Abstract
Glypican-3 (GPC-3) is predominantly found in the placenta and fetal liver, with limited expression in adult tissues. Its re-expression in hepatocellular carcinoma (HCC) and secretion into the serum highlights its potential as a diagnostic marker. GPC-3 is involved in important cellular processes such as proliferation, metastasis, apoptosis, and epithelial–mesenchymal transition through various signaling pathways including Wnt, IGF, YAP, and Hedgehog. To review the structure, biosynthesis, and post-translational modifications of GPC-3, and to elucidate its signaling mechanisms and role as a pro-proliferative protein in HCC, emphasizing its diagnostic and therapeutic potential. A comprehensive literature review was conducted, focusing on the expression of GPC-3 in various tumors, with a special emphasis on HCC. The review synthesized findings from experimental studies and clinical trials, analyzing the overexpression of GPC-3 in HCC, its differentiation from other liver diseases, and its potential as a diagnostic and therapeutic target. GPC-3 overexpression in HCC is linked to aggressive tumor behavior and poor prognosis, including shorter overall and disease-free survival. Additionally, GPC-3 has emerged as a promising therapeutic target. Ongoing investigations, including immunotherapies such as monoclonal antibodies and CAR-T cell therapies, demonstrate potential in inhibiting tumor growth and improving clinical outcomes. The review details the multifaceted roles of GPC-3 in tumorigenesis, including its impact on tumor-associated macrophages, glucose metabolism, and epithelial–mesenchymal transition, all contributing to HCC progression. GPC-3’s re-expression in HCC and its involvement in key tumorigenic processes underscore its value as a biomarker for early diagnosis and a target for therapeutic intervention. Further research is warranted to fully exploit GPC-3’s diagnostic and therapeutic potential in HCC management.
Keywords: Apoptosis, Glypican-3, Hepatocellular carcinoma, Oncogenesis, Signal transduction
Introduction
According to global cancer statistics from 2020, primary liver cancer is the sixth most commonly diagnosed cancer and the third leading cause of cancer-related mortality worldwide. HCC is the most common form of primary liver cancer, representing 75–85% of cases. [1]. The high mortality rate associated with HCC is partly due to the late-stage diagnosis and the lack of effective systemic therapies [2].(GPC-3, a cell surface heparan sulfate proteoglycan, has emerged as a promising diagnostic and therapeutic target in HCC due to its re-expression in malignant tissues and absence in healthy liver. GPC-3’s overexpression in HCC is associated with aggressive tumor behavior and poorer outcomes, including reduced overall survival and disease-free survival. These findings suggest that GPC-3 is not only a valuable diagnostic marker, but also holds therapeutic potential. Recent advancements in targeted therapies, such as monoclonal antibodies and chimeric antigen receptor (CAR)-T cells targeting GPC-3, have shown encouraging results in preclinical models, underlining the molecule’s significance in HCC prognosis and therapy [3–5]. Even though liver resection, ablation, and transplantation are potentially curative, HCC requires diagnosis sufficiently early. Chemotherapy, including first-line agents such as sorafenib and lenvatinib, often encounters issues of resistance [3]. Thus, the early diagnosis of HCC is important to enable early therapeutic intervention and prolong survival [4]. In this context, GPC-3 has emerged as a promising biomarker for hepatocellular carcinoma [5]. GPC-3 is a member of the heparan sulfate proteoglycan family and a glycosylphosphatidylinositol (GPI)-anchored cell surface oncofetal protein [6, 7]. Currently, six glypican proteins (GPC 1–6) have been identified in mammals, along with two homolog proteins (Daily and Dally-like protein) in flies [8]. GPC-3 is primarily expressed in embryonic tissues, with notable variation in expression across healthy adult tissues. While GPC-3 is present during development in tissues such as the digestive tract, gonads, kidneys, liver, oral cavity, nervous tissue, tongue, and vertebrae, its expression is silenced in most adult tissues due to promoter region hypermethylation [9, 10]. As a key member of the glypican family, GPC-3 plays important roles in various cellular processes, including adhesion, apoptosis, proliferation, migration, and survival, by interacting with pathways such as Wnt, Hedgehog, fibroblast growth factors, and bone morphogenetic proteins [6, 7, 11]. Mutations in GPC-3 can lead to Simpson–Golabi–Behmel syndrome, an X-linked disorder characterized by pre- and post-natal overgrowth and an increased risk of embryonal tumors [12]. This syndrome is linked to excessive activation of the Hedgehog signaling pathway, with GPC-3 serving as a potent negative regulator [13]. The loss of GPC-3 function leads to organ enlargement, underscoring its role in regulating human growth and development [14]. Recent studies also highlight the involvement of GPC-3 in tumorigenesis [13]. GPC-3 is highly expressed in various cancers, including liver, gastric, breast, ovarian, penile, prostate, and gallbladder cancers, with particularly high levels in HCC [6, 9]. Notably, GPC-3 is absent in normal and benign liver tissues, as well as in patients with hepatitis. Immunostaining for GPC-3 can help differentiate hepatocellular carcinoma from dysplastic changes in cirrhotic livers and cholangiocarcinoma, making it a promising therapeutic target for liver cancer [15]. Overexpression of GPC-3 in HCC has been confirmed through functional genomic mRNA profiling, which found GPC-3 overexpression in 77% of cases [16]. Additionally, soluble Glypican-3 (sGPC-3), formed through enzymatic lysis of GPC-3, is detectable in the blood of HCC patients, making it a potentially valuable diagnostic marker [17]. Prognostically, increased GPC-3 expression in HCC is associated with poor outcomes, including reduced overall and disease-free survival [5, 18]. Given its role as a cancer antigen, GPC-3 has emerged as an effective therapeutic target in treating HCC [19, 20]. This review provides a comprehensive synthesis of the multifaceted roles of GPC-3 in HCC, focusing on its diagnostic, prognostic, and therapeutic potential. It explores the intricate interactions of GPC-3 within complex signaling networks such as Wnt, YAP, and growth factor pathways, highlighting its regulatory mechanisms in HCC progression and metastasis. The review also addresses the heterogeneity of HCC and the challenges it poses for GPC-3-targeted therapies, emphasizing the need for personalized approaches in evaluating GPC-3’s therapeutic potential. Moreover, this review discusses the dual nature of GPC-3, functioning both as a cell-surface co-receptor and in its soluble form, adding complexity to its role in HCC. By examining GPC-3's roles in the tumor microenvironment and its therapeutic implications, this review offers new insights and directions for future research.
Review methodology
A comprehensive literature review was undertaken, focusing on Glypican-3, hepatocellular carcinoma, signaling pathways, and tumorigenic mechanisms. The search strategy incorporated specific keywords and Medical Subject Headings (MeSH) terms, including ‘Glypican-3’, ‘HCC’, ‘Signal Transduction’, and ‘Tumorigenesis’, utilizing Boolean operators for precision. This approach was applied across PubMed/MedLine, Scopus, and Web of Science. Inclusion criteria were English-language articles published since 2008, directly addressing Glypican-3's role in HCC. Exclusion criteria applied to articles focusing on Glypican-3 in non-HCC cancers. Out of 170 initially identified articles, 138 met the inclusion criteria, resulting in a focused dataset for analysis.
Structure, biosynthesis and post-translational modification
GPC-3 is a protein encoded by the GPC-3 / OCI-5 gene. It was first isolated by Filmus et al. in 1998 [6]. The GPC-3 gene is located on the X chromosome at Xq26 [13]. The single-chain GPC-3 forms a core protein of 70 kDa, consisting of 580 amino acids and eight exons [20]. Furin cleaves the GPC-3 core protein at the Arg358-Cys359 position, generating mature GPC-3 composed of two subunits: a 40-kDa soluble N-terminal subunit and a 30-kDa hydrophobic C-terminal subunit [6, 9]. Following cleavage, these two subunits are connected by seven disulfide bonds, forming a conserved structure with 14 cysteine residues [20]. Furin-mediated convertase processing is essential for GPC-3-induced cell survival and Wnt signaling in zebrafish, although it is not required for HCC cell proliferation [21]. The C-terminal of GPC-3 contains two heparan sulfate chains (HSC), each attached to specific insertion sites within the last 55 amino acids of the carboxy terminus, at positions S495 and S504. This positioning brings the HSCs in close proximity to the cell membrane. [9, 11, 13, 22]. The synthesis of HS GAG (glycosaminoglycan) begins in the cytoplasm, where five uridine diphosphate (UDP)-derived activated sugars—UDP-glucuronic acid, UDP-N-acetyl glucosamine, UDP-xylose, UDP-galactose, and UDP-N-acetyl galactosamine—are produced. An antiporter transmembrane transporter moves these activated sugars from the cytoplasm to the Golgi apparatus [23, 24]. The addition of heparan sulfate chains (HSC) to the GPC-3 core protein requires a glycosaminoglycan (GAG)–protein tetrasaccharide linkage at specific serine residues on the core proteins. The synthesis of this tetrasaccharide region begins with the attachment of xylose to serine (catalyzed by two xylosyltransferases), followed by the sequential addition of two galactose residues (by galactosyltransferases I and II) and glucuronic acid (by glucuronosyltransferase I) from UDP sugars, forming the Xyl-Gal-Gal-Glc structure. N-acetylglucosaminyl transferase I then adds GlcNAc (N-acetylglucosamine) to this bond. The HS chains are copolymerized by the alternating addition of GlcA and GlcNAc residues, which undergo epimerization and sulfation. Some of these modified sugars undergo O-sulfation during chain assembly [25], determining their bioactive functions [23]. The HS chains possess a negative charge, which facilitates binding to positively charged growth factors, including hepatocyte growth factors (HGFs), fibroblast growth factors (FGFs), Wnts, Hedgehog, and bone morphogenetic proteins (BMP) [11]. As a result, HSCs act as docking sites for these growth factors. In HCC, however, the HS chains do not exhibit a cell-specific function, as neither the HSC nor the convertase processing by furin is necessary for GPC-3 and Wnt-mediated tumorigenic activity [11]. Another key feature of the C-terminal region is the lipid anchor known as glycosylphosphatidylinositol (GPI), which, upon maturation, facilitates the attachment of GPC-3 to the cell surface. A series of enzymatic processes in the endoplasmic reticulum leads to the assembly of the GPI anchor on a phosphatidylinositol lipid, which is covalently attached to the carboxyl terminus (omega-site) of the protein. During the attachment of the GPI anchor, the COOH terminal of the hydrophobic domain is removed and replaced with a pre-assembled GPI anchor through a transamidation reaction (a chemical process where one functional group is replaced by another) [26, 27]. By analyzing the amino acid sequence of GPC-3 using the big-PI predictor, serine 560 was identified as the cleavage site for the GPI anchor in GPC-3 [11]. Notum, an extracellular lipase, is responsible for GPI anchor cleavage in other proteins, but it does not cleave GPC-3; instead, it functions as a Wnt deacetylase, according to recent studies [21, 28]. In contrast, membrane-bound sheddase enzymes, such as phospholipase D, are suspected to be involved in the cleavage of GPC-3’s GPI anchor [6]. This cleavage results in the shedding of GPC-3 from tumor cells into the extracellular environment, forming soluble Glypican-3 (sGPC-3) [6, 29]. Since GPC-3 must be attached to the cell membrane to exert its growth-promoting effects, the formation of sGPC-3 acts as a dominant-negative form by competing with membrane-bound GPC-3 for binding to Wnt and other growth factors [11]. The physiochemical properties of GPC-3 were assessed using the online tool ProtParam. The analysis revealed that the mature GPC-3 protein has a molecular weight of 65,967.09 Da and a molecular formula of C2950H4609N779O858S39, with an isoelectric point of 6.01, indicating its acidic nature. Leucine was found to be the most prevalent amino acid, accounting for 10.3% of the total amino acids [13].
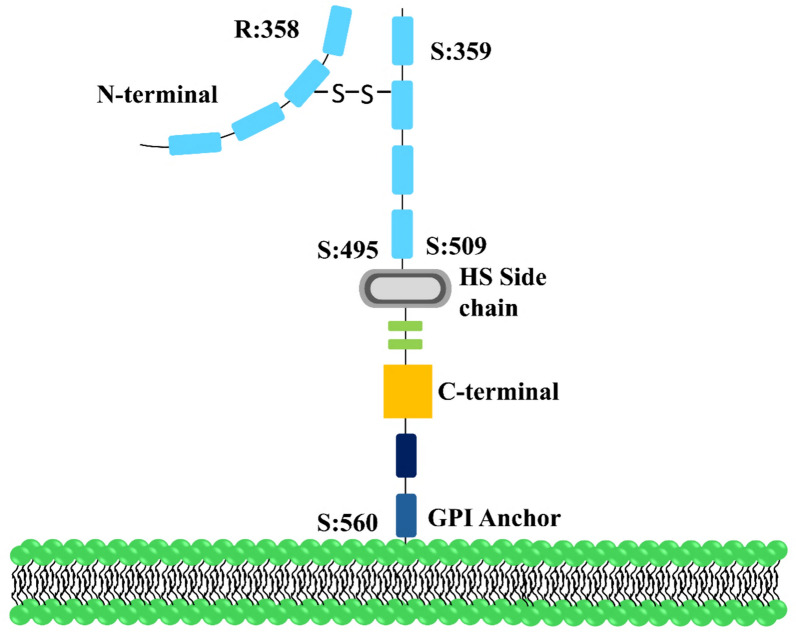
GPC-3 undergoes several post-translational modifications, including the attachment of two O-linked HS side chains and intracellular cleavage by furin, which generates two peptide fragments linked by disulfide bridges. The protein is anchored to the cell surface via a GPI anchor and is later released by cleavage of the anchor, forming soluble GPC-3. These chemical modifications are essential for GPC-3's functional activity [30]. Glypican-3 plays a significant role in regulating cell proliferation, which is important for its involvement in HCC progression (Fig. 1). Table 1 summarizes the key structural, biosynthetic, and post-translational modifications of Glypican-3 in the context of HCC.
Fig. 1.
Glypican 3 structure: this represents the structural illustration of GPC-3 with 70-kDa core protein, attached to the cell surface via GPI anchor. The core protein is cleaved by furin at the Arg 358Cys 359 bond to generate two subunits: a 40-kDa N-terminal subunit and a 30-kDa C-terminal subunit with two HS side chains as appendages at S495 and S509. Both these subunits are linked to each other after cleavage by disulfide bonds. Ser560 is the cleavage site for GPI anchor and releases GPC-3 from the cell surface into the extracellular environment after cleavage by phospholipase D, a membrane-bound sheddase enzyme. GPC-3 Glypican-3, GPI glycosylphosphatidylinositol, HS heparan sulfate
Table 1.
Structural insights and post-translational modifications of GPC-3 in HCC
| Feature | Description | Key processes | Molecular interactions | References |
|---|---|---|---|---|
| Gene and location | GPC-3 encoded by GPC-3/OCI-5 gene on X chromosome at Xq26 | – | – | [6, 13] |
| Core protein | Single-chain core protein of 70 kDa with 580 amino acids | – | – | [20] |
| Cleavage and maturation | Furin cleaves at Arg 358—Cys 359; forms 40-kDa N-terminal and 30-kDa C-terminal subunits | Furin-mediated convertase processing | Linked by seven disulfide bonds | [6, 9, 20, 21] |
| HSC | Two HSCs attached at the C-terminal within the last 55 amino acids | Synthesis involves UDP-sugars, xylosyltransferases, galactosyltransferase, glucuronosyltransferase, N-acetyl glucosaminyl transferase | Binds growth factors like HGFs, FGFs, Wnts | [9, 11, 13, 22–25] |
| GPI anchor | C-terminal GPI anchor attaches GPC-3 to cell membrane | Enzymatic processes in the endoplasmic reticulum; transamidation reaction for anchor assembly | Notum acts as Wnt deacetylase; phospholipase D suspected for cleavage | [6, 11, 21, 26–29] |
| Soluble GPC-3 (sGPC-3) | Formation of sGPC-3 by GPI anchor cleavage | Competes with cell-bound GPC-3 for binding to Wnt and other growth factors | Acts as a dominant-negative form of GPC-3 | [6, 11, 29] |
| Physiochemical properties | Mature GPC-3 has molecular weight of 65,967.09 Da | Assessed using ProtParam | Leucine is the most prevalent amino acid | [13, 30] |
C-terminal carboxy terminal, FGFs fibroblast growth factors, GAG glycosaminoglycan, GPI glycosylphosphatidylinositol, GPC-3 Glypican-3, HCC hepatocellular carcinoma, HGFs hepatocyte growth factors, HSC heparan sulfate chains, N-terminal amino terminal, sGPC-3 soluble Glypican 3, UDP uridine diphosphate, Wnts Wnt proteins
Mechanism of tumorigenesis regulated by GPC-3
Recruitment of tumor-associated macrophages
Macrophages are immune cells that can have both pro-tumor and anti-tumor effects, acting as a double-edged sword [31]. The M2 phenotype of macrophages, in particular, promotes HCC progression and is associated with poor prognosis in HCC patients [22, 32]. GPC-3 expression on the cell membrane has been shown to reactivate the recruitment of macrophages. In xenograft models derived from SK-HEP-1 and SK03 cell lines, GPC-3 expression was either undetectable or elevated. Genome-array analysis indicated that the recruitment of M2-polarized macrophages into SK03 xenografts is associated with GPC-3 overexpression in tumor cells. These findings highlight the role of intratumoral M2 macrophages, also known as tumor-associated macrophages (TAMs) [33], which act as pro-tumorigenic agents by promoting invasion and metastasis [34]. TAMs are a prominent cell type in the tumor microenvironment (TME), and their immunosuppressive nature is inversely correlated with patient survival and tumor progression [35]. GPC-3 overexpression in HCC cells enhances TAM recruitment in HCC tissues through binding to CCL5 and CCL3. Targeting GPC-3 with antibodies has been shown to reduce the recruitment of M2-polarized TAMs in advanced HCC [31].
Glucose metabolism
By reprogramming glucose metabolism, GPC-3 promotes liver carcinogenesis and metastasis [36]. In HCC, elevated levels of GPC-3 enhance glucose uptake and lactate production, supporting biomass regeneration and maintaining an acidic environment that fosters cell proliferation and metastasis [36, 37]. GPC-3 acts as a regulator of the Warburg effect by upregulating the HIF-1α-mediated activation of glycolytic enzymes, while concurrently downregulating PGC-1α-mediated mitochondrial oxidative phosphorylation [36]. To help cancer cells survive under hypoxic conditions, GPC-3 on the cell surface interacts with monocarboxylate transporter 4 (MCT4) and glucose transporter 4 (GLUT4) in liver cancer cells [38]. The co-localization of CD147 and MCT4 further promotes tumor migration and invasion through the activation of matrix metallopeptidases [37, 38] (Table 2).
Table 2.
Impact of Glypican 3 on tumorigenesis mechanisms in HCC
| Mechanism | Effect on HCC | Key interactions/outcomes | References |
|---|---|---|---|
| Recruitment of TAMs | ↑ HCC progression |
GPC-3 overexpression reactivates macrophage recruiting ↑ Association with poor prognosis |
[22, 31–33] |
| Glucose metabolism | ↑Liver carcinogenesis ↑Metastasis |
GPC-3 reprograms metabolism favoring glucose absorption and lactate synthesis acts as a Warburg effect regulator |
[36, 37] |
| EMT | ↑Correlation with metastasis |
GPC-3 elevation linked to EMT via ERK signaling; ↓ E-cadherins ↑Invasion, ↑metastasis |
[10, 39–43] |
| CXCL12/CXCR4 axis | ↑Cellular migration ↑Angiogenesis |
↑CXCL12/CXCR4 signaling ↑HCC cell proliferation and migration |
[20, 44–46] |
| Bax/Bcl-2 Pathway | ↑Apoptosis resistance |
↓Bax/Bcl-2 ↑Cell survival |
[47] |
| SULF-2 interaction | ↑Oncogenic effect |
↑SULF-2 expression ↑Proliferation ↓Apoptosis |
[48–51] |
TAMs tumor-associated macrophages, GPC-3 Glypican 3, HCC hepatocellular carcinoma, EMT epithelial–mesenchymal transition, ERK extracellular receptor kinase, CXCL12 C-X-C motif chemokine ligand 12, CXCR4 C-X-C chemokine receptor type 4, SULF-2 sulfatase 2. Symbol: ↑increase, ↓decrease
EMT
Elevated GPC-3 levels are strongly correlated with the epithelial–mesenchymal transition (EMT) process and metastasis via the ERK (extracellular receptor kinase) signaling pathway [39]. EMT is a highly coordinated biological program that downregulates epithelial cell markers while upregulating mesenchymal markers [40]. Various proteins regulate EMT, including E-cadherin, Snail1, Snail2 (Slug), and matrix metallopeptidases 2 and 9 (MMP-2 and MMP-9) [10, 41]. E-cadherin, a cell adhesion molecule, plays an important role in maintaining epithelial integrity, and its loss is associated with the transition from benign lesions to invasive metastatic cancer [42]. GPC-3 and Snail act as negative regulators of E-cadherin in HCC [10, 39]. Silencing GPC-3 in HepG2 cells results in downregulation of Snail and Slug while increasing E-cadherin levels [10]. The progression of HCC from Barcelona Clinic Liver Cancer (BCLC) stages A or B to stage C shows a parallel rise in GPC-3 expression, EMT markers, and vascular invasion [43]. Additionally, β-catenin and Wnt signaling-related proteins also contribute to the EMT process, which is sequentially regulated by GPC-3. Two mechanisms have been proposed for the role of β-catenin in EMT. One involves the formation of a complex with E-cadherin, depleting E-cadherin levels at adherens junctions and releasing β-catenin into the cytosol, where it activates transcription necessary for cell proliferation. The other mechanism suggests that β-catenin-mediated transcription may upregulate the EMT program by inducing Snail and Slug expression [10]. Thus, there is a tripartite relationship between β-catenin, EMT, and GPC-3 in regulating invasion and metastasis in HCC [10].
CXCL12/CXCR 4
C-X-C chemokine receptor type 4 (CXCR4) interacts with its ligand CXCL12 (also known as stromal-derived factor 1 alpha, SDF-1α) to promote cellular migration, angiogenesis, tumor growth, and metastasis in HCC [44, 45]. Immunohistochemical (IHC) staining has revealed the presence of CXCR4 in HCC tissues, but not in normal liver tissues. CXCR4 levels are particularly elevated at the tumor border and in perivascular areas, which are indicative of invasive behavior [46].
The increased expression of CXCR4 in HCC cells can lead to enhanced matrix stiffness and the promotion of epithelial-to-mesenchymal transition (EMT), further driving cell proliferation [44]. GPC-3 indirectly modulates the CXCL12/CXCR4 signaling pathway by inhibiting CD26, a proteolytic enzyme responsible for degrading CXCL12 [20]. Moreover, CXCR4 enhances cancer cell migration through interactions with transforming growth factor-beta (TGF-β) and matrix metalloproteinases (MMPs) [46]. GPC-3 positively influences TGF-β signaling and EMT [10], thereby potentially amplifying CXCR4 activity.
Bax/Bcl-2
GPC-3 plays an important role in carcinogenesis by enhancing resistance to apoptosis. Since avoiding apoptosis and maintaining cell proliferation is a primary strategy employed by cancer cells, GPC-3 promotes this process by disrupting the Bax/Bcl-2/cytochrome c/caspase-3 signaling pathway. Bax is a pro-apoptotic protein homologous to Bcl-2, and it induces the release of cytochrome c, which activates caspase enzymes. Therefore, the Bax/Bcl-2 ratio is critical in regulating cell apoptosis. In HepG2 cells, silencing GPC-3 using RNA interference (RNAi) led to an upregulation of the Bax/Bcl-2 ratio, resulting in the release of cytochrome c from mitochondria into the cytoplasm, sequential activation of caspase-3, and ultimately, cell death. These findings suggest that overexpression of GPC-3 in HCC cells disrupts the Bax/Bcl-2 signaling pathway, thereby increasing resistance to apoptosis [47].
Sulfatase 2 (SULF 2)
SULF-2, a heparan-degrading endosulfatase [48], has a malignant impact on HCC by upregulating FGF signaling and GPC-3 expression [49], along with promoting an anti-apoptotic effect via activation of the PI3K/Akt signaling pathway [50]. An immunohistochemical analysis of 30 HCC patients was conducted to assess cell proliferation and apoptosis rates. The tumors were categorized into two subclasses: subclass A (poor prognosis) and subclass B (favorable prognosis), corresponding to high and low SULF-2 expression, respectively. HCCs with high SULF-2 expression showed increased proliferation and reduced apoptosis [51].
Elevated SULF-2 expression was associated with increased Erk and Akt phosphorylation, accompanied by a proportional increase in phosphorylation of the anti-apoptotic Akt substrate GSK-3β. Additionally, SULF-2 upregulated the expression of the anti-apoptotic molecule Bcl-2, further diminishing apoptotic activity [51]. GPC-3 may enhance this effect by also increasing Bcl-2 expression, thereby preventing the release of proteolytic enzymes and caspases [47]. Through its 6-O-desulfatase activity on the GPC-3 heparan sulfate side chains, SULF-2 releases stored Wnt and other heparin-binding growth factors, such as FGF and HGF, from their binding sites on GPC-3 [50]. As a result, the associated signaling pathways mediated by their receptors are activated [6]. Altogether, these findings suggest that SULF-2 exerts an oncogenic effect in HCC, in contrast to the tumor suppressor-like role of SULF-1 [51], [56].
Signal transduction pathways regulated by GPC-3
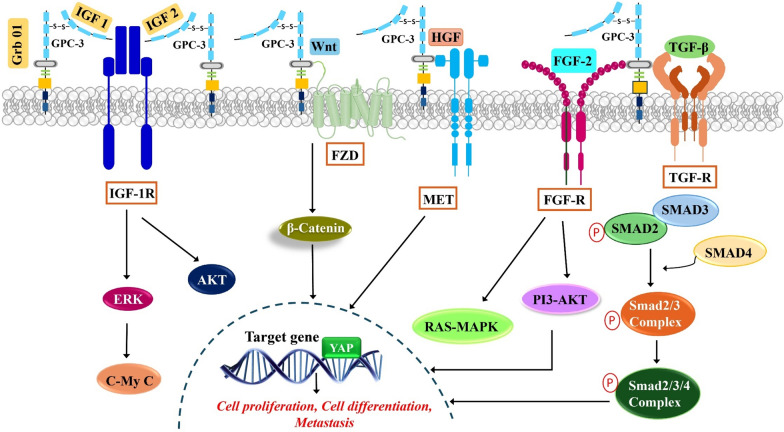
Multiple signalling networks orchestrate the development of HCC and among these, the signalling mechanisms activated by Glypican 3 have emerged as key players (Fig. 2) (Table 3).
Fig. 2.
Schematic diagram of signal transduction pathways regulated by GPC-3 in HCC: GPC-3 serves as a co-receptor to attract Wnt, IGF-1&2 and growth factors such as HGF, FGF-2 and TGF to corresponding receptors namely FZD, IGF-1R, FGFR, TGFR on the cell surface and activate respective signalling pathways to stimulate HCC proliferation, migration, survival and differentiation. Glypican-3 is a target gene of YAP and they together exhibit a positive correlation in HCC. GPC-3 Glypican-3, HCC hepatocellular carcinoma, IGF-1 insulin-like growth factor-1, IGF-2 insulin-like growth factor-2, IGF-1R insulin-like growth factor-1 receptor, Grb10 growth factor receptor-bound protein 10, FZD frizzled, HGF hepatocyte growth factor, MET mesenchymal epithelial transition factor, FGF-2 fibroblast growth factor -2, FGF-R - fibroblast growth factor receptor, TGF-β2 transforming growth factor-β2, TGF-R transforming growth factor-receptor, YAP Yes-associated protein
Table 3.
Influence of GPC-3 on key signaling pathways in HCC
| Signaling pathway | Role of GPC-3 | Mechanism | Impact on HCC | Key findings | References |
|---|---|---|---|---|---|
| Wnt signaling | ↑ autocrine/paracrine canonical Wnt signaling | ↑ Wnt concentration at receptor sites, facilitating Wnt-receptor interaction and stabilizing the Wnt-FZD complex | ↑HCC cell proliferation; associated with dysregulation in 95% of HCC cases |
GPC-3 acts as a bridge to stabilize Wnt and FZD through its HS chains; mutation in the Wnt-binding groove of GPC-3 reduces Wnt activation |
[15, 48, 52–57] |
| YAP signaling | Target gene of YAP; positive correlation with YAP activation | Downregulation of GPC-3 reduces YAP signaling; HN3 antibody targeting GPC-3 blocks YAP signaling | Promotes liver tumorigenesis and HCC progression | ↑YAP which plays a role in the recruitment of M2 macrophages | [58–62] |
| Growth factor signaling | Interacts with growth factors via HS chains | Facilitates signaling cascades by acting as a co-receptor; binds to growth factors, enhancing oncogenicity and cell migration | Stimulates cell growth, survival, and metastasis in HCC |
GPC-3 interaction with FGF, IGF, and HGF enhances signaling pathways like RAS-MAPK, PI3K-AKT ↑Cell migration ↑Invasion |
[63–71] |
GPC-3 Glypican 3, HCC hepatocellular carcinoma, Wnt wingless/Int-1, YAP Yes-associated protein, FGF fibroblast growth factor, IGF insulin-like growth factor, HGF hepatocyte growth factor, TGF-β2 transforming growth factor beta 2, HS heparan sulfate, FZD frizzled. Symbol: ↑increase
Wnt signaling
GPC-3 enhances HCC cell proliferation both in vitro and in vivo by promoting autocrine/paracrine canonical Wnt signaling [20]. Approximately 95% of HCC cases exhibit Wnt/β-catenin dysregulation [48]. In the canonical pathway, Wnt binds to frizzled (FZD) receptors and low-density lipoprotein receptor-related protein 5/6 (LRP5/6) co-receptors, leading to β-catenin accumulation in the cytoplasm, followed by its translocation into the nucleus, where it activates target genes like cyclin D1, which controls cell division. In the absence of Wnt, β-catenin is degraded by the destruction complex and proteasomes, preventing its nuclear translocation and possibly inducing apoptosis [53, 57]. To date, 19 Wnt ligands and 10 frizzled receptors have been identified in humans [48]. Although GPC-3 lacks a cytoplasmic domain to directly transduce signaling, it interacts with Wnt at the cell membrane, increasing Wnt concentration at receptor sites and lowering the activation threshold [8, 11]. Co-immunoprecipitation and cell-binding assays have demonstrated GPC-3’s ability to bind Wnt, underscoring its potential to facilitate Wnt–receptor interactions [15]. Initially, it was believed that heparan sulfate chains (HSC) mediated Wnt-GPC-3 binding, but subsequent studies suggest that the GPC-3 core protein itself engages in Wnt binding [48]. When FZD receptors cluster, and the Wnt/GPC-3/FZD complex forms, GPC-3 may stabilize Wnt and FZD through its HS chains [48].
Both the GPC-3 core protein and its HSCs promote Wnt binding and activation, acting as extracellular co-receptors. The progression of HCC can be correlated with GPC-3 and FZD receptor levels. Healthy liver tissue typically shows low concentrations of both GPC-3 and FZD, whereas advanced HCC exhibits high levels of both. High GPC-3 and low FZD levels are indicative of early-stage HCC [48]. A recent structural model by Li et al. (2019) revealed that GPC-3 contains a frizzled-like cysteine-rich domain (CRD), forming a Wnt-binding groove in the N-lobe of GPC-3. The middle region of Wnt3a interacts with this groove, and mutations to this region result in decreased Wnt binding and reduced HCC tumor growth in mice [8]. Importantly, GPC-3 must remain membrane-bound to activate Wnt signaling [20]. Once GPC-3 is secreted into the extracellular environment, it can sequester Wnt from the cell surface, with soluble GPC-3 (sGPC-3) inhibiting Wnt-mediated HCC cell proliferation [29].
YAP signalling
The Hippo pathway functions as a tumor suppressor in the liver and is predominantly inactivated in the hepatic stem cell (HS) subtype during hepatocarcinogenesis [58]. One of its downstream effectors, Yes-associated protein (YAP), is activated in liver tumorigenesis and is overexpressed in HCC, contributing to the progression of the disease [59, 60]. GPC-3 is a target gene of YAP, and their expressions are positively correlated, with higher YAP activation corresponding to increased GPC-3 levels [61]. In cell culture, reducing GPC-3 expression results in decreased YAP signaling. HCC cell proliferation can be inhibited by a human monoclonal antibody (HN3), which targets a conformational epitope in GPC-3, blocking YAP signaling. Moreover, tumor-infiltrating type II (M2) macrophages promote carcinogenesis by suppressing immune clearance, enhancing proliferation, and increasing angiogenesis. YAP plays a key role in their recruitment [62]. There appears to be potential crosstalk between GPC-3, YAP, and M2 macrophages in promoting hepatocellular carcinoma progression.
Growth factor signalling
GPC-3 has been shown to link with growth factors via its HS chains, resulting in cell growth stimulation [63]. Include GPC-3 interaction with growth factors, including FGF, IGF, and receptors.
-
i.
IGF
By expressing Myc and Akt1 in the liver, IGF/IGF-1R signaling controls cell growth, survival, mobility, and protein synthesis while limiting apoptosis, thereby promoting tumor cell invasion and metastasis. IGF-2 expression is upregulated in HCC patients, where it binds to IGF-1R, further stimulating cancer cell proliferation [64]. GPC-3 interacts with IGF-2 and IGF-1R through its N-terminal domain, leading to ERK phosphorylation, c-Myc expression, and enhanced oncogenicity. Additionally, GPC-3 inhibits IGF-1R ubiquitination and degradation, likely by binding to and sequestering growth factor receptor-bound protein 10 (Grb10), a mediator of ligand-induced receptor ubiquitination. This mechanism prolongs IGF-1R signaling, further promoting tumor growth and survival [65].
-
ii.
HGF and c-Met
Hepatocyte growth factor (HGF) and its high-affinity receptor, mesenchymal–epithelial transition factor (c-Met), are key players in the initiation, proliferation, invasion, and metastasis of HCC. GPC-3, through its interaction with the HGF/c-Met pathway mediated by its heparan sulfate chains, has been implicated in promoting HCC cell migration and motility. Inhibition of GPC-3’s heparan sulfate chains using a human monoclonal antibody targeting GPC-3 was shown to block c-Met activation in HGF-treated HCC cells, reducing cell migration and the formation of 3D-cultured spheroids [66].
-
iii.
FGF
The fibroblast growth factor (FGF)/fibroblast growth factor receptor (FGFR) pathway is increasingly recognized for its role in the carcinogenesis of HCC. Overexpression of at least one FGF and/or FGFR, which are involved in oncogenesis, cell proliferation, and neo-angiogenesis, is found in more than 80% of HCC cases [69]. FGF2 expression, in particular, has been detected in HCC, and binding of FGF to its receptor activates multiple downstream pathways, including RAS–MAPK, PI3K–AKT, and PLCγ [67]. GPC-3 interacts with FGF-2 via its heparan sulfate (HS) chains in HCC cells, acting as a co-receptor and facilitating these signaling cascades [68].
-
iv.
TGF beta 2
The transforming growth factor-beta (TGF-β) signaling pathway regulates a variety of cellular processes, including cell growth, differentiation, migration, and apoptosis. TGF-β binds to its receptors, activating downstream SMAD proteins and related complexes [69]. In addition to their pro-apoptotic and cytoprotective roles, TGF-β family members serve as major tumor suppressors during the early stages of HCC [70]. Genetic studies have identified TGF-β receptor type II (TBRII) as a functional suppressor in HCC formation. Inhibition of GPC-3 in HCC cells has been shown to increase TGF-β2 levels, leading to decreased cell proliferation, cessation of growth, and the promotion of replicative senescence [71, 72].
Limitations
This review elucidates the multifaceted roles of GPC-3 in HCC, highlighting its significance in diagnostic, prognostic, and therapeutic contexts, but several limitations must be acknowledged. Firstly, the current understanding of GPC-3's interactions within complex signaling networks, such as Wnt, YAP, and growth factor pathways, remains incomplete. The interplay between GPC-3 and these pathways suggests a sophisticated regulatory mechanism that warrants further exploration to fully elucidate its role in HCC progression and metastasis. Secondly, the heterogeneity of HCC presents a significant challenge, as the tumor microenvironment's complexity can vary widely among patients. This variability may influence the expression and function of GPC-3, thereby affecting the efficacy of GPC-3-targeted therapies. Consequently, the findings from studies utilizing cell lines and animal models may not fully translate to the clinical setting, underscoring the need for more personalized approaches in evaluating GPC-3's therapeutic potential. Although GPC-3 has shown promise as a target for immunotherapy, the response rates and overall effectiveness of such treatments in HCC patients have yet to be optimized; also the development of resistance to GPC-3-targeted therapies and the potential for off-target effects remain significant concerns that necessitate ongoing research and the development of more sophisticated therapeutic strategies. Additionally, the role of soluble forms of GPC-3 (sGPC-3) in HCC progression and its implications for therapy are not fully understood. The dual nature of GPC-3, acting both as a cell-surface co-receptor and in a soluble form, adds another layer of complexity to its function in HCC. This duality poses challenges in designing therapeutic interventions that can effectively target the relevant form of GPC-3 without inducing adverse effects. Glypican 3 presents a promising target in hepatocellular carcinoma management, but further studies are essential to overcome the current limitations and these studies should aim to elucidate the intricate mechanisms of GPC-3 within the tumor microenvironment, improving the translation of preclinical findings to the clinic and develop more effective, personalized therapeutic strategies for HCC patients.
Current and future perspectives
In the present review, it has been discussed the promising candidate, GPC-3 and its multilateral roles in HCC. Even though there remains much to be discovered about the mechanism involved in the GPC-3 function in HCC, its association with some molecular pathways is now evident. Studies point out how GPC-3 modulates the various cell signalling pathways and its reason for re-expression during HCC to be more explored in detail. According to preclinical and clinical studies, the oncofoetal antigen GPC-3 proved itself as a commendable target antigen for the diagnosis, treatment and prognosis of HCC. Even with its secretory forms, GPC-3 also proved to be a reliable target for the same aspects. Future works revealing the biochemistry of serum GPC-3 will be valuable. Because of the tumor-regressive properties elucidated by sGPC-3, the development of molecules that facilitate the activation of specific phospholipases for cleaving GPC-3 from the cell surface will be a significant piece of investigation. The elevated Glypican 3 expressions during HCC make it an eminent diagnostic marker and correspond to a poor patient prognosis. Estimating Glypican 3 levels helps differentiate from benign mimickers and metastatic neoplasm to the liver. GPC-3specific imaging modalities provided an accurate assessment of tumor response aiding in the timely treatment. Being a prevalent molecular marker expressed very early in the disease line and maintaining high expression during median and advanced tumors stages enhances its therapeutic utility in HCC. Studies suggest that the anti-GPC three treatments alone are insufficient to resolve HCC. Therefore, further exploration is needed to strengthen the data obtained to utilize GPC-3 as a combined treatment strategy with anti-cancer drugs and immunotherapeutic agents. Furthermore, it is required to find out how GPC 3 expression should be regulated to avoid the emergence of drug-resistant clones.
Acknowledgements
ARD, BN, GKP, RA, BSV, LRN acknowledge the support from Amrita Vishwa Vidyapeetham. We express our sincere gratitude to Dr Shanthi Kumar V Nair, Dean of Research, Amrita Vishwa Vidyapeetham and Dr Sabitha M, Principal, Amrita School of Pharmacy for all the facilities provided. The authors also would like to express their gratitude to Dr. Irina Zamfir, MD, RCP London, Basildon University Hospital UK, for providing professional English editing of this manuscript and for editorial support.
Abbreviations
- BMP
Bone morphogenetic proteins
- CRD
Cysteine-rich domain
- EMT
Epithelial–mesenchymal transition
- FGF
Fibroblast growth factor
- FGFR
Fibroblast growth factor receptor
- FZD
Frizzled
- GAG
Glycosaminoglycan
- GPI
Glycosylphosphatidylinositol
- GPC-3
Glypican-3
- Grb10
Growth factor receptor-bound protein 10
- HCC
Hepatocellular carcinoma
- HGF
Hepatocyte growth factor
- HS
Heparan sulfate
- HSC
Heparan sulfate chains
- IGF
Insulin-like growth factor
- IGF-1R
Insulin-like growth factor 1 receptor
- LRP 5/6
Low-density lipoprotein-receptor-related protein 5/6
- M2
Macrophage type 2
- MET
Mesenchymal–epithelial transition factor
- MMP
Matrix metallopeptidase
- sGPC-3
Soluble glypican 3
- TAMs
Tumor-associated macrophages
- TGF-β2
Transforming growth factor beta 2
- TGFR
Transforming growth factor receptor
- TME
Tumor microenvironment
- Wnt
Wingless-Int
- YAP
Yes-associated protein
Author contributions
A.R.D., B.N., G.K.P., R.A., B.S.V., L.R.N., D.C., J.S.-R., made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas. That is revising or critically reviewing the article; giving final approval of the version to be published; agreeing on the journal to which the article has been submitted; and, confirming to be accountable for all aspects of the work.
Funding
We acknowledge the support from the Amrita Vishwa Vidyapeetham SEED Grant to LRN (Project ID: K-PHAR-22-662).
Availability of data and material
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Aswathy R. Devan, Bhagyalakshmi Nair, Govind K. Pradeep and Roshini Alexander contributed equally to this work.
Contributor Information
Lekshmi R. Nath, Email: lekshmirnath@aims.amrita.edu
Daniela Calina, Email: calinadaniela@gmail.com.
Javad Sharifi-Rad, Email: javad.sharifirad@gmail.com.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2.Likhitsup A, Razumilava N, Parikh ND. Treatment for advanced hepatocellular carcinoma: current standard and the future. Clin Liver Dis. 2019;13(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang A, Yang XR, Chung WY, Dennison AR, Zhou J. Targeted therapy for hepatocellular carcinoma. Signal Transduct Target Ther. 2020;5(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grandhi MS, Kim AK, Ronnekleiv-Kelly SM, Kamel IR, Ghasebeh MA, Pawlik TM. Hepatocellular carcinoma: from diagnosis to treatment. Surg Oncol. 2016;25(2):74–85. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu Y, Mizuno S, Fujinami N, Suzuki T, Saito K, Konishi M, Takahashi S, Gotohda N, Tada T, Toyoda H, Kumada T. Plasma and tumoral glypican-3 levels are correlated in patients with hepatitis C virus-related hepatocellular carcinoma. Cancer Sci. 2020;111(2):334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishida T, Kataoka H. Glypican 3-targeted therapy in hepatocellular carcinoma. Cancers. 2019;11(9):1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moudi B, Heidari Z, Mahmoudzadeh-Sagheb H. Meta-analysis and systematic review of prognostic significance of Glypican-3 in patients with hepatitis B-related hepatocellular carcinoma. Virusdisease. 2019;30(2):193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li N, Wei L, Liu X, Bai H, Ye Y, Li D, Li N, Baxa U, Wang Q, Lv L, Chen Y. A frizzled-like cysteine-rich domain in glypican-3 mediates wnt binding and regulates hepatocellular carcinoma tumor growth in mice. Hepatology. 2019;70(4):1231–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montalbano M, Georgiadis J, Masterson AL, McGuire JT, Prajapati J, Shirafkan A, Rastellini C, Cicalese L. Biology and function of glypican-3 as a candidate for early cancerous transformation of hepatocytes in hepatocellular carcinoma. Oncol Rep. 2017;37(3):1291–300. [DOI] [PubMed] [Google Scholar]
- 10.Qi XH, Wu D, Cui HX, Ma N, Su J, Wang YT, Jiang YH. Silencing of the glypican-3 gene affects the biological behavior of human hepatocellular carcinoma cells. Mol Med Rep. 2014;10(6):3177–84. [DOI] [PubMed] [Google Scholar]
- 11.Capurro M, Martin T, Shi W, Filmus J. Glypican-3 binds to Frizzled and plays a direct role in the stimulation of canonical Wnt signaling. J Cell Sci. 2014;127(7):1565–75. [DOI] [PubMed] [Google Scholar]
- 12.Reischer T, Laccone F, Kasprian GJ, Yerlikaya-Schatten G. Simpson–Golabi–Behmel-syndrome in dichorionic-diamniotic twin pregnancy. Clin Pract. 2021;11(1):75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filmus J, Capurro M. The role of glypicans in Hedgehog signaling. Matrix Biol. 2014;1(35):248–52. [DOI] [PubMed] [Google Scholar]
- 14.Kolluri A, Ho M. The role of glypican-3 in regulating Wnt, YAP, and hedgehog in liver cancer. Front Oncol. 2019;2(9):708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shih TC, Wang L, Wang HC, Wan YJ. Glypican-3: a molecular marker for the detection and treatment of hepatocellular carcinoma. Liver Res. 2020;4(4):168–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moek KL, Fehrmann RS, van der Vegt B, de Vries EG, de Groot DJ. Glypican 3 overexpression across a broad spectrum of tumor types discovered with functional genomic mRNA profiling of a large cancer database. Am J Pathol. 2018;188(9):1973–81. [DOI] [PubMed] [Google Scholar]
- 17.Chen C, Huang X, Ying Z, Wu D, Yu Y, Wang X, Chen C. Can glypican-3 be a disease-specific biomarker? Clin Transl Med. 2017;6(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cartier F, Indersie E, Lesjean S, Charpentier J, Hooks KB, Ghousein A, Desplat A, Dugot-Senant N, Trézéguet V, Sagliocco F, Hagedorn M. New tumor suppressor microRNAs target glypican-3 in human liver cancer. Oncotarget. 2017;8(25):41211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimizu Y, Suzuki T, Yoshikawa T, Endo I, Nakatsura T. Next-generation cancer immunotherapy targeting glypican-3. Front Oncol. 2019;10(9):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaur SP, Cummings BS. Role of glypicans in regulation of the tumor microenvironment and cancer progression. Biochem Pharmacol. 2019;1(168):108–18. [DOI] [PubMed] [Google Scholar]
- 21.Zhou F, Shang W, Yu X, Tian J. Glypican-3: a promising biomarker for hepatocellular carcinoma diagnosis and treatment. Med Res Rev. 2018;38(2):741–67. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Gao F, Jiang L, Jia M, Ao L, Lu M, Gou L, Ho M, Jia S, Chen F, Gao W. 32A9, a novel human antibody for designing an immunotoxin and CAR-T cells against glypican-3 in hepatocellular carcinoma. J Transl Med. 2020;18(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casale J, Crane JS. Biochemistry, Glycosaminoglycans. [PubMed]
- 24.Ghiselli G. Drug-mediated regulation of glycosaminoglycan biosynthesis. Med Res Rev. 2017;37(5):1051–94. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L. Glycosaminoglycan (GAG) biosynthesis and GAG-binding proteins. Prog Mol Biol Transl Sci. 2010;1(93):1–7. [DOI] [PubMed] [Google Scholar]
- 26.Kinoshita T. Glycosylphosphatidylinositol (GPI) anchors: biochemistry and cell biology: introduction to a thematic review series. J Lipid Res. 2016;57(1):4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Müller G. Novel applications for glycosylphosphatidylinositol-anchored proteins in pharmaceutical and industrial biotechnology. Mol Membr Biol. 2011;28(3):187–205. [DOI] [PubMed] [Google Scholar]
- 28.Kakugawa S, Langton PF, Zebisch M, Howell SA, Chang TH, Liu Y, Feizi T, Bineva G, O’Reilly N, Snijders AP, Jones EY. Notum deacylates Wnt proteins to suppress signalling activity. Nature. 2015;519(7542):187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zittermann SI, Capurro MI, Shi W, Filmus J. Soluble glypican 3 inhibits the growth of hepatocellular carcinoma in vitro and in vivo. Int J Cancer. 2010;126(6):1291–301. [DOI] [PubMed] [Google Scholar]
- 30.Saad A, Liet B, Joucla G, Santarelli X, Charpentier J, Claverol S, Grosset CF, Trézéguet V. Role of glycanation and convertase maturation of soluble glypican-3 in inhibiting proliferation of hepatocellular carcinoma cells. Biochemistry. 2018;57(7):1201–11. [DOI] [PubMed] [Google Scholar]
- 31.Tian Z, Hou X, Liu W, Han Z, Wei L. Macrophages and hepatocellular carcinoma. Cell Biosci. 2019;9(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeung OW, Lo CM, Ling CC, Qi X, Geng W, Li CX, Ng KT, Forbes SJ, Guan XY, Poon RT, Fan ST. Alternatively activated (M2) macrophages promote tumour growth and invasiveness in hepatocellular carcinoma. J Hepatol. 2015;62(3):607–16. [DOI] [PubMed] [Google Scholar]
- 33.Takai H, Ashihara M, Ishiguro T, Terashima H, Watanabe T, Kato A, Suzuki M. Involvement of glypican-3 in the recruitment of M2-polarized tumor-associated macrophages in hepatocellular carcinoma. Cancer Biol Ther. 2009;8(24):2329–38. [DOI] [PubMed] [Google Scholar]
- 34.Yang L, Zhang Y. Tumor-associated macrophages: from basic research to clinical application. J Hematol Oncol. 2017;10(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parayath N, Padmakumar S, Nair SV, Menon D, Amiji MM. Strategies for targeting cancer immunotherapy through modulation of the tumor microenvironment. Regen Eng Transl Med. 2020;6(1):29–49. [Google Scholar]
- 36.Yao G, Yin J, Wang Q, Dong R, Lu J. Glypican-3 enhances reprogramming of glucose metabolism in liver cancer cells. BioMed Res Int. 2019;2019. [DOI] [PMC free article] [PubMed]
- 37.Mossenta M, Busato D, Dal Bo M, Toffoli G. Glucose metabolism and oxidative stress in hepatocellular carcinoma: role and possible implications in novel therapeutic strategies. Cancers. 2020;12(6):1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yorita K, Ohno A, Nishida T, Kondo K, Ohtomo T, Kataoka H. Intratumoral reciprocal expression of monocarboxylate transporter 4 and glypican-3 in hepatocellular carcinomas. BMC Res Notes. 2019;12(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu Y, Liu H, Weng H, Zhang X, Li P, Fan CL, Li B, Dong PL, Li L, Dooley S, Ding HG. Glypican-3 promotes epithelial–mesenchymal transition of hepatocellular carcinoma cells through ERK signaling pathway. Int J Oncol. 2015;46(3):1275–85. [DOI] [PubMed] [Google Scholar]
- 40.Chaffer CL, San Juan BP, Lim E, Weinberg RA. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016;35(4):645–54. [DOI] [PubMed] [Google Scholar]
- 41.Giannelli G, Koudelkova P, Dituri F, Mikulits W. Role of epithelial to mesenchymal transition in hepatocellular carcinoma. J Hepatol. 2016;65(4):798–808. [DOI] [PubMed] [Google Scholar]
- 42.Sawada Y, Yoshikawa T, Ofuji K, Yoshimura M, Tsuchiya N, Takahashi M, Nobuoka D, Gotohda N, Takahashi S, Kato Y, Konishi M. Phase II study of the GPC-3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. Oncoimmunology. 2016;5(5): e1129483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu Y, Liu H, Ding H. GPC-3 in hepatocellular carcinoma: current perspectives. J Hepatocellular Carcinoma. 2016;3:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang N, Chen T, Wang L, Liu R, Niu Y, Sun L, Yao B, Wang Y, Yang W, Liu Q, Tu K. CXCR4 mediates matrix stiffness-induced downregulation of UBTD1 driving hepatocellular carcinoma progression via YAP signaling pathway. Theranostics. 2020;10(13):5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu J, Liang J, Meng YM, Yan J, Yu XJ, Liu CQ, Xu L, Zhuang SM, Zheng L. Vascular CXCR4 expression promotes vessel sprouting and sensitivity to sorafenib treatment in hepatocellular carcinoma. Clin Cancer Res. 2017;23(15):4482–92. [DOI] [PubMed] [Google Scholar]
- 46.Jeng KS, Jeng CJ, Jeng WJ, Chang CF, Sheen I. Role of CXC chemokine ligand 12/CXC chemokine receptor 4 in the progression of hepatocellular carcinoma. Oncol Lett. 2017;14(2):1905–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu S, Li Y, Chen W, Zheng P, Liu T, He W, Zhang J, Zeng X. Silencing glypican-3 expression induces apoptosis in human hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2012;419(4):656–61. [DOI] [PubMed] [Google Scholar]
- 48.Zheng X, Liu X, Lei Y, Wang G, Liu M. Glypican-3: a novel and promising target for the treatment of hepatocellular carcinoma. Front Oncol. 2022;16(12): 824208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Gayyar MM, Abbas A, Hamdan AM. Chemopreventive and hepatoprotective roles of adiponectin (SULF2 inhibitor) in hepatocelluar carcinoma. Biol Chem. 2016;397(3):257–67. [DOI] [PubMed] [Google Scholar]
- 50.Lai JP, Oseini AM, Moser CD, Yu C, Elsawa SF, Hu C, Nakamura I, Han T, Aderca I, Isomoto H, Garrity-Park MM. The oncogenic effect of sulfatase 2 in human hepatocellular carcinoma is mediated in part by glypican 3–dependent Wnt activation. Hepatology. 2010;52(5):1680–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lai JP, Sandhu DS, Yu C, Moser CD, Hu C, Shire AM, Aderca I, Murphy LM, Adjei AA, Sanderson S, Roberts LR. Sulfatase 2 protects hepatocellular carcinoma cells against apoptosis induced by the PI3K inhibitor LY294002 and ERK and JNK kinase inhibitors. Liver Int. 2010;30(10):1522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu P, Ke C, Guo X, Ren P, Tong Y, Luo S, et al. Both glypican-3/Wnt/β-catenin signaling pathway and autophagy contributed to the inhibitory effect of curcumin on hepatocellular carcinoma. Dig Liver Dis. 2019;51(1):120–6. [DOI] [PubMed] [Google Scholar]
- 53.Dong Z, Yao M, Wang L, Yang J, Yao D. Down-regulating glypican-3 expression: molecular-targeted therapy for hepatocellular carcinoma. Mini Rev Med Chem. 2014;14(14):1183–93. [DOI] [PubMed] [Google Scholar]
- 54.Madan B, Virshup DM. Targeting Wnts at the source—new mechanisms, new biomarkers, new drugs. Mol Cancer Ther. 2015;14(5):1087–94. [DOI] [PubMed] [Google Scholar]
- 55.Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36(11):1461–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loh KM, van Amerongen R, Nusse R. Generating cellular diversity and spatial form: Wnt signaling and the evolution of multicellular animals. Dev Cell. 2016;38(6):643–55. [DOI] [PubMed] [Google Scholar]
- 57.Kafri P, Hasenson SE, Kanter I, Sheinberger J, Kinor N, Yunger S, Shav-Tal Y. Quantifying β-catenin subcellular dynamics and cyclin D1 mRNA transcription during Wnt signaling in single living cells. Elife. 2016;23(5): e16748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sohn BH, Shim JJ, Kim SB, Jang KY, Kim SM, Kim JH, Hwang JE, Jang HJ, Lee HS, Kim SC, Jeong W. Inactivation of Hippo pathway is significantly associated with poor prognosis in hepatocellular carcinoma. Clin Cancer Res. 2016;22(5):1256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hong L, Cai Y, Jiang M, Zhou D, Chen L. The Hippo signaling pathway in liver regeneration and tumorigenesis. Acta Biochim Biophys Sin. 2015;47(1):46–52. [DOI] [PubMed] [Google Scholar]
- 60.Mi L, Kuang H. Melatonin regulates cisplatin resistance and glucose metabolism through hippo signaling in hepatocellular carcinoma cells. Cancer Manag Res. 2020;12:1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li H, Wolfe A, Septer S, Edwards G, Zhong X, Bashar Abdulkarim A, Ranganathan S, Apte U. Deregulation of Hippo kinase signalling in human hepatic malignancies. Liver Int. 2012;32(1):38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo X, Zhao Y, Yan H, Yang Y, Shen S, Dai X, Ji X, Ji F, Gong XG, Li L, Bai X. Single tumor-initiating cells evade immune clearance by recruiting type II macrophages. Genes Dev. 2017;31(3):247–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li N, Spetz MR, Ho M. The role of Glypicans in cancer progression and therapy. J Histochem Cytochem. 2020;68(12):841–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ngo MH, Jeng HY, Kuo YC, Diony Nanda J, Brahmadhi A, Ling TY, Chang TS, Huang YH. The role of igf/igf-1r signaling in hepatocellular carcinomas: stemness-related properties and drug resistance. Int J Mol Sci. 2021;22(4):1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheng W, Huang PC, Chao HM, Jeng YM, Hsu HC, Pan HW, Hwu WL, Lee YM. Glypican-3 induces oncogenicity by preventing IGF-1R degradation, a process that can be blocked by Grb10. Oncotarget. 2017;8(46):80429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang J, Roncal OA, Ittmann MM, Ye Y. Evaluation of Glypican-3 expression in poorly differentiated carcinomas of lung origin. North Am J Med Sci. 2020;13(1).
- 67.Lee JJ, Choo SP. The fibroblast growth factor receptor pathway in hepatocellular carcinoma. Hepatoma Res. 2018;13:4. [Google Scholar]
- 68.Zheng N, Wei W, Wang Z. Emerging roles of FGF signaling in hepatocellular carcinoma. Transl Cancer Res. 2016;5(1):1. [PMC free article] [PubMed] [Google Scholar]
- 69.Brar G, Greten TF, Brown ZJ. Current frontline approaches in the management of hepatocellular carcinoma: the evolving role of immunotherapy. Ther Adv Gastroenterol. 2018;11:1756284818808086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fabregat I, Moreno-Càceres J, Sánchez A, Dooley S, Dewidar B, Giannelli G, Ten Dijke P. TGF-β signalling and liver disease. he FEBS J. 2016;283(12):2219–32. [DOI] [PubMed] [Google Scholar]
- 71.Nair B, Nath LR. Inevitable role of TGF-β1 in progression of nonalcoholic fatty liver disease. J Recept Signal Transduction. 2020;40(3):195–200. [DOI] [PubMed] [Google Scholar]
- 72.Tang Y, Kitisin K, Jogunoori W, Li C, Deng CX, Mueller SC, Ressom HW, Rashid A, He AR, Mendelson JS, Jessup JM. Progenitor/stem cells give rise to liver cancer due to aberrant TGF-β and IL-6 signaling. Proc Natl Acad Sci. 2008;105(7):2445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.