L.-H., Moore C.I. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nat. Protoc. 2010;5:247–254. doi: 10.1038/nprot.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berndt A., Schoenenberger P., Mattis J., Tye K.M., Deisseroth K., Hegemann P., Oertner T.G. High-efficiency channelrhodopsins for fast neuronal stimulation at low light levels. Proc. Natl. Acad. Sci. USA. 2011;108:7595–7600. doi: 10.1073/pnas.1017210108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haddad R., Lanjuin A., Madisen L., Zeng H., Murthy V.N., Uchida N. Olfactory cortical neurons read out a relative time code in the olfactory bulb. Nat. Neurosci. 2013;16:949–957. doi: 10.1038/nn.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bitzenhofer S.H., Ahlbeck J., Hanganu-Opatz I.L. Methodological Approach for Optogenetic Manipulation of Neonatal Neuronal Networks. Front. Cell. Neurosci. 2017;11 doi: 10.3389/fncel.2017.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
-
•
The original datasets and analysis code for the current study are available from the corresponding authors upon request.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the corresponding authors upon request.