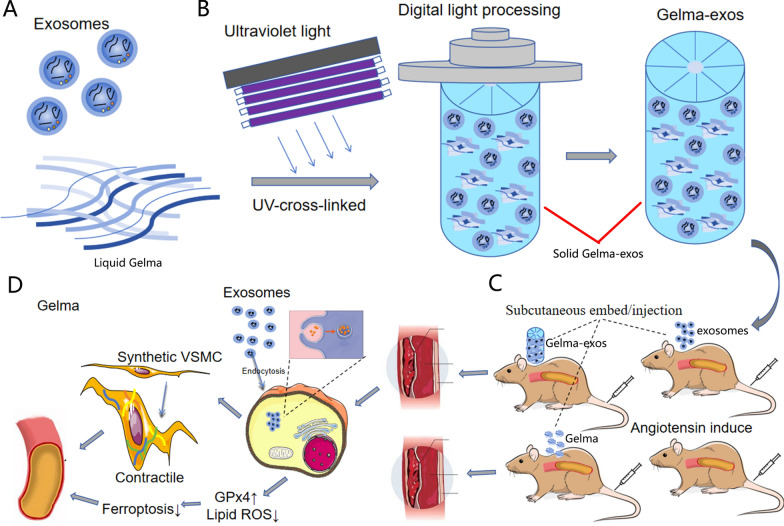
Abstract
Aortic dissection (AD) is a devastating disease with a high mortality rate. Exosomes derived from mesenchymal stem cells (exo-MSCs) offer a promising strategy to restore aortic medial degeneration and combat ferroptosis in AD. However, their rapid degradation in the circulatory system and low treatment efficiency limit their clinical application. Methylacrylated gelatin (Gelma) was reported as a matrix material to achieve controlled release of exosomes. Herein, exo-MSCs-embedded in Gelma hydrogels (Gelma-exos) using ultraviolet light and three-dimensional (3D) printing technology. These Gelma-exos provide a sustained release of exo-MSCs as Gelma gradually degrades, helping to restore aortic medial degeneration and prevent ferroptosis. The sustained release of exosomes can inhibit the phenotypic switch of vascular smooth muscle cells (VSMCs) to a proliferative state, and curb their proliferation and migration. Additionally, the 3D-printed Gelma-exos demonstrated the ability to inhibit ferroptosis in vitro, in vivo and ex vivo experiments. In conclusion, our Gelma-exos, combined with 3D-printed technology, offer an alternative treatment approach for repairing aortic medial degeneration and ferroptosis in AD, potentially reducing the incidence of aortic dissection rupture.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12951-024-02821-w.
Keywords: 3D-printed technolgy, Gelma, Mesenchymal stem cell, Exosome, Vascular smooth muscle cells, Phenotypic switch
Introduction
Aortic dissection (AD) is a devastating disease that requires rapid diagnosis and treatment [1–3]. Surgical outcomes for this highly lethal condition remain poor due to the complexity of the procedures, which often involve prolonged extracorporeal circulation and even deep hypothermic circulatory arrest [4]. Because the molecular pathogenesis of AD is not well understood, targeted therapy remains limited. Aortic medial degeneration, which weakens the aortic wall, is a key factor in the onset of AD [5]. Therefore, designing ideal materials and drugs to prevent aortic medial degeneration is of paramount importance.
Bone Mesenchymal stem cells (MSCs) have been used as a treatment option for many diseases, including autoimmune and inflammatory diseases [6]. They represent a promising tissue engineering material for repairing aortic medial degeneration. MSCs are widely used in various fields due to their abundant availability and low cost. However, ethical controversies, low survival rates of transplanted MSCs, immune rejection reactions, and the risk of infection have limited the widespread application of this technology. Recently, the therapeutic efficacy of MSC treatment has been linked to exosomes derived from mesenchymal stem cells (exo-MSC) [7]. Exo-MSCs offer several advantages, including high stability, ease of procurement, storage, and transportation, abundant sources, and low immunogenicity, making them a promising alternative for tissue-engineering therapies [8]. Moreover, exosomes can encapsulate various bioactive substances, such as proteins, nucleic acids, lipids, and miRNAs, which are taken up by target cells to facilitate cellular communication, tissue repair and regeneration [9].
Exosomes, which originates from MSC secretion, are extracellular vesicles with an average diameter of approximately 100 nm [10]. They contain bioactive substances such as proteins, non-coding RNAs, and lipids, which can be taken up by target cells within the microenvironment. These exosomal contents play crucial roles in cell-to-cell communication, regulating cell proliferation, migration, autophagy, self-repair, and aging [11]. However, exogenous exosomes are rapidly cleared by the circulatory system and excreted from the body, leading to low utilization efficiency, which is a significant limitation for widespread use [12]. To enable the sustained release of exo-MSCs during in vivo application, hydrogels present a viable biomaterial carrier option due to their favorable thixotropy, biocompatibility, mechanical performance, and biodegradability [13]. Gelatin methacrylate (Gelma) hydrogel is an injectable biomaterial with UV-cross-linking properties, and 3D-printed Gelma has been explored in tissue repair and regenerative medicine. However, there have been no reports on its application in repairing aortic medial degeneration and ferroptosis during AD. In this work, we aim to incorporate exo-MSC into Gelma hydrogel before UV-cross-linking to create Gelma-exo, which can achieve sustained release of exosomes as the hydrogel degrades.
Ferroptosis is a novel form of programmed cell death caused by the accumulation of iron-dependent lipid peroxides [14–16]. Increasing evidence suggests that ferroptosis is triggered by the incorporation of polyunsaturated fatty acids into cell membranes. Its susceptibility is closely linked to several biological processes, including the metabolic pathways of amino acids, iron, and polyunsaturated fatty acids, as well as the synthesis pathways of GSH, phospholipids, NADPH, and CoQ10. Therefore, ferroptosis is considered an iron-dependent form of metabolic cell death due to excessive lipid peroxidation. Targeting ferroptosis presents a new therapeutic approach for addressing aortic medial degeneration in AD.
In this study, we successfully synthesized Gelma-exos for the first time, which allows for the sustained release of exosomes as the Gelma hydrogel degrades. The exosomes are taken up by vascular smooth muscle cells (VSMCs), where they reverse the excessive proliferative phenotype of VSMCs, reduce the migration and proliferation of damaged VSMCs during aortic medial degeneration, and inhibit ferroptosis in AD. By successfullky reversing aortic medial degeneration and ferroptosis, Gelma-exos offer a promising therapeutic strategy for reducing the rupture rate in AD.
Materials and methods
Development of the AD model in mice and perivascular delivery of exosomes
ApoE−/− mice (C57BL/6) were housed at the animal care facility of the Jilin university. The animal study was approved by the Institutional Animal Research Committee of Jilin university. Four weeks old male ApoE−/− mice were randomly divided into normal group, Ang II + Gelma group, Ang II + Gelma-exos group and Ang II + exosomes group, respectively. 4 weeks old male mice were fed on a regular diet and administered BAPN (Sigma-Aldrich, St. Louis, MO, USA) dissolved in drinking water (1 g/kg per day) for 4 weeks. Exosomes (50 ug) was administered via subcutaneous injection for another consecutive 7 days at the 4 weeks. Gelma or Gelma-exos were embed subcutaneously at the 4 weeks. Mice were infused via osmotic mini pumps (Alzet, Cupertino, CA) with either saline or 2500 ng•kg-1•min-1 Ang II (Sigma-Aldrich, St. Louis, MO) for 7 days at the 6 weeks.The ascending aorta was acquired after animal sacrifice. If the mice sudden death, anatomy was performed immediately. Aorta rupture with bleeding was considered as dissection. We calculate the incidence of AD by using the number of AD mice dividing the total.
Gelma materials
Gelatin was purchased from Sigma-Aldrich (St. Louis, MO, USA). Methacrylic anhydride (MA, 97%) and lithium phenyl-2,4,6-trimethylbenzoyl phosphinate (LAP) were purchased from J&K (Beijing, China).
Bioinks were prepared from gelatin dissolved in phosphate buffered saline (PBS) at 40 ℃ to prepare a 10% gelatin solution. After adding methacrylic anhydride (MA) dropwise into the gelatin solution, the solution was stirred with magnetic force for three hours at 40℃ and 300 rpm. A white porous foam was prepared after dialysis against distilled water for 5 days at 40 ℃ and lyophilized. Gelma [5% (w/v)] was dissolved in PBS supplemented with lithium phenyl (2,4,6-trimethylbenzoyl) phosphinate [LAP, 0.25% (w/v)] and tartrazine [0.05% (w/v)]. Then, a bioink composed of 5% Gelma was prepared.
Fabrication of scaffolds
The computer aided project model was designed as a cylinder with interconnected pores, with a diameter of 16 mm, height of 8 mm, and pore size of 600 μm. The digital lightprocessing printer (Longer Orange 30, China) was used to prepare the scaffolds and the parameters were adjusted for printing. Then, the scaffolds were strengthened under ultraviolet light for 3 min (kernel parameters: layer height, 100 μm; light intensity, 20 mW/cm2; exposure time, 4 s; temperature, 29 °C).
Characterization
The microstructure of the 3D-Printed scaffolds were observed using a scanning electron microscope.
Compressive Tests: A compression test was performed using a physical MCR 301 (Anton Paar, AUSTRIA) at room temperature. Nuclear magnetic resonance: nuclear magnetic resonance was performed using a BRUKER 300 UltraShield.
Degradation rate: The different scaffolds were lyophilized, and their weights (W0) were measured. Then, the lyophilized scaffolds were placed in PBS solution and soaked at 37 °C. The PBS was changed every day. After rinsing twice with deionized water, the samples were lyophilized and weighed (Wt). The remaining weight was calculated as (Wt/W0) × 100%.
Exosomes realease and cumulative release: the lyophilized scaffolds were placed in PBS solution and soaked at 37 °C. The PBS was changed every day. The exosomes release amount in PBS was measure by BCA method.
Swelling rate: The different scaffolds were placed into PBS and soaked for 24 h at 37 °C, and their weights (Ws) were measured after sufficient swelling. Then, the scaffolds were freeze-dried to obtain their dry weight (Wd). The swelling ratio was calculated as (Ws − Wd)/Wd.
RT-qPCR
RNA was extracted from normal VSMCs and tissues using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.). A NanoDrop ND-1000 (NanoDrop; Thermo Fisher Scientific, Inc.) was used to detect the integrity and concentration of the RNA samples. Total RNA was reverse transcribed to cDNA using PrimeScript RT reagent kit with gDNA Eraser (Takara Bio, Inc.) according to the manufacturer’s instructions. RT-qPCR was performed using FastStart Universal SYBR Green Master (ROX) (Roche Diagnostics) and an Applied Biosystems 7500 Fast Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The relative expression levels of genes were calculated using the 2−ΔΔCq method after normalization to GAPDH.
Western blotting analysis
Cells or tissues proteins were extracted by lysate (RIPA Lysis Buffer) and the concentrations were calculated by BCA protein assay kit (Thermo Fisher). Equal amounts of protein was isolated by SDS-PAGE, and transferred to nitrocellulose membrane. Blots were blocked with 5% nonfat milk with 0.1% Tween 20. Diluted antibodies: GPx4 (1:5000, Abcam), COX2 (1:500, Abcam), ACSL4 (1:25,000, Abcam), FTH1 (1:500, Abcam), SM22a (1:500, Abcam), SMA (1:800, bioss), calponin (1:1,000, bioss), MYH11, (1:1,000, bioss), followed by secondary antibodies and visualized with a Bio-Rad (Hercules, CA) imaging system.
Exosomes isolation and identification
Exosomes were isolated from BMSCs medium supernatant after 48 h cultured. Culture medium supernatant was centrifuged at 300 g for 10 min, at 2000 g for 10 min, at 10,000 g for 30 min, at 100,000 g for 70 min and resuspended in PBS. Exosomes was identified through transmission electron microscope, nanoparticle tracking analysis and western blotting (CD9, CD63, CD81, Tsg101). Exosomes were fixed in 4% glutaraldehyde for 2 h, washed 3 times with PBS and fixed with 1% osmium tetroxide for 2 h. The exosomes were dehydrated, followed by impregnated, embedded, polymerized, and observed.
Cell counting kit 8
A total of 100 μl of cell suspension (3 × 104 cells) were seed in 96-well plate for 24 h. Cell Counting Kit (CCK) Solution 2 ul (TransDetect, Beijing, China) was putted into medium and incubated with DMEM. The absorbance at 450 nm were collected.
EdU staining assay
VSMCs were cocultured with Gelma, exosomes and Gelma-exos, incubated with EdU (20 mmol/L) for 1, 3, 5, 7 days. The cells were fixed with 4% paraformaldehyde for 20 min at room temperature. EdU‐positive cells were analysed in different group.
Live/dead assay
The cells were cocultured with Gelma, exosomes and Gelma-exos, incubated with EdU (20 mmol/L) for 1, 3, 5, 7 days. Cells were then stained with Live/Dead assay kit (Invitrogen R37601). The images were taken using an Olympus fluorescent microscope (Olympus, FV300).
Measurement of intracellular ROS
VSMCs cell culture medium was stained with 10 µm DCHF‑DA (Invitrogen; Thermo Fisher Scientific, Inc.) for 2 h at 37 °C. The samples were collected, washed twice with PBS and assayed by fluorescence microscope.
Transwell migration assay
The VSMCs cells (3 × 104) cultured in serum-free medium were seeded in the upper chamber of Transwell inserts, while 500 μL DMEM with 20% FBS was added into the lower chambers. A cotton swab was used to scraped the cells remaining in the upper chamber after 36 h. The polycarbonate films were carefully removed from the upper chambers, and the cells on bottom side were fixed with methanol and stained with 0.1% crystal violet. The number of migratory cells was counted under a microscope.
Wound healing assay
Cells (2.5 × 105 cells/well) were seeded into a 6-well plate. Cells underwent serum starvation in medium containing 0.5% FBS for 48 h, after which, a linear wound was created in the center of the cell layer using a 200-µl pipette tip. Cells were then incubated in serum-free medium for 48 h. The floating cells were removed by washing the cells with PBS. The distance of cell migration was determined by the mean value of the width of the gap between the top, middle and bottom of the wound. The wounds were observed in five fields of view using a light microscope.
Human diseased ascending arteries collection
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Second Hospital of Jilin University. Ascending aortic specimens near the intimal tear were obtained from AD patients undergoing surgical repair if informed consent was obtained. Patients diagnosed with AD were confirmed by computed tomography angiography, and hereditary AD was excluded. The AD ascending aortic tissues were cultured in PBS. Gelma, exosomes and Gelma-exos was added into the PBS for 5 days, respectively.
Immunohistochemistry staining
All samples were fixed in 4% neutral formaldehyde solution and embedded in paraffin. Tissue blocks were sliced into 2 μm sections and dewaxed, hydrated and antigen-repaired by PT link (Dako, Agilent Technologies, USA). Specifically, the slices were placed in repair solution preheated to 65 ℃ and incubated for 30 min by heating to 90 ℃, then cooled to 70 ℃. Subsequently, the slices were washed by PBS. Incubation with primary and secondary antibodies and DAB coloring solution was automated by an Autostainer Link 48 (Dako, Agilent Technologies, USA). Specifically, the slices were incubated with hydrogen peroxide for 10 min, primary antibody for 30 min, and secondary antibody for 20 min at room temperature, followed by counterstaining with hematoxylin, routine dehydration, transparency, and sealing.
Human vascular smooth muscle cell line
Human normal VSMCs line was purchased from Shanghai Fusheng Biotechnology Co., Ltd. (Shanghai, China). VSMCs were cultured in DMEM in 10% fetal bovine serum. Culture medium was replaced every 2 days. VSMCs were serum starved (0.5% fetal bovine serum) for 24 h and stimulated by Ang II (1 ug/ml) for 24 h before further experiments.
Fluorescent labeling of exosomes
Exosomes derived from BMSCs were labeled with lipophilic dye PKH26 liquor (MINI26-1KT, Sigma, USA). Briefly, exosomes resuspended by 25 ul PBS was mixed with 1 ml dilution C. At the same time, 4 μl PKH26 was mixed with 1 ml dilution C. then these two mixture were blend together and incubator at 37 ℃ with 5% CO2 for 3 min. 2 ml FBS was added into the mixed liquid to termination the reaction. Finally, the labeled exosomes were centrifuged at 100,000 g for 70 min and rinsed by 5 ml PBS. 1 ml PBS with exosomes was added into the VSMCs medium supernatant and co-culture for 24 h. The VSMCs was washed by PBS twice and stained with 4,6-diamidino-2-phenylindole (DAPI). At last, the internalization of exosomes was measured by a confocal microscope.
Ferroptosis relate assays
GSH level, NADPH level, GSH/GSSG ratio, GPx4 activity, MDA level, LPO level, ROS level, iron level were detected as the manufacturer’s instructions.
Statistical analysis
All statistical analyses were performed by SPSS 20.0. The data are presented as the mean ± standard deviation. One-way analysis of variance (ANOVA) was performed between-group differences and multiple groups. Comparisons among multiple groups were performed using one-way ANOVAs, followed by post hoc Tukey tests. p < 0.05 was considered to indicate statistically significant.
Results
Preparation and properties of 3D-printing hydrogels loaded exosomes
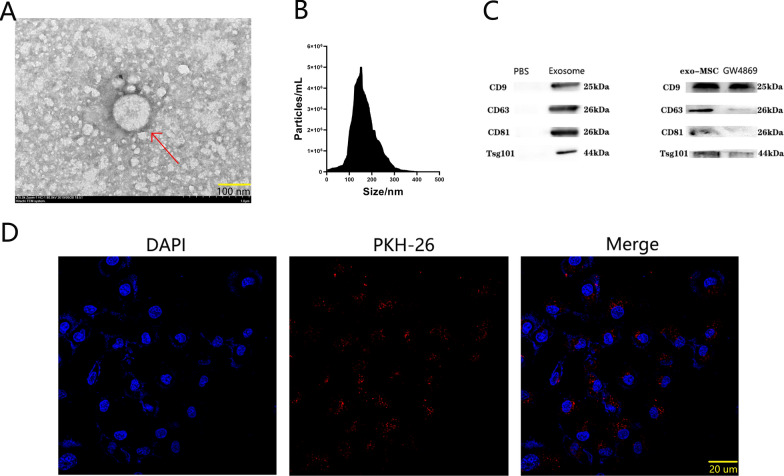
The exosomes used in this study was extracted from BMSCs (Bone Marrow Mesenchymal Stem Cells). Transmission electron microscope revealed that the secretions extracted via ultracentrifugation had bilayer membrane with a diameter of 100 nm (ranging from 30 to 150 nm) (Fig. 1A). The size distribution and surface marker proteins were confirmed by dynamic light scattering (Fig. 1B) and western blotting (Fig. 1C). GW4869, an exosomal inhibitor, was added in the culture medium, which resulted in a reduction of surface marker proteins compared to the PBS control (Fig. 1C). These results confirmed that the secretions extracted from the BMSCs culture medium supernatant were exosomes. After labeling with PKH26, the exosomes were cocultured with VSMCs (Vascular Smooth Muscle Cells). Confocal microscopy showed red fluorescence of PKH26-labeled exosomes within VSMCs after 8 h of co-culture (Fig. 1D), indicating that the exosomes were successfully taken up by VSMCs.
Fig. 1.
Basic characteristic of exo-BMSC. A TEM images of exo-BMSC. The red arrow represents exosomes. Scale bar = 100 nm. B The particle size distribution of exo-BMSC nanoparticles by DLS. C The expression of exosomal surface marker proteins CD9, CD81, CD63 and TSG101 by western blot analysis. (n = 3). D Representative fluorescence micrograph of PKH-26 (red)-labelled exosomes. DAPI (nuclear; in blue), PKH-26 (exosomes; in red). Scale bar = 20 um
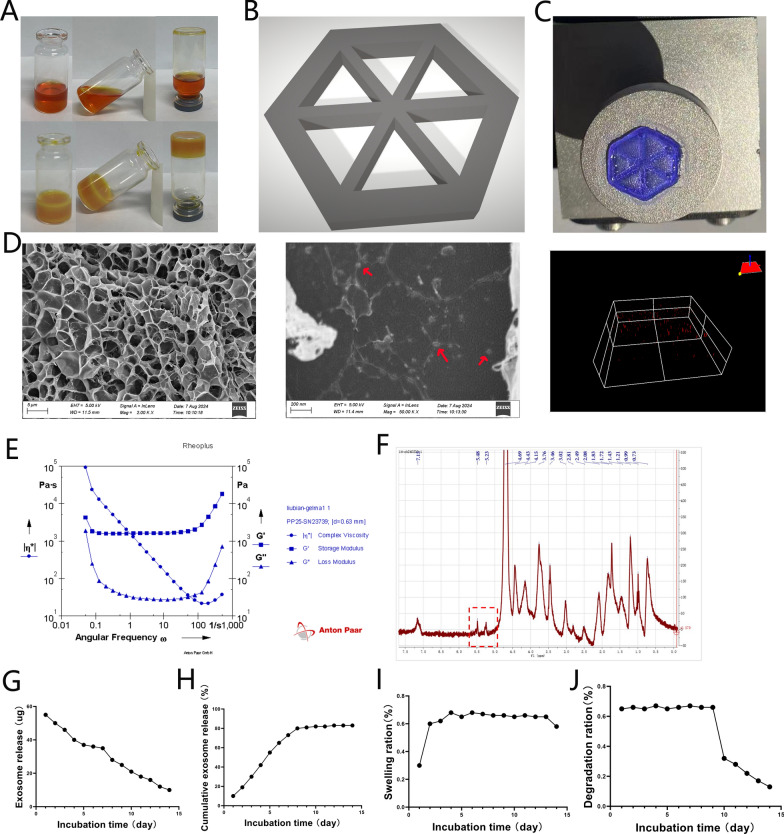
To enhance the mechanical and biological properties of the Gelma hydrogel achieve sustained exosome release, LAP photoinitiator was added, followed by 2 min of UV radiation. The mechanism of hydrogel formation is shown in Fig. 2A. The hydrogel scaffold was then prepared using a 3D printing system (Fig. 2B). Before UV radiation, Gelma was mixed with exosomes, and the Gelma-exos was solubilized to a final concentration of 5%. The Gelma-exos hydrogel was synthesized using a 3D printing system, followed by visible light-induced crosslinking in the presence of LAP (Fig. 2C). The Gelma-exos displayed a 3D porous structure, as observed under a scanning electron microscope and confocal microscope (Fig. 2D), confirming the successful preparation of Gelma-exos.
Fig. 2.
3D-printing Gelma-exos bioink preparation. A Images of the hydrogel before and after crosslinking. B Hydrogels scaffold was designed by 3D printing system. C Solid Gelma-exos (The blue hexagon) synthesis by 3D printing system. D Representative scanning electron microscope of Gelma-exos hydrogel, Scale bar = 5 μm or 200 nm. The red arrow represents exosomes in Gelma-exos hydrogel. Representative confocal microscope three-dimensional reconstruction pictures of Gelma-exos hydrogel. The red represents exosomes in Gelma. E Rheological analysis of Gelma-exos hydrogel. F NMR characterization of methacrylated gelatin. G Release mass. H Cumulative release rate. I Swelling ration J Degradation rate
Rheological analysis, including elastic modulus (G′) and loss modulus (Gʺ), demonstrated that Gelma-exos possess good mechanical strength (Fig. 2E). The nuclear magnetic resonance characterization of methacrylated gelatin (Fig. 2F). The release rate, cumulative release rate, swelling ration, and degradation rate are presented in Fig. 2G–J, respectively. These results indicate that the 3D-printed Gelma-exos can programmatically release exosomes over time.
Biocompatibility of Gelma-exos in VSMCs
Good biocompatibility is a prerequisite for clinical application in cardiovascular tissue engineering. We evaluated the cytocompatibility of 3D-printed Gelma-exos using the CCK-8 assay, live/dead viability assay, and EdU assay. Gelma was prepared at different concentrations (3%, 5%, and 7%) for VSMC culture, with each concentration containing the same amount of exosomes (50 mg). After the first 24 h, VSMCs cell numbers were almost identical across the different Gelma concentrations (Supplementary Fig. 1 A–C). Additionally, there were no significant differences in VSMCs cell morphologies among the various Gelma concentration groups (Supplementary Fig. 1 B, C). Moreover, neither scaffold concentrations (3%, 5%, or 7%) had a notable impact on VSMC cell number or morphology (Supplementary Fig. 1 A–C). These results demonstrate that Gelma has good biocompatibility with VSMCs. Given that a low Gelma concentration could reduce mechanical properties while a high Gelma concentration might alter the hydrogel’s microstructure, we chose the 5% Gelma concentration for subsequent experiments.
Aortic dissection VSMCs cell models
As we previously reported, VSMCs in AD exhibit enhanced phenotype switching, as well as increased proliferation and migration abilities. Angiotensin II (Ang II) is one of the most effective inducers in AD VSMC cell models. However, different studies have shown varying doses and times for Ang II induction. Furthermore, few studies have reported the definitive Ang II dose and time required to induce ferroptosis. Our concentration-dependent curve showed that the expression of contractile phenotypic markers did not decrease until the Ang II concentration reached 1 μmol/L (Supplementary Fig. 2A). The time-dependent curve showed that the expression of these markers did not significantly decrease until 24 h of Ang II induction (Supplementary Fig. 2 B). Therefore, in our study, the induction conditions were set at 1 umol/L Ang II for 24 h. Under these conditions, VSMCs exhibited enhanced migration (Supplementary Fig. 2C, D) and proliferation abilities (Supplementary Fig. 2 E–G). RT-qPCR and western blotting showed that two positive regulators of ferroptosis (ACSL4 and COX2) were upregulated in Ang II VSMCs compared to normal VSMCs. Meanwhile, the levels of two negative regulators (GPX4 and FTH1) were reduced in Ang II-induced VSMCs. Additionally, Ang II inducing led to a reduction in GSH level, NADPH levels, the GSH/GSSG ratio, and GPx4 activity, while increasing MDA, LPO, and ROS levels (Supplementary Fig. 2I). Consistent with these findings, iron, another essential factor for ferroptosis, showed significant cytoplasmic accumulation in the Ang II-induced VSMCs (Supplementary Fig. 2I). These results suggest that Ang II induces changes in ferroptosis-related factors, which may sensitize AD to ferroptosis during hypertension.
Capacity of Gelma-exos for VSMCs phenotype switching in vitro
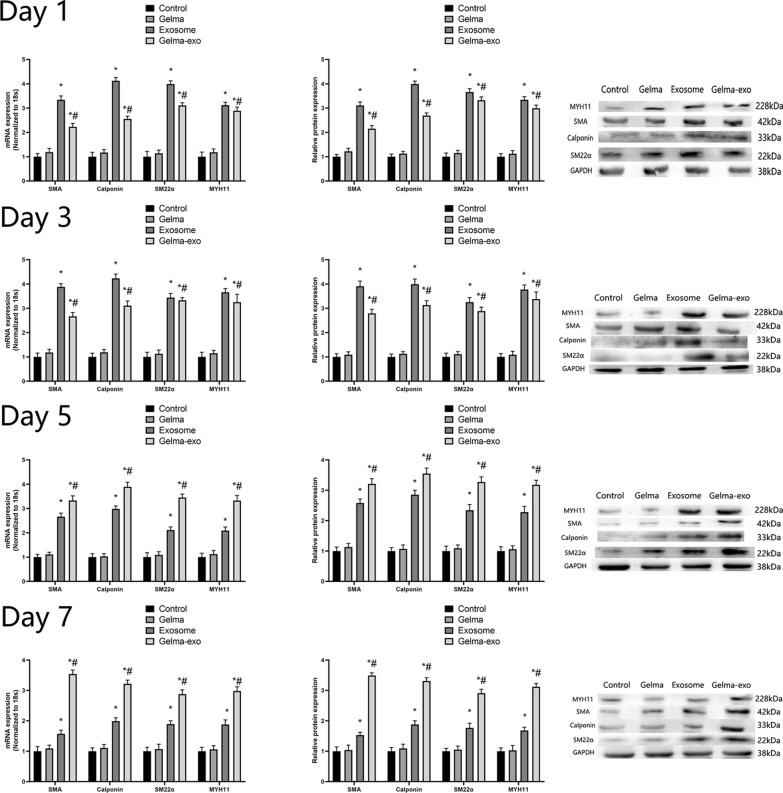
To assess the effects of Gelma-exos on VSMCs phenotype switching, we introduced Gelma, exosomes, and Gelma-exos into the VSMC culture medium. The expression of VSMC contractile phenotypic markers (MYH11, SM22α, Calponin and SMA) was measured using RT-qPCR and western blotting on days 1, 3, 5, and 7 days (Fig. 3). The expression levels of these markers was similar between the control and Gelma group from the first day 1 to day 7. During the first 3 days, both the exosomes and Gelma-exos groups showed enhanced expression of contractile phenotypic markers compared to the control group, with the exosome group showing a greater effect than the Gelma-exos group. However, after day 5, the Gelma-exos group exhibited a more pronounced enhancement in the expression of contractile phenotypic markers compared to the exosome control (Fig. 3). These results indicate that exosomes loaded in the Gelma hydrogel provide a sustained release, offering better therapeutic efficacy than a single full injection after 5 days.
Fig. 3.
Capacity of Gelma-exos for VSMCs phenotype switching. The expression of contractile phenotypic markers (MYH11, SM22α, Calponin and SMA) in VSMCs co culture with Gelma, exosomes, and Gelma-exos. RT-qPCR and western blotting results at the 1, 3, 5, 7 days. All data are shown as the mean ± SEM from at least three independent experiments. *P < 0.05, compare with Control or Gelma group. #: P < 0.05, compare with Exosome group, Student’s t test
Capacity of Gelma-exos for VSMCs proliferation and migration In Vitro
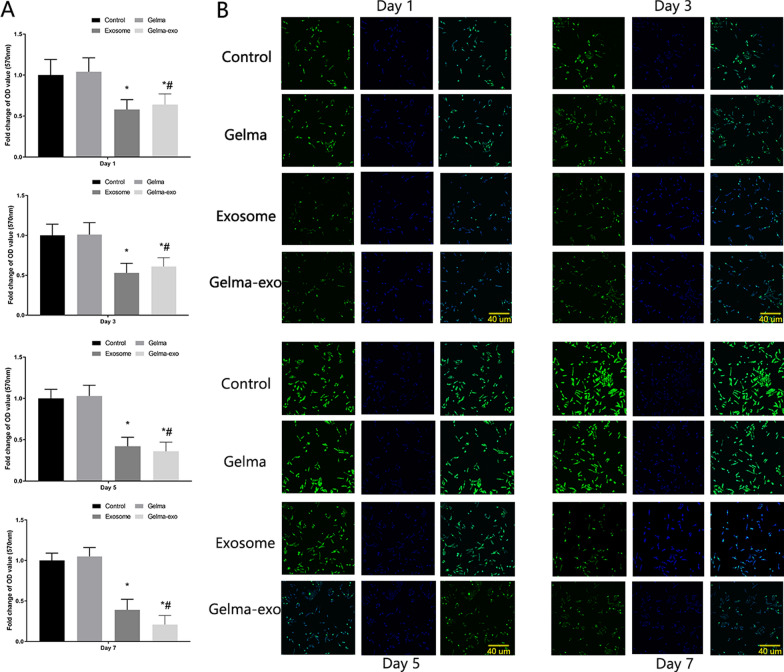
To explore the impact of Gelma-exos on VSMC proliferation and migration, we added Gelma, exosomes and Gelma-exos to the VSMC culture medium. We performed the CCK-8 assay (Fig. 4A), live/dead viability assay (Supplementary Fig. 3), EdU assay (Fig. 4B), transwell assay (Fig. 5) and wound healing assay (Supplementary Fig. 4) on days 1, 3, 5, and 7. The results showed that Gelma alone did not affect VSMC proliferation and migration. However, weakened proliferation and migration were observed in VSMCs co-cultured with exosomes and Gelma-exos, suggesting that exosomes can inhibit VSMC proliferation and migration. During the first 3 days, the exosome group exhibited a stronger inhibitory effect on proliferation and migration than the Gelma-exos group. These results showed that Gelma-exos could sustain the release of exosomes from the hydrogel, achieving better therapeutic efficacy than a single exosome injection.
Fig. 4.
Capacity of Gelma-exos for VSMCs proliferation. A CCK-8 assay in VSMCs co culture with Gelma, exosomes, and Gelma-exos. B EdU assay in VSMCs co culture with Gelma, exosomes, and Gelma-exos at the 1, 3, 5, 7 days. All data are shown as the mean ± SEM from at least three independent experiments. *P < 0.05, compare with Control or Gelma group. #: P < 0.05, compare with Exosome group, Student’s t test. Scale bar = 40 um. DAPI (nuclear; in blue), EdU (EdU positive cells, in green)
Fig. 5.
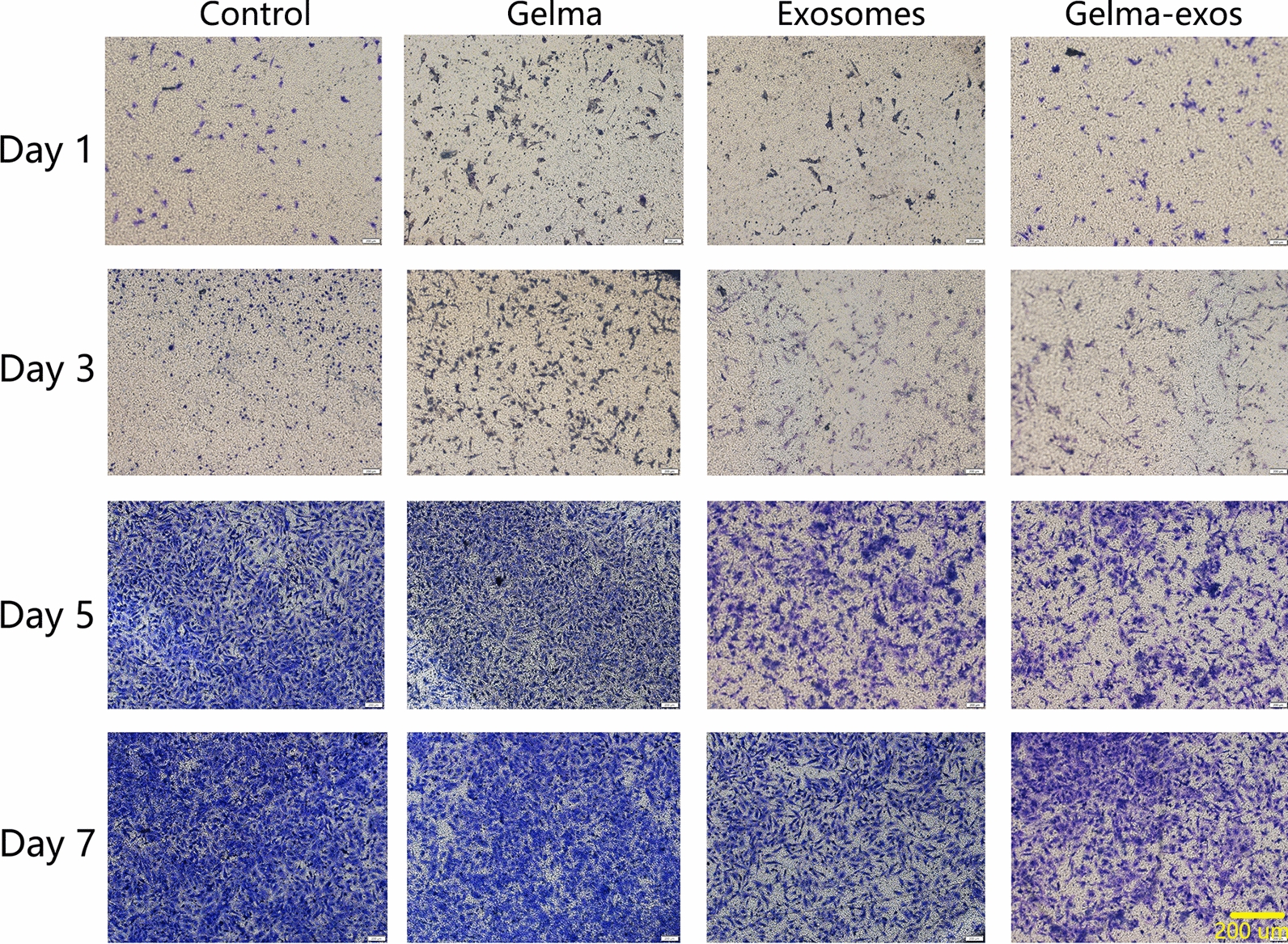
Capacity of Gelma-exos for VSMCs migration. Transwell assay in VSMCs co culture with Gelma, exosomes, and Gelma-exos at the 1, 3, 5, 7 days. Scale bar = 200 um
Capacity of Gelma-exos for VSMCs ferroptosis In Vitro
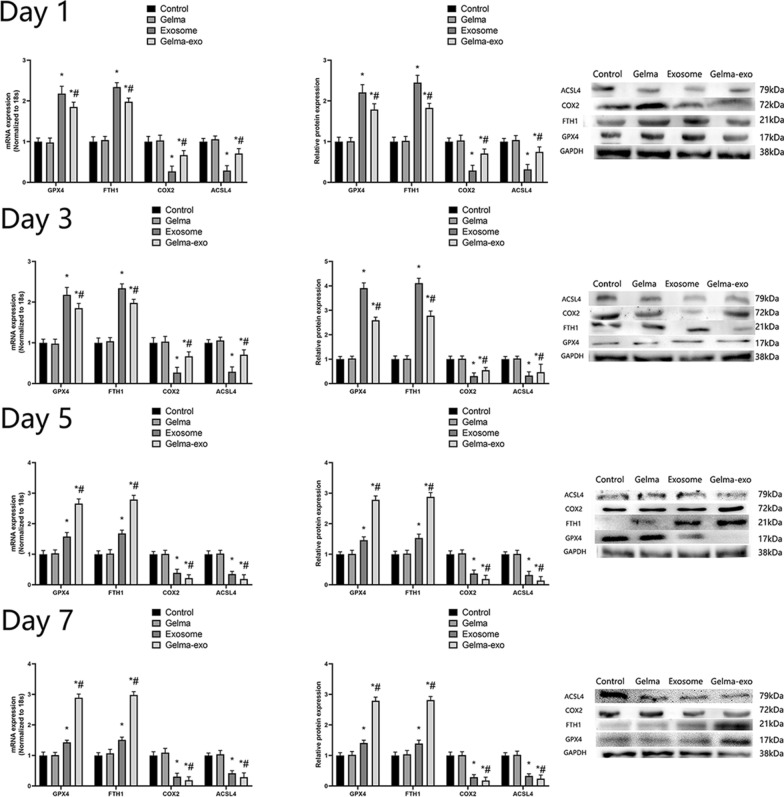
To explore the effect of Gelma-exos on ferroptosis in VSMCs, we added Gelma, exosomes, and Gelma-exos to the VSMCs culture medium. We measured the expression of ferroptosis-related genes and proteins (ACSL4, COX2, GPX4 and FTH1) using RT-qPCR and western blotting. Additionally, we assessed GSH level, NADPH levels, GSH/GSSG ratio, GPx4 activity, MDA level, LPO level, and ROS levels on days 1, 3, 5, and 7. The results showed that Gelma alone did not influence ferroptosis in VSMCs compared with the control group. However, in both the exosome and Gelma-exos groups, there was a decrease in the positive regulators of ferroptosis (ACSL4 and COX2) (Fig. 6). These was also an increase in GSH levels, NADPH levels, GSH/GSSG ratio, and GPx4 activity (Supplementary Fig. 5), along with a decrease in MDA level, LPO levels (Supplementary Fig. 6), and ROS levels (Supplementary Fig. 7) in these groups. The exosomes initially showed a greater effect on these ferroptosis-related indices compared to Gelma-exos at the first 3 days. However, Gelma-exos provided a sustained release of exosomes, achieving better effects than a single exosome injection after 5 days. Consistent with these findings, iron expression showed significant cytoplasmic degradation in Gelma-exos group (Supplementary Fig. 5). These results indicate that the exosomes loaded into Gelma can be continuously released, thereby inhibiting Ang II-induced ferroptosis in VSMCs.
Fig. 6.
Capacity of Gelma-exos for VSMCs ferroptosis. The expression of erroptosis related genes and proteins (ACSL4, COX2, GPX4 and FTH1) in VSMCs co culture with Gelma, exosomes, and Gelma-exos. RT-qPCR and western blotting results at the 1, 3, 5, 7 days. All data are shown as the mean ± SEM from at least three independent experiments. *P < 0.05, compare with Control or Gelma group. #: P < 0.05, compare with Exosome group, Student’s t test
Capacity of Gelma-exos for Ang II induced aortic dissection mice In Vivo
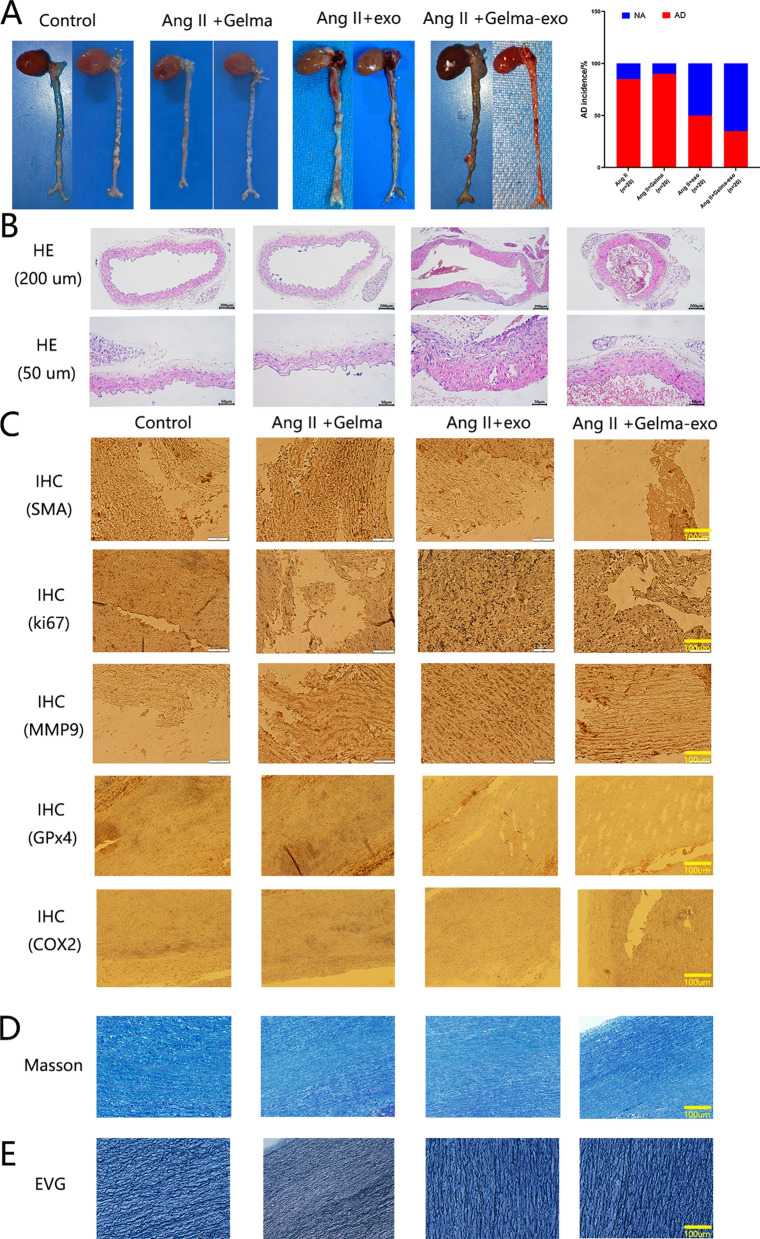
To explore the effect of the Gelma-exos on AD, Gelma and Gelma-exos were embedded subcutaneously, while exosomes were administered through a single subcutaneous injection in Ang II-induced AD mice. The biocompatibility of Gelma-exos was assessed by evaluating liver function (alanine aminotransferase, aspartate aminotransferase, bilirubin), renal function (creatinine, blood urea nitrogen), blood parameters (white blood cells, red blood cells, platelets) (Supplementary Fig. 8). The results showed that Gelma-exos, Gelma, and exosomes were non-toxic to the mice. In the Ang II-induced model, 85% (17/20) of the mice experienced sudden death, and 90% (18/20) of the mice treated with Gelma died, with ascending aortic dissection and rupture found upon anatomical injection. In contrast, in the grouo x exosomes through a single subcutaneous injection, 50% (10/20) of mice died suddenly due to ascending aortic dissection rupture and bleeding. Notably, the incidence of AD and sudden death was significantly lower in the Gelma-exos group, with only 35% (7/20) mortality (P < 0.05; Fig. 7A). These data suggest that Gelma-exos can provide sustained release of exosomes, helping to prevent AD rupture. To further assess AD in the ascending aorta from AD mice, H&E staining was performed (Fig. 7B). IHC showed the expression of SMA and GPx4 in AD mice treated with Gelma-exos compared to the exosome group, while the expression of Ki-67, MMP9, COX2 was lower in Gelma-exos group (Fig. 7C). Masson staining indicated collagen accumulation in the aortic media of control AD mice (Fig. 7D), while Verhoeff’s van Gieson (EVG) staining showed increased collagen deposition and fragmented elastic fibers (Fig. 7E). These results suggest that Gelma-exos might help reduce the occurrence of AD.
Fig. 7.
Capacity of Gelma-exos for Ang II induced aortic dissection mice In Vivo. A Representative aorta images from, Gelma, Ang II + exosomes and Ang II + Gelma-exos. Quantification of AD incidence in the aorta. B Representative H&E staining images in the ascending aortia. Scale bar = 50um and 200 um. C Protein expression of SMA, ki-67, MMP9, GPx4, COX2 by immunohistochemical staining in aortas. n = 3. Scale bar = 100 um. D Representative pictures of aortic media with Masson staining of the tissues from the ascending aortia. n = 3. Scale bar = 100 um. E EVG staining of the tissues from the ascending aortia. n = 3. Scale bar = 100 um
Capacity of Gelma-exos for human aortic dissection in ex vivo
To translate our findings from mice model to humans, we investigated the effect of the Gelma-exos on human AD tissues ex vivo. The human AD tissues were co- cultured with Gelma, exosomes, and Gelma-exos for 7 days. IHC releaved that the expression of SMA and GPx4 was higher in the Gelma-exos group compared to the exosome group, while the expression of Ki-67, MMP9, and COX2 was lower in Gelma-exos group (Supplementary Fig. 9A). Masson staining indicated collagen degradation in the aortic media of Gelma-exos group (Supplementary Fig. 9B), and Verhoeff’s van Gieson (EVG) staining showed fewer fragmented elastic fibers and decreased collagen deposition (Supplementary Fig. 9C). RT-qPCR and western blotting showed that the expression of the two positive regulators of ferroptosis (ACSL4 and COX2) decreased in the Gelma-exos co-culture with human AD tissues. Meanwhile, the levels of two negative regulators (GPX4 and FTH1) increased (Supplementary Fig. 9D). Addionally, there was an increase in GSH levels, NADPH levels, GSH/GSSG ratio, GPx4 activity, alongside a decrease in MDA levels, LPO levels, ROS levels in Gelma-exos group (Supplementary Fig. 9E). These results suggest that Gelma-exos have potential therapeutic in human AD.
Discussion
AD is an extremely dangerous condition caused by medial degeneration, VSMC remodeling and inflammation [17–21]. To date, the precise molecular pathogenesis of AD remains unclear, and no molecular targeted therapies are available for AD. In this study, we synthesized a hydrogel material containing Gelma and exosomes. This specialized hydrogel allows for the programmed, time-released delivery of exosomes from Gelma-exos, which can restore aortic medial degeneration by inhibiting ferroptosis. Our finding demonstrate that sustained-release exosomes can promote the transition of synthetic phenotype VSMCs to a contractile phenotype, inhibit VSMCs proliferation and migration, and suppress VSMC ferroptosis in the aorta. Collectively, these effects contribute to the reduction of AD development, progression and rupture.
Despite the significant advancement in understanding the pathogenesis of AD and the development of comprehensive treatment strategies, molecular targeted therapy has limited progress in treating this lethal disease. Exosomes derived from bone marrow stromal cells (BMSC) are considered a promising therapeutic approach that can delay the progression of AD [22]. However, exosomes administered intravenously are quickly cleared from the bloodstream, limited their sustained presence and therapeutic effect [12]. A suitable carrier for exosomes is needed to a controlled release through gradual degradation. Hydrogels present an attrative option as a biomaterial carrier due to their excellent thixotropy, desirable biocompatibility, mechanical performance and biodegradability. Combined 3D printing technology, hydrogels can be designed to encapsulate various bioactive substances, allowing for their sustained release as the hydrogel degrades. Although 3D-printing hydrogels are primarily used in orthopedic [23] and dental [24] applications, their potential in treating cardiovascular diseases, especially AD, has not been fully explored.
In this study, we developed a novel hydrogel that encapsulates exosomes using 3D printing technology, aiming to restore aortic medial degeneration by inhibiting ferroptosis in AD. This research is the first to demonstrate that Gelma-exos can reduce the rupture rate of AD. Exosomes derived from MSC play crucial roles in cell-to-cell communication [9], regulatory cell proliferation, migration, autophagy, self-repair and senescence. However, the rapid clearance of exogenous exosomes by the circulatory system and their subsequent excretion limit their utilization efficiency, hindering their widespread application [12]. Gelma, with its favorable biocompatibility and slow degradation rates, is reported in tissue repair and regenerative medicine. Gelma hydrogels have demonstrated their potential for various tissue engineering applications due to their numerous desirable properties [25]. This injectable biomaterial can combined with a variety of bioactive substances, which are released continuously as Gelma degrades following UV cross-linking.
In this study, exosomes were added to Gelma as matrix, resulting in a material with satisfactory printing and mechanical properties. The exosomes encapsulated within Gelma were released into the surrounding tissue in a controlled manner. Nuclear magnetic resonance (NMR) analysis confirmed that the hydrogel is Gelma. Moreover, no significant cytotoxicity was observed in the printed scaffolds over 1, 3, 5, and 7 days. Prior to 3D printing the hydrogel, the pre-solution was concentrated, and the pore size was selected. Research indicated that a pore size ranging from 400 to 700 μm is optimal for cells and tissue regeneration [26–31]. In this study, the pore size of the scaffold was set to 600 μm, which exhibited excellent blood vessel repair capability. We observed that the pore size of the hydrogel decreased due to the increase in the concentration of the pre-solution and the cross-linking density. Scanning electron microscopy and confocal microscopy confirmed the successful incorporation of exosomes into the Gelma matrix. Degradation tests demonstrated that Gelma-exos could gradually release exosomes into the environment, which is beneficial for vascular repair. The Gelma-exos synthesized in this study provided an intelligent drug delivery system that the limitations of rapid exosome clearance by the circulatory system, offering sustained therapeutic effects with low toxicity both in vitro and in vivo.
The advantage of Gelma-exos in regulating VSMCs proliferation, migration and ferroptosis were investigated in this study. Studies showed that Gelma could promote bone cell proliferation [32]. However, our findings revealed no significant difference in VSMC proliferation, migration and ferroptosis between the control group and Gelma group. Notably, both the exosomes and Gelma-exos effectively inhibited VSMCs proliferation, migration and ferroptosis induced with Ang II. While a single injection of exosomes exhibited a more pronounced effect that than Gelma-exos during the first three days, Gelma-exos demonstrated superior efficacy after five days due to the sustained release of exosomes. These findings suggest that Gelma provides a suitable microenvironment that supports exosomes-induced VSMC repair.
VSMCs hsve the ability to switch from a contractile to a synthetic phenotype in response to external stimuli, such as inflammation, oxidative stress, and hypertension. Contractile VSMCs expressed higher levels of contractile markers, which are essential for maintaining aorta function. An imbalance in VSMCs phenotypic switching contributes to pathological processes leading to cardiovascular diseases. In AD, the balance between contractile and synthetic VSMC is disrupted, with studies showing decreased expression of contractile markers like SMA, SM22α, MYH11, and Calponin, and increased expression of synthetic markers like osteopontin increased [33]. Therefore, some studies aimed to reverse the excessive synthetic phenotype, and reduce the rupture rate of AD. Currently, ALDH2 [34], Anxa1 [35], miR-134 [36], miR-193 [19] and linc01278 [18] have been reported to regulate the this phenotypic switch Recently, exosomes have emerged as potential carriers, for bioactive substances, capable of inhibiting the synthetic phenotype of VSMCs. However, the transient presence of exosomes in the bloodstream limits their ability to provide continuous therapeutic treatment [12]. In this study, we synthesized a novel hydrogel containing Gelma and exosomes, which enabled the controlled-release of exosomes as the Gelma degraded. Our results demonstrated that both exosomes and Gelma-exos could inhibit the synthetic phenotype of VSMCs by promoting the expression of contractile markers. Additionally, the proliferation and migration of VSMCs were also reduced. While a single injection of exosomes showed enhanced capability in repairing aortic medial degeneration during the first three days, Gelma-exos proved to be more effective in inhibiting VSMC proliferation and migration over time due to its controlled release mechanism.
VSMCs constitute the main component of the aortic wall's middle layer. Aortic medial degeneration, driven by VSMC apoptosis and extracellular matrix (ECM) degradation, weakens the aortic wall, making apoptosis and necrosis closely associated with the pathogenesis of AD [37]. Recently, several novel pathological and physiological changes, including cuproptosis, ferroptosis, necroptosis, and autophagy, have been identified as relevant to AD progression. Ferroptosis, a form of regulated cell death, offers new insights into the molecular pathogenesis of AD [38]. Previous studies have shown that ferroptosis is activated in AD by the overexpression of SLC7A11 and the downregulation of GPx4 [39, 40]. Moreover, targeting ferroptosis and related genes could potentially alleviate AD [41]. In our study, we investigated ferroptosis-related genes and conducted ferroptosis assays in vitro, vivo and ex vivo. We found that exosomes and Gelma-exos inhibited the expression of two positive regulators of ferroptosis (ACSL4 and COX2) while promoting the expression of two negative regulators (GPx4 and FTH1). Increases in GSH levels, NADPH levels, GSH/GSSG ratios, and GPx4 activity, along with decreases in MDA levels, LPO levels, and ROS levels, were observed in both the exosomes and Gelma-exos groups. While a single injection of exosomes showed a stronger initial effect in inhibiting ferroptosis during the first three days, Gelma-exos provided superior long-term protection against ferroptosis due to its controlled-release.
This study has several limitations. While Gelma, as an extracellular matrix, could theoretically inhibit VSMC phenotypic transformation, our results did not show significant differences between the control and Gelma groups. Additionally, further experiments are need to elucidate the molecular mechanism by which Gelma-exo influence VSMC phenotypic transformation and ferroptosis. A comprehensive understanding of the therapeutic effects of Gelma-exos in AD is warranted in future studies.
In conclusion, we identified Gelma-exos as a novel targeted treatment for regulating VSMC phenotype switching and ferroptosis during AD. This study marks the first time Gelma-exos have been used to reverse VSMC phenotype switching, aortic remodeling, and ferroptosis. These findings could have significant implications for the future treatment of human AD.
Supplementary Information
Acknowledgements
None.
Abbreviations
- AD
Aortic dissection
- MSCs
Mesenchymal stem cells
- exo-MSCs
Exosomes derived from mesenchymal stem cells
- Gelma
Methylacrylated gelatin
- Gelma-exos
exo-MSCs-embedded GelMA hydrogels
- 3D
Three-dimensional
- VSMC
Vascular smooth muscle cell
- RT-qPCR
Reverse transcription -quantitative polymerase chain reaction
- CCK
Cell Counting Kit
- EVG
Verhoeff’s van Gieson
Author contributions
Weitie Wang and Kexiang Liu designed and supervised the study; Dongdong Zheng, Yale Su, Hulin Piao and Jinyu Xu, performed the analysis work; Weitie Wang, Qing Liu, Qiweiyang, Songning Fu and Kexiang Liu organized, designed, and wrote the paper. All authors reviewed the final manuscript.
Funding
This work was supported by the project of the National Natural Science Foundation, China (Grant no. 81970399). Basic Department of Jilin Provincial Science and Technology Department, China (Grant no. 20190901008JC). Jilin Province Health Science and Technology Capability Enhancement Project, China (Grant no. 2021JC043). Jilin Province Science and Technology Development Plan Project, China (Grant no. 212553HJ010288477).
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Second Hospital of Jilin University. The animal study was approved by the—Institutional Animal Research Committee of Jilin university.
Consent for publication
All authors agreed to publish this manuscript.
Competing interests
The authors declare no competing financial and/or non-financial interests in relation to the work described.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Weitie Wang and Qing Liu have contributed equally to this work.
References
- 1.Wang W, Piao H, Wang Y, Li B, Wang T, Xu R, et al. Long-term outcomes of hybrid technique of complicated type B aortic dissection. Ann Thorac Surg. 2019;107(5):1319–25. [DOI] [PubMed] [Google Scholar]
- 2.Wang W, Piao H, Wang Y, Li B, Zhu Z, Wang T, et al. Early outcomes with a hybrid technique for repair of a non-A non-B aortic dissection. J Thorac Cardiovasc Surg. 2022;163(5):1766–74. [DOI] [PubMed] [Google Scholar]
- 3.Wang W, Wang Y, Piao H, Zhu Z, Li D, Wang T, et al. Ministernotomy approach to aortic arch inclusion and frozen elephant trunk in the treatment of acute Stanford A aortic dissection. Front Cardiovasc Med. 2022;9: 944612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu Y, Lingala B, Baiocchi M, Tao JJ, Toro Arana V, Khoo JW, et al. Type A aortic dissection-experience over 5 decades: JACC historical breakthroughs in perspective. J Am Coll Cardiol. 2020;76(14):1703–13. [DOI] [PubMed] [Google Scholar]
- 5.Zhong X, Wu Q, Wang Z, Zhang M, Zheng S, Shi F, et al. Iron deficiency exacerbates aortic medial degeneration by inducing excessive mitochondrial fission. Food Funct. 2022;13(14):7666–83. [DOI] [PubMed] [Google Scholar]
- 6.Shen Z, Huang W, Liu J, Tian J, Wang S, Rui K. Effects of mesenchymal stem cell-derived exosomes on autoimmune diseases. Front Immunol. 2021;12: 749192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ha DH, Kim HK, Lee J, Kwon HH, Park GH, Yang SH, et al. Mesenchymal stem/stromal cell-derived exosomes for immunomodulatory therapeutics and skin regeneration. Cells. 2020;9(5):1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lotfy A, AboQuella NM, Wang H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res Ther. 2023;14(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Q, Piao H, Wang Y, Zheng D, Wang W. Circulating exosomes in cardiovascular disease: novel carriers of biological information. Biomed Pharmacother. 2021;135: 111148. [DOI] [PubMed] [Google Scholar]
- 10.Rezaie J, Feghhi M, Etemadi T. A review on exosomes application in clinical trials: perspective, questions, and challenges. Cell Commun Signal. 2022;20(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin S, Lin S, Xu J, Yang G, Chen H, Jiang X. Dominoes with interlocking consequences triggered by zinc: involvement of microelement-stimulated MSC-derived exosomes in senile osteogenesis and osteoclast dialogue. J Nanobiotechnology. 2023;21(1):346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi Y, Nishikawa M, Shinotsuka H, Matsui Y, Ohara S, Imai T, et al. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol. 2013;165(2):77–84. [DOI] [PubMed] [Google Scholar]
- 13.Zhao C, Zhou L, Chiao M, Yang W. Antibacterial hydrogel coating: strategies in surface chemistry. Adv Colloid Interface Sci. 2020;285: 102280. [DOI] [PubMed] [Google Scholar]
- 14.Liang D, Minikes AM, Jiang X. Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol Cell. 2022;82(12):2215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11(2):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22(4):266–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang W, Wang T, Wang Y, Piao H, Li B, Zhu Z, et al. Integration of gene expression profile data to verify hub genes of patients with stanford A aortic dissection. Biomed Res Int. 2019;2019:3629751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W, Liu Q, Wang Y, Piao H, Zhu Z, Li D, et al. LINC01278 sponges miR-500b-5p to regulate the expression of ACTG2 to control phenotypic switching in human vascular smooth muscle cells during aortic dissection. J Am Heart Assoc. 2021;10(9): e018062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang W, Wang Y, Piao H, Li B, Zhu Z, Li D, et al. Bioinformatics analysis reveals microRNA-193a-3p regulates ACTG2 to control phenotype switch in human vascular smooth muscle cells. Front Genet. 2021;11: 572707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W, Liu Q, Wang Y, Piao H, Li B, Zhu Z, et al. Verification of hub genes in the expression profile of aortic dissection. PLoS ONE. 2019;14(11): e0224922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang W, Wang Y, Piao H, Li B, Huang M, Zhu Z, et al. Circular RNAs as potential biomarkers and therapeutics for cardiovascular disease. PeerJ. 2019;7: e6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chai T, Yang X, Zhuang X, Qiu Z, Zheng H, Cai M, et al. Upregulation of miR-222-3p alleviates the symptom of aortic dissection through targeting STAT3. Life Sci. 2022;310: 121051. [DOI] [PubMed] [Google Scholar]
- 23.Turnbull G, Clarke J, Picard F, Riches P, Jia L, Han F, et al. 3D bioactive composite scaffolds for bone tissue engineering. Bioact Mater. 2017;3(3):278–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paczkowska-Walendowska M, Koumentakou I, Lazaridou M, Bikiaris D, Miklaszewski A, Plech T, et al. 3D-printed chitosan-based scaffolds with Scutellariae baicalensis extract for dental applications. Pharmaceutics. 2024;16(3):359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao S, Zhao T, Wang J, Wang C, Du J, Ying L, et al. Gelatin methacrylate (GelMA)-based hydrogels for cell transplantation: an effective strategy for tissue engineering. Stem Cell Rev Rep. 2019;15(5):664–79. [DOI] [PubMed] [Google Scholar]
- 26.Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26(27):5474–91. [DOI] [PubMed] [Google Scholar]
- 27.Roosa SM, Kemppainen JM, Moffitt EN, Krebsbach PH, Hollister SJ. The pore size of polycaprolactone scaffolds has limited influence on bone regeneration in an in vivo model. J Biomed Mater Res A. 2010;92(1):359–68. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Sun N, Zhu M, Qiu Q, Zhao P, Zheng C, et al. The contribution of pore size and porosity of 3D printed porous titanium scaffolds to osteogenesis. Biomater Adv. 2022;133: 112651. [DOI] [PubMed] [Google Scholar]
- 29.Chen Z, Yan X, Yin S, Liu L, Liu X, Zhao G, et al. Influence of the pore size and porosity of selective laser melted Ti6Al4V ELI porous scaffold on cell proliferation, osteogenesis and bone ingrowth. Mater Sci Eng C Mater Biol Appl. 2020;106: 110289. [DOI] [PubMed] [Google Scholar]
- 30.Luo C, Wang C, Wu X, Xie X, Wang C, Zhao C, et al. Influence of porous tantalum scaffold pore size on osteogenesis and osteointegration: a comprehensive study based on 3D-printing technology. Mater Sci Eng C Mater Biol Appl. 2021;129: 112382. [DOI] [PubMed] [Google Scholar]
- 31.Chang B, Song W, Han T, Yan J, Li F, Zhao L, et al. Influence of pore size of porous titanium fabricated by vacuum diffusion bonding of titanium meshes on cell penetration and bone ingrowth. Acta Biomater. 2016;33:311–21. [DOI] [PubMed] [Google Scholar]
- 32.Yuan Z, Yuan X, Zhao Y, Cai Q, Wang Y, Luo R, et al. Injectable GelMA cryogel microspheres for modularized cell delivery and potential vascularized bone regeneration. Small. 2021;17(11): e2006596. [DOI] [PubMed] [Google Scholar]
- 33.Rombouts KB, van Merrienboer TAR, Ket JCF, Bogunovic N, van der Velden J, Yeung KK. The role of vascular smooth muscle cells in the development of aortic aneurysms and dissections. Eur J Clin Invest. 2022;52(4): e13697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang K, Ren J, Li X, Wang Z, Xue L, Cui S, et al. Prevention of aortic dissection and aneurysm via an ALDH2-mediated switch in vascular smooth muscle cell phenotype. Eur Heart J. 2020;41(26):2442–53. [DOI] [PubMed] [Google Scholar]
- 35.Zhou C, Lin Z, Cao H, Chen Y, Li J, Zhuang X, et al. Anxa1 in smooth muscle cells protects against acute aortic dissection. Cardiovasc Res. 2022;118(6):1564–82. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Dong CQ, Peng GY, Huang HY, Yu YS, Ji ZC, et al. MicroRNA-134-5p regulates media degeneration through inhibiting VSMC phenotypic switch and migration in thoracic aortic dissection. Mol Ther Nucleic Acids. 2019;16:284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu T, Xu J, Guo JL, Lin CY, Luo WM, Yuan Y, et al. YAP1 up-regulation inhibits apoptosis of aortic dissection vascular smooth muscle cells. Eur Rev Med Pharmacol Sci. 2017;21(20):4632–9. [PubMed] [Google Scholar]
- 38.Song W, Chen Y, Qin L, Xu X, Sun Y, Zhong M, et al. Oxidative stress drives vascular smooth muscle cell damage in acute Stanford type A aortic dissection through HIF-1α/HO-1 mediated ferroptosis. Heliyon. 2023;9(12): e22857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li N, Yi X, He Y, Huo B, Chen Y, Zhang Z, et al. Targeting ferroptosis as a novel approach to alleviate aortic dissection. Int J Biol Sci. 2022;18(10):4118–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y, Yi X, Huo B, He Y, Guo X, Zhang Z, et al. BRD4770 functions as a novel ferroptosis inhibitor to protect against aortic dissection. Pharmacol Res. 2022;177: 106122. [DOI] [PubMed] [Google Scholar]
- 41.Yang M, Luo H, Yi X, Wei X. Jiang DS (2023) The epigenetic regulatory mechanisms of ferroptosis and its implications for biological processes and diseases. MedComm. 2020;4(3): e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.