Abstract
Interferon (IFN) type I (alpha/beta IFN [IFN-α/β]) is very important in directly controlling herpes simplex virus type I (HSV-1) replication as well as in guiding and upregulating specific immunity against this virus. By contrast, the roles of IFN type II (IFN-γ) and antibodies in the defense against HSV-1 are not clear. Mice without a functional IFN system and no mature B and T cells (AGR mice) did not survive HSV-1 infection in the presence or absence of neutralizing antibodies to the virus. Mice without a functional IFN type I system and with no mature B and T cells (AR129 mice) were unable to control infection with as little as 10 PFU of HSV-1 strain F. By contrast, in the presence of passively administered neutralizing murine antibodies to HSV-1, some AR129 mice survived infection with up to104 PFU of HSV-1. This acute immune response was dependent on the presence of interleukin-12 (IL-12) p75. Interestingly, some virus-infected mice stayed healthy for several months, at which time antibody to HSV-1 was no longer detectable. Treatment of these virus-exposed mice with dexamethasone led to death in approximately 40% of the mice. HSV-1 was found in brains of mice that did not survive dexamethasone treatment, whereas HSV-1 was absent in those that survived the treatment. We conclude that in the presence of passively administered HSV-1-specific antibodies, the IL-12-induced IFN-γ-dependent innate immune response is able to control low doses of virus infection. Surprisingly, in a significant proportion of these mice, HSV-1 appears to persist in the absence of antibodies and specific immunity.
Control of acute as well as persistent herpes simplex virus type I (HSV-1) is believed to require interferons (IFN), natural killer (NK) cells, virus-specific CD4+ and CD8+ T cells, and virus-specific antibodies (8, 29). IFN type I (alpha/beta IFN [IFN-α/β]), which is produced by many cells, is crucial for the immediate control of initial HSV-1 replication and powerfully initiates innate and specific immune responses (12, 19, 28). IFN-γ, which is secreted mostly by NK and T cells, can also directly interfere with virus replication, but the main role of IFN-γ is believed to be indirect by regulating more than 200 genes (3). For IFN-γ secretion, NK cells need to be activated by IFN-α/β, which is induced directly by virus infection or by IL-12 or tumor necrosis factor alpha secreted by infection of monocytes/macrophages (6, 10, 33). In addition, NK cells can be activated by binding immunoglobulins through their Fc receptor (CD16) located on the cell surface (13, 27).
In vitro the biological effect of antibody specific to HSV-1 can be neutralization to prevent virus infection, whereas in vivo the biological effect may further include aggregation of antigen followed by complement activation. The antibody-aggregated virus facilitates uptake by phagocytes. Antigen-bound antibodies may help to activate macrophages or NK cells (16, 17). However, in the absence of IFN, the effect of neutralizing antibodies in defending against HSV-1 is unknown.
To directly address these questions, we made use of mice with the IFN receptor and recombination-activating gene (RAG) deleted. Animals termed AGR129 mice have no functional receptors for IFN type I (IFN-α/β) or IFN type II (IFN-γ) and carry a third deletion (RAG) that does not allow these mice to produce mature T and B cells (12). By contrast, AR129 mice have a functional IFN-γ system but a deleted IFN type I system and no mature T and B cells (10). Using these two mouse strains without specific immunity, the biological effect of neutralizing antibody to HSV-1 in the absence of a functional IFN system in AGR129 mice or in the presence of IFN-γ in AR129 mice was analyzed.
(This publication is in partial fulfillment of the requirements for Sabine Vollstedt's doctoral thesis from the Faculty of Veterinary Medicine, University of Zurich.)
MATERIALS AND METHODS
Animals and virus.
Six- to 8-week-old mice with gene-targeted disruptions of IFN receptor types I and II as well as RAG (AGR129) and congenic 129Sv/Ev mice with targeted disruptions of IFN receptor type I and RAG (AR129) were used (10, 12). Thus, AGR129 mice have no functional IFN system and no mature T and B cells, whereas AR129 mice have no functional type I IFN (IFN-α/β) system and no mature T and B cells but have an intact IFN-γ system. Mice were bred and maintained under specific-pathogen-free conditions in the Labortierkunde, Universität Zurich, Zurich, Switzerland.
The HSV-1 F strain was originally obtained from B. Roizman (University of Chicago) and was propagated on Vero cells (9). For all experiments purified virus particles from the same batch were used. Purification of infectious particles was performed by ultracentrifugation on a sucrose density gradient, and the virus titer was determined as described previously (32).
Production of neutralizing murine antibodies, serology, and passive immunizations.
For the production of neutralizing murine antibodies, groups of C57BL/6 and 129Sv/Ev mice were injected with a sublethal dose of 106 PFU of the HSV-1 F strain. At 3 to 4 weeks after immunization mice were bled, and the sera were pooled and analyzed for in vitro virus neutralization capacity and enzyme-linked immunosorbent assay (ELISA) titer as described previously, using peroxidase-conjugated polyclonal anti-mouse immunoglobulin G1 and immunoglobulin G2a antibody (Southern Biotechnology, Birmingham, Ala.) (32).
For the calibration of passive immunization with antibodies, different amounts of immune sera were administered intraperitoneally (i.p.) to naive AGR or AR129 mice to obtain a neutralization titer of 60 to 80/ml and an ELISA titer of approximately 104/ml in the sera of these mice as determined 24 h later. For some experiments, sera from passively immunized animals were analyzed at later time points (see Table 1 for details). For controls, sera from naive C57BL/6 specific-pathogen-free animals, or no serum, were used. To determine the biological significance of IL-12 p75, 100 μg of neutralizing monoclonal antibody (10F6) specific to this cytokine was administered i.p. to mice on days −1, +1, +3, and +5 of the infection experiment (23). Monoclonal antibody 10F6 used under these conditions is not toxic but can neutralize nanogram amounts of IL-12 p70 to prevent death after induction of shock (reference 23 and unpublished observations).
TABLE 1.
Influence of antibody titer on survival of HSV-1-infected AR129 micea
| Day after passive immunization | Neutralization titer | ELISA titer | % Surviving animals |
|---|---|---|---|
| 1 | 80 | 12,800 | 50 |
| 7 | NDb | 4,500 | 16 |
| 21 | ND | 1,125 | 0 |
| 42 | ND | <50 | ND |
AR129 mice were injected with murine antibodies against HSV-1 (see Materials and Methods). At 1 to 42 days after passive immunization of mice, the mean serum neutralization titer and ELISA titer were determined from pooled sera of three mice collected at each time point. Six mice each were infected at the given time point after passive immunization with 102 PFU of HSV-1, and the percentage of surviving animals was determined.
ND, not determined.
HSV-1 infection and reactivation protocol.
Before infection, mice were injected with neutralizing antibodies or sera from naive animals or were left untreated (see above). Twenty-four hours later, AGR129 or AR129 mice were infected i.p. with 102 or 104 PFU of HSV-1 F strain, or in some experiments this was done 1 or 3 weeks after passive immunization. After 3 to 4 months, mice that survived HSV-1 infection were treated with corticosteroids. Mice were given dexamethasone (Veterinaria AG, Zurich, Switzerland) in two doses of 3 mg each within 2 days. Three milligrams of dexamethasone contains 1 mg of dexamethasone sodium phosphate and 2 mg of dexamethasone phenylpropionate. Dexamethasone sodium phosphate is immediately released and lasts for 48 h, whereas dexamethasone phenylpropionate lasts for 8 days. This dose is not lethal to HSV-1-negative control mice but enables virus reactivation of HSV-1-infected wild-type animals.
Resection of trigeminal and spinal ganglia and PCR.
Trigeminal and spinal ganglia were isolated to probe for HSV-1 DNA by PCR. Trigeminal ganglia were separated from brain tissue after opening and removing the skull. Spinal ganglia were surgically removed by opening the spinal cord under microscopic control. To detect the HSV-1 genome in spleen, liver, lung, kidney, or brain, DNA was isolated by standard procedures and HSV-specific genes were amplified by PCR. Primers were directed to the HSV glycoprotein B (gB) gene. The forward primer was 5′-TCC CGG TAC GAA GAC CAG, and the reverse primer was 5′-AGC AGG CCG CTG TCC TTG. Conditions for PCR were as described previously (30). Under the PCR conditions used, the lower limit of detection is approximately 5 to 10 copies of gB per assay.
RESULTS
Neutralizing antibodies specific to HSV-1 are unable to protect AGR129 mice against infection with HSV-1.
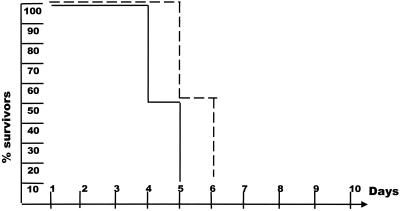
AGR129 mice, which lack functional IFN (34) as well as mature T and B cells, are very susceptible to exposure to various viruses (12). When infected with 102 PFU of HSV-1, these mice died within 6 to 7 days (Fig. 1). Virus was reisolated from several organs, or the viral genome was detected by PCR (data not shown). Animals infected with less than 10 PFU of HSV-1 also died, indicating that mice without IFN and mature T and B cells have no resistance against infection with HSV-1. We next tested whether neutralizing murine antibodies with a titer of 80 (Table 1) could induce resistance against virus infection. Mice were first injected with neutralizing antibodies specific to HSV-1 at 24 h before infection with 102 PFU of virus, and the effect of the antibodies was analyzed by clinical observation. The data shown in Fig. 1 illustrate that the onset of the disease was delayed somewhat, but protection was not achieved. Infection of AGR129 mice with approximately 10 PFU of virus and more antibodies (up to a titer of 180) delayed the onset of the disease but did not significantly protect mice from a deadly infection (data not shown). Hence, virus-specific antibody and complement naturally present in AGR mice were not able to directly protect mice against infection with HSV-1. In addition, in mice without functional IFN, elements of the innate immunity such as macrophages or NK cells were not able to cooperate with antibodies in virus clearance, because they appeared to need activation by IFN-α/β or IFN-γ (20, 27).
FIG. 1.
Neutralizing antibodies specific to HSV-1 are unable to protect AGR129 mice against infection with HSV-1. Six AGR129 mice were infected with 102 PFU of HSV-1 in the absence (—) or presence (- - - ) of HSV-1-specific antibody. The survival of mice after virus infection is shown. Data are from a representative experiment of three.
AR129 mice supplemented with neutralizing antibodies to HSV-1 are resistant to infection with small amounts of virus.
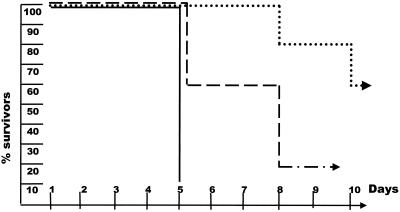
In a next step, the IFN-γ system was analyzed for its effect on influencing the course of HSV-1 infection. AR129 mice have a functional IFN-γ system but lack the IFN type I system and specific T and B cells. Groups of mice were infected with 102 PFU of virus, and the animals were analyzed for resistance against the infection. Similar to the case for AGR129 mice, no AR129 mouse survived the virus infection. Members of both mouse strains succumbed to the infection within the same time frame (Fig 2). Similar to the case for AGR129 mice, infection with less virus led to an extended survival time of the AR129 mice, but none survived longer than 2 months (data not shown). We next analyzed whether pretreatment of AR129 mice with neutralizing antibody specific to HSV-1 antigen allowed survival after infection with 102 PFU of virus. Interestingly, about 50% of the infected mice survived. Increasing the virus dose to 104 PFU led to survival of about 15% of the mice (Fig. 2). Mice pretreated with normal mouse serum and then exposed to HSV-1 did not survive the infection. In mice that succumbed after HSV-1 infection, virus was found in brain, trigeminal ganglion, spleen, kidney, and lung, and serum antibody specific to the virus was absent at this time point.
FIG. 2.
AR129 mice supplemented with neutralizing antibodies to HSV-1 are resistant to infection with small amounts of virus. Groups of 10 AR129 mice were each infected with 102 or 104 PFU of HSV-1 in the absence (—) or presence (--;····) of HSV-1-specific antibody. The survival of mice after virus infection is shown. The data shown were pooled from two different experiments.
In the next experiment, two groups of mice were injected with the standard amount of antiserum to HSV-1 antigen. Sera from mice of group 1 were analyzed periodically for the presence of antibody to HSV-1, whereas mice of group 2 were infected with HSV-1 at 24 h or at weekly intervals after passive immunization, and the health status of the animals was monitored (Table 1). The antibody titer to HSV-1 in sera taken weekly from mice of group 1 decreased gradually and was undetectable 6 weeks after passive administration. Fifty percent of the mice infected with 102 PFU of virus 24 h after infusion of antibodies survived the infection, whereas only 16% of the mice infected 1 week after infusion and none of the mice infected 2 weeks after passive administration of antibodies survived. Mice that received HSV-1 had no detectable virus-specific antibodies 1 week after infection, irrespective of whether the mice survived the infection or not. Therefore, a critical level of antibody to HSV-1 was required to confer protection against acute infection with a given amount of virus.
IL-12 is essential for the protection of AR129 mice exposed to HSV-1 in the presence of neutralizing antibodies.
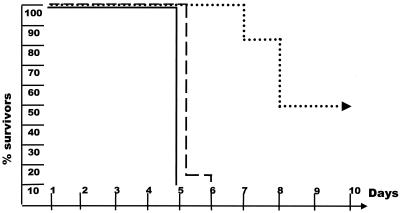
After virus infection, IFN-α/β may activate cells of the innate immune system directly, whereas in the absence of IFN-α/β, induction of IFN-γ requires the presence of IL-12 (7, 26). Because AR129 mice have no functional type I IFN system, the role of IL-12 after HSV-1 infection was investigated (14). Groups of mice were given the standard amount of antiserum to HSV-1 together with neutralizing antibodies specific to IL-12 (23). Twenty-four hours later, the mice were infected with 102 PFU of virus. All mice died within 7 days (Fig. 3), similar to the case for AR129 mice not receiving antiserum specific to HSV-1. Therefore, critical amounts of neutralizing antibody to HSV-1 together with the IL-12-dependent IFN-γ system are crucial for the resistance against HSV-1 infection in these immunocompromised mice.
FIG. 3.
IL-12 is essential for the protection of AR129 mice exposed to HSV-1 in the presence of neutralizing antibodies. Groups of 10 AR129 mice were each infected with 102 PFU of HSV-1 in the absence (—) or presence (--;····) of HSV-1-specific antibody or, in addition to these antibodies, with monoclonal antibody that neutralizes IL-12p70 (····). The survival of mice after virus infection is shown. The data shown were pooled from two different experiments.
Elimination or control of persistent virus in long-term survivor HSV-1-infected AR129 mice.
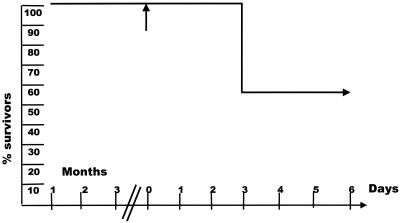
In the presence of both sufficient amounts of neutralizing antibody to HSV-1 and the IL-12-dependent IFN-γ system, about 50% of AR129 mice were able to resist acute infection with small amounts of virus. HSV-1-infected animals were devoid of antibody shortly after infection, and detectable antibody was present in noninfected animals for no more than 6 weeks (Table 1). However, some HSV-1-infected animals survived for up to 15 weeks, the latest time point analyzed. It was possible that the mice that survived the infection had eliminated the virus or that persistent virus infection occurred in AR129 mice. To test for these two possibilities, six mice that survived 102 PFU and one that survived 104 PFU of HSV-1 infection were treated with dexamethasone. Within 4 days after the treatment, two of the six mice infected with 102 PFU of HSV-1 and the one mouse infected with 104 PFU of HSV-1 died (Fig. 4). HSV-1 DNA was detected in brain but not in other organs of the diseased animals by amplification of HSV-1 gB-specific gene fragments by PCR. Reisolation of replicating virus was not reproducible. Animals that resisted dexamethasone treatment were sacrificed, and brain, ganglia, and peripheral organs were analyzed for the presence of HSV-1 gB by PCR. All organs tested were negative. The data indicate that in the presence of antibodies, the IL-12-induced IFN-γ system, possibly aided by activated monocytes/macrophages and NK cells, appeared to eliminate HSV-1 in four out of seven cases, whereas in the remaining three cases infection seemed to be controlled by the immune system.
FIG. 4.
Elimination or control of persistent virus in long-term survivor HSV-1-infected AR129 mice. AR129 mice were infected with 102 or 104 PFU of HSV-1 in the presence of HSV-1-specific antibody. Three months after infection, the surviving animals were treated with glucocorticosteroids (↑). The survival of animals after treatment with glucocorticoids is shown.
DISCUSSION
In mice without a functional IFN system or mature T and B cells, neutralizing antibodies to HSV-1 antigen had no significant effect in impeding infection or spread of the virus, even though in vitro these antibodies had a neutralizing titer of 80 (Table 1). In fact, there was no difference in the death rate when AGR129 mice were infected with 103 PFU of HSV-1 in the presence or absence of antibodies. In vivo, HSV-1 may be able to bind to far more receptors than in vitro and direct virus neutralization by antibodies may thus be difficult (5). Simple mass law considerations illustrate this point: [ligand] + [receptor] ⇄ [ligand-receptor]. This equation predicts that the amount of receptors on the cells is directly proportional to the amount of virus available for interaction ([ligand-receptor]). In addition, in vivo the diversity and perhaps the density of the receptors per cell may be higher than in vitro (5). This makes cooperative binding of the virus to cells more likely but direct neutralization by antibodies more difficult in vivo than in vitro, where one defined cell line adherent to plastic as a monolayer is used for virus neutralization by antibodies (25). Therefore, the biological effect of antibodies bound to HSV-1 in vivo may be more indirect by stimulating innate immune responses. This is illustrated in newborn mice, where large amounts of antibody are required to protect animals from relatively small amounts of virus (2, 15, 22). In these mice, the IFN system is immature and supplementation of monocytes/macrophages or NK cells from adult animals increased protection against virus infection significantly (4). The data presented here suggest that in AGR129 mice without a functional IFN system at all or in newborn mice with an immature IFN system, antibody is not efficient in directly neutralizing HSV-1 infection (1). Therefore, indirect mechanisms that are dependent on IFN support the biological activity of these molecules.
Because the IFN type I system has a profound direct effect on HSV-1 replication (19), we have analyzed whether IFN type II (IFN-γ) may indirectly support the biological activity of neutralizing antibodies. AR129 mice have a functional IFN-γ system but were unable to resist HSV-1 infection of as low as 10 PFU, possibly because this virus can downregulate immune responses, at least in vitro (18, 31). Interestingly, neutralizing antibodies passively administered to these mice allowed them to resist acute infection, depending on the dose of HSV-1 and the amount of passively administered antibodies, but normal mouse serum was ineffective (Table 1). Therefore, additional activation signals, possibly delivered by antibody-antigen complexes together with IFN-γ, were necessary to induce protection against HSV-1. In the absence of IFN-α/β, the IFN-γ system needs activation by IL-12 (26). Treatment of AR129 mice with neutralizing antibodies against IL-12 clearly indicated that this cytokine was necessary for protection against HSV-1. Production of IL-12 by dendritic cells or granulocytes after HSV-1 infection in mice has previously been demonstrated (14). We thus speculate that virus-containing cells that secrete IL-12 may activate and/or attract cells able to produce IFN-γ, enabling control of HSV-1 replication (24). However, the temporal order in which the postulated HSV-1 antigen-antibody complexes, IL-12, and IFN-γ might operate in concert with other cytokines and chemokines as well as the cellular members of the innate immune system is not clear. The need for immediate cytokine production to control herpesvirus infection was shown previously (12). Because IL-12 is a cytokine that is produced by monocytes/macrophages early in immune responses, we consider it likely that HSV-1 antigen-antibody may enable these cells to produce IL-12, activate nearby NK cells, and recruit other cells to the site of infection (24). Antibody may further be used to focus and/or activate NK cells against virus-infected targets (21, 27). In addition, we have previously provided evidence that CD8 α+ dendritic cells from AR129 mice activate NK cells by direct cell-to-cell contact to produce IFN-γ and to kill major histocompatibility complex-negative tumor cells in vitro and possible in vivo (10). Determination of whether a similar mechanism is operating in HSV-1-infected AR129 mice requires further analysis.
Animals that succumbed to virus infection as well as those that survived had no detectable antibodies to HSV-1 antigen as analyzed by ELISA. Interestingly, animals that survived acute HSV-1 infection lived for up to 15 weeks, the latest time analyzed. Treatment of these mice with corticosteroids revealed two possible mechanisms of virus control in AR129 mice. In the majority of these animals corticosteroids did not affect the health status of the mice, and no evidence of virus was found in the organs by reisolation or PCR. We can conclude that in these mice virus had been eliminated by the innate immune system. In some cases animals succumbed after treatment with corticosteroids. HSV-1 gB DNA was found by PCR in ganglia and in brain of the diseased animals but not in peripheral organs. In these animals HSV-1 appeared to persist and hence needed control by the innate immune system provided by the AR129 mice, because AGR129 mice without the IFN-γ system all died after infection with HSV-1. Thus, immune cells, possibly NK cells activated by monocytes/macrophages, may be able to control persistent virus infection in the absence of specific immunity. Immune histological analysis and infection experiments with mouse strains without mature T and B cells as well as NK may allow us to address these questions (1).
These data have significance for neonatal immune responses against intracellular pathogens that depend on IFN for defense. The effect of maternal or colostral antibodies may be enhanced by specifically or nonspecifically inducing IL-12 or IFN (11).
ACKNOWLEDGMENTS
We thank Cornelia Schwerdel for skillful technical assistance and Hanspeter Nägeli, Institute for Veterinary Pharmacology, University of Zurich, for assistance in the use of corticosteroids.
This work was supported by the Canton of Zurich and a grant from the BBW (no. 96.0046-1; EU concerted action B104-CT 96-0398). G.A. was supported by a Socrates fellowship.
REFERENCES
- 1.Ashkar A A, Di Santo J P, Croy B A. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med. 2000;192:259–270. doi: 10.1084/jem.192.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baron S, Worthington M G, Williams J, Gaines J W. Postexposure serum prophylaxis of neonatal herpes simplex virus infection of mice. Nature. 1976;261:505–506. doi: 10.1038/261505a0. [DOI] [PubMed] [Google Scholar]
- 3.Boehm U, Klamp T, Groot M, Howard J C. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 4.Bukowski J F, Welsh R M. The role of natural killer cells and interferon in resistance to acute infection of mice with herpes simplex virus type 1. J Immunol. 1986;136:3481–3485. [PubMed] [Google Scholar]
- 5.Campadelli-Fiume G, Cocchi F, Menotti L, Lopez M. The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells. Rev Med Virol. 2000;10:305–319. doi: 10.1002/1099-1654(200009/10)10:5<305::aid-rmv286>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 6.Cousens L P, Orange J S, Su H C, Biron C A. Interferon-alpha/beta inhibition of interleukin 12 and interferon-gamma production in vitro and endogenously during viral infection. Proc Natl Acad Sci USA. 1997;94:634–639. doi: 10.1073/pnas.94.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cousens L P, Peterson R, Hsu S, Dorner A, Altman J D, Ahmed R, Biron C A. Two roads diverged: interferon alpha/beta- and interleukin 12-mediated pathways in promoting T cell interferon gamma responses during viral infection. J Exp Med. 1999;189:1315–1328. doi: 10.1084/jem.189.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daheshia M, Feldman L T, Rouse B T. Herpes simplex virus latency and the immune response. Curr Opin Microbiol. 1998;1:430–435. doi: 10.1016/s1369-5274(98)80061-1. [DOI] [PubMed] [Google Scholar]
- 9.Ejercito P M, Kieff E D, Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez N C, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, Perricaudet M, Tursz T, Maraskovsky E, Zitvogel L. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 11.Franchini M, Abril C, Schwerdel C, Ruedl C, Ackermann M, Suter M. Protective T-cell-based immunity induced in neonatal mice by a single replicative cycle of herpes simplex virus. J Virol. 2001;75:83–89. doi: 10.1128/JVI.75.1.83-89.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grob P, Schijns V E M, van den Broek F, Cox S P, Ackermann M, Suter M. Role of the individual interferon systems and specific immunity in mice in controlling systemic dissemination of attenuated pseudorabies virus infection. J Virol. 1999;73:4748–4754. doi: 10.1128/jvi.73.6.4748-4754.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guidotti L G, Chisari F V. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu Rev Immunol. 2001;19:65–91. doi: 10.1146/annurev.immunol.19.1.65. [DOI] [PubMed] [Google Scholar]
- 14.Kanangat S, Thomas J, Gangappa S, Babu J S, Rouse B T. Herpes simplex virus type 1-mediated up-regulation of IL-12 (p40) mRNA expression. Implications in immunopathogenesis and protection. J Immunol. 1996;156:1110–1116. [PubMed] [Google Scholar]
- 15.Kohl S. Neonatal herpes simplex virus infections. J Pediatr. 1982;101:794–795. doi: 10.1016/s0022-3476(82)80328-4. [DOI] [PubMed] [Google Scholar]
- 16.Kohl S. Role of antibody-dependent cellular cytotoxicity in neonatal infection with herpes simplex virus. Rev Infect Dis. 1991;13(Suppl. 11):S950–S952. doi: 10.1093/clind/13.supplement_11.s950. [DOI] [PubMed] [Google Scholar]
- 17.Kohl S, Tang J P, Loo L S. Antibody-dependent cellular cytotoxicity and natural killer cytotoxicity of peritoneal cells from nude mice to herpes simplex virus-infected cells. Microbiol Immunol. 1984;28:439–449. doi: 10.1111/j.1348-0421.1984.tb00695.x. [DOI] [PubMed] [Google Scholar]
- 18.Kruse M, Rosorius O, Kratzer F, Stelz G, Kuhnt C, Schuler G, Hauber J, Steinkasserer A. Mature dendritic cells infected with herpes simplex virus type 1 exhibit inhibited T-cell stimulatory capacity. J Virol. 2000;74:7127–7136. doi: 10.1128/jvi.74.15.7127-7136.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leib D A, Harrison T E, Laslo K M, Machalek M A, Moorman N J, Virgin H W. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J Exp Med. 1999;189:663–672. doi: 10.1084/jem.189.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leibson P J. Signal transduction during natural killer cell activation: inside the mind of a killer. Immunity. 1997;6:655–661. doi: 10.1016/s1074-7613(00)80441-0. [DOI] [PubMed] [Google Scholar]
- 21.Lin S J, Roberts R L, Ank B J, Nguyen Q H, Thomas E K, Stiehm E R. Effect of interleukin (IL)-12 and IL-15 on activated natural killer (ANK) and antibody-dependent cellular cytotoxicity (ADCC) in HIV infection. J Clin Immunol. 1998;18:335–345. doi: 10.1023/a:1023290932154. [DOI] [PubMed] [Google Scholar]
- 22.Luyet F, Samra D, Soneji A, Marks M I. Passive immunization in experimental herpesvirus hominis infection of newborn mice. Infect Immun. 1975;12:1258–1261. doi: 10.1128/iai.12.6.1258-1261.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattner F, Ozmen L, Podlaski F J, Wilkinson V L, Presky D H, Gately M K, Alber G. Treatment with homodimeric interleukin-12 (IL-12) p40 protects mice from IL-12-dependent shock but not from tumor necrosis factor alpha-dependent shock. Infect Immun. 1997;65:4734–4737. doi: 10.1128/iai.65.11.4734-4737.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melero I, Mazzolini G, Narvaiza I, Qian C, Chen L, Prieto J. IL-12 gene therapy for cancer: in synergy with other immunotherapies. Trends Immunol. 2001;22:113–115. doi: 10.1016/s1471-4906(00)01824-x. [DOI] [PubMed] [Google Scholar]
- 25.Menotti L, Lopez M, Avitabile E, Stefan A, Cocchi F, Adelaide J, Lecocq E, Dubreuil P, Campadelli-Fiume G. The murine homolog of human Nectin1delta serves as a species nonspecific mediator for entry of human and animal alpha herpesviruses in a pathway independent of a detectable binding to gD. Proc Natl Acad Sci USA. 2000;97:4867–4872. doi: 10.1073/pnas.97.9.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orange J S, Biron C A. An absolute and restricted requirement for IL-12 in natural killer cell IFN-gamma production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infections. J Immunol. 1996;156:1138–1142. [PubMed] [Google Scholar]
- 27.Ravetch J V, Bolland S. IgG Fc Receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 28.Riffault S, Eloranta M L, Carrat C, Sandberg K, Charley B, Alm G. Herpes simplex virus induces appearance of interferon-alpha/beta-producing cells and partially interferon-alpha/beta-dependent accumulation of leukocytes in murine regional lymph nodes. J Interferon Cytokine Res. 1996;16:1007–1014. doi: 10.1089/jir.1996.16.1007. [DOI] [PubMed] [Google Scholar]
- 29.Roizman B, Batterson W. Herpesviruses and their replication. In: Fields B N, Knipe D M, editors. Fundamental virology. New York, N.Y: Raven Press; 1986. pp. 607–636. [Google Scholar]
- 30.Ryncarz A J, Goddard J, Wald A, Huang M L, Roizman B, Corey L. Development of a high-throughput quantitative assay for detecting herpes simplex virus DNA in clinical samples. J Clin Microbiol. 1999;37:1941–1947. doi: 10.1128/jcm.37.6.1941-1947.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salio M, Cella M, Suter M, Lanzavecchia A. Inhibition of dendritic cell maturation by herpes simplex virus. Eur J Immunol. 1999;29:3245–3253. doi: 10.1002/(SICI)1521-4141(199910)29:10<3245::AID-IMMU3245>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 32.Suter M, Lew A M, Grob P, Adema G J, Ackermann M, Shortman K, Fraefel C. BAC-VAC, a novel generation of (DNA) vaccines: a bacterial artificial chromosome (BAC) containing a replication-competent, packaging-defective virus genome induces protective immunity against herpes simplex virus 1. Proc Natl Acad Sci USA. 1999;96:12697–126702. doi: 10.1073/pnas.96.22.12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trinchieri G. Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFN-gamma) Curr Opin Immunol. 1997;9:17–23. doi: 10.1016/s0952-7915(97)80154-9. [DOI] [PubMed] [Google Scholar]
- 34.van den Broek M, Muller F U, Huang S, Zinkernagel R M, Aguet M. Immune defence in mice lacking type 1 and/or type 2 interferon receptors. Immunol Rev. 1995;148:5–18. doi: 10.1111/j.1600-065x.1995.tb00090.x. [DOI] [PubMed] [Google Scholar]