Abstract
Background
Oral squamous cell carcinoma (OSCC) remains a major death cause in head and neck cancers, but the exact pathogenesis mechanisms of OSCC are largely unclear.
Results
Saliva derived from OSCC patients but not healthy controls (HCs) significantly promotes OSCC development and progression in rat models, and metabolomic analyses reveal saliva of OSCC patients but not HCs and OSCC tissues but not adjacent non-tumor tissues contain higher levels of kynurenic acid (KYNA). Furthermore, large amounts of Streptococcus mutans (S. mutans) colonize in OSCC tumor tissues, and such intratumoral S. mutans mediates KYNA overproductions via utilizing its protein antigen c (PAc). KYNA shifts the cellular types in the tumor microenvironment (TME) of OSCC and predominantly expedites the expansions of S100a8highS100a9high neutrophils to produce more interleukin 1β (IL-1β), which further expands neutrophils and induces CD8 + T cell exhaustion in TME and therefore promotes OSCC. Also, KYNA compromises the therapeutic effects of programmed cell death ligand 1 (PD-L1) and IL-1β blockades in oral carcinogenesis model. Moreover, KYNA-mediated immunosuppressive program and aryl hydrocarbon receptor (AHR) expression correlate with impaired anti-tumor immunity and poorer survival of OSCC patients.
Conclusions
Thus, aberration of oral microbiota and intratumoral colonization of specific oral bacterium such as S. mutans may increase the production of onco-metabolites, exacerbate the oral mucosal carcinogenesis, reprogram a highly immunosuppressive TME, and promote OSCC, highlighting the potential of interfering with oral microbiota and microbial metabolism for OSCC preventions and therapeutics.
Video Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s40168-024-01907-9.
Keywords: Oral squamous cell carcinoma, Oral microbiota, Tumor microenvironment
Introduction
Oral squamous cell carcinoma (OSCC) remains a major cause of morbidity and mortality in patients with head and neck squamous cell carcinoma (HNSCC) [1]. Despite recent advances in surgical techniques, adjuvant therapy, molecular targeted therapy, and immunotherapy, outcomes are still unsatisfactory, particularly in advanced OSCC patients with a 5-year survival rate less than 20% [2]. Although genetic factors, tobacco, betel quid, alcohol, and poor oral hygiene are considered predominant risk factors for OSCC, the exact pathogenesis mechanisms of OSCC remain largely unclear [3]. Oral carcinogenesis is a stepwise evolutionary process that initiates from normal to dysplasia and then carcinoma. Oral leukoplakia (OLK) shows typical histologic feature of dysplasia, which is regarded as the most common type of oral precancerous lesion with rates of 1.1–40.8% for malignant transformation to OSCC [4, 5]. Therefore, interrogating the molecular mechanisms driving the development, progression, and poor prognosis of OSCC is fundamentally important for the discovery of potential new prevention and therapeutic targets of OSCC.
The oral cavity is the first encounter with life-sustaining substances such as food, air and water, and becomes one of the most important dynamic anatomic interaction windows between human and environment, harboring billions of bacteria, viruses, fungi and other unidentified microbes, which were called oral microbiota [6, 7]. The oral microbiota is the second most diverse microbial community colonizing in the human body, after the intestinal microbiota [8]. However, compared with intestinal microbiota, oral microbiota is believed to be compositionally distinct and more stable and resilient to external perturbations such as dietary changes and antibiotic treatments [9]. Thus, oral microbiota is a particularly active architect driving constant interactions with multilayer oral squamous cell epitheliaum and immune cells to engineer a sustainable niche, which fundamentally shapes and is also reshaped by both oral microenvironment and systemic health.
The finely tuned crosstalk among the oral microbiota, mucosal immune responses and epithelial barrier plays a critical role in maintaining the oral mucosal architecture and homeostasis [10]. In contrast, the altered oral microbial community may promote the release of toxins and metabolites, propel the immune imbalance, and damage the integrity and tolerance of the epithelial barrier, which may therefore contribute to the aberration of inflammatory microenvironment and host signaling pathways that may promote the exacerbation of oral mucosal cell viability, proliferation and differentiation [11]. Particularly, oral bacteria show highly invasive nature and ability to facilitate the formation of large-scale bacterial biofilm on tooth pellicle and oral epithelium, which may designate some bacteria superior capability in spatial translocation and tissue colonization in the oral mucosa and further mediate the aberrated crosstalk among the oral microbiota, mucosal immune responses, and the epithelial barrier [7]. However, it is largely unknown regarding exact mechanistic pathways and consequences on oral microbiota-host crosstalk that may orchestrate the metabolic and immune balance, which may further determine the pathogenesis underlying the development, progression and prognosis of OSCC.
We hypothesized that oral microbiota is a key contributor to metabolically modulate aberrant oral mucosal immune microenvironment, thereby aggravating oral carcinogenesis. In this study, we aimed to integrate in vitro, in vivo, and in silico approaches to gain an ecosystem-level mechanistic crosstalk of microbiota-epithelia-metabolite-immunity axis in OSCC pathogenesis. Our work may shed light on targeting multifaceted interactions to develop an innovative microbiota-based therapeutic paradigm.
Results
Saliva derived from patients with OSCC significantly promotes 4NQO-induced oral carcinogenesis in rats
As an initial step to determine the role and underlying mechanism of oral microbiota in the pathogenesis of OSCC, we hypothesized that altered oral microbial community might change the composition and abundance of microbiota and metabolites in saliva, which may therefore lead to the disruption of the metabolic and immune homeostasis of oral microenvironment during the development and progression of OSCC. To address this hypothesis, 4-Nitroquinoline N-oxide (4NQO)-treated rats were co-administrated with saliva derived from OSCC and OLK patients and healthy controls (HCs) (Fig. 1A). Although all the tongues derived from rats showed granular hyperplasia, white flakes and spot plaques, the tongue lesions in the rats treated with OLK and OSCC saliva remarkably showed larger-scale erosion, ulcer, and endogenous growth with unclear boundaries at week 20 (a represented tongue lesion in each group was shown in Fig. 1B and more tongue lesions were shown in Fig. S1). Importantly, numbers of invasive carcinoma were significantly increased in rats received saliva of OLK and OSCC compared with those received saliva of HCs (Fig. 1C and D). Also, tongues derived from rats treated with OLK and OSCC saliva displayed much higher pathological scores, as revealed by hematoxylin and eosin (H&E) staining-based quantitative analyses of oral carcinogenesis pathology (Fig. 1E). To preliminarily examine the general immune features of TME, immunohistochemistry (IHC) analyses were performed to analyze the expression of Ki67, CD11b, CD3 and programmed cell death protein 1 (PD-1). IHC results showed that tongues derived from rats treated with OSCC saliva displayed much larger numbers of infiltrated Ki67-, CD11b- and PD-1-, but less CD3-expressing cells in tongue lesions than rats treated with saliva of HCs and saline (Fig. 1F and G), suggesting that tongues treated with OSCC saliva showed increased CD11b + myeloid cells, insufficient CD3 + T cell infiltrations, and more suppressive or exhausted immune features. Collectively, these findings suggested that transplantations of OLK and OSCC saliva promoted the oral carcinogenesis.
Fig. 1.
Saliva derived from OSCC patients promotes oral carcinogenesis in 4NQO-induced rat models. A Schematic illustration of the experimental protocol in 4NQO-induced carcinogenesis model. Rats were randomly assigned to four groups: saline, HCs saliva (rats treated with saliva from healthy controls), OLK saliva (rats treated with saliva from OLK patients), and OSCC saliva (rats treated with saliva from OSCC patients). HCs, healthy controls; OLK, oral leukoplakia; OSCC, oral squamous cell carcinoma. B Representative images of tongue lesions in rats with indicated treatments at weeks 8, 16, and 20. C Representative gross observation and H&E images of the tongues derived from rats treated with saline, saliva from HCs, OLK and OSCC patients at endpoint. Scale bars: 2.5 mm for original magnification, 500 µm for 5 × magnification and 125 µm for 20 × magnification. D Quantification of the histological degree of low-grade dysplasia, high-grade dysplasia, and carcinoma in rats with indicated treatments. E H&E scores of the histopathologic diagnoses in rats with indicated treatments. H&E, hematoxylin and eosin. F Representative IHC images of Ki67, CD11b, CD3, and PD-1 in rats with indicated treatments. Scale bars: 200 µm for 10 × magnification and 50 µm for 40 × magnification. PD-1, programmed cell death 1. G Quantification IHC analysis of Ki67, CD11b, CD3, and PD-1 in rats with indicated treatments. IHC, immunohistochemistry. In D, Fisher’s exact test; E, Kruskal–Wallis test; G, one-way ANOVA
Saliva of OSCC patients contains significantly higher levels of kynurenic acid (KYNA) than HCs and OLK patients, and elevated KYNA levels in saliva contribute to oral carcinogenesis
Since saliva derived from OLK and OSCC patients promoted the oral carcinogenesis, it was reasonable to postulate that OSCC saliva might contain pathological components such as proteins, carbohydrates, metabolites and DNA/RNA, and altered abundance levels of these pathological components might induce disruption of oral epithelial homeostasis, favoring the malignant transformation. To determine which saliva component is the primary driving force leading to malignant transformation of dysplasia, OSCC patients (n = 12), OLK patients (n = 15), and healthy controls (HCs, n = 6) were enrolled. There was no statistical difference in terms of demographic factors (age, sex, and tobacco use) among the three groups (Table S1).
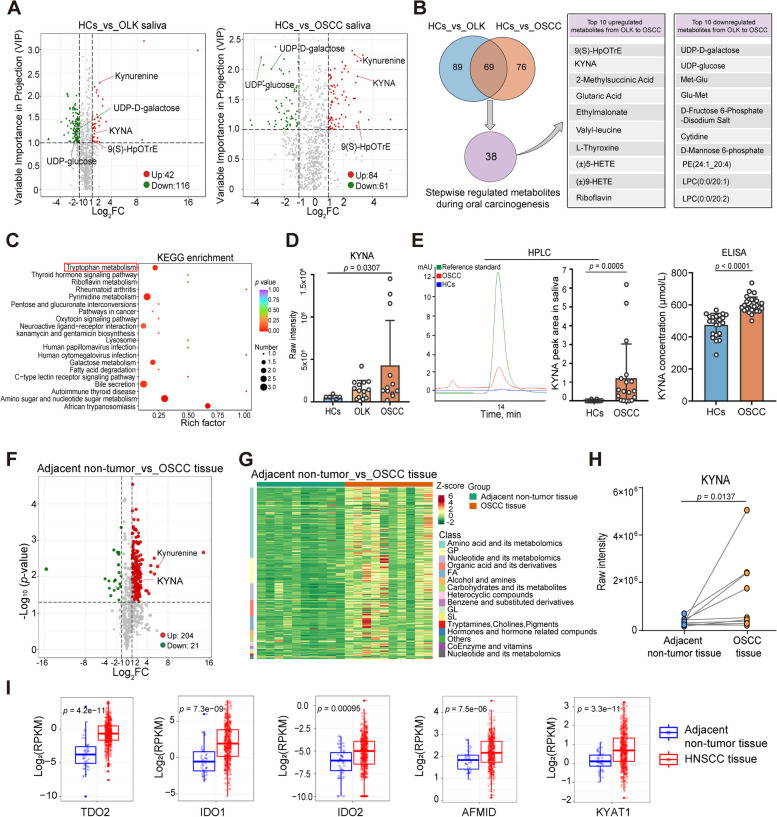
Widely targeted metabolomic analyses of saliva derived from these individuals (Table S1) were performed using ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). The total ion current (TIC) diagram curves of quality control (QC) samples were highly overlapped, and the retention time and peak intensity were consistent, indicating that the signal was stable throughout the detection process (Fig. S2A). Principal component analysis (PCA) and orthogonal partial least squares-discriminant analysis (OPLS-DA) showed that saliva metabolites significantly varied among OSCC and OLK patients and HCs (Fig. S2B-C). Further, after filtering the data using criteria including absolute Log2FC (fold change) ≥ 1 and variable importance in projection (VIP) score ≥ 1, we found that the abundance levels of 42 metabolites increased, and 116 metabolites decreased in saliva derived from OLK patients compared with those in HCs. Furthermore, 84 upregulated and 61 downregulated metabolites, respectively, were identified in the saliva of OSCC patients, compared with the saliva of HCs (Fig. 2A). Venn diagram analysis showed that there were 69 overlapping differentially abundant metabolites between HCs vs. OLK and HCs vs. OSCC patients (Fig. 2B). Due to the stepwise evolutionary process of oral carcinogenesis, after selecting the 69 overlapping differentially abundant metabolites between HCs vs. OLK and HCs vs. OSCC patients, we further screened the stepwise upregulated or downregulated (sustainable increase or decrease) metabolites from normal to OLK, and from OLK to OSCC saliva based on the abundance level of each metabolite. Consequently, 38 compounds showed the most significant differential abundance levels during oral carcinogenesis. Notably, the top 10 most significantly upregulated metabolites in epithelial malignant transformation (from OLK to OSCC) included 9(S)-HpOTrE, KYNA 2-methylsuccinic acid, etc., and KYNA exhibited the greatest increase among filtered hydrophilic compounds. The top 10 most significantly downregulated metabolites in epithelial malignant transformation included UDP-D-galactose, UDP-glucose, Met-Glu, etc. (Fig. 2B). Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis revealed that these 38 differentially expressed metabolites were mainly enriched in biosynthetic and metabolic processes such as tryptophan metabolism (Fig. 2C and Fig. S2D). Importantly, compared with saliva derived from HCs and OLK, OSCC saliva contained significantly higher levels of KYNA, a key product involving in kynurenine pathway of tryptophan metabolism (Fig. 2D).
Fig. 2.
KYNA concentration is significantly elevated in saliva of OSCC patients than HCs and OLK patients, and KYNA concentrations in tumors are much higher than non-tumor tissues derived from the same OSCC patients. A Volcano plots comparing metabolites of HCs (n = 6) vs. OLK patients (n = 15) and HCs (n = 6) vs. OSCC patients (n = 12) in whole saliva by widely targeted metabolomics. KYNA, kynurenic acid. B Venn diagram showing overlapping, differentially abundant metabolites between HCs vs. OLK and HCs vs. OSCC to screen the stepwise regulated metabolites during oral carcinogenesis (left). Top 10 upregulated and downregulated metabolites ranked by the fold change of OSCC/OLK in the process of malignant transformation (right). ( ±)5-HETE, ( ±)5-hydroxy-6E,8Z,11Z,14Z-eicosatetraenoic acid; ( ±)9-HETE, ( ±)-9-hydroxy-5Z,7E,11Z,14Z-eicosatetraenoic acid; PE, phosphatidyl ethanolamine; LPC, lysophosphatidyl choline. C KEGG pathway analysis of 38 significantly stepwise altered metabolites during oral carcinogenesis. Diagram displaying the top 20 significant pathways ranked by the p-value. KEGG, Kyoto Encyclopedia of Genes and Genomes. D The relative concentrations of KYNA in whole saliva by UPLC-MS/MS in HCs, OLK, and OSCC groups. E Representative chromatogram and the relative concentration of KYNA (left) in whole saliva by HPLC in HCs (n = 10) and OSCC patients (n = 18) from an independent cohort. KYNA concentration determination in saliva by ELISA kits (right) in HCs (n = 22) and OSCC patients (n = 27) from an independent cohort. F Volcano plots comparing metabolites between tumor and adjacent non-tumor tissues from OSCC patients (n = 10 per group). G Heatmap and cluster analysis illustrating the metabolite profiles in tumor and adjacent non-tumor tissues from OSCC patients. H The relative concentration of KYNA by UPLC-MS/MS in paired adjacent non-tumor and tumor tissues from the same OSCC patients (n = 10). I Expression of tryptophan-catabolic enzymes in tumor and adjacent non-tumor tissues from HNSCC patients in TCGA dataset (n = 430). RPKM, reads per kilobase million. In D, Kruskal–Wallis test; E, Mann–Whitney U test; F, paired test; H, Wilcoxon matched-pairs signed rank test; I, unpaired Student’s t-test
To validate that KYNA content was indeed elevated in the saliva of OSCC patients, KYNA level was further detected by high-performance liquid chromatography (HPLC) and enzyme-linked immunosorbent assay (ELISA) from an independent cohort (demographic characteristics of these participants were shown in Table S2). Consistently, KYNA was significantly elevated in saliva from OSCC patients than those from HCs (Fig. 2E). Moreover, compared with saliva from HCs, OLK and OSCC patients showed a significant increase of kynurenine, an upstream metabolite of KYNA (Fig. S2E). Thus, the metabolomics analysis suggested saliva of OSCC patients contained much higher level of KYNA than that of OLK patients and HCs.
We then analyzed whether KYNA was indeed involved in modulating the pathogenesis of OSCC. To address this, OSCC tumor tissues and adjacent non-tumor tissues from the same patients were subjected for analysis of KYNA concentration by UPLC-MS/MS, and we found OSCC tissues contained much higher levels of KYNA and kynurenine than adjacent non-tumor tissues derived from the same patients (Figs. 2F–H and S2F–S2I). Notably, tryptophan-catabolic enzymes (TCEs), such as tryptophan-2,3-dioxygenase (TDO2), indoleamine-2,3-dioxygenase 1 and 2 (IDO1 and IDO2), arylformamidase (AFMID) and kynurenine aminotransferases 1 (KYAT1), which mediated tryptophan degradation to produce bioactive downstream metabolites [12], were significantly upregulated in HNSCC tumor tissues compared with adjacent non-tumor tissues in the cancer genome atlas (TCGA) dataset (Fig. 2I). These data suggested higher concentrations of KYNA were indeed associated with OSCC.
Tumor-colonized Streptococcus mutans (S. mutans) utilizes A-region of protein antigen c (PAc) to mediate higher KYNA concentration during oral carcinogenesis
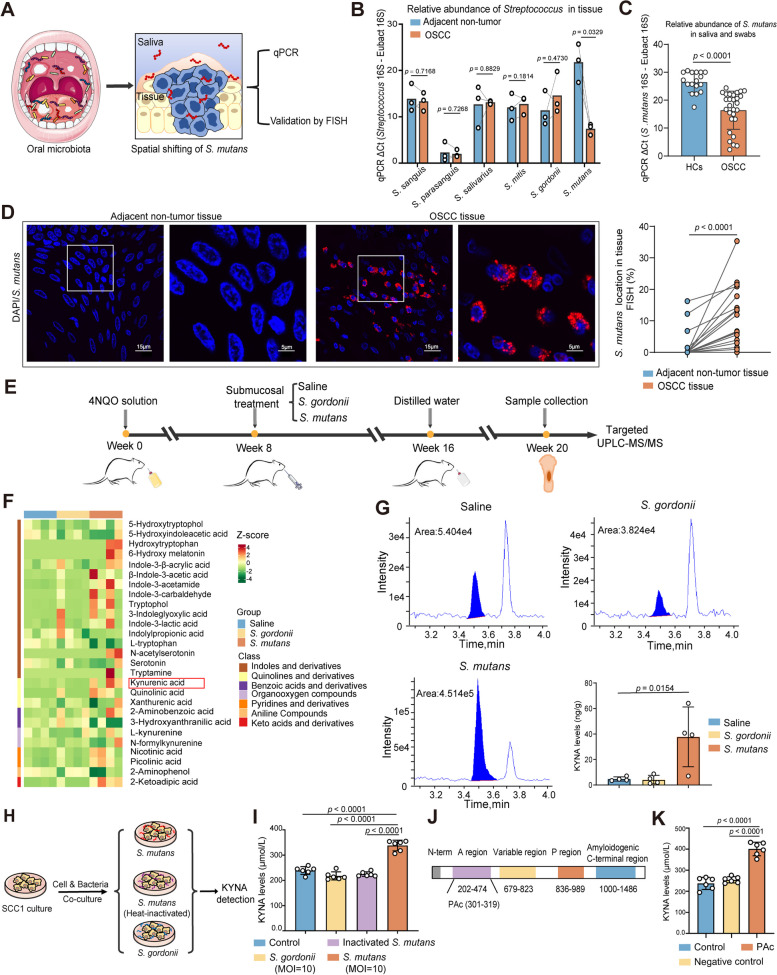
We then sought to explore the mechanisms underlying the increased concentration of KYNA in the saliva of OSCC. Tumor-associated microbiota is an intrinsic component of the TME, which critically shapes the metabolic patterns and immune statuses in the TME. Although emerging evidences have revealed that Streptococci were significantly associated with high carcinogenesis risk and advanced disease, the centrality of Streptococci to OSCC is still debated [13, 14]. We hypothesized that tumor-resident Streptococci might induce higher abundance levels of KYNA and further promoted oral carcinogenesis. To explore the potential abundance shifts of Streptococci species, the relative abundance of major oral Streptococci species [15] (including S. sanguis, S.parasanguis, S. salivarius, S. mitis, S. gordonii, and S. mutans) were analyzed by fluorescence quantitative polymerase chain reaction (qPCR) in the oral tumor and adjacent non-tumor tissues derived from OSCC patients (Fig. 3A). We found the S. mutans displayed the most significant increase of abundance level in the tumor tissues compared with non-tumor tissues in OSCC patients (Fig. 3B). The qPCR product visualization of S. mutans on 2% agarose gel electrophoresis revealed approximately 110 bp bands, and subsequent DNA sequencing and BLAST analysis confirmed that DNA bands originated from S. mutans (Fig. S3A-B). This data suggested that S. mutans was a predominant oral bacterial species colonized in tumor tissues of OSCC patients, but also implicated that S. mutans might translocate from saliva or superficial of oral epithelia to tumor tissues.
Fig. 3.
A significant amount of S. mutans colonizes in OSCC tissues and induces overproduction of KYNA using its A-region of PAc. A Experimental diagram for determining the relative abundance and spatial distribution of S. mutans in saliva and tumor tissues. S. mutans, Streptococcus mutans; qPCR, quantitative polymerase chain reaction; FISH, fluorescence in situ hybridization. B Relative abundance of representative Streptococci species in tumor and adjacent non-tumor tissues from OSCC patients detected by qPCR. C Relative abundance of S. mutans in saliva and swabs from HCs (n = 17) and OSCC patients (n = 28) detected by qPCR. D Representative FISH images (left) and quantification analysis (right) of S. mutans in paired adjacent non-tumor and OSCC tissues (n = 24) using a Cy3-conjugated 16S rDNA-directed probe (red: S. mutans colonization). Scale bars: 15 µm for 100 × magnification and 5 µm for 300 × magnification. E Schematic illustration of the experimental protocol in 4NQO-induced rat model of OSCC. Rats were randomly assigned to three groups: saline, S. gordonii (treated with S. gordonii), and S. mutans (treated with S. mutans). UPLC-MS/MS, ultra-performance liquid chromatography-tandem mass spectrometry. F Heatmap illustrating the tryptophan metabolite profiles of tongue tissues in saline, S. mutans-treated and S. gordonii-treated rats by UPLC-MS/MS-based targeted metabolomics. G Representative chromatograms of KYNA detected by targeted UPLC-MS/MS and quantitative analysis in rats with indicated treatments. H Schematic diagram depicting coculture strategy of live S. mutans UA159 strain, heat-inactivated S. mutans, and control bacterium S. gordonii with SCC1 for KYNA detection. I KYNA concentration in SCC1 supernatant after coculturing with S. gordonii, heat-inactivated S. mutans, and live S. mutans detected by ELISA. MOI, multiplicity of infection. J Schematic diagram of relevant domains within the primary sequence of A-region of PAc. PAC, surface protein antigen c. K KYNA concentration in SCC1 supernatant after stimulation using PAc peptide (residue 301–319) and negative control (ftsZ: residue 409–428) peptide detected by ELISA. In B, paired Student’s t-test; C, Mann–Whitney U test; D, Wilcoxon matched-pairs signed rank test; G, I, and K, one-way ANOVA
Additionally, saliva and swabs were also collected from OSCC patients (n = 28) and HCs (n = 17) to investigate the abundance of S. mutans in oral microenvironment. Remarkably, the relative abundance levels of S. mutans in OSCC were significantly higher than those in HCs (Fig. 3C), suggesting that S. mutans might have translocated from saliva or superficial of oral epithelium and penetrated into tumor tissue during carcinogenesis. To further validate this, sections of paired tumor and adjacent non-tumor tissues (n = 24) derived from OSCC patients were subjected to fluorescence in situ hybridization (FISH) analyses with a S. mutans 16S rDNA-specific probe. It was shown that S. mutans exhibited much higher levels of colonization and abundance in OSCC tumor tissues compared with the adjacent non-tumor tissues (Fig. 3D). Therefore, these pieces of data suggested that significant amounts of S. mutans penetrated and colonized in tumor tissues of OSCC.
We then further analyzed whether intratumoral colonization of S. mutans in OSCC tissues indeed induced an increase of KYNA. Live S. mutans strain (UA159) and S. gordonii (NCTC 7865) with no significant colonization in tumor tissues as negative control were orally incubated underneath oral mucosal epithelia of rats bearing 4NQO-induced spontaneous OSCC, and tongues of rats at week 20 were subjected for tryptophan and its metabolites analyses based on UPLC-MS/MS (Fig. 3E). It was shown that the levels of tryptophan metabolites in tumor tissues of rats treated with S. mutans were significantly different from those of rats treated with S. gordonii control or saline (Fig. 3F). Oral administration of S. mutans underneath oral mucosal epithelia induced much higher levels of KYNA production than the control bacterium S. gordonii and saline did (Fig. 3G). These data suggested that S. mutans could penetrate and colonize underneath oral mucosal epithelia to promote the KYNA productions in vivo.
We then investigated which bacterial components of S. mutans mechanically mediated the increased productions of KYNA. To address this, live S. mutans, heat-inactivated S. mutans, and the control bacterium S. gordonii were co-cultured with tongue epithelium-derived OSCC cells (SCC1 cell line) for quantitative analyses of KYNA production (Fig. 3H). Exposure of SCC1 cells to the live S. mutans resulted in much higher KYNA production in culture supernatants than heat-inactivated S. mutans and control bacterium did (F ig. 3I). As heat treatments might preserve the biological activity of the most bacterial lipids and polysaccharides but inactivate most outer membrane proteins by altering bacterial structure and further affect the functions of bacteria, this result implicated S. mutans-specific protein components might be the primary driving force mediating the high KYNA production by OSCC cells.
Since PAc, particularly A-region of PAc [16], is one of the major surface proteins of S. mutans but not S. gordonii [17], which mediates the critical adherence interactions with glucans on the biofilm and oral epithelia [18], we further postulate that PAc might be a major virulence factor of S. mutans driving the production of KYNA. Thus, A-region of PAc was selected to determine whether PAc drove the productions of KYNA and the following inflammatory responses. To determine this, peptide spanning the A-region of PAc (residue 301–319) and negative control peptide (ftsZ: residue 409–428) with similar length were incubated with SCC1 cells, and culture supernatants were subjected for analyses of KYNA productions (Fig. 3J). We found the PAc peptide significantly induced elevated KYNA concentration compared with the control peptide (Fig. 3K). Thus, these data collectively suggested S. mutans utilized the A-region of PAc to activate the tryptophan metabolism pathway, leading to abundant KYNA release via OSCC cells.
S. mutans-induced KYNA promotes immunosuppression of TME and tumor progression of OSCC
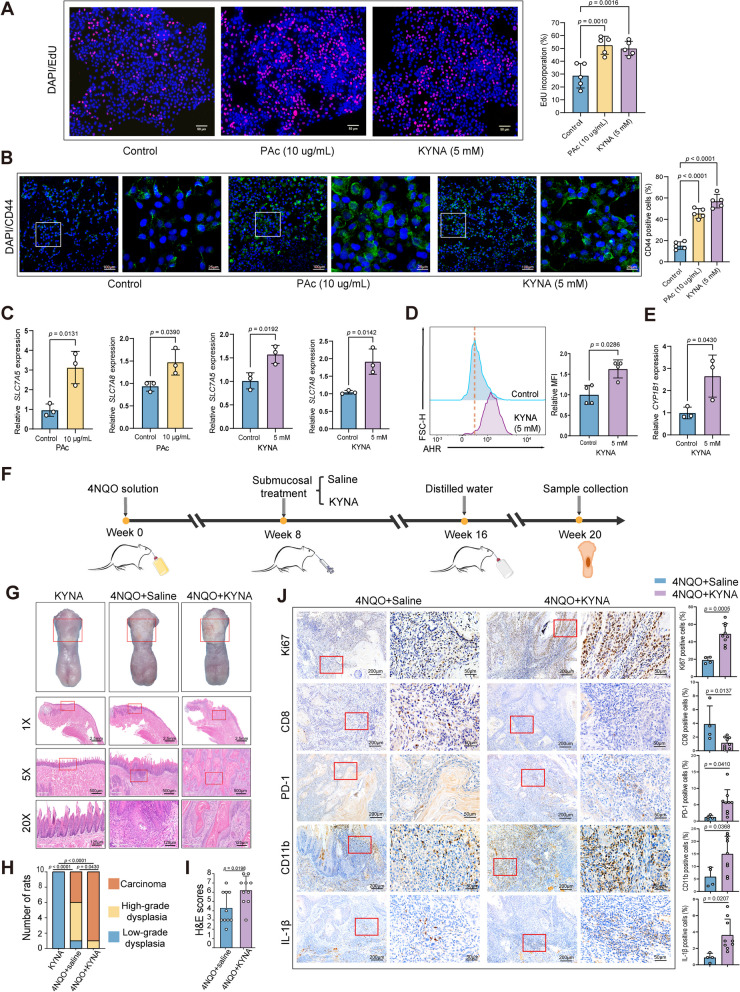
We then evaluated whether PAc and KYNA could really promote the tumorigenesis of oral mucosal epithelia. To address this, PAc and KYNA were co-cultured with SCC1 cells to evaluate the proliferation of SCC1 cells. We found that treatments with PAc and KYNA significantly promoted the proliferation of OSCC cells (Fig. 4A). Immunofluorescence staining (IF) showed that treatments of PAc and KYNA favored cancer stemness with promoting CD44 expression (Fig. 4B). KYNA is a bioactive compound produced along the kynurenine pathway during tryptophan degradation [19]. Solute carrier family 7 member 5 (SLC7A5) and solute carrier family 7 member 8 (SLC7A8) have been implied to play a role in transporting tryptophan metabolites into cells [20, 21]. Therefore, we hypothesized that KYNA could upregulate the SLC7A5 and SLC7A8 transporters. Also, transcriptional analyses revealed that PAc and KYNA treatments significantly upregulated the gene expression of SLC7A5 and SLC7A8 (Fig. 4C). Also, SCC1 cells exposed to KYNA showed significantly elevated expressions of aryl hydrocarbon receptor (AHR) by flow cytometry (Fig. 4D), as revealed by mRNA expression level of CYP1B1 (Fig. 4E), a hallmark gene for AHR activation [22]. AHR is a ligand-activated transcription factor that can be activated by endogenous metabolites including KYNA [23], which also showed higher expression levels in OSCC tumor tissues compared with adjacent non-tumor tissues in TCGA dataset (Fig. S4A). Interleukin 1β (IL-1β) has long been known for its pleiotropic effects on inflammation that plays a complex role in tumor progression [24]. Significantly, KYNA upregulated the IL-1β expression in SCC1 cells (Fig. S4B). Thus, these data showed that KYNA upregulated the expression of kynurenine transporters, activated AHR, promoted tumor progression, and exacerbated inflammation in OSCC cells.
Fig. 4.
KYNA promotes malignant phenotypes of OSCC cells and oral carcinogenesis in rats with 4NQO-induced spontaneous OSCC. A The proliferation ability of SCC1 after stimulation using PAc and KYNA detected by EdU assays. Scale bar = 50 µm. B Representative immunofluorescent (IF) images (left) and statistical analysis (right) of CD44 + SCC1 cells after stimulations using PAc and KYNA, respectively. Scale bars: 100 µm for 10 × magnification and 25 µm for 40 × magnification. C The relative expression of SLC7A5 and SLC7A8 in SCC1 after PAc and KYNA treatment detected by RT-qPCR. SLC7A5, solute carrier family 7 member 5; SLC7A8, solute carrier family 7 member 8. D AHR expression after KYNA treatment detected by flow cytometry. AHR, aryl hydrocarbon receptor; MFI, mean fluorescence intensity. E CYP1B1 mRNA expression after KYNA treatment detected by RT-qPCR. F Schematic illustration of the experimental protocol in 4NQO-induced rat model. Rats exposed to 4NQO were randomly assigned to two groups: saline and KYNA (rats treated with KYNA). Rats not exposed to 4NQO but only treated with KYNA were used as controls. G Representative gross observation and H&E images of the rat tongues with indicated treatments at endpoint. Scale bars: 2.5 mm for original magnification, 500 µm for 5 × magnification and 125 µm for 20 × magnification. H Distribution of the histological degree of low-grade dysplasia, high-grade dysplasia, and carcinoma tissues in rats with indicated treatments. I H&E scores of the histopathologic diagnoses for the saline and KYNA-treated rats. J Representative IHC staining images and quantification analysis of Ki67, CD8, PD-1, CD11b, and IL-1β in rats with indicated treatments. Scale bars: 200 µm for 10 × magnification and 50 µm for 40 × magnification. In A and B, one-way ANOVA; C, E, I, and J, unpaired Student’s t-test; D, Mann–Whitney U test; H, Fisher’s exact test
To further validate that KYNA promoted oral carcinogenesis, KYNA was administrated in 4NQO-treated rat models by tongue submucosal injection (Fig. 4F). H&E staining indicated that rats with KYNA treatments induced more severe oral pathological impairments (Figs. 4G–I and S4C). In rats with 4NQO-KYNA co-administrations, nine (90%) rats developed into oral carcinoma, while only four (40%) rats progressed into oral carcinoma in rats treated with 4NQO only (Fig. 4H). IHC examination revealed that the Ki67 expression was upregulated significantly in tumors after treatment with KYNA (Fig. 4J). Also, KYNA administration led to lower numbers of CD8 + cells and higher numbers of immunosuppressive PD-1 + , CD11b + , and IL-1β + cells in the tumors, indicating that KYNA played a crucial role in modulating tumor immune microenvironment (Fig. 4J). Collectively, these results suggested that S.mutans and PAc stimulated OSCC cells to produce higher amounts of KYNA, which further increased the proliferation and stemness of OSCC, induced a more immunosuppressive TME, and exacerbated oral carcinogenesis.
KYNA predominantly expands neutrophils and shifts the cellular types at single-cell level in the TME of OSCC
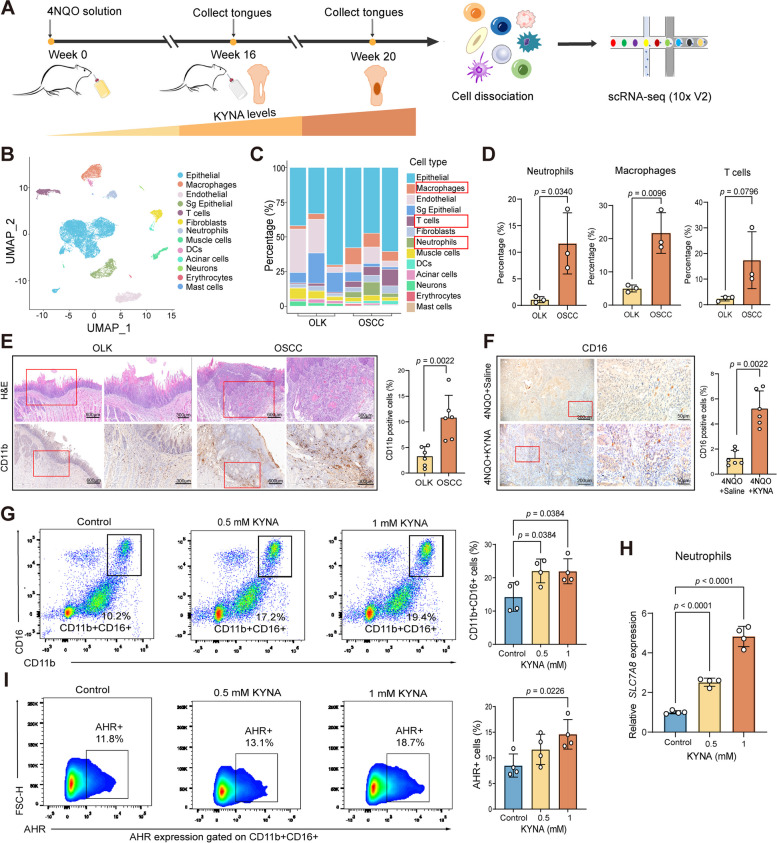
Since both KYNA-enriched saliva and KYNA promoted OSCC, and such KYNA-promoted OSCC was characterized with increased infiltrations of CD11b + cells, decreased infiltrations of CD8 + T cells, and higher PD-1 and IL-1β expression, these data implicated that KYNA might promote OSCC via modulating tumor immune microenvironment. To gain the in-depth mechanistic insights by which KYNA regulated the OSCC TME, we performed single-cell RNA sequencing (scRNA-seq) analyses to reveal more comprehensive compositions of cell types and transcriptome atlas of such KYNA-promoted oral carcinogenesis.
To address this, tongues derived from rats with OLK and OSCC were subjected for scRNA-seq analyses (Fig. 5A). After initial quality control, 20,326 cells were retained, and partitioned into 13 clusters by the documented differential gene expressions, which comprised of epithelial, endothelial, fibroblast, and immune cells (Figs. 5B and S5A). scRNA-seq analyses revealed that, as compared with tongue tissues derived from OLK, neutrophils, macrophages, and T cells significantly increased in OSCC tongue tissues containing higher concentration of KYNA during malignant transformation (Fig. 5C). Particularly, the numbers of neutrophils increased approximately 10 times, which were much larger than macrophages and T cells in terms of increase times (Fig. 5D), implicating that neutrophils might be the predominant regulatory targets in the KYNA-mediated TME.
Fig. 5.
Single-cell transcriptomic landscape reveals the shift of the cellular types during oral carcinogenesis, and KYNA is a predominant driving force for expansions and infiltrations of neutrophils in the TME of OSCC. A Overview of the workflow for scRNA-seq analyses of OLK and OSCC rat tongue tissues. B UMAP plot of the clustering results for 13 major cell types from OLK and OSCC tissues. C Stacked histogram of the percentages of different cells from OLK and OSCC tissues. D Quantitative analysis of neutrophils, macrophages, and T cells in non-epithelial cells in OLK and OSCC tissues. E H&E and IHC staining for CD11b in OLK and OSCC tissues of experimental rats. Scale bar: 600 µm for 4 × magnification and 300 µm for 10 × magnification. F Representative IHC staining images and statistical analysis of CD16 in saline and KYNA-treated 4NQO rats. Scale bars: 200 µm for 10 × magnification and 50 µm for 40 × magnification. G Representative flow cytometry dot plots and statistical analysis of CD11b + CD16 + neutrophils from the peripheral blood of OSCC patients after KYNA treatment. H Relative SLC7A8 expression in neutrophils from OSCC patients after KYNA treatment detected by RT-qPCR. I Representative flow cytometry dot plots and statistical analysis of AHR + neutrophils from OSCC patients after treatments with KYNA in indicated concentrations. In D, unpaired Student’s t-test; E and F, Mann–Whitney U test; G, H, and I, one-way ANOVA
Indeed, IHC analyses suggested that there were larger amounts of CD11b + cells in tongue tissues derived from OSCC rats than those from OLK rats (Fig. 5E). Also, IHC analyses of tumor tissues derived from tongues of KYNA-treated rats showed larger proportions of neutrophils than saline-treated rats (Fig. 5F). To validate that neutrophils are also the major target of KYNA in humans, peripheral blood from OSCC patients was stimulated ex vivo with KYNA and subjected for flow cytometric analyses. It was shown that neutrophil populations from OSCC patients were significantly increased by ex vivo KYNA treatments (Fig. 5G). Additionally, neutrophils were markedly expanded after treatment with S. mutans supernatants and lysates (Fig. S5B and S5C). We further found that the expression of SLC7A8 (a transporter of KYNA) and AHR in neutrophils was upregulated by KYNA (Fig. 5H, I), suggesting that KYNA activated the KYNA transporter and AHR to facilitate neutrophils to take up more KYNA for further expansion. Thus, KYNA predominantly expanded and increased the infiltration of neutrophils in OSCC tumor tissues, which therefore significantly changed the compositions of cellular types in the TME of OSCC.
scRNA-seq shows that KYNA-reprogrammed neutrophils display highly immunosuppressive S100a8highS100a9high phenotype and superior IL-1β production capability in the TME of OSCC
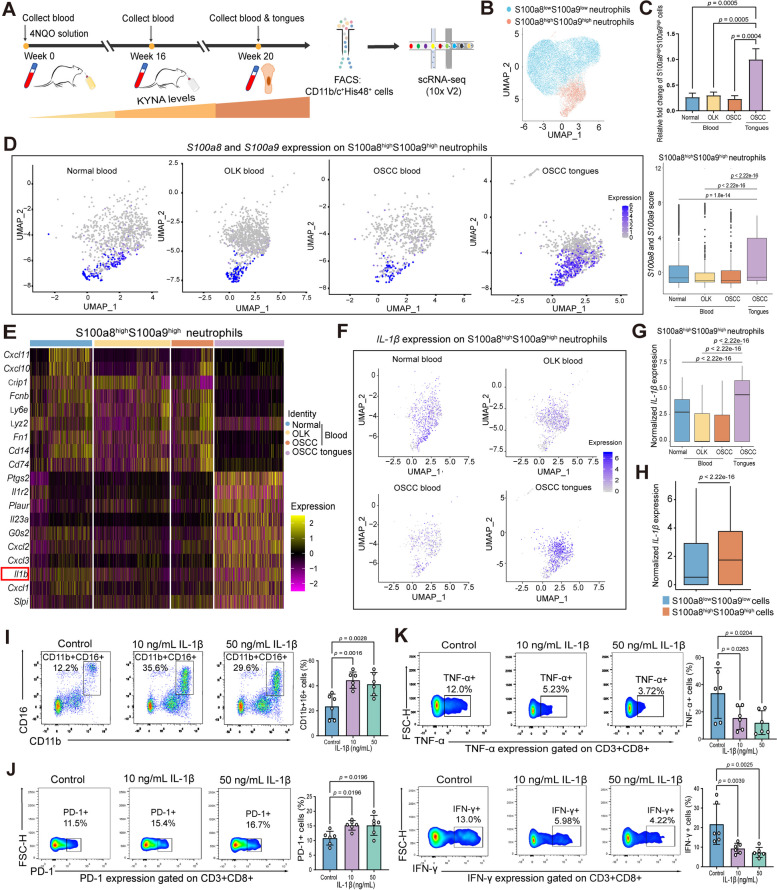
Since KYNA predominantly expanded and increased the infiltration of neutrophils in the OSCC TME, we collected peripheral blood at the development and progression stages of oral carcinogenesis and the OSCC tissues of rats to sort CD11b/c + His48 + neutrophils for further scRNA-seq analyses to explore the dynamic cellular state and functional evolution of neutrophils in the progression of OSCC (Fig. 6A). By applying stringent quality control methods, 18,645 high-quality neutrophils were obtained. To better evaluate the immune phenotypes and functional changes of neutrophils during oral carcinogenesis, S100a8 and S100a9 (also known as MRP8 and MRP14, respectively) were used to characterize the inflammatory status of these neutrophils in the TME of OSCC [25]. The sorted neutrophils during OSCC progression could be classified into two populations characterized by S100a8highS100a9high and S100a8lowS100a9low (Figs. 6B and S6A). Expressions of S100a8 and S100a9 may confer neutrophils highly pro-inflammatory, myeloid-derived suppressor cells (MDSC)-like immunosuppressive features [26]. We found that, compared with neutrophils isolated from peripheral blood, neutrophils derived from the TME preferentially showed S100a8highS100a9high pro-inflammatory, MDSC-like immunosuppressive phenotypes (Fig. 6C). Moreover, the expression of S100a8 and S100a9 in S100a8highS100a9high neutrophils was much higher in OSCC tissues than those in peripheral blood (Fig. 6D). We further comparatively analyzed the gene expression patterns of S100a8highS100a9high and S100a8lowS100a9low neutrophils. The characteristic gene expressions of these two group neutrophils showed significant differences (Figs. 6E and S6B). Particularly, S100a8highS100a9high neutrophils expressed a broader array of chemokine ligands (CXCL1, 2, 3) as well as cytokines (Ptgs2, IL1r2, IL23, IL-1β) (Fig. 6E). Also, Gene Ontology (GO) analysis of S100a8highS100a9high neutrophils in the TME of OSCC was highly enriched in inflammatory responses and immune regulation pathways, such as response to IL-1 pathway (Fig. S6C).
Fig. 6.
Single-cell transcriptomic landscape reveals higher KYNA concentrations drive preferential S100a8highS100a9high phenotype and superior ability of IL-1β expression by neutrophils during oral carcinogenesis. A Flow chart of scRNA-seq of CD11b/c + His48 + cells in peripheral blood and OSCC tissues during carcinogenesis in 4NQO rats. FACS, fluorescence-activated cell sorting. B UMAP plot of the clusters of S100a8highS100a9high and S100a8lowS100a9low neutrophils based on the S100a8 and S100a9 expression. C Statistical analysis of the S100a8highS100a9high neutrophils in peripheral blood and tissues during oral carcinogenesis process. “OSCC tongue” group as the “reference group” to calculate the fold change. D UMAP plot (left) and statistical analysis (right) of the S100a8 and S100a9 score in S100a8highS100a9high neutrophils across different periods. Color representing the log-normalized expression level of the marker genes across multiple stages of cells. Gray to purple: low to high expression. E Heatmap of gene profiling of S100a8highS100a9high neutrophils in peripheral blood and OSCC tissues of 4NQO-induced rats. Color representing the scaled normalized expression level of the marker genes across all cells. Yellow: high expression; purple: low expression. F Expressions of IL-1β on S100a8highS100a9high neutrophils in normal blood, OLK blood, OSCC blood, and OSCC tissues during the carcinogenesis process. Note that OSCC tissues contained much higher IL-1β + S100a8highS100a9high neutrophils. Color representing the log-normalized expression level of the marker genes across multiple stages of cells. Gray to purple: low to high expression. G Quantitative analyses of IL-1β expressions in normal blood, OLK blood, OSCC blood, and OSCC tissues during the carcinogenesis process on S100a8highS100a9high neutrophils. H Comparison of IL-1β expression between S100a8highS100a9high and S100a8lowS100a9low neutrophils from all the identified cells in the scRNA-seq. Note that IL-1β was significantly higher in S100a8highS100a9high neutrophils than their S100a8lowS100a9low counterparts. I Representative flow cytometry dot plots and statistical analysis of CD11b + CD16 + neutrophils from OSCC patients after treatments of IL-1β in indicated concentrations. J Representative flow cytometry dot plots and statistical analysis of the expression of PD-1 in CD3 + CD8 + T cells from OSCC patients after treatments of IL-1β in indicated concentrations. K Representative flow cytometry dot plots and statistical analysis of the expression of TNF-α and IFN-γ on CD3 + CD8 + T cells from OSCC patients after treatments of IL-1β in indicated concentrations. In C, I, J, and K, one-way ANOVA; D and G, Kruskal–Wallis test; H, Mann–Whitney U test
Because of the critical tumor-promoting role of IL-1β signaling in the TME [24, 27, 28], the dynamic expression of IL-1β was further analyzed. First, S100a8highS100a9high neutrophils expressed much higher levels of IL-1β in OSCC tissues than those isolated from peripheral blood derived from normal, OLK and OSCC stage (Fig. 6F, G). Also, S100a8highS100a9high neutrophils of the four groups expressed much higher levels of IL-1β than their counterparts with S100a8lowS100a9low phenotypes during oral carcinogenesis (Fig. 6H). Meanwhile, we found that KYNA increased IL-1β levels in neutrophils of peripheral blood from OSCC patients during in vitro culture (Fig. S6D). Moreover, treatments with recombinant IL-1β enhanced the expansion of neutrophils (Fig. 6I), increased the expression of PD-1 (Fig. 6J), and inhibited the anti-tumor effector functions with decreased expressions of tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) of CD8 + T cells (Fig. 6K). Also, scRNA-seq analyses revealed that there existed significant interaction events of immune inhibition/exhaustion between S100a8highS100a9high neutrophils and T/NK cells derived from the TME of OSCC (Fig. S6E-F). Notably, the expression of CD274 (PD-L1) in S100a8highS100a9high neutrophils were much higher in OSCC tissues than that in peripheral blood (Fig. S6G). Thus, these pieces of data collectively suggested that KYNA-expanded neutrophils with preferential expression of S100a8/S100a9, which designated neutrophils with highly immunosuppressive features and stronger ability to express IL-1β, and IL-1β increased the expression of PD-1 and inhibited the anti-tumor effector functions of CD8 + T cells with more functional impairment and exhaustion in the TME of OSCC.
KYNA impedes the therapeutic effects of IL-1β and programmed cell death ligand 1 (PD-L1) blockades in 4NQO-induced oral carcinogenesis model
The responsiveness of PD-1/PD-L1 blockade therapeutics in OSCC ranged from only 13 to approximately 18% [29, 30], which needs to be significantly improved. Since S.mutans-mediated KYNA induced a highly immunosuppressive TME in OSCC, particularly increased infiltrations of neutrophils and higher expression of IL-1β and PD-L1, we then postulated whether KYNA was a primary pathogenesis driving force for poor responsiveness or unresponsiveness in IL-1β and PD-1/PD-L1 blockade therapeutics of OSCC.
To address this, we further examined the effects of KYNA on the IL-1β and PD-L1 blockade therapeutics in 4NQO-induced carcinogenesis model. 4NQO-treated rats were treated with KYNA in the presence or absence of blockade anti-IL-1β monoclonal antibody (IL-1β mAb) or anti-PD-L1 mAb (PD-L1 mAb) (Fig. 7A). Microscopic observation of H&E staining-based pathological analyses suggested that the rats with treatments of IL-1β mAb or PD-L1 mAb exhibited milder mucosal lesions, while KYNA treatments significantly compromised the beneficial effects conferred by treatments of IL-1β or PD-L1 mAb or even dramatically exacerbated the pathological impairment of OSCC (Figs. 7B–D and S7). IHC analyses also suggested that KYNA treatments significantly decreased the numbers of CD8 + T cells in the presence of IL-1β mAb and increased the expression of PD-1 and CD11b in the presence of PD-L1 mAb treatment in the TME of OSCC (Fig. 7E, F). These data suggested that KYNA might serve as a pathogenic onco-metabolite underlying poor responsiveness in IL-1β and PD-1/PD-L1 blockade therapeutics of OSCC.
Fig. 7.
KYNA is a primary pathogenesis driving force for poor responsiveness of IL-1β and PD-L1 blockade therapeutics in 4NQO-induced carcinogenesis models. A Schematic illustration of the experimental protocol in 4NQO-induced oral carcinogenesis model. Rats exposed to 4NQO were randomly assigned to five groups: IgG, IL-1β monoclonal antibody (treated with IL-1β mAb), KYNA + IL-1β mAb, PD-L1 mAb (treated with PD-L1 mAb), and KYNA + PD-L1 mAb. B Representative gross observation and H&E images of the rat tongues in the rats with indicated treatments at endpoint. Scale bars: 2.5 mm for original magnification, 500 µm for 5 × magnification and 125 µm for 20 × magnification. C Quantification of the histological ratio of low-grade dysplasia, high-grade dysplasia, and carcinoma tissues in the rats with indicated treatments. Significant differences were displayed with p-values. D H&E scores of the histopathologic diagnoses in the rats with indicated treatments. E Representative IHC staining images of CD8, PD-1, CD11b, and IL-1β in the rats with indicated treatments. Scale bars: 200 µm for 10 × magnification and 50 µm for 40 × magnification. F Quantification analysis of IHC scores of CD8, PD-1, CD11b, and IL-1β in the OSCC tumors of rats with indicated treatments. In C, Fisher’s exact test; D and F, one-way ANOVA
KYNA-mediated metabolic reprogramming correlates with impaired anti-tumor immune activity and poor survival in OSCC patients
Finally, we analyzed whether KYNA-induced reprogramming of the OSCC TME was clinically relevant. We found that larger numbers of CD11b + , PD-1 + , PD-L1 + , and IL-1β + cells were existed in OSCC compared with OLK (non-tumor) tissues (Fig. 8A). Furthermore, IF staining exhibited more spatial co-localized cells of CD11b with PD-L1 and CD8 with PD-1 in the OSCC tissues than OLK (non-tumor) tissue (Fig. 8B). IHC staining suggested that AHR was predominantly expressed in OSCC, whereas it was nearly absent in OLK tissues (Fig. 8C). These data collectively suggested malignant transformation of oral mucosa was associated with increased infiltrations of CD11b + cells and highly immune inhibitory features.
Fig. 8.
KYNA-mediated signaling pathways correlate with impairment of anti-tumor immunity and poor survival in OSCC patients. A Representative IHC images (up) and quantitative analysis (down) of CD11b, PD-1, PD-L1, and IL-1β in human OLK (non-tumor) and OSCC tissues. Scale bar: 200 µm for 10 × magnification and 50 µm for 40 × magnification. B Representative IF images (up) and quantitative analysis (down) of spatial colocalizations of CD11b with PD-L1, and CD8 with PD-1 in human OLK (non-tumor) and OSCC tissues. Scale bar: 20 µm. C Representative IHC images and quantitative analysis of AHR in human OLK and OSCC tissues. Scale bar: IHC, 200 µm for 10 × magnification and 50 µm for 40 × magnification. D Correlation analysis (Spearman correlation) between CD274 and IDO1, IDO2, and TDO2 in TCGA dataset. E Kaplan–Meier survival curves of overall survival based on OSCC patients with high and low expression of IL-1β, CD274 and AHR in TCGA dataset. F Schematic illustration depicted that S. mutans-mediated KYNA might increase the amounts and infiltrations of neutrophils, remodel the functionality of neutrophils, and reprogram the TME to promote OSCC progression. S. mutans, Streptococcus mutans; PAc, protein antigen c; KYNA, kynurenic acid; AHR, aryl hydrocarbon receptor; SLC7A8, solute carrier family 7 member 8; IL-1β, interleukin-1β; PD-1: programmed cell death protein 1; TNF-α, tumor necrosis factor-α; IFN-γ: interferon-γ. In A, B, and C, unpaired Student’s t-test; D, Spearman correlation analysis; E, log-rank test
We then explored the correlation between TCE expression and immune activity in TCGA. The expression of IDO1, IDO2, and TDO2 was significantly associated with that of CD274 (p = 1.43 × 10−58, 2.56 × 10−22, 8.96 × 10−6), and showed a strong (r = 0.65), moderate (r = 0.42), and mild (r = 0.2) positive correlation. Meanwhile, AHR in HNSCC had a positive slight-correlation with CD274 expression level (r = 0.13, p = 4.42 × 10−3) (Figs. 8D and S8A). We further investigated the clinical prognostic relevance of FCGR3B (CD16), IL-1β, CD274 (PD-L1), and AHR for OSCC patients in TCGA, GSE41613 and GSE65858. Patients were dichotomized into high and low expression groups by R package “survminer.” Kaplan–Meier analysis indicated that the higher expression of FCGR3B, IL-1β, CD274, and AHR predicted poorer overall survival (OS) (Figs. 8E and S8B-S8C). Patients with high expression of KYAT1, which is pivotal to the conversion from kynurenine to KYNA, showed a poorer OS than those with low KYAT1 expression in TCGA (Fig. S8D). These data suggested that KYNA-related signal pathways were associated with poor outcomes of OSCC patients.
Collectively, these data presented a new pathogenic mechanism of OSCC by which tumor-colonized S.mutans increased KYNA productions, which predominantly expanded neutrophils and remodeled the functionality of neutrophils, promoted the immune inhibition or exhaustion of CD8 + T cells in the TME, and further drove the development, progression, and poor prognosis of OSCC (as summarized in Fig. 8F).
Discussion
Oral microbiota plays a critical role in shaping oral and systemic health and disease [6, 7]. This work provides new insights into how compositionally altered microbiota, particularly increased abundance of S. mutans, manipulates intercellular interactions and cell functionality through metabolic-immune crosstalk involving in microbiota, metabolites, immune cells and oral mucosa in the development, progression, and prognosis of OSCC. Specifically, we found tumor-colonized S. mutans stimulated the productions of high levels of KYNA by OSCC cells via its surface adhesion protein PAc, and then KYNA activated the AHR and promoted the expansion and infiltration of neutrophils characterized with highly immune suppressive features in the TME, which further increased the production of IL-1β and promoted CD8 + T cell exhaustion and OSCC progression. Such interaction axis of oral bacteria-oncometabolite-neutrophil expansions -immune exhaustion induced a poor responsiveness against PD-1/PD-L1 blockade immunotherapy in OSCC rat models and linked with poor survival of OSCC patients.
OSCC leads to an approximately 50% 5-year mortality rate, considerable physical disfigurement and decreased life quality [1, 31]. Although genetics, virus infection, environmental factors, and dietary habitats may lead to the development and exacerbate the development and progression of OSCC, approximately 15% of OSCC have unclear risk factors [32]. Oral microbiota is linked with periodontitis, caries, and other systemic diseases such as inflammatory bowel disease (IBD) and colorectal cancer (CRC) [33–35], but the exact interplay components and mechanisms among oral microbes, malignant and non-malignant cells in the TME, which critically mediate the development, progression, and poor prognosis of OSCC, are largely unclear. Thus, this work presents a previously unrecognized pathogenic mechanism of OSCC by which a highly immunosuppressive TME of OSCC contributing to poor responsiveness for immunotherapy is mediated by an onco-metabolite overly induced by a tumor-colonized oral bacterium.
Chemotherapy and immunotherapy have made significant progresse against OSCC and other HNSCC [31], but the responsiveness rates of OSCC to pembrolizumab immunotherapy are only approximately 16%, with strong side effects and high relapse rates [36, 37]. Particularly, the mechanisms underlying such low responsiveness rates of OSCC to immunotherapy are unclear. This work shows that over productions of a metabolite (e.g., KYNA) induced by tumor-colonized oral bacteria (e.g., S. mutans) significantly compromised the immunotherapeutic effects of PD-1/PD-L1 blockade. Thus, this work presents a leading evidence suggesting that oral tumor-colonized bacteria promote the production of specific onco-metabolites and reprogram a highly immunosuppressive OSCC TME, which therefore serves as a critical mediator compromising the effects of anti-tumor immunotherapy.
Although emerging evidence has revealed that S. mutans was significantly associated with poor differentiation, advanced disease, and high carcinogenesis risk [38, 39], the microbiota-host interactions and molecular mechanisms of S. mutans-mediated OSCC are largely unexplored. Oral streptococci, which encode a family of serine-rich repeat-containing glycoproteins and antigen I/II family of adhesins binding to the tooth pellicle and epithelium, are the most frequently pioneer microbial colonizers of the oral cavity [7, 40]. S. mutans is a vital contributor to dental biofilm associated with the formation of dental caries, and plays a major role in the development and establishment of extracellular polysaccharide matrices, which increases the virulence of the biofilm and facilitates the persistence of an acidic and anaerobic environment [41, 42].
However, traditionally, our understanding of the exact roles of S. mutans and actually most of other oral bacteria in oral health and disease is mostly limited in dental caries [43]. This work showed that S. mutans spatially relocated from the superficial of the oral mucosa to OSCC tissues, and served as a “keystone pathogen” colonized in OSCC tissues, which promoted OSCC by generating extremely high amounts of some onco-metabolites (e.g., KYNA) and reprogramming the oral microenvironment favoring OSCC development and progression. Possibly, the unique microenvironment will further aggravate the colonization of other bacteria such as Fusobacterium nucleatum, invasion, and metastasis of malignant cells, and prevent cytotoxic T cells from acquiring optimal anti-tumor effector functions [13]. However, the clinical correlation between the tumor-colonized S. mutans and OSCC outcomes should be further explored in larger cohorts of OSCC patients.
It has been implicated that tumor-colonized bacteria may serve as a fundamental component in the tumor tissue and contribute to the initiation, progression, and poor prognosis of OSCC and other cancers [13, 44–48], but the exact mechanisms by which tumor-colonized bacteria interacts with host and regulates the tumor development and progression are largely unknown. It is previously presumed that invasive bacteria and suppressive metabolites are responsible for recruiting myeloid cells to induce an inflammatory response, promoting T cell exhaustion and tumor growth by secreting specific interleukins and chemokines into the surrounding environment [49, 50]. Our findings revealed that at least some of S. mutans underwent spatial translocations from oral epithelium surface to OSCC tumor tissues through mechanisms that required further in-depth investigations. Furthermore, KYNA, an endogenous tryptophan metabolite, is induced by tumor-colonized S. mutans, which activates AHR signaling, resulting in pro-tumorigenic phenotypes and immune suppression in the TME. KYNA has been considered an immunosuppressive metabolite that induces T cell arrest and differentiation preferentially into Foxp3 + Treg [51, 52]. In this work, we found that overproduction of KYNA significantly reprogrammed the TME through increasing the expression of KYNA transporter and S100a8/S100a9 on neutrophils, enhancing the expansion and infiltration of neutrophils, decreasing the infiltrations of CD8 + T cells, and elevating the production of IL-1β and exhaustion of CD8 + T cells in the TME. Thus, KYNA was a primary metabolic barrier or molecular driving force of such reprogrammed TME promoting the impairment of anti-tumor immunity, heightened proliferation, and stemness of OSCC, which favored OSCC development and progression. Indeed, KYNA strikingly compromised or reversed the anti-tumor therapeutic effects of IL-1β and PD-L1 blockades in the OSCC carcinogenesis model, which reinforced the carcinogenic role of KYNA as a highly potent onco-metabolite. Notably, elucidating such expansions and infiltrations of neutrophils contributing to a more immunosuppressive TME, were mediated by oral bacteria (e.g., S. mutans here) or onco-metabolites (e.g., KYNA here) derived from or induced by oral bacteria, may present a new opportunity to develop novel prevention and therapeutic targets against OSCC and potentially other HNSCC.
Alternation of cell types and functions in the microenvironment of oral mucosal in periodontitis have been shown [53], and the critical roles of neutrophils in oral mucosal immunity have been implicated [54, 55], but the exact atlas and the functional characteristics and mechanisms of neutrophils in OSCC remain unclear. Our work suggested that tumor-colonized S. mutans utilized its surface protein antigen PAc to stimulate the production of KYNA by OSCC cells, which further mediated expansions and infiltrations of neutrophils. scRNA-seq analyses suggested that these S. mutans-PAc-KYNA-expanded neutrophils preferentially presented highly inflammatory S100a8highS100a9high phenotypes with extraordinary ability to produce pro-inflammatory IL-1β, which further expanded neutrophils and induced exhaustion of CD8 + T cells in the TME of OSCC. However, the exact molecular mechanisms by which KYNA increased the expressions of S100a8 and S100a9 on neutrophils and the precise roles of S100a8 and S100a9 regulating the TME of OSCC needed to be further elucidated.
Our current work suggests that S. mutans indeed serves as a highly potent mediator in the development and progression of OSCC. However, there are profound, complex, dynamic interactions or communications for commensal microbes and microbial metabolites, which may spatiotemporally and metabolically govern oral carcinogenesis [6, 56]. Hence, a more complete picture of complex microbiota and their impact on tumorigenesis will be realized by the use of combined methods such as 16S rRNA and metagenomic sequencing in larger-scale clinical samples. In addition, it is useful to further explore the clinical correlation of such multiple-dimensional interactions of oral and other microbiota/metabolites in regulating the development and progression of OSCC.
Also, the detailed mechanisms underlying KYNA production mediated by PAc of S. mutans were not fully elucidated. To more specifically define the mechanism of KYNA production mediated by PAc of S. mutans, genome editing, small molecules or neutralizing antibodies targeting PAc may inhibit or interfere the expression, activation, and amount of PAc, which will be helpful to further validate the functional specificity and characteristics of PAc in mediating production of KYNA and other metabolites.
In summary, this work presented a leading evidence showing how a compositional and spatially shift of oral microbiota might reprogram the metabolic patterns and functionality of neutrophils, reshape the TME, drive the oral carcinogenesis, and impair the immunotherapeutic effects of OSCC. These findings may provide new insights for the prevention and treatment of OSCC.
Materials and Methods
Human specimen collection
Oral samples (including oral whole saliva, swabs, and tissue specimens) and peripheral anticoagulation venous blood were collected from patients at the first diagnosis and healthy controls (HCs). The HCs were defined as individuals who did not have detectable periodontal inflammation, visible carious lesions, or oral mucosal diseases. Participants who received antibiotics or topical steroids treatment one month prior to sampling, and individuals who had malignant tumor and systemic disease were excluded. Oral leukoplakia (OLK) and oral squamous cell carcinoma (OSCC) patients were subjected to the same aforementioned criteria, and their diagnoses were confirmed by biopsy and pathological findings. HCs and cases were recruited in the Sun Yat-sen University (SYSU) Hospital of Stomatology and SYSU Cancer Center. The collection of clinical samples was approved by the Ethics Committee of Hospital of Stomatology, SYSU (KQEC-2021-62-01), and written informed consent was obtained from all participants.
The saliva samples were collected for animal intervention experiments, metabolic analyses, and DNA isolation; swab samples were collected for DNA isolation by rubbing the oral mucosa five to seven times with a specified cotton swab (collecting superficial swabs of oral epithelia and fluid saliva of oral cavity samples from the same individual can acquire as many bacteria as possible to get more comprehensive and accurate quantification of bacteria in oral microenvironment); peripheral blood was collected for flow cytometry experiments; fresh tissue samples were isolated for metabolic analyses and DNA isolation, and paraffin sections were collected for immunohistochemical (IHC), immunofluorescence (IF) and fluorescence in situ hybridization (FISH) staining.
Metabolomics and metabolite analyses
Saliva from OSCC patients, OLK patients and HCs, and adjacent non-tumor and tumor tissues from OSCC patients were collected for widely targeted metabolomics by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). Saliva metabolomics and tissue metabolomics were two independent experiments, and the samples of saliva and tissue were collected and processed separately. Tongues of 4-Nitroquinoline N-oxide (4NQO) rats treated with S. mutans and S. gordonii were collected for targeted metabolomics (tryptophan and its metabolites analysis). The samples were prepared according to the operating instructions and subjected to subsequent UPLC-MS/MS analysis by Wuhan Metware Biotechnology Co., Ltd, which were processed and analyzed by the same experimental procedures (same time, operator, and instrument) in an uninterrupted manner to eliminate inter-batch effects.
Centrifuged saliva supernatants and fresh tissues were snap frozen in liquid nitrogen and then stored at − 80 ℃ until use. To detect as many metabolites as possible in the saliva, both hydrophilic and hydrophobic metabolites were extracted and analyzed. Briefly, saliva samples were thawed on ice, and the hydrophilic and hydrophobic metabolites were extracted with ice methanol and lipid extract, respectively. Tissue samples were weighed, homogenized, and extracted with ice methanol for hydrophilic metabolite analysis based on experimental needs. The sample extracts were further analyzed using an LC-electrospray ionization (ESI)-MS/MS system. Quality control (QC) samples were prepared for detecting the intra-batch variability, which were obtained by mixing all the sample extracts of the same batch, and analyzed after every 10–15 samples in the running sequence, which was examined by the total ion current (TIC) overlapping of QC samples to evaluate the stability of UPLC-MS/MS analysis.
Regarding the analysis of metabolomic data, the unsupervised principal component analysis (PCA) was conducted by statistical function prcomp in R (www.r-project.org), and W∗d-test was applied to assess the significance [57]. Z-score normalized signal intensities of metabolites (unit variance scaling) were visualized as color spectrum for hierarchical cluster analysis (HCA) by R package ComplexHeatmap. Variable importance in projection (VIP) values were extracted from orthogonal partial least squares discriminant analysis (OPLS-DA), which included score plots and permutation plots by applying R package MetaboAnalystR. The permutation test (200 permutations) was conducted to avert overfitting. VIP ≥ 1 and absolute Log2FC (fold change) ≥ 1 denote the remarkable metabolites. The determined metabolites were annotated by applying the Kyoto Encyclopedia of Genes and Genomes (KEGG) compound database (http://www.kegg.jp/kegg/compound/), and then the annotated metabolites were mapped to the KEGG pathway database (http://www.kegg.jp/kegg/pathway.html). The substantially enriched pathways were determined by applying the p-value of the hypergeometric test and adjusted p-value of Benjamini–Hochberg FDR correction in the given list of metabolites. The details of metabolomics and data analyses were fully explained in the Supplementary Materials.
Saliva from OSCC patients and HCs in an independent cohort was analyzed by high-performance liquid chromatography (HPLC; Agilent1260, USA) to validate the concentration levels of kynurenic acid (KYNA). The standard substance of KYNA was purchased from Sigma-Aldrich (K3375, Saint Louis, MO, USA). The reactions were quenched with 25 mM sodium acetate. The solvents used for HPLC were tri-distilled water (solvent A) and acetonitrile (solvent B). The conversion rate of substrate to product was < 10% for kinetic assays. In addition, the saliva supernatants were also subjected to KYNA concentration determination by ELISA kits according to the manufacturer’s protocols (BS-E7827H1, JSBOSSEN, China). Each experiment was independently repeated at least three times.
Single-cell RNA sequencing
In panel one, OLK and OSCC tissues from 4NQO rats were dissociated into single-cell droplets with collagenase type I (0.5 mg/mL; Sigma-Aldrich, SCR103) and collagenase type IV (1 mg/mL; Sigma-Aldrich, C5138). All the droplets were subjected to sequencing.
In panel two, blood from the normal, OLK and OSCC stages was collected from the angular vein of rats, and neutrophils were isolated from blood using Polymorphprep™ (AS1114683, Axis-Shield, Norway) density gradient centrifugation. OSCC tissues were processed to acquire single cells as above. Collected cells and neutrophils were stained with CD11b/c-APC (MA5-17,507, Thermo Fisher Scientific, Waltham, USA) and His48-FITC antibodies (11–0570-82, Thermo Fisher Scientific, Waltham, USA) at the same time, and incubated on ice and protected from light for 30 min to perform fluorescence-activated cell sorting (FACS, LSRFortessa, BD Biosciences) and sort CD11b/c + and His48 + double positive cells.
Cell concentration was adjusted to 700–1200 cells/µL and processed by a Chromium Controller instrument (10X Genomics) and a Chromium™ Single Cell 3′ Library & Gel Bead Kit v2 (10X Genomics, PN-120237/PN-120267). Sequencing was performed on the BGISEQ-500 sequencing platform. Raw sequencing data were processed using 10 × CellRanger 3.0.0, and each sample was aligned to an indexed Rnor_6.0 genome using Cell Ranger Count. For data quality control, SoupX package was applied for background correction, and Scrublet package was applied to exclude remaining cells predicted to be doublets that were still present in the dataset. Data were combined in R (version 3.4.1), and converted to a Seurat object using the Seurat R package (version 3.0.2) for downstream analyses including normalization, scaling, clustering of cells, and identifying cluster marker genes (https://github.com/satijalab/seurat). To normalize the library size effect in each cell, we scaled UMI counts using scale.factor = 10,000. The expression matrix was then log-normalized using the “NormalizeData” function. Other factors, including “percent.mt,” “nCount_RNA,” and “nFeature_RNA,” were corrected for variation regression using the ScaleData function in Seurat (version 3.0.2). The details of single-cell RNA sequencing and data processing were elaborated in the Supplementary Materials.
FISH assays
FISH of adjacent non-tumor and tumor tissues from OSCC patients was performed using a specific probe MUT590 against S. mutans [58]. Tissue paraffin sections from OSCC patients were probed with 5 mg/mL S. mutans 16S RNA-specific oligonucleotide POGI 5′-ACTCCAGACTTTCCTGAC-3′ labeled with Cy3 dye (Takara, Japan). Images were assessed using a laser confocal fluorescence microscope and quantitative analysis was performed. Three representative fields (at least 50 cells per field) of sections were randomly selected from each sample under 100 × magnification. The positive and total cells were calculated using ImageJ v2 software, and then the average positive rates were evaluated.
Bacteria and cell culture
Streptococcus mutans UA159 and Streptococcus gordonii NCTC 7865 were obtained from Guangdong Microbiology Culture Center (Guangzhou, China). Bacteria were grown in Brain Heart Infusion (BHI) at 37 °C, 5% O2, and 95% N2. S. mutans was harvested at the appropriate growth phase based on the predetermined growth curve. Bacteria suspension with OD600 = 1.0 corresponded to approximately 109 colony-forming units per milliliter (CFU/mL).
The supernatant was collected by centrifugation (12,000 rpm, 10 min, 4 °C) of bacteria suspension. To collect the lysates of S. mutans, ultrasonication of the bacteria was performed with Ultrasonic homogenizer (Qsonica, Misonix, Sonicator USA). Five milliliters of bacterial suspension on ice was sonicated for 50 circulations with 20 s working time at 15 W amplitude and 60 Hz frequency, and 20 s pause. The mixture of bacteria lysate was filtered with a 0.22 µm filter unit for 2 times. For heat sterilization of S. mutans, the bacteria were harvested, washed and suspended in saline, then incubated at 56 °C in a water bath for 30 min. S. mutans supernatants, lysates, and the heat-inactivated S. mutans were stored at −80 °C for use.
Human tongue squamous cell carcinoma cells (SCC1) were purchased from American Type Culture Collection (ATCC) cell bank and maintained in Dulbecco’s modified eagle medium (DMEM, Gibco, USA), supplemented with 10% fetal bovine serum (FBS, Gibco, USA) in a humidified incubator with 5% CO2 at 37 °C.
Synthetic peptides
The peptide sequence of PAc (301–319) (ANAANEADYQAKLTAYQTE) was derived from the sequence of the PAc gene from S. mutans [59], as reported by previous studies [16, 60]. The sequence of the ftsZ peptide (20 aa: residue 409–428; QLKMSSFSADSDDDDELETP) was designed (https://esbl.nhlbi.nih.gov/AbDesigner/) as negative control (https://www.genome.jp/entry/smu:SMU_552). The peptides were synthesized using a stepwise solid-phase procedure [61]. Synthesized peptide samples were subsequently purified by reversed-phase HPLC on a TSK-GEL column (1 × 30 cm) (TOSO, Tokyo, Japan) with a 10–45% acetonitrile gradient in 0.1% trifluoroacetic acid (TFA) and developed over 50 min at a flow rate of 5 mL/min. Purity was determined to be greater than 95% in each tube by HPLC analysis. To confirm the amino acid sequences of the synthetic peptides, samples were analyzed using a System 7300 Amino Acid Analyzer (Beckman, USA) and a Model 477A Protein Sequencer (Applied Biosystems, Foster City, USA).
Bacteria/PAc coculture with OSCC cells for KYNA detection
SCC1 cells were seeded at 2 × 105/mL in wells of 6-well plates overnight, and bacteria were prepared at 37 °C, 5% O2. Live S. mutans, heat-inactivated S. mutans, and live S. gordonii were added to cells at a multiplicity of infection (MOI) of 10, respectively. SCC1 cells were treated with PAc and negative control peptides (10 µg/mL). As controls, SCC1 were also cultured in medium alone. Plates were incubated at 37 °C with 5% CO2 for 24 h. The supernatants were harvested and stored at −80 °C until KYNA concentration determination by ELISA kits according to the manufacturer’s protocols (BS-E7827H1, JSBOSSEN, China).
Intervention experiments on 4NQO-induced rat oral carcinogenesis model
Male Sprague–Dawley (SD) rats (4 weeks) were fed daily with 0.002% 4NQO (Sigma-Aldrich, Saint Louis, MO, USA) solution in their drinking water. After the 16-week carcinogen treatment, the drinking water was switched to distilled water. The rats intervened by different methods were described as follows.
(1) Saliva intervention (10 rats/group): (A) 4NQO + OSCC patient saliva; (B) 4NQO + OLK patient saliva; (C) 4NQO + HCs saliva; (D) 4NQO + saline. Rats were treated with 200 µL saliva by submucosal injection twice a week at the start of week 8 and ended at week 16. At week 20, the rats were sacrificed, and the tongues were dissected, and a longitudinal mid-lingual incision was made.
(2) S. mutans/S. gordonii intervention (4 rats/group): (A) 4NQO + S. mutans (CFU = 109); (B) 4NQO + S. gordonii (CFU = 109); (C) 4NQO + saline. Rats were treated with 200 µL live S. mutans/S. gordonii by submucosal injection every 5 days at the start of week 8 and ended at week 16. At week 20, the rats were sacrificed and the tongues were dissected for mucosal epithelium and stored at − 80 °C for further metabolic analyses.
(3) KYNA intervention (10 rats/group): (A) 4NQO + KYNA (28 mg/kg); (B) 4NQO + saline; (C) Normal water + KYNA. Rats were treated with KYNA (70 mg/mL) by submucosal injection every 5 days at the start of week 8 and ended at week 16. At week 20, the rats were sacrificed, and the tongues were dissected, and a longitudinal mid-lingual incision was made.
(4) IL-1β/PD-L1monoclonal antibody (mAb) intervention (8 rats/group): (A) 4NQO + IL-1β mAb (200 µg/rat, BE0246, cloneB122, Bioxcell, USA); (B) 4NQO + KYNA + IL-1β mAb; (C) 4NQO + PD-L1 mAb (200 µg/rat, BE0383, clone 368A.4H1, Bioxcell, USA); (D) 4NQO + KYNA + PD-L1 mAb; (E) 4NQO + control IgG (200 µg/rat, BE0091, Bioxcell, USA). Rats were treated with 200 µL KYNA (28 mg/kg) by submucosal injection every 5 days at the start of week 8 and ended at week 16. After that, rats were treated with indicated IL-1β mAb or PD-L1 mAb by submucosal injection twice a week from week 16 to week 20. At the end of week 20, the rats were sacrificed, and the tongues were dissected, and a longitudinal mid-lingual incision was made. Half of the specimens were fixed in 10% buffered formalin, embedded in paraffin, and cut into 5 μm sections for hematoxylin and eosin (H&E) staining to confirm the pathological diagnosis and immunohistochemistry assays. The other half of the specimens were stored at − 80 °C.
All of the animal procedures were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Sun Yat-sen University (2017-015A and KQEC-2022-07-01).
Histology and pathological analysis
H&E staining was performed on the tongue sections based on the pathological characteristics of the whole slides. Lesions of rats were histopathologically classified into three categories: low-grade dysplasia (normal, hyperplasia, and mild dysplasia), high-grade dysplasia (moderate and severe dysplasia), and carcinoma (in situ, mild, moderate, severe invasive), and scored on a 9-degree scale [62, 63]. The 9-degree histopathological scoring (normal = 0; hyperplasia = 1; mild dysplasia = 2; moderate dysplasia = 3; severe dysplasia = 4; carcinoma in situ = 5; invasive carcinoma within 1/3 of the tongue = 6; invasive carcinoma involving 1/3 to 2/3 of the tongue = 7; invasive carcinoma over 2/3 of the tongue = 8) was performed by two certified pathologists. A high level of concordance (90%) was achieved.
Immunohistochemistry assays
Formalin-fixed, paraffin-embedded specimens were collected, and a routine H&E slide was first evaluated. Immunohistochemical staining was done on 5-μm-thick paraffin-embedded sections using Ki67 (ab15580, Abcam, Cambridge, UK), CD3 (GB11014, Servicebio, China), CD8 (ab237709, Abcam, Cambridge, UK), CD11b (ab133357, Abcam, Cambridge, UK), PD-1 (66,220–1, Proteintech, Rosemont, USA), IL-1β (ab283818, Abcam, Cambridge, UK), CD16 (16,559–1; Proteintech, Rosemont, USA), PD-L1 (66,248–1; Proteintech, Rosemont, USA), and AHR (67,785–1; Proteintech, Rosemont, USA) antibodies, with a standard avidin–biotin HRP detection system according to manufacturer’s instructions (R&D Systems, MN, USA). Tissues were counterstained with hematoxylin, dehydrated and mounted.
For statistical analysis, three representative fields (at least 300 cells per field) of sections were randomly selected from each sample under 40 × magnification. The positive and total cells were calculated using ImageJ v2 software, and then the average positive rates were evaluated for each sample.
Immunofluorescence assays
Paraffin sections were deparaffinized in xylene, rehydrated, exposed to 3% hydrogen peroxide, and permeabilized with 0.5% TritonX-100. Samples were stained with primary antibodies against CD8 (ab237709, Abcam, Cambridge, UK), PD-1 (66,220–1, Proteintech, Rosemont, USA), CD11b (ab133357, Abcam, Cambridge, UK), and PD-L1 (66,248–1; Proteintech, Rosemont, USA) at 4 ℃ overnight. Then, sections were incubated with the secondary antibody labeled with Alexa Fluor 488 (A-11008, Invitrogen, Thermo Fisher Scientific, Waltham, USA) and Alexa Fluor 594 (A-11005, Invitrogen, Thermo Fisher Scientific, Waltham, USA) for an hour, followed by DAPI (62,248, Thermo Fisher Scientific, Waltham, USA) staining.
Tumor cell culture samples were fixed with 4% paraformaldehyde and stained with primary antibodies against CD44 (ab254530, Abcam, Cambridge, UK) at 4 ℃ overnight. Samples were incubated with the secondary antibody labeled with Alexa Fluor 488 (A-11001, Invitrogen, Thermo Fisher Scientific, Waltham, USA) for an hour, followed by DAPI (62,248, Thermo Fisher Scientific, Waltham, USA) staining. Images were assessed using a laser confocal fluorescence microscope.
Three representative fields (at least 50 cells per field for colocation under 100 × magnification and at least 30 cells per field for CD44 under 40 × magnification) were randomly selected from each sample. The colocalization (CD11b with PD-L1 and CD8 with PD-1) or CD44 positive cells and total cells were calculated using ImageJ v2 software, and then the average positive rates were evaluated for each sample.
Cell proliferation assays
Cell proliferation was detected by EdU assay kit (C10310, Ribobio, Guangzhou, China). The SCC1 cells (1.5 × 104 cells/well) were seeded into 48-well plates overnight. After treatment with KYNA (5 mM) or PAc (10 µg/mL) for 48 h, cells were performed EdU staining according to the manufacturer’s protocols. Images were captured with a fluorescence microscope (Zeiss Inverted Microscope). For each EdU experiment, three random fields (at least 500 cells per field) of view from each well were imaged at 10 × magnification, and the images were analyzed in ImageJ v2 software. The EdU incorporation rate was calculated as the ratio of the number of EdU‐incorporated cells to the number of Hoechst 33342‐staining cells.
DNA extraction, RNA extraction, fluorescence quantitative polymerase chain reaction (qPCR), and reverse transcription quantitative PCR (RT-qPCR)
Bacterial DNA was extracted from oral saliva and swab samples with Genomic DNA Kit (ZP321T, Zomanbio, Beijing, China) and tissue samples with Microbiome DNA Isolation Kit (DC502, Vazyme, Nanjing, China) for qPCR. Total RNA of SCC1 and neutrophils was extracted using RNA-quick purification kit (RN001, ESscience Biotech, Shanghai, China) for RT-qPCR, and subsequently, cDNA was synthesized using a HiScript III RT SuperMix for qPCR kit (R323-01, Vazyme, Nanjing, China).
Fluorescence quantification was performed using a SYBR Green Master Mix (11201ES08, Yeasen, Shanghai, China) according to the manufacturer’s protocol with an Applied LightCycler quantitative PCR system (Roche). In human oral bacteria samples, the Ct values for Streptococci species were normalized using a primer set for the total bacteria, and the relative abundance was calculated by the ΔCt method. Five microliters qPCR product of S. mutans was analyzed by using 2.0% TAE agarose gel and visualized under UV light, which was further confirmed by DNA sequencing and BLAST analysis (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Relative mRNA concentration of SCC1 and neutrophils was normalized to GAPDH using the 2−ΔΔCt method. The details of primer sequences for each assay are summarized in Table S3 in the Supplementary Materials.
Isolation, culture, activation, and treatment of immune cells
Whole blood leukocytes were isolated using red blood cell lysis buffer (420,301, Biolegend, USA) from anticoagulant peripheral bloods of enrolled OSCC patients. Neutrophils were isolated from peripheral blood using Polymorphprep™ (AS1114683, Axis-Shield, Norway) density gradient centrifugation. Cells were plated (5 × 105 per well) in a 96-well plate and cultured in RPMI 1640 containing 10% fetal bovine serum and 1 × penicillin/streptomycin at 37 °C, followed by treatment with (1) KYNA (0.5 mM and 1 mM) for 48 h in the presence of recombinant human IL-2 and GM-CSF (Peprotech, USA; final concentration, 10 ng/mL); (2) IL-1β (10 ng/mL and 50 ng/mL, Peprotech, USA) for 48 h in the presence of 1 µg/mL anti-CD3 (317,326, clone OKT3, BioLegend, USA) and 2 µg/mL anti-CD28 (302,934, clone CD28.2, BioLegend, USA); (3) S. mutans supernatant or lysate (5% and 10%) for 48 h in the presence of recombinant human IL-2 and GM-CSF (10 ng/mL).
Flow cytometry of immune cells and tumor cells
Before intracellular cytokine staining, cells were re-stimulated with Cell Activation Cocktail (423,304, BioLegend, USA) containing phorbol 12-myristate 13-acetate (PMA), ionomycin and Brefeldin A for 4 h. Cells were blocked with Human TruStain FcX (422,301, Fc Receptor Blocking Solution, BioLegend, USA) on ice for 10 min. Cells were then centrifuged at 500 g for 5 min at 4 °C and washed once with FACS buffer (1 × PBS with 3% FBS).
For cell surface staining, cells were incubated for 30 min in the dark at 4 °C with preconjugated fluorescence-labeled antibodies including anti-CD11b-PerCP-Cy5.5 (clone ICRF44, BioLegend), anti-CD16-APC (clone 3G8, BioLegend), anti-CD3-BV421 (clone SK7, BD Biosciences), anti-CD8-BV605 (clone SK1, BioLegend), and anti-PD-1-PE (clone A17188A, BioLegend). Then cells were washed once with FACS buffer (420,508, BioLegend). For intracellular cytokine staining, following the washing step, cells were fixed with Fixation Buffer (420,801, BioLegend) for 30 min in the dark at 4 °C and then washed twice with Intracellular Staining Permeabilization Wash buffer (421,002, BioLegend). Cells were stained with anti-IFN-γ-BV421 (clone B27; BioLegend), anti-TNF-α-PE (clone MAb11, BioLegend), and anti-IL-1β-AF647 (clone JK1B-1, BioLegend) for 30 min at 4 °C in the dark. Cells were then washed twice with Permeabilization Wash buffer.
For intracellular transcription factor staining, cells were fixed with Fix/Perm buffer (562,574, Transcription Factor Buffer Set, BD Biosciences, USA) for 50 min, stained with anti-AHR-PE (clone T49-550; BD Biosciences) for 50 min and washed twice with Perm/Wash buffer (Transcription Factor Buffer Set, 562,574, BD Biosciences).
All the stained cells were suspended in 300 µL PBS and then measured on a LSRFortessa (BD Biosciences) using application settings, and at least 20,000 events were collected. Data were then analyzed with FlowJo software (Version 10.0, USA).
TCGA dataset collection and analyses
Reads/Fragments per kilobase million (RPKM/FPKM) and clinical information of head and neck squamous cell carcinoma (HNSCC) from The Cancer Genome Atlas (TCGA) (https://gdc.cancer.gov/) were downloaded. OSCC data from Gene Expression Omnibus (GEO) datasets (https://www.ncbi.nlm.nih.gov/geo/) (GSE41613 and GSE65858) were downloaded. The FPKM values were converted to transcripts per million (TPMs), and log2 was transformed for normalization.
The expression of tryptophan metabolism pathway-related enzymes (TDO2, IDO1, IDO2, AFMID, and KYAT1) and AHR between adjacent non-tumor and tumor tissues was analyzed using the normalized count matrix of the TCGA dataset and determined by Student’s t-test. Spearman correlation analysis was conducted between the expression of CD274 and IDO1, IDO2, TDO2, and AHR. The optimal cut-off values of gene expression were determined by the “survminer” R packages (Version 4.1.0). Kaplan–Meier analysis was performed to assess the survival difference between the high and low expression groups, and log-rank tests were used to determine the significance using “lifelines” Python package (Version 3.7.0). The bioinformatics analyses were conducted using Python version 3.7.0 and R version 4.1.0.
Statistical analysis
All the experiments were replicated at least three times. For measurement data, Shapiro–Wilk test was used to evaluate the normality. Student’s t or ANOVA test was used to evaluate the statistical significance for normally distributed data, and Mann–Whitney U or Kruskal–Wallis test was used for non-normally distributed data. For categorical data, chi-square test or Fisher’s exact test was applied to assess the difference. All data were analyzed with GraphPad Prism 9 software (Dotmatics, San Diego, USA) and SPSS 25.0 (IBM, Armonk, NY, USA). All statistical tests were two-sided.
Supplementary Information
Additional file 1: Figure S1 to S8 and Table S1 to S3
Acknowledgements
Not applicable.
Authors’ contributions
Conceptualization: G.Z., B.C., and T.W.; methodology: J.Z., Z.H., L.W., Q.H., Z.C., T.L., Q.S., and R.Z.; investigation: J.Z., Z.H., and G.Z.; formal analysis: Y.Y., Z.W., G.L., and C.Z.; resources: H.L. and K.S.; visualization: Z.Z., J.Z., Z.H., L.Z., and Y.C.; funding acquisition: J.Z., T.W., G.Z., and B.C.; supervision: B.C.; project administration: G.Z., B.C., and T.W.; writing—original draft: J.Z., Z.H., and L.W.; writing—review and editing: G.Z., B.C., and T.W. All authors read and approved the final manuscript.
Funding
This study was partially supported by the National Natural Science Foundation of China (Grant Nos. 82370960, 823B2017, 32370983, and 82072250), Science and Technology Project of Guangzhou, China (Grant No. 202206080009), Guangdong Provincial Key Laboratory of Human Disease Genomics (Grant No. 2020B1212070028), and Shenzhen Key Laboratory of Single-Cell Omics (Grant No. ZDSYS20190902093613831).
Availability of data and materials
The data of HNSCC and OSCC for bioinformatics analysis were downloaded from The Cancer Genome Atlas (TCGA) (https://gdc.cancer.gov/) and Gene Expression Omnibus (GEO) datasets (https://www.ncbi.nlm.nih.gov/geo/). The data supporting the findings of single-cell sequencing have been deposited into CNGB Sequence Archive (CNSA) [64] of China National GeneBank DataBase [65] (CNGBdb; https://db.cngb.org/) with accession number CNP0000468. Data supporting the metabolomic findings have been deposited into MetaboLights (https://www.ebi.ac.uk/metabolights/) with accession numbers MTBLS10162, MTBLS10171, and MTBLS10209.
Declarations
Ethics approval and consent to participate
This study protocol about human specimens was approved by the Ethics Committee of the Hospital of Stomatology, Sun Yat-sen University (KQEC-2021-62-01), and was conducted in agreement with the Helsinki Declaration, and written informed consent was obtained from all study participants. All of the animal procedures were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Sun Yat-sen University (2017-015A and KQEC-2022-07-01).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiaying Zhou, Zixuan Hu, Lei Wang, Qinchao Hu and Zixu Chen contributed equally to this work.
Contributor Information
Gucheng Zeng, Email: zenggch@mail.sysu.edu.cn.
Bin Cheng, Email: chengbin@mail.sysu.edu.cn.
Tong Wu, Email: wutong23@mail.sysu.edu.cn.
References
- 1.Johnson DE, et al. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020;6:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chinn SB, Myers JN. Oral cavity carcinoma: current management, controversies, and future directions. J Clin Oncol. 2015;33:3269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chamoli A, et al. Overview of oral cavity squamous cell carcinoma: risk factors, mechanisms, and diagnostics. Oral Oncol. 2021;121:105451. [DOI] [PubMed]
- 4.Srivastava S, et al. The making of a precancer atlas: promises, challenges, and opportunities. Trends Cancer. 2018;4:523–36. [DOI] [PubMed] [Google Scholar]
- 5.Aguirre-Urizar JM, Lafuente-Ibanez de Mendoza I, Warnakulasuriya S. Malignant transformation of oral leukoplakia: systematic review and meta-analysis of the last 5 years. Oral Dis. 2021;27:1881–95. [DOI] [PubMed]
- 6.Tuganbaev T, Yoshida K, Honda K. The effects of oral microbiota on health. Science. 2022;376:934–6. [DOI] [PubMed] [Google Scholar]
- 7.Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018;16:745–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freire M, Nelson KE, Edlund A. The oral host-microbial interactome: an ecological chronometer of health? Trends Microbiol. 2021;29:551–61. [DOI] [PubMed] [Google Scholar]
- 9.Kitamoto S, et al. The bacterial connection between the oral cavity and the gut diseases. J Dent Res. 2020;99:1021–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zubeidat K, Hovav AH. Shaped by the epithelium - postnatal immune mechanisms of oral homeostasis. Trends Immunol. 2021;42:622–34. [DOI] [PubMed] [Google Scholar]
- 11.Stasiewicz M, Karpinski TM. The oral microbiota and its role in carcinogenesis. Semin Cancer Biol. 2022;86:633–42. [DOI] [PubMed] [Google Scholar]
- 12.Abd E-F, E. IDO/kynurenine pathway in cancer: possible therapeutic approaches. J Transl Med. 2022;20:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galeano NJ, L., et al. Effect of the intratumoral microbiota on spatial and cellular heterogeneity in cancer. Nature. 2022;611:810–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston CD, Bullman S. The tumour-associated microbiome. Nat Rev Gastroenterol Hepatol. 2022;19:347–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abranches J, et al. Biology of oral Streptococci. Microbiol Spectr. 2018;6:10. [DOI] [PMC free article] [PubMed]
- 16.Takahashi I, et al. Genetic control of immune responses in mice to synthetic peptides of a Streptococcus mutans surface protein antigen. Infect Immun. 1992;60:623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okahashi N, et al. Cloning of a surface protein antigen gene from serotype c Streptococcus mutans. Mol Microbiol. 1989;3:221–8. [DOI] [PubMed] [Google Scholar]
- 18.Koga T, et al. Surface hydrophobicity, adherence, and aggregation of cell surface protein antigen mutants of Streptococcus mutans serotype c. Infect Immun. 1990;58:289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cervenka I, Agudelo LZ, Ruas JL. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science. 2017;357:eaaf9794. [DOI] [PubMed]
- 20.Iizuka T, et al. Mono-(2-ethyl-5-hydroxyhexyl) phthalate promotes uterine leiomyoma cell survival through tryptophan-kynurenine-AHR pathway activation. Proc Natl Acad Sci U S A. 2022;119:e2208886119. [DOI] [PMC free article] [PubMed]
- 21.Liu Y, et al. Tumor-repopulating cells induce PD-1 expression in CD8(+) T cells by transferring kynurenine and AhR activation. Cancer Cell. 2018;33:480-494.e7. [DOI] [PubMed]
- 22.Mescher M, Haarmann-Stemmann T. Modulation of CYP1A1 metabolism: from adverse health effects to chemoprevention and therapeutic options. Pharmacol Ther. 2018;187:71–87. [DOI] [PubMed] [Google Scholar]
- 23.Murray IA, Patterson AD, Perdew GH. Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nat Rev Cancer. 2014;14:801–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garlanda C, Mantovani A. Interleukin-1 in tumor progression, therapy, and prevention. Cancer Cell. 2021;39:1023–7. [DOI] [PubMed] [Google Scholar]
- 25.Ryckman C, et al. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J Immunol. 2003;170:3233–42. [DOI] [PubMed] [Google Scholar]
- 26.Scott NR, et al. S100A8/A9 regulates CD11b expression and neutrophil recruitment during chronic tuberculosis. J Clin Invest. 2020;130:3098–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplanov I, et al. Blocking IL-1beta reverses the immunosuppression in mouse breast cancer and synergizes with anti-PD-1 for tumor abrogation. Proc Natl Acad Sci U S A. 2019;116:1361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Briukhovetska D, et al. Interleukins in cancer: from biology to therapy. Nat Rev Cancer. 2021;21:481–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larkins E, et al. FDA approval summary: pembrolizumab for the treatment of recurrent or metastatic head and neck squamous cell carcinoma with disease progression on or after platinum-containing chemotherapy. Oncologist. 2017;22:873–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kujan O, van Schaijik B, Farah CS. Immune checkpoint inhibitors in oral cavity squamous cell carcinoma and oral potentially malignant disorders: a systematic review. Cancers (Basel). 2020;12:1937. [DOI] [PMC free article] [PubMed]
- 31.Cramer JD, et al. The changing therapeutic landscape of head and neck cancer. Nat Rev Clin Oncol. 2019;16:669–83. [DOI] [PubMed] [Google Scholar]
- 32.Knippel RJ, Drewes JL, Sears CL. The cancer microbiome: recent highlights and knowledge gaps. Cancer Discov. 2021;11:2378–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sears CL, Garrett WS. Microbes, microbiota, and colon cancer. Cell Host Microbe. 2014;15:317–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ray K. The oral-gut axis in IBD. Nat Rev Gastroenterol Hepatol. 2020;17:532. [DOI] [PubMed] [Google Scholar]
- 35.Flemer B, et al. The oral microbiota in colorectal cancer is distinctive and predictive. Gut. 2018;67:1454–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyauchi S, et al. Immune modulation of head and neck squamous cell carcinoma and the tumor microenvironment by conventional therapeutics. Clin Cancer Res. 2019;25:4211–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauml J, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J Clin Oncol. 2017;35:1542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai MS, et al. Streptococcus mutans promotes tumor progression in oral squamous cell carcinoma. J Cancer. 2022;13:3358–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu L, et al. The oral bacterium Streptococcus mutans promotes tumor metastasis by inducing vascular inflammation. Cancer Sci. 2022;113:3980–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yumoto H, et al. The pathogenic factors from oral Streptococci for systemic diseases. Int J Mol Sci. 2019;20:4571. [DOI] [PMC free article] [PubMed]
- 41.Barbour A, et al. Metabolites of the oral microbiome: important mediators of multikingdom interactions. FEMS Microbiol Rev. 2022;46:fuab039. [DOI] [PubMed]
- 42.de Soet JJ, Nyvad B, Kilian M. Strain-related acid production by oral Streptococci. Caries Res. 2000;34:486–90. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi N, Nyvad B. Caries ecology revisited: microbial dynamics and the caries process. Caries Res. 2008;42:409–18. [DOI] [PubMed] [Google Scholar]
- 44.Fu A, et al. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell. 2022;185:1356–1372.e26. [DOI] [PubMed]
- 45.Riquelme E, et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell. 2019;178:795-806.e12. [DOI] [PMC free article] [PubMed]
- 46.Nejman D, et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science. 2020;368:973–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sepich-Poore GD, et al. The microbiome and human cancer. Science. 2021;371:eabc4552. [DOI] [PMC free article] [PubMed]
- 48.Zheng DW, et al. Biomaterial-mediated modulation of oral microbiota synergizes with PD-1 blockade in mice with oral squamous cell carcinoma. Nat Biomed Eng. 2022;6:32–43. [DOI] [PubMed] [Google Scholar]
- 49.DePeaux K, Delgoffe GM. Metabolic barriers to cancer immunotherapy. Nat Rev Immunol. 2021;21:785–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matson V, Chervin CS, Gajewski TF. Cancer and the microbiome-influence of the commensal microbiota on cancer, immune responses, and immunotherapy. Gastroenterology. 2021;160:600–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walczak K, et al. Kynurenic acid and cancer: facts and controversies. Cell Mol Life Sci. 2020;77:1531–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pires AS, et al. Recent advances in clinical trials targeting the kynurenine pathway. Pharmacol Ther. 2022;236:108055. [DOI] [PubMed]
- 53.Kitamoto S, et al. The intermucosal connection between the mouth and gut in commensal pathobiontdriven colitis. Cell. 2020;182:447-462.e14. [DOI] [PMC free article] [PubMed]
- 54.Williams DW, et al. Human oral mucosa cell atlas reveals a stromal-neutrophil axis regulating tissue immunity. Cell. 2021;184:4090-4104.e15. [DOI] [PMC free article] [PubMed]
- 55.Silva LM, et al. Fibrin is a critical regulator of neutrophil effector function at the oral mucosal barrier. Science. 2021;374:eabl5450. [DOI] [PMC free article] [PubMed]
- 56.Cai Y, et al. The role of gut microbiota in infectious diseases. WIREs Mech Dis. 2022;14:e1551. [DOI] [PubMed]
- 57.Hamidi B, et al. W*d -test: robust distance-based multivariate analysis of variance. Microbiome. 2019;7:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thurnheer T, et al. Automated fluorescent in situ hybridization for the specific detection and quantification of oral streptococci in dental plaque. J Microbiol Methods. 2001;44:39–47. [DOI] [PubMed] [Google Scholar]
- 59.Ajdic D, et al. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc Natl Acad Sci U S A. 2002;99:14434–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsuha Y, et al. Role of peptide antigen for induction of inhibitory antibodies to Streptococcus mutans in human oral cavity. Clin Exp Immunol. 2004;137:393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Behrendt R, White P, Offer J. Advances in Fmoc solid-phase peptide synthesis. J Pept Sci. 2016;22:4–27. [DOI] [PMC free article] [PubMed]
- 62.Hu S, et al. TDO2+ myofibroblasts mediate immune suppression in malignant transformation of squamous cell carcinoma. J Clin Invest. 2022;132:e157649. [DOI] [PMC free article] [PubMed]
- 63.Li Y, et al. Tacrolimus inhibits oral carcinogenesis through cell cycle control. Biomed Pharmacother. 2021;139:111545. [DOI] [PubMed]
- 64.Guo X, et al. CNSA: a data repository for archiving omics data. Database (Oxford). 2020;2020:baaa055. [DOI] [PMC free article] [PubMed]
- 65.Chen F, et al. CNGBdb: China National GeneBank DataBase. Hereditas. 2020;42:799–809. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1 to S8 and Table S1 to S3
Data Availability Statement
The data of HNSCC and OSCC for bioinformatics analysis were downloaded from The Cancer Genome Atlas (TCGA) (https://gdc.cancer.gov/) and Gene Expression Omnibus (GEO) datasets (https://www.ncbi.nlm.nih.gov/geo/). The data supporting the findings of single-cell sequencing have been deposited into CNGB Sequence Archive (CNSA) [64] of China National GeneBank DataBase [65] (CNGBdb; https://db.cngb.org/) with accession number CNP0000468. Data supporting the metabolomic findings have been deposited into MetaboLights (https://www.ebi.ac.uk/metabolights/) with accession numbers MTBLS10162, MTBLS10171, and MTBLS10209.