Abstract
Metabolic dysfunction-associated steatohepatitis (MASH) and progression to hepatocellular carcinoma (HCC) exhibits distinct molecular and immune characteristics. These traits are influenced by multiple factors, including the gut microbiome, which interacts with the liver through the "gut–liver axis". This bidirectional relationship between the gut and its microbiota and the liver plays a key role in driving various liver diseases, with microbial metabolites and immune responses being central to these processes. Our review consolidates the latest research on how gut microbiota contributes to MASH development and its progression to HCC, emphasizing new diagnostic and therapeutic possibilities. We performed a comprehensive literature review across PubMed/MedLine, Scopus, and Web of Science from January 2000 to August 2024, focusing on both preclinical and clinical studies that investigate the gut microbiota’s roles in MASH and HCC. This includes research on pathogenesis, as well as diagnostic and therapeutic advancements related to the gut microbiota. This evidence emphasizes the critical role of the gut microbiome in the pathogenesis of MASH and HCC, highlighting the need for further clinical studies and trials. This is to refine diagnostic techniques and develop targeted therapies that exploit the microbiome’s capabilities, aiming to enhance patient care in liver diseases.
Keywords: Gut microbiota, Metabolic dysfunction-associated steatohepatitis/non-alcoholic steatohepatitis, Hepatocellular carcinoma, Immune modulation
Introduction
The human gut consists of an abundant and diverse microbial population and the bacterial density in the colon has been estimated to be 1011–1012 per ml [1]. The molecular techniques involving metabolomic, lipidomic, metatranscriptomic and metagenomic deciphered the impact of gut microbial populations in different organs [2]. The alterations in the microbial composition in the gut lead to the development of various diseases including inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), type-2 diabetes, atopy, autoimmune diseases (ulcerative colitis, lupus, psoriasis, multiple sclerosis, and Crohn’s disease), hepatic steatosis and several types of carcinomas (oral cancer, gastric cancer, colorectal cancer, lung cancer, cervical cancer, gall bladder cancer, hepatocellular carcinoma, etc.) [3–8]. Gut microbiota consists of several microorganisms belonging to the category of bacteria, viruses and yeast. The most prominent bacterial phyla in the composition of gut microbiota are Firmicutes and Bacteroidetes [9, 10]. Other microbes including Actinobacteria, Fusobacteria, Proteobacteria, and Verrucomicrobia are also present. The Firmicutes phylum mainly possesses 200 different genera, for example, Lactobacillus, Clostridium, Bacillus, Ruminococcus and Enterococcus. Clostridium genera are most prominent in (around 95%) the Firmicutes phyla. Bacteroidetes are composed of prime genera such as Bacteroides and Prevotella [11, 12]. The clinical study revealed that the presence of bacteria named Ruminococcus obeum and Alistipes was reduced while Dorea, Lactobacillus, and Megasphaera were enriched in NAFLD patients compared to healthy individuals [1, 13, 14]. Compared to fatty liver patients, NASH patients possess higher levels of Firmicutes but lower levels of Bacteroidetes at the phylum level [15]. Studies demonstrated that the patients with cirrhosis showed higher levels of Enterobacteriaceae and Streptococcus and low levels in Akkermansia. In HCC patients, the presence of Bacteroides and Ruminococcaceae was elevated, while Bifidobacterium was reduced. Bacteria such as Akkermansia and Bifidobacterium were inversely correlated with calprotectin concentration (cellular inflammatory markers). The fecal microbial diversity is evident in cirrhosis to early HCC, and the presence of phylum Actinobacteria was high in the early stage of HCC. Similarly, Gemmiger and Parabacteroides were higher in early HCC than in cirrhosis. On the other hand, butyrate-producing genera declined and lipopolysaccharide-producing genera were enriched in early HCC than healthy individuals [16, 17]. The abnormal increase of Bacteroidetes/Firmicutes ratio in NASH patients may increase the occurrence of HCC [18]. Evidence suggests that the changes in the composition and diversity of gut microbiome may lead to the development and progression of different liver diseases. Metabolic dysfunction-associated steatotic liver disease (MASLD), previously known as non-alcoholic fatty liver disease (NAFLD), refers to a range of liver conditions characterized by the accumulation of excess fat in the liver (≥ 5% hepatic steatosis). This spectrum of disease begins with steatotic liver disease (SLD), which represents the early stage of liver fat accumulation [19]. As the condition progresses, it may develop into metabolic MASH, formerly known as non-alcoholic steatohepatitis (NASH), which involves liver inflammation and damage, potentially with or without fibrosis. In more advanced stages, MASLD can lead to cirrhosis, liver failure, and even liver cancer [20]. Epidemiological evidence shows that NAFLD, along with its more severe form, non-alcoholic steatohepatitis (NASH), is increasingly recognized as a major contributor to hepatocellular carcinoma (HCC). Dysbiosis in the gut microbiome can disrupt homeostasis, exacerbating liver cell injury by triggering various immune-mediated responses.Several genomic factors interrupt the gut–liver axis and increase microbial exposure to the liver [21]. Evidences indicate that microbial metabolites such as secondary bile acids, trimethylamine, short-chain fatty acids, etc., are responsible for the onset and progression of liver diseases [22]. Improper microbial production and the entry of microbial products into the liver via the portal vein can cause hepatic inflammation and lead to the development of NAFLD to NASH progression [23]. The gut microbiota influences NASH to HCC progression, via modulating different factors, such as gut epithelial permeability, hepatic Toll-like receptor (TLR) endogenous alcohol production, choline metabolism, bile acid metabolism, and release of inflammatory cytokines [24–30]. The present review elaborates on the importance of gut microbiota-mediated influences in MASH and HCC. Also, it highlights the diagnostic and therapeutic importance of gut microbiota in MASH and HCC (Fig. 1) (Table 1).
Fig. 1.
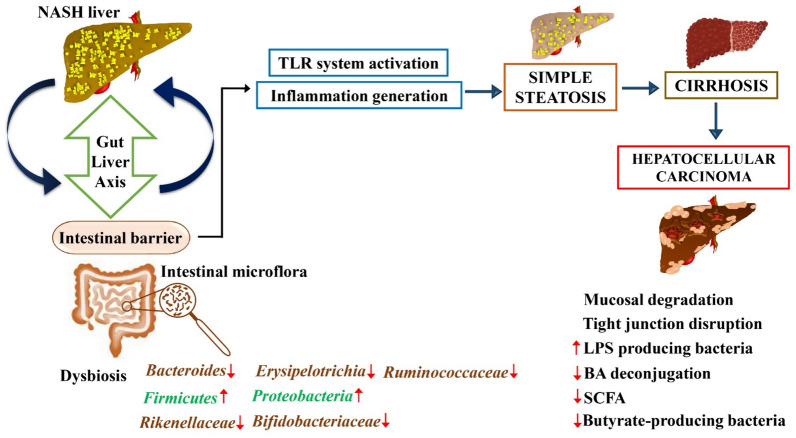
Progression of liver disease from MASH to hepatocellular carcinoma via gut–liver axis dysregulation. The figure illustrates the pathological progression from MASH to HCC through disruptions in the gut–liver axis. The MASH liver exhibits alterations in the intestinal barrier and gut microbiota, leading to dysbiosis characterized by fluctuations in key bacterial populations. The dysregulated gut microbiota affects the intestinal barrier's integrity, fostering mucosal degradation and tight junction disruption. This breakdown facilitates the systemic infiltration of lipopolysaccharides (LPS) and other bacterial metabolites into the liver through the portal circulation. Increased TLR (Toll-like receptor) activation in the liver induces inflammation, progressing from simple steatosis to cirrhosis and ultimately culminating in hepatocellular carcinoma. Key changes in microbial populations include increased Firmicutes and Proteobacteria, with a decrease in Bacteroides, Erysipelotrichia, Ruminococcaceae, Rikenellaceae, and Bifidobacteriaceae. The figure also notes a decrease in bile acid (BA) deconjugation, short-chain fatty acids (SCFA), and butyrate-producing bacteria, which are critical to maintaining hepatic and intestinal health. Symbols: ↑increase, ↓decrease
Table 1.
Expression of different microbiome in MASH-induced HCC
| Sl. No | Gut microbiome | Status in MASH | Status in HCC |
|---|---|---|---|
| 1 | Bacteroides | Bacteroides ↑ [104] | Bacteroides ↑ [105] |
| 2 | Enterococcus | Enterococcus ↑ [104] | Enterococcus ↑ [105] |
| 3 | Ruminococcaceae | Ruminococcaceae ↑ [104] | Ruminococcaceae ↑ [105] |
| 4 | Bifidobacterium | Bifidobacterium ↓ [104] | Bifidobacterium ↓ [105] |
| 5 | Oscillospira | Oscillospira ↓ [104] | Oscillospira ↑ [105] |
| 6 | Lachnospiraceae | Lachnospiraceae↑ [104] | Lachnospiraceae↑ [105] |
Methodology
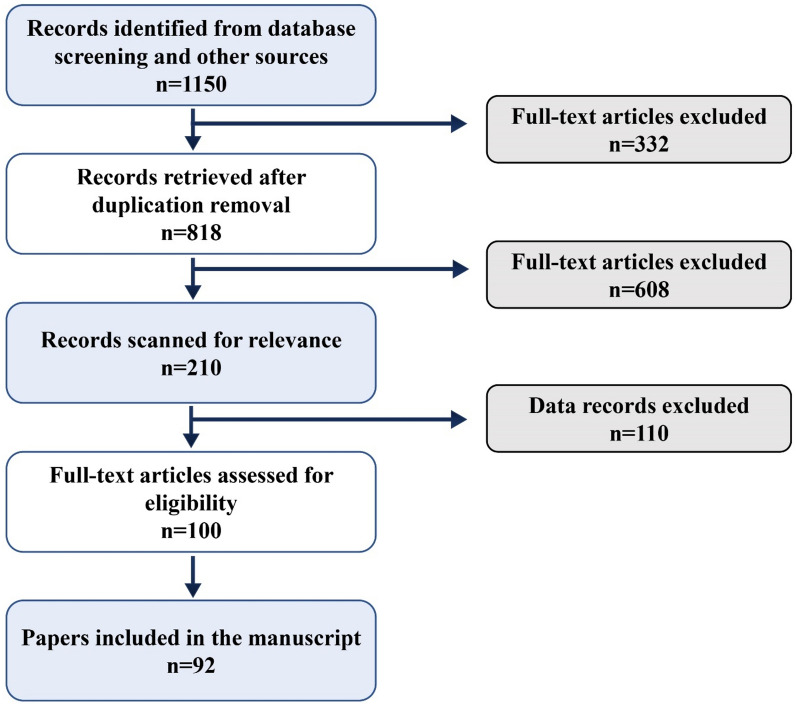
This review was conducted by searching electronic databases to identify studies that report on the role of gut microbiota in the development of metabolic dysfunction-associated steatohepatitis /non-alcoholic steatohepatitis (MASH/NASH) and its progression to hepatocellular carcinoma (HCC). The databases included PubMed/MedLine, Scopus, and Web of Science. The search was conducted from January 2000 to August 2024 to encompass recent developments in the field (Fig. 2). The search strategy employed both Medical Subject Headings (MeSH) and free-text terms to ensure comprehensive coverage of the literature. The MeSH terms used were: "Gut Microbiota" [MeSH]; "Hepatocellular Carcinoma" [MeSH]; " Metabolic dysfunction-associated fatty liver disease/Non-alcoholic Fatty Liver Disease" [MeSH]; " Metabolic dysfunction-associated steatohepatitis/Non-alcoholic Steatohepatitis" [MeSH]; "Microbiome" [MeSH]; These terms were combined with additional keywords and phrases relevant to the study topic, such as "intestinal microbiome", "liver cancer", "MASH/NASH", "immune modulation", and "therapeutic implications". Boolean operators (AND, OR) were used to combine these terms effectively.
Fig. 2.
Flow diagram of the study selection
Inclusion criteria:
Studies published in English.
Studies that directly investigated the impact of gut microbiota on the pathogenesis, progression, or treatment of MASH/NASH and HCC.
Both preclinical and clinical studies.
Reviews, meta-analyses, randomized controlled trials, cohort studies, and case–control studies.
Exclusion criteria:
Studies published before the year 2000.
Studies not in English.
Studies focusing on alcoholic liver disease or other forms of liver disease not directly related to MASH/NASH or HCC.
Commentaries, editorials, and expert opinions without original data or systematic analysis.
Studies with incomplete data or unclear methodologies.
The most representative data are summarized in tables and figures.
Immune modulatory role of gut microbiota in MAFLD/MASH to HCC progression
Numerous preclinical and clinical studies have established that abnormal expression of gut microbiota and its metabolites are closely associated with liver diseases such as MAFLD, MASH (formerly known as NAFLD and NASH), Cirrhosis and HCC [31, 32]. The alternations in the gut microflora can facilitate the synthesis of free fatty acids (FFA) in the intestine and increase the permeability of FFA across the intestinal area which may lead to the development of NAFLD [33, 34]. The studies indicated that the high-fat diet could increase the number of alcohol-producing bacteria such as Escherichia genus members of the Proteobacteria phylum in the gut, which may produce acetate and acetaldehyde via the oxidation of ethanol and facilitate the synthesis of fatty acids and contributes to the development of NAFLD [35]. Recent studies suggest that the gut microflora is altered due to genetic predisposition and improper diet which may affect the lipid and hepatic carbohydrate metabolism and also influence the activities of anti-inflammatory and pro-inflammatory agents in the liver and may lead to the development of NAFLD and its progression to NASH [32]. The consumption of obesogenic foods such as a high-fat diet may disrupt the Gram-negative bacteria that are present in the intestinal tract and increase the level of lipopolysaccharides (LPS), which can act as a key regulator for producing inflammatory responses in the liver tissue and produce liver injury via activating TLR4 signaling and cause the development of NAFLD and its progression [34]. Moreover, the high-fat diet can also modulate the enzyme produced by the gut microbiome, it may act as a catalyst for the conversion of choline into toxic metabolites, known as dimethylamine and trimethylamine. These metabolites can be converted into trimethylamine oxide (TMAO) in the liver, which produces inflammation in the hepatocytes and the progression of NAFLD into NASH [36]. The clinical studies indicated that the overgrowth of bacteria in the small intestine due to high-fat diet and genetic factors in NAFLD patients may increase the risk for the development of NASH [37–39]. Small intestinal bacterial overgrowth (SIBO) prominently affects the progression of NASH in NAFLD patients. Wigg et al. [41] carried out a comparative study to evaluate the presence of SIBO in NASH patients as well as in healthy controls [40]. The report indicated that 50% of NASH patients were observed with SIBO whereas SIBO was limited up to 22% in healthy controls. They evaluated the mean levels of tumor necrosis factor TNF-α in NASH patients and healthy people and it was found to be 14.2 and 7.5 pg/ml, respectively. The amount of intestinal bacteria was quantified via glucose hydrogen breath test and quantitative jejunal aspirate culture (the removal and culture of a sample of intestinal fluid) and the outcome indicated that low-grade SIBO around ≥ 103 CFU/ml in NASH patients compared with that of controls. These data established that patients with NASH have the highest prevalence of SIBO [41]. Shanab et al. [43] reported that an increased level of SIBO in NASH patients increases the hepatic release of interleukin-8 (IL-8) and increases the expression of Toll-like receptors-4 (TLR-4) which facilitates the development and progression of NASH [42]. The imbalance in toll-like receptor (TLR) also contributes to the progression of NAFLD to NASH. Abnormal bacterial DNA, LPS and other endogenous substances can activate the innate immune system via TLR 4 and TLR 9 which will facilitate the production of kuffer cells and interleukin-1β (IL-1β). IL-1β can uphold the accumulation of lipids and also increase the cell death of hepatocytes followed by inflammation and steatosis [43]. Moreover, microbial pathogen-associated molecular patterns can activate different inflammasome such as NLRP1 (NALP1), NLRP3 (NALP3, cryporin), NLRC4 (IPAF), AIM2 and NLRP6 can facilitate the progression of NAFLD and contribute to the initiation of steatosis [44]. The evidence suggests that TLR 4 and microbacteria-derived LPS are the key factors that lead to the progression of cirrhosis [45]. Gut dysbiosis may cause systemic inflammation and immunodeficiency by impairing the functions of immune cells such as T cells, B cells, macrophages, etc., and cause the development of cirrhosis-associated immune dysfunction (CAID). CAID can promote the translocation of bacterial products into the bloodstream and facilitate the intensity of inflammation in the body [46]. Moreover, gut dysbiosis can disrupt the bacterial flora and facilitate the LPS/TLR4-mediated signaling. It can also initiate the cirrhotic to cancer progression via increased secretion of chemokines from HSCs and chemotaxis of Kupffer cells which stimulate the profibrogenic cytokine TGF-b [47]. The cirrhosis to HCC progression is driven by different inflammatory pathways, initiated via the crosstalk between the intestinal bacteria, immune system, and liver. The inflammatory process mainly encompasses the interplay between the macrophages, Kupffer cells, and PAMPs in the liver cells. These Kupffer cells, macrophages, and PAMPs can elicit the NF-κB pathway through binding with nucleotide-binding oligomerization domain-like receptors (NOD-like receptors) and TLRs, particularly TLR-4 and TLR-9 [48]. The gut microbiota-mediated TLR-4 signaling pathway participates in the pro-inflammatory response in the liver and promotes HCC development [49]. The inflammatory chain reactions may elicit excess cytokine release and inflammation in the liver in turn causing dysbiosis of the microbiota. This increases Kupffer cells mediated secretion of inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-8 (IL-8), and IL-1β. Excessive cytokines may elicit lipid accumulation and apoptosis in hepatocytes and can cause steatosis and inflammation. Increased levels of pro-inflammatory cytokines due to the abnormal regulation of gut microbiota were observed in almost all NAFLD and NASH patients, which promote the development and progression of NASH through TLR-triggered pathways [50, 51]. Thus, gut microbiota-mediated cytokines play a key role in the initiation and progression of NAFLD to NASH to HCC [52–54]. Dysbiosis induced by cirrhosis, coupled with increased intestinal permeability, may trigger the release of pathogen-associated molecular patterns (PAMPs) and metabolites mediated by the gut microbiome, leading to enhanced inflammation, damage, and fat production in the liver [55]. Research indicates that PAMPs initiate the release of cytokines and chemokines such as IL8, IL-17, and IL1β through TLR activation, intensifying immune cell presence in the liver [56, 57]. Continuous production of these cytokines can lead to DNA damage and oxidative stress, thereby initiating and advancing hepatocellular carcinoma (HCC) [57, 58]. Furthermore, metabolites produced by the microbiota, including bile acids, short-chain fatty acids, PAMPs, lipoteichoic acid (LTA), and branched-chain amino acids, are known to activate hepatic stellate cells (HSCs) via the senescence-associated secretory phenotype (SASP), promoting hepatocyte proliferation and increasing susceptibility to HCC [59]. Clinical and preclinical studies demonstrated that bile acid (BA) metabolism also plays a significant role in NASH to HCC progression. [60, 61]. Increased levels of bile acids in the liver can prompt inflammation, hepatocyte DNA damage, and apoptosis; hence tumorigenesis will occur in the liver [62]. Furthermore, dysbiosis in NASH will increase the abundance of Gram-positive microorganisms in the microflora, thus stimulating HCC through an increase in the synthesis of secondary BA including deoxycholic acid (DCA), which restricts the activation of liver sinusoidal endothelial cell (LSEC) and facilitates the suppression of chemokine ligand 6 (CXCL6), natural killer T cell recruitment (NK), and produce tumorigenesis [63, 64]. Additionally, secondary bile acids directly initiate the development of HCC from NASH via stimulating mTOR signaling [65]. Guerra Ruiz et al. [64] demonstrated that the serum levels of lipopolysaccharides (LBP) were significantly increased in NASH patients compared with healthy patients with simple steatosis. The augmented serum LBP level was connected with abnormal expression of tumor necrosis factor (TNF-α) in the liver tissue. The increased level of TNF-α plays a key role in the development of HCC [66, 67]. Obesity is another risk factor that influences the changes in the composition of the microbiota and its metabolites such as LPS or PAMPs [68]. The injured hepatocytes can produce damage-associated molecular patterns (DAMPs) which prompt the inflammatory molecules via TLR and activation of target immune cells and arouse the transition from NAFLD–NASH–HCC [69]. Current evidence indicates that gut microbiome can also have an impact on antitumor responses, which may provide a novel perspective on refining the effectiveness of cancer immunotherapy [70]. Emerging evidence established that the abnormal characterization of gut microbiota can elicit immunosuppression by inducing M2 (pro-tumor)-like tumor-associated macrophage (TAM). Dysbacteriosis associated with IL-25-persuaded activation of M2 macrophages can augment HCC progression by secreting C-X-C motif chemokine ligand 10 (CXCL10) and augment epithelial–mesenchymal transition pathway (EMT) [71]. Studies revealed that gut microbiota can produce oncogenesis and cancer progression in myeloid-derived suppressor cells (MDSC) dependent manner [72]. Abnormal gut microbiome-mediated dysbiosis can disturb homeostasis, consequently prompting immune-mediated hepatocyte injury which further prompts the HCC progression. Metabolomics and metagenomic studies related to gut** microbiota discovered that the gut microbiota causes T cell-mediated immune suppression via increasing the level of regulatory T cells (T reg) and reducing the level of CD8 + T cells including cytotoxic T cells [73]. Moreover, Kang, Y et al., 2021 demonstrated that the gut microbiota can increase the generation of prostaglandin E2 (PGE2) and cyclooxygenase-2 (COX-2) enzymes which inhibits the antitumor immune responses through Prostaglandin E2 receptor 4 (EP4 receptor), hence facilitate HCC progression [29]. The expression of different proteins such as CD68 (Cluster of Differentiation 68) is considered a marker of macrophages and TLR (TLR-2, TLR-4, TLR-5 and TLR-9) plays a negative role in the activation of the innate immune system. Studies demonstrated that CD68 is a tumor-associated macrophage and it leads to the development of NAFLD and NASH-HCC progression [31]. It also indicates that a leaky gut can result in the overproduction of gut microbiota-derived metabolites, potentially impacting the hepatic immune system and increasing the risk of HCC [74] (Fig. 3).
Fig. 3.
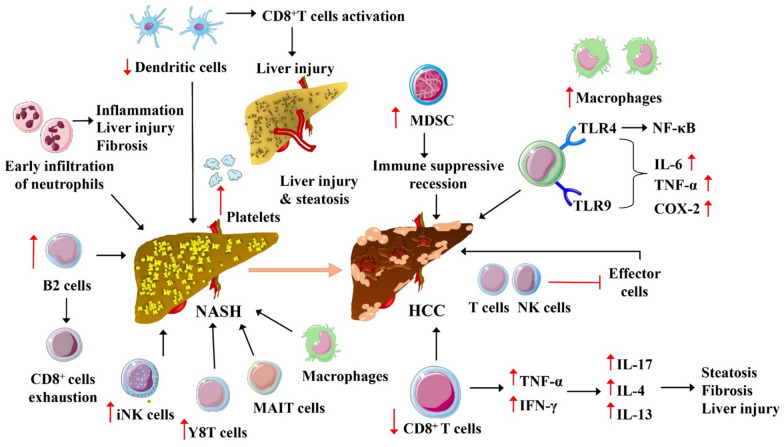
Potential mechanisms for gut microbiota-associated immune modulation in MASH–HCC progression. This figure illustrates the complex immune interactions and cellular transformations involved in the progression from MASH to HCC. Key features include the early infiltration of neutrophils leading to inflammation, liver injury, and fibrosis, and the role of B2 cells and CD8 + T cells in modulating the immune response. Activation of CD8 + T cells contributes to further liver injury and steatosis, while the presence of myeloid-derived suppressor cells (MDSC) indicates an immune suppressive state facilitating cancer progression. The diagram also highlights the activation of macrophages through toll-like receptors (TLR4 and TLR9) leading to an increase in inflammatory cytokines (IL-6, TNF-α) and COX-2, which are important in the development of HCC. Additionally, the impact of various cytokines such as TNF-α, IFN-γ, IL-17, IL-4, and IL-13 on the hepatic environment, promoting steatosis, fibrosis, and liver injury, is depicted. Abbreviations and symbols: B2 cells: a type of B cell involved in immune response; CD8 + T cells: cytotoxic T cells which are a part of the immune system that kills cancer cells, virus-infected cells, and other damaged cells; COX-2: cyclooxygenase-2, an enzyme that plays a crucial role in inflammation; HCC: hepatocellular carcinoma; IFN-γ: interferon gamma, a cytokine critical for innate and adaptive immunity; IL-4: interleukin 4, a cytokine involved in the regulation of immune responses; IL-6: interleukin 6, a cytokine involved in inflammation and maturation of B cells; IL-13: interleukin 13, involved in inflammatory responses; IL-17: interleukin 17, a pro-inflammatory cytokine; iNK cells: invariant natural killer T cells, a component of the immune system that recognizes lipid antigens; MAIT cells: mucosal-associated invariant T cells, involved in the mucosal immunity; MDSC: myeloid-derived suppressor cells, regulate immune responses in cancer; NASH: non-alcoholic steatohepatitis
Table 2 summarizes the roles of microbial and immune factors in the progression from MASH to HCC.
Table 2.
Impact of gut microbiota and immune factors on MAFLD/MASH to HCC progression
| Microbial and immune factors | Impact on MAFLD/MASH | Impact on HCC progression | Evidence | References |
|---|---|---|---|---|
| Small intestinal bacterial overgrowth (SIBO) |
Associated with 50% of NASH patients Linked to higher levels of TNF-α |
↑ Hepatic inflammation and cytokine production ↑ IL-8, ↑ TLR-4 |
Clinical study | [41, 42] [43] |
| TLR-4-mediated pathway |
Augments hepatic inflammation by enhancing secretion of pro-inflammatory cytokines (↑) ↑ level of LPS Increase the expression of CD68 (tumor-associated macrophage) |
Directly linked to the development of HCC via sustained inflammatory responses (↑) NAFLD and NASH-HCC progression |
Preclinical study Germ-free animal model |
[31, 48, 49] |
| Pathogen-associated molecular patterns (PAMPs) |
↑Iintestinal permeability allow microbial metabolites to promote liver inflammation (↑) |
↑ IL-8, ↑ IL-17, ↑ IL-1β ↑chemokines release, exacerbating liver injury and initiating HCC (↑) |
Germ-free animal model Clinical study |
[55, 56] [56] |
| Bile acid metabolism | Dysregulated metabolism contributes to liver inflammation (↑) | Altered metabolism leads to increased secondary bile acids, promoting HCC via mTOR signaling and inflammation (↑) | Clinical study | [58–60] |
| Prostaglandin E2 (PGE2) and COX-2 enzymes | Not directly mentioned in NAFLD/NASH context | Suppress antitumor immune responses, facilitating HCC progression (↑) | Preclinical study | [30] |
| DAMPs and cytokine production | Activates inflammatory pathways through TLR stimulation (↑) |
↑ Chronic cytokine release ↑DNA damage ↑Oxidative stress ↑HCC progression |
Clinical study | [67] |
| Microbial dysbiosis |
This leads to SIBO and imbalances in key bacterial populations, aggravating liver conditions (↑) Promote steatohepatitis via modulating Toll-like receptor 4 (TLR4) and TLR9 |
Increases the risk of HCC by altering liver immune responses and metabolic functions (↑) HCC was developed due to hepatic inflammation |
Clinical study germ-free animal model | [75] |
↑: indicates an increase in activity; ↓: indicates a decrease in activity. COX-2: cyclooxygenase-2; DAMPs: damage-associated molecular patterns; HCC: hepatocellular carcinoma; IL-1β: interleukin-1 beta; IL-8: interleukin-8; IL-17: interleukin-17; mTOR: mechanistic target of rapamycin; NAFLD: non-alcoholic fatty liver disease; NASH: non-alcoholic steatohepatitis; PAMPs: pathogen-associated molecular patterns; PGE2: prostaglandin E2; SIBO: small intestinal bacterial overgrowth; TLR-4: toll-like receptor 4; TNF-α: tumor necrosis factor-alpha
Immunotherapeutic significance of modulating gut microbiota in MASH and HCC
NASH-induced dysbiosis in the gut microbiota leads to an increase in intestinal permeability, thereby increasing exposure to bacterial metabolites in the liver and causing severe inflammation, contributing to HCC [76]. Modulating gut microbiota-mediated bile acids (BAs) metabolism, toll-like receptor (TLR) activity, regulating farnesoid X receptor (FXR)/Takeda G protein-coupled receptor 5 (TGR5) activation, choline metabolism, and targeting inflammatory cytokines are considered novel therapeutic options against NASH and NASH-associated HCC [18]. Therapeutic targeting of the gut microbiota against NASH and HCC is highly attractive; meanwhile, these treatment modalities show a low risk of adverse effects and a high safety profile, including fecal microbiota transplantation (FMT), probiotics, prebiotics, synbiotics (combination of prebiotics and probiotics), antibiotics, and immunotherapies [29]. The predominant mechanisms behind the gut microbiota-targeted therapies are as follows: controlling the T helper 17 (Th17) cell proliferation which may raise the secretion of interleukin-17 (IL-17); decreasing the level of metastasis through reduction of the overexpression of vascular endothelial growth factor (VEGF) limiting the angiogenesis lymphangiogenesis, and inflammations [77]. Additionally, alterations in regulating gut microbiota may stimulate the synthesis of short-chain fatty acids (SCFAs) and eventually restrict the progression of NASH to HCC. Modulations in the composition of gut microbiota may upsurge the production of propionate that may help the patients to recover from HCC through cyclic adenosine 3′,5′-monophosphate (cAMP) level-dependent pathway and the stimulation of G protein-coupled receptors 43 (GPR43) [78]. Moreover, regulation of gut microbiota may accomplish an anti-HCC effect by increasing the level of hepatic CXCR6 + NKT cells and elevating the level of interferon-gamma (IFN-γ). Simultaneously, CXCR6 + NKT cell accumulation was controlled through the expression of CXCL16 in the liver sinusoidal endothelial cells, which was connected with microbiome-triggered primary-to-secondary bile acid conversion [79].
Probiotics are primarily utilized to correct microbial imbalances [65]. Clinical applications of probiotic bacteria have demonstrated effectiveness in decelerating the progression of NASH and reducing the spread of HCC cells by diminishing the activation of inflammation mediated by toll-like receptors (TLRs). Pathogen-associated molecular patterns (PAMPs) contribute to the development of NASH and HCC by activating inflammatory responses through TLRs. Using microbial agents, particularly probiotic bacteria, has proven to mitigate liver metastasis by curtailing the excessive inflammatory responses triggered by TLRs [80]. In experiments, rats with liver cirrhosis treated with Lactiplantibacillus plantarum exhibited reduced TLR4 expression and minimal liver damage. Additionally, sterilizing the gut and deactivating the TLR4 receptor significantly slowed HCC progression by 80–90%, suggesting their potential as a preventative measure against HCC [81]. Research by Li et al. in 2016, confirmed that a combination of probiotics could limit NASH and its progression to HCC by lowering the levels of pro-inflammatory cytokines like IL-17 in mouse models. It also indicated that probiotics could reduce liver fat and aspartate aminotransferase (AST) levels in NASH patients [82], [NCT00870012, NCT01791959]. Probiotics triggered the growth of gut microbiota composition towards specific beneficial bacteria including Prevotella and Oscillibacter. Prevotella and Oscillibacter elicit anti-inflammatory metabolites, which afterward diminished the T helper 17 cells (Th17) polarization and increase the differentiation of regulatory T cells ((Treg)/Type 1 regulatory T (Tr1)) cells in the gut and produce anti-inflammatory responses in the cancer cells. Besides, probiotics can control the abnormal growth of segmented filamentous bacteria (SFB), which are the foremost bacteria to increase the level of Th17 in the body. Thus, the administration of probiotics intensely decreases the level of SFB which leads to reduce the production of pro-inflammatory cytokines such as IL-17. The IL-17A formed from Th17 could favor angiogenesis, thus reduction of Th17 and IL-17 level may reduce HCC progression. Different clinical and preclinical studies have shown that probiotics are effective against NASH and HCC [83]. Furthermore, Helicobacter sp. was found in the surroundings of NASH cells and its translocation might be possible to elicit HCC. To this end, the intestinal microbial profile might be prominently exhibit the beneficial rates in HCC patients experiencing treatment with immunotherapy such as immune checkpoint inhibitors (ICIs), indicating that the gut micro flora targeted immunotherapy could be beneficial for liver cancer [84]. A double-blind, randomized, placebo-controlled trial of probiotics in patients with Child–Pugh A-B cirrhosis was conducted to evaluate the predictive role and risk of the microbiome in HCC development. In this particular study, they evaluate the role of probiotics towards the presence of endotoxins (LPS) and different cytokines (IL-6 and TNF-α) in the tumor microenvironment (TME) and also evaluate the expression of TLR4 in mononuclear cells (NCT03853928) [85].
Prebiotics are non-absorbent oligosaccharide substances that accelerate the growth of bacteria. It can also reduce the growth of harmful bacteria and maintain the balance of gut microbiota. They mainly initiate the production of SCFAs and regulate the immune responses in the liver cells. Hence, prebiotics alter the gut microbiota to reduce the progression of NASH as well as NASH-associated HCC [86]. Dietary polyphenols are significant prebiotics used in this modern era due to their great therapeutic value. They mainly include flavonoids including lignins, and phenolic acids found in tea, vegetables, nuts, fruits and wine. One of the significant prebiotic polyphenols is ellagic acid which is an antioxidant having anti-cancer properties. The ellagic acid is metabolized by micro-flora present in the colon producing urolithins that are abundantly present in certain nuts and berries [87]. Urolithins can suppress the COX-2-associated inflammation in liver cells [88]. Another polyphenol such as resveratrol which is naturally found in grapes can also reduce or prevent NASH [89, 90] and HCC progression by destroying the metastatic invasion and tumor cell migration in liver cancer [91, 92]. Resveratrol acts as an immunomodulatory agent by either stimulating the immune cells situated in the tumor microenvironment (TME) or by sensitizing tumor cells toward the cytotoxic signaling of immune cells [93]. Quercetin is another dietary flavonoid that works as a prebiotic through the suppression of activated nuclear factor kappa B (NF-kB) in hepatocytes [94, 95]. A prospective cohort study demonstrated that increasing the consumption of tree nuts such as almonds, hazelnuts, pistachios, macadamias, cashews and pecans was related to a reduced risk of NASH and HCC [96–98]. The combinatorial effects of pectins and fructo-oligosaccharide (FOS) with raspberry polyphenols on microbial fermentation and modulation of inflammation and lipid metabolism in the liver was evaluated and thus suggests, FOS and pectins improved the action of the raspberry polyphenolic extract against NASH and HCC [99, 100]. Moreover, a study on hepatocytes demonstrated that polyphenols extracted from raspberries also control immunometabolic signals connected with the development of obesity [90, 101]. Supplementation with prebiotics will also help the activation of AMPK [102, 103]. Astragalus polysaccharides, grifolan, lentinan, and krestin (PSK) display anti-cancer properties by regulating the activity of the immune system and eliciting direct actions against cancer cells [104]. Clinical studies revealed that Omega 3 fatty acid and EPA (eicosapentaenoic acid) are active against HCC [NCT04682665]. Some of the clinical trials also established the effectiveness of synbiotic and prebiotics against NASH [NCT02530138, NCT01791959, NCT03184376 and NCT03897218].
Antibiotics can be also used to reduce or remove the altered gut microbial content; this can help restrict the inflammatory signals from leaky guts. Different preclinical evidences suggest that different antibiotics such as vancomycin, metronidazole, ampicillin, and neomycin significantly decrease HCC proliferation [105]. Antibiotic cocktails (ABX, including vancomycin, primaxin, and neomycin) produce anti-HCC effect. These antibiotics can increase the hepatic CXCR6 + NKT cells and also enhance the level of INF-γ and inhibit cancer cell growth [105]. A phase 2, interventional study was also evaluate the safety and efficacy of solithromycin against NASH without cirrhosis [NCT02510599]. A randomized interventional clinical trial established the effect of rifaximin on the lipopolysaccharides (LPS) and related cytokine levels in NAFLD and NASH [NCT02009592]. The prolonged antibiotics belonging to β-lactams, tetracyclines, fluoroquinolones, sulfonamides, and aminoglycosides impacts human gut flora. They can modify the diversity of bacterial flora and composition leading to the occurrence of various metabolic alterations in the body that contribute to the onset and progression of NAFLD [106]. Deregulated metabolism in the body especially in the metabolism of SCFA may lead to obesity, metabolic syndrome, and diabetes. Moreover, studies indicated that the continuous use of antibiotics can cause the depletion in gut bacterial diversity and may increase the susceptibility to infections [107]. The continuous use of antibiotics may increase the level of the antibiotic-resistant gene in the microbiome. These pools of resistant genes can initiate antibiotic resistance [108]. In this scenario, the major challenge is to facilitate the growth of beneficial microorganisms, meanwhile reducing the proportion of microorganisms that are responsible for dysbiosis to promote the patient’s health. Thus, the development of novel antibiotics can be personalized for a patient based on intestinal and biochemical individuality. The use of selective antibiotics will minimize the negative impact of antibiotics on human health due to changes in the gut microbiome [109]. Fecal microbiota transplantation (FMT) is a medical procedure for the transfer of a small sample of stool (faces) from a healthy person to a diseased person [110]. The healthy stool sample comprises trillions of beneficial microbiomes that can ameliorate the health of the diseased person. Studies suggest that FMT can restore the healthy bacteria in the lower intestine, which will also help to terminate the growth of Clostridium difficile from the intestinal area [111, 112]. As mentioned earlier a healthy intestinal tract possesses a large number of healthy bacteria, but in certain conditions, the use of antibiotics may restrict the growth of good bacteria, and it may promote the development of unhealthy bacteria in the colon. FMT is usually preferred to treat Clostridioides difficile infection (CDI) and also in patients who suffer from IBD [114, 115]. Based on clinical trial of FMT is extended to irritable bowel syndrome, hepatic encephalopathy, diabetes mellitus, refractory diarrhea, fatty liver disease, metabolic syndrome, neurological disease (parkinsonism), and neuropsychiatric disease (autism spectrum disorder) [115, 116]. FMT procedure can be achieved via using different techniques such as colonoscopy, enema, nasogastric (NG) tube and oral capsules (VOWST, SER-109) [117, 118]. Currently, the U.S. Food and Drug Administration (FDA) approves FMT only for the treatment of recurrent CDI that is not responsive to standard antibiotic therapy. Two different FMT therapies have been approved by the FDA REBYOTA (fecal microbiota, live—JSLM) and VOWST [119]. Studies revealed that FMT elicits around 80–90% in preventing CDI from recurring after antibiotics. Nevertheless, there are several short-term and long-term adverse effects are also associated with FMT [120]. Thus, rigorous donor screening and testing should be mandated to minimize the risk of FMT.
Table 3 outlines the impact of gut microbiota modulation on immune responses in MASH and HCC, detailing therapeutic strategies and their outcomes.
Table 3.
Immunotherapeutic effect of gut microbiota modulation in MASH and HCC
| Category | Agent | Mechanism of Action | Outcome and Benefits | References |
|---|---|---|---|---|
| Prebiotics | Kappaphycus striatum | Carrageenan polysaccharide with different molecular weights | Immunostimulating activities and antitumor effect via increasing NK cell activity | [72] |
| Ganoderma lucidum | Elicits immune regulation, decreases blood sugar and lipid levels | Upsurge anti-inflammatory and anti-hypoxia effects; scavenges free radicals | [121] | |
| Antrodia cinnamomea | Stimulates immune modulatory action via TLR5 and NLRP3 | ↑Immune response, potentially beneficial against cancer | [122] | |
| Hirsutella sinensis |
↑Cytotoxic T cells ↓Regulatory T cell production in the TME |
↑Anti-cancer effects by stimulating T cell activity and inhibiting immune inhibitors | [123] | |
| Polyphenols | ↑immune cells in the TME | ↑ immune cells to potentially counteract cancerous growth | [124] | |
| Lactobacillus acidophilus, Lactobacillus reuteri + Inulin | ↑Secretion of Th1 mediated T cells | Augments cytokine secretion (e.g., IFN-γ), ↓angiogenesis, ↑cytotoxicity and antigen presentation | [125] | |
| Probiotics | Lactobacillus rhamnosus | Modulates gut microbiota; ↓endotoxemia | ↓TNF-α expression, ↓inflammation and liver damage | [126] |
| Streptococcus thermophilus |
↑Gut homeostasis ↓Intestinal and hepatic inflammation |
Helps restrict progression of cirrhosis to HCC | [127] | |
| Lactiplantibacillus plantarum | ↓TLR4 expression, gut sterilization and TLR4 receptor inactivation | Abridges the progression of HCC by 80% to 90% | [56] | |
| Antibiotics | Vancomycin | Inhibits fermentable fiber-induced liver cancer by downregulating TLR | Potentially reduces liver cancer progression | [29, 128] |
| Metronidazole | ↓Butyrate-producing bacteria | ↓Occurrence of HCC by impacting bacterial profiles linked to cancer progression | [129] | |
| Ampicillin | Interferes with TLR protein | ↓HCC progression | [130] | |
| Neomycin | ↓IL-6, ↓TNF-α,↓Ki67 | ↓Inflammatory and proliferative markers associated with HCC | [130] | |
| Fecal microbiota transplantation | REBYOTA and VOWST | The spore suspension is produced by treating fecal matter with ethanol to kill live organisms that are not spores | Helps to destroy Clostridioides difficile from the digestive tract | [113] |
HCC: hepatocellular carcinoma; IL-6: interleukin 6; Ki67: a proliferation marker; NASH: non-alcoholic steatohepatitis; NK cells: natural killer cells; NLRP3: NLR family pyrin domain containing 3; TME: tumor microenvironment; TLR: toll-like receptor; TNF-α: tumor necrosis factor alpha; VEGF: vascular endothelial growth factor. Symbols: ↓decrease; ↑increase
Limitations and challenges
Despite the comprehensive analysis of the role of gut microbiota in the development of non-alcoholic steatohepatitis (NASH) and its progression to hepatocellular carcinoma (HCC) presented in this manuscript, several limitations and challenges remain that need to be addressed:
-
i.
The majority of studies discussed are preclinical, involving animal models or in vitro systems. These studies provide valuable insights, but may not fully replicate the complex interactions and environmental factors influencing human gut microbiota and liver disease progression. Thus, translating these findings into clinical practice requires much attention, as human studies are more variable and complex.
-
ii.
The gut microbiome is extraordinarily complex, with a vast number of microbial species that have not been fully characterized. This complexity makes it challenging to determine causal relationships between specific microbial changes and disease states. The functional roles of many species within the microbiome and their interactions with host metabolism and immunity are still poorly understood.
-
iii.
There is significant variability in microbiota composition among different populations due to factors such as diet, genetics, lifestyle, and antibiotic use. This variability can affect the reproducibility and applicability of findings across different demographic and geographic groups.
-
iv.
Current methodologies for analyzing the gut microbiome, such as 16S rRNA sequencing and metagenomic sequencing, have limitations in resolution, and accuracy and may not capture the full spectrum of microbial diversity or the functional potential of the microbiome. Additionally, these methods are susceptible to contamination and other technical issues that can affect data quality and interpretation.
-
v.
Modulating the gut microbiota presents a promising therapeutic avenue, but developing effective microbiota-based therapies is challenging. Issues include ensuring the stability and survival of probiotic strains, the unpredictability of prebiotic effects on the existing gut flora, and the potential for adverse effects from broad-spectrum antibiotics.
-
vi.
The regulatory pathway for microbiota-targeted therapies is not fully established, which may pose challenges in clinical trial design, approval, and market access. Safety concerns also remain, particularly regarding the long-term impacts of altering the gut microbiome on immune function and susceptibility to other diseases.
-
vii.
The interactions between gut microbiota-modulating therapies and existing treatments for NASH and HCC are not well understood. These interactions could affect the efficacy and safety profiles of treatments.
-
viii.
The cost of developing microbiota-targeted therapies and the technological demands of such treatments may limit their accessibility, especially in low-resource settings where NASH and HCC are increasingly prevalent.
These limitations underscore the need for further research to better understand the gut microbiome's role in liver diseases and to develop safe, effective, and accessible therapies. Future studies should aim to incorporate larger, more diverse human cohorts, utilize advanced technologies for microbiome analysis, and explore the mechanistic pathways connecting the gut microbiome to liver disease outcomes.
Conclusions
Unhealthy gut microbiota and its metabolites lead to the generation of improper immune signaling in the liver leading to the initiation and progression of different kinds of liver diseases such as MAFLD, MASH, and especially HCC progression. Probiotics, prebiotics, and synbiotics may exemplify advanced, safe, and affordable treatment strategies against these diseases. However, preclinical and well-designed human trials prove that the modifications in the gut microbiota elicit immune modulations in the TME along with anti-tumor response. Understanding the pivotal role of the gut microbiota in the cancer progression may empower the discovery of more effective diagnostic and prevention modalities against HCC. Thus, the treatment of MASH and HCC via targeting the gut microbiota will be an effective research direction in the future for treating MASH-induced HCC. Gene sequencing and machine learning-based data analysis help to identify a key biomarker for the detection of liver illnesses, particularly in MASH-associated HCC. In these circumstances, more numbers of laboratory-based mechanistic evaluations and detailed clinical trials are needed to estimate the composition of gut microflora and this will help to select appropriate useful bacterial strains for the treatment of cancer. Hence, more evidence is needed to translate the existing knowledge relating to the functional aspects of the gut microbiome into diagnostic, prognostic, and therapeutic strategies in patients suffering with HCC. However, evidence suggests that modulation of gut microbiota paves way to a promising therapeutic strategy for the treatment and prevention of MASH and MASH-associated HCC.
Acknowledgements
LRN acknowledge the support of the Amrita Vishwa Vidyapeetham SEED grant. The authors would like to express their gratitude to Dr. Irina Zamfir, MD, RCP London, Basildon University Hospital UK for providing professional English editing of this manuscript and for editorial support.
Abbreviation lists
- ABX
Antibiotic cocktails
- AMPK
AMP-activated protein kinase
- AST
Aspartate aminotransferase
- BA
Bile acid
- BAs
Bile acids
- COX-2
Cyclooxygenase-2
- cAMP
Cyclic adenosine monophosphate
- CXCL16
Chemokine (C-X-C motif) ligand 16
- CXCR6
C-X-C chemokine receptor type 6
- DCA
Deoxycholic acid
- DAMPs
Damage-associated molecular patterns
- EPA
Eicosapentaenoic acid
- FMT
Fecal microbiota transplantation
- FXR
Farnesoid X receptor
- GPR43
G protein-coupled receptor 43
- HCC
Hepatocellular carcinoma
- HSCs
Hepatic stellate cells
- ICIs
Immune checkpoint inhibitors
- IFN-γ
Interferon gamma
- IL-1β
Interleukin-1 beta
- IL-6
Interleukin 6
- IL-8
Interleukin 8
- IL-17
Interleukin 17
- LPS
Lipopolysaccharides
- LSEC
Liver sinusoidal endothelial cell
- MAFLD
Metabolic dysfunction-associated fatty liver disease
- MASLD
Metabolic dysfunction-associated steatotic liver disease
- MASH
Metabolic dysfunction-associated steatohepatitis
- MDSC
Myeloid-derived suppressor cells
- NAFLD
Non-alcoholic fatty liver disease
- NASH
Non-alcoholic steatohepatitis
- NF-κB
Nuclear factor kappa B
- NK
Natural killer
- NKT
Natural killer T
- NLRP3
NLR family pyrin domain containing 3
- PAMPs
Pathogen-associated molecular patterns
- PGE2
Prostaglandin E2
- PSK
Polysaccharide krestin
- RCTs
Randomized controlled trials
- SASP
Senescence-associated secretory phenotype
- SCFAs
Short-chain fatty acids
- SFB
Segmented filamentous bacteria
- SIBO
Small intestinal bacterial overgrowth
- TGR5
Takeda G protein-coupled receptor 5
- Th17
T helper 17
- TLR
Toll-like receptor
- TLR4
Toll-like receptor 4
- TME
Tumor microenvironment
- TNF-α
Tumor necrosis factor-alpha
- Treg
Regulatory T cells
- Tr1
Type 1 regulatory T
- VEGF
Vascular endothelial growth factor
Author contributions
A.R.K., B.N., A.J.K., L.R.N., D.C., J.S.-R. made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas. That is revising or critically reviewing the article; giving final approval of the version to be published; agreeing on the journal to which the article has been submitted; and, confirming to be accountable for all aspects of the work.
Funding
Not applicable.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lekshmi R. Nath, Email: lekshmirnath@aims.amrita.edu
Daniela Calina, Email: calinadaniela@gmail.com.
Javad Sharifi-Rad, Email: javad.sharifirad@gmail.com.
References
- 1.Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GA, Gasbarrini A, Mele MC. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Vos WM, Tilg H, Van Hul M, Cani PD. Gut microbiome and health: mechanistic insights. Gut. 2022;71(5):1020–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan AA, Sirsat AT, Singh H, Cash P. Microbiota and cancer: current understanding and mechanistic implications. Clin Transl Oncol. 2022;24(2):193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Shen J, Shi X, Du Y, Niu Y, Jin G, Wang Z, Lyu J. Gut microbiome analysis as a predictive marker for the gastric cancer patients. Appl Microbiol Biotechnol. 2021;105:803–14. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Y, Liu Y, Li S, Peng Z, Liu X, Chen J, Zheng X. Role of lung and gut microbiota on lung cancer pathogenesis. J Cancer Res Clin Oncol. 2021;147(8):2177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sánchez-Alcoholado L, Ramos-Molina B, Otero A, Laborda-Illanes A, Ordóñez R, Medina JA, Gómez-Millán J, Queipo-Ortuño MI. The role of the gut microbiome in colorectal cancer development and therapy response. Cancers. 2020;12(6):1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma J, Huda F, Naithani M, Singh SK, Kumar N, Basu S. Role of gut microbiome and enteric bacteria in gallbladder cancer. In immunology of the GI tract-recent advances 2022. London: IntechOpen; 2022. [Google Scholar]
- 8.Park SY, Hwang BO, Lim M, Ok SH, Lee SK, Chun KS, Park KK, Hu Y, Chung WY, Song NY. Oral–gut microbiome axis in gastrointestinal disease and cancer. Cancers. 2021;13(9):2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen YH, Wu WK, Wu MS. Microbiota-associated therapy for non-alcoholic steatohepatitis-induced liver cancer: a review. Int J Mol Sci. 2020;21(17):5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang C, Xu J, Xu X, Xu W, Tong B, Wang S, Ji R, Tan Y, Zhu Y. Characteristics of gut microbiota in patients with metabolic associated fatty liver disease. Sci Rep. 2023;13(1):9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang F, Lyu B, Xie F, Li F, Xing Y, Han Z, Lai J, Ma J, Zou Y, Zeng H, Xu Z. From gut to liver: unveiling the differences of intestinal microbiota in NAFL and NASH patients. Front Microbiol. 2024;4(15):1366744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren Z, Li A, Jiang J, Zhou L, Yu Z, Lu H, Xie H, Chen X, Shao L, Zhang R, Xu S. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut. 2019;68(6):1014–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caligiuri A, Gentilini A, Marra F. Molecular pathogenesis of NASH. Int J Mol Sci. 2016;17(9):1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474(11):1823–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ponziani FR, Nicoletti A, Gasbarrini A, Pompili M. Diagnostic and therapeutic potential of the gut microbiota in patients with early hepatocellular carcinoma. Therapeut Adv Med Oncol. 2019;11:1758835919848184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cholankeril G, Patel R, Khurana S, Satapathy SK. Hepatocellular carcinoma in non-alcoholic steatohepatitis: Current knowledge and implications for management. World J Hepatol. 2017;9(11):533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobhonslidsuk A, Chanprasertyothin S, Pongrujikorn T, Kaewduang P, Promson K, Petraksa S, Ongphiphadhanakul B. The association of gut microbiota with nonalcoholic steatohepatitis in Thais. Biomed Res Int. 2018;16:2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–57. [DOI] [PubMed] [Google Scholar]
- 20.Nair B, Kamath AJ, Tergaonkar V, Sethi G, Nath LR. Mast cells and the gut-liver Axis: implications for liver disease progression and therapy. Life Sci. 2024;10: 122818. [DOI] [PubMed] [Google Scholar]
- 21.Yu J, Marsh S, Hu J, Feng W, Wu C. The pathogenesis of nonalcoholic fatty liver disease: interplay between diet, gut microbiota, and genetic background. Gastroenterol Res Pract. 2016;2016(1):2862173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albillos A, De Gottardi A, Rescigno M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol. 2020;72(3):558–77. [DOI] [PubMed] [Google Scholar]
- 23.Gupta H, Youn GS, Shin MJ, Suk KT. Role of gut microbiota in hepatocarcinogenesis. Microorganisms. 2019;7(5):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang C, Yang M, Ericsson AC. The potential gut microbiota-mediated treatment options for liver cancer. Front Oncol. 2020;14(10): 524205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohtani N, Hara E. Gut-liver axis-mediated mechanism of liver cancer: a special focus on the role of gut microbiota. Cancer Sci. 2021;112(11):4433–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu H, Williams B, Schnabl B. Gut microbiota, fatty liver disease, and hepatocellular carcinoma. Liver Res. 2018;2(1):43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young VB. The role of the microbiome in human health and disease: an introduction for clinicians. BMJ. 2017;15:356. [DOI] [PubMed] [Google Scholar]
- 28.Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, Glickman JN, Siebert R, Baron RM, Kasper DL, Blumberg RS. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336(6080):489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loo TM, Kamachi F, Watanabe Y, Yoshimoto S, Kanda H, Arai Y, Nakajima-Takagi Y, Iwama A, Koga T, Sugimoto Y, Ozawa T. Gut microbiota promotes obesity-associated liver cancer through PGE2-mediated suppression of antitumor immunitygut microbiota promotes obesity-linked HCC via immune escape. Cancer Discov. 2017;7(5):522–38. [DOI] [PubMed] [Google Scholar]
- 30.Kang Y, Cai Y, Yang Y. The gut microbiome and hepatocellular carcinoma: Implications for early diagnostic biomarkers and novel therapies. Liver Cancer. 2021;11:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Roy T, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, Martin P, Philippe C, Walker F, Bado A, Perlemuter G. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62(12):1787–94. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Zhang L, Dong B. Molecular mechanisms in MASLD/MASH related HCC. Hepatology. 2024;13:10–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolodziejczyk AA, Zheng D, Shibolet O, Elinav E. The role of the microbiome in NAFLD and NASH. EMBO Mol Med. 2019;11(2): e9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdul-Hai A, Abdallah A, Malnick SD. Influence of gut bacteria on development and progression of non-alcoholic fatty liver disease. World J Hepatol. 2015;7(12):1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chakraborti CK. New-found link between microbiota and obesity. World J Gastrointest Pathophysiol. 2015;6(4):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arslan N. Obesity, fatty liver disease and intestinal microbiota. World J Gastroenterol WJG. 2014;20(44):16452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazzotti A, Caletti MT, Sasdelli AS, Brodosi L, Marchesini G. Pathophysiology of nonalcoholic fatty liver disease: lifestyle-gut-gene interaction. Dig Dis. 2016;34(Suppl. 1):3–10. [DOI] [PubMed] [Google Scholar]
- 38.Augustyn M, Grys I, Kukla M. Small intestinal bacterial overgrowth and nonalcoholic fatty liver disease. Clin Exp Hepatol. 2019;5(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wijarnpreecha K, Lou S, Watthanasuntorn K, Kroner PT, Cheungpasitporn W, Lukens FJ, Pungpapong S, Keaveny AP, Ungprasert P. Small intestinal bacterial overgrowth and nonalcoholic fatty liver disease: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2020;32(5):601–8. [DOI] [PubMed] [Google Scholar]
- 40.Gudan A, Jamioł-Milc D, Hawryłkowicz V, Skonieczna-Żydecka K, Stachowska E. The prevalence of small intestinal bacterial overgrowth in patients with non-alcoholic liver diseases: NAFLD, NASH, fibrosis, cirrhosis—a systematic review, meta-analysis and meta-regression. Nutrients. 2022;14(24):5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor α in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48(2):206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghoshal UC, Baba CS, Ghoshal U, Alexander G, Misra A, Saraswat VA, Choudhuri G. Low-grade small intestinal bacterial overgrowth is common in patients with non-alcoholic steatohepatitis on quantitative jejunal aspirate culture. Indian J Gastroenterol. 2017;36:390–9. [DOI] [PubMed] [Google Scholar]
- 43.Shanab AA, Scully P, Crosbie O, Buckley M, O’Mahony L, Shanahan F, Gazareen S, Murphy E, Quigley EM. Small intestinal bacterial overgrowth in nonalcoholic steatohepatitis: association with toll-like receptor 4 expression and plasma levels of interleukin 8. Dig Dis Sci. 2011;56:1524–34. [DOI] [PubMed] [Google Scholar]
- 44.Thuy S, Ladurner R, Volynets V, Wagner S, Strahl S, Königsrainer A, Maier KP, Bischoff SC, Bergheim I. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J Nutr. 2008;138(8):1452–5. [DOI] [PubMed] [Google Scholar]
- 45.Tranah TH, Edwards LA, Schnabl B, Shawcross DL. Targeting the gut–liver–immune axis to treat cirrhosis. Gut. 2021;70(5):982–94. [DOI] [PubMed] [Google Scholar]
- 46.Odena G, Chen J, Lozano JJ, Altamirano J, Rodrigo-Torres D, Affo S, Morales-Ibanez O, Matsushita H, Zou J, Dumitru R, Caballeria J. LPS-TLR4 pathway mediates ductular cell expansion in alcoholic hepatitis. Sci Rep. 2016;6(1):35610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soares JB, Pimentel-Nunes P, Roncon-Albuquerque R, Leite-Moreira A. The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases. Hep Intl. 2010;4:659–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chassaing B, Gewirtz AT. Gut microbiota, low-grade inflammation, and metabolic syndrome. Toxicol Pathol. 2014;42(1):49–53. [DOI] [PubMed] [Google Scholar]
- 49.Liu J, Zhuang ZJ, Bian DX, Ma XJ, Xun YH, Yang WJ, Luo Y, Liu YL, Jia L, Wang Y, Zhu ML. Toll-like receptor-4 signalling in the progression of non-alcoholic fatty liver disease induced by high-fat and high-fructose diet in mice. Clin Exp Pharmacol Physiol. 2014;41(7):482–8. [DOI] [PubMed] [Google Scholar]
- 50.Nobili V, Carpino G, Alisi A, Franchitto A, Alpini G, De Vito R, Onori P, Alvaro D, Gaudio E. Hepatic progenitor cells activation, fibrosis, and adipokines production in pediatric nonalcoholic fatty liver disease. Hepatology. 2012;56(6):2142–53. [DOI] [PubMed] [Google Scholar]
- 51.Yoon HJ, Cha BS. Pathogenesis and therapeutic approaches for non-alcoholic fatty liver disease. World J Hepatol. 2014;6(11):800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brandi G, De Lorenzo S, Candela M, Pantaleo MA, Bellentani S, Tovoli F, Saccoccio G, Biasco G. Microbiota, NASH, HCC and the potential role of probiotics. Carcinogenesis. 2017;38(3):231–40. [DOI] [PubMed] [Google Scholar]
- 53.Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, Olefsky JM, Brenner DA, Seki E. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1β in mice. Gastroenterology. 2010;139(1):323–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47(4):571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Del Chierico F, Nobili V, Vernocchi P, Russo A, De Stefanis C, Gnani D, Furlanello C, Zandonà A, Paci P, Capuani G, Dallapiccola B. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology. 2017;65(2):451–64. [DOI] [PubMed] [Google Scholar]
- 56.Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A, Bataller R, Lefkowitch JH. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21(4):504–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan D, Huang S, Berger E, Liu L, Gross N, Heinzmann F, Ringelhan M, Connor TO, Stadler M, Meister M, Weber J. Kupffer cell-derived Tnf triggers cholangiocellular tumorigenesis through JNK due to chronic mitochondrial dysfunction and ROS. Cancer Cell. 2017;31(6):771–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang X, Fu X, Van Ness C, Meng Z, Ma X, Huang W. Bile acid receptors and liver cancer. Curr Pathobiol Reports. 2013;1:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen T, Xie G, Wang X, Fan J, Qiu Y, Zheng X, Qi X, Cao Y, Su M, Wang X, Xu LX. Serum and urine metabolite profiling reveals potential biomarkers of human hepatocellular carcinoma. Mol Cell Proteom. 2011. 10.1074/mcp.M110.004945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jansen PL. Endogenous bile acids as carcinogens. J Hepatol. 2007;47(3):434–5. [DOI] [PubMed] [Google Scholar]
- 61.Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako V, Ritz T. Gut microbiome–mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360(6391):eaan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jia B. Commentary: gut microbiome–mediated bile acid metabolism regulates liver cancer via NKT cells. Front Immunol. 2019;20(10):282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamada S, Takashina Y, Watanabe M, Nagamine R, Saito Y, Kamada N, Saito H. Bile acid metabolism regulated by the gut microbiota promotes non-alcoholic steatohepatitis-associated hepatocellular carcinoma in mice. Oncotarget. 2018;9(11):9925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guerra Ruiz A, Casafont F, Crespo J, Cayón A, Mayorga M, Estebanez A, Fernadez-Escalante JC, Pons-Romero F. Lipopolysaccharide-binding protein plasma levels and liver TNF-alpha gene expression in obese patients: evidence for the potential role of endotoxin in the pathogenesis of non-alcoholic steatohepatitis. Obes Surg. 2007;17:1374–80. [DOI] [PubMed] [Google Scholar]
- 65.Shao G, Liu Y, Lu L, Zhang G, Zhou W, Wu T, Wang L, Xu H, Ji G. The pathogenesis of HCC driven by NASH and the preventive and therapeutic effects of natural products. Front Pharmacol. 2022. 10.3389/fphar.2022.944088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duseja A, Chawla YK. Obesity and NAFLD: the role of bacteria and microbiota. Clin Liver Dis. 2014;18(1):59–71. [DOI] [PubMed] [Google Scholar]
- 67.Arrese M, Cabrera D, Kalergis AM, Feldstein AE. Innate immunity and inflammation in NAFLD/NASH. Dig Dis Sci. 2016;61:1294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu X, Chen Y, Zhang S, Dong L. Gut microbiota-mediated immunomodulation in tumor. J Exp Clin Cancer Res. 2021;40(1):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Q, Ma L, Shen S, Guo Y, Cao Q, Cai X, Feng J, Yan Y, Hu T, Luo S, Zhou L. Intestinal dysbacteriosis-induced IL-25 promotes development of HCC via alternative activation of macrophages in tumor microenvironment. J Exp Clin Cancer Res. 2019;38:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Q, Ma C, Duan Y, Heinrich B, Rosato U, Diggs LP, Ma L, Roy S, Fu Q, Brown ZJ, Wabitsch S. Gut Microbiome directs hepatocytes to recruit MDSCS and promote cholangiocarcinoma the gut microbiome controls hepatic MDSCs. Cancer Discov. 2021;11(5):1248–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Behary J, Amorim N, Jiang XT, Raposo A, Gong L, McGovern E, Ibrahim R, Chu F, Stephens C, Jebeili H, Fragomeli V. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat Commun. 2021;12(1):187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou A, Tang L, Zeng S, Lei Y, Yang S, Tang B. Gut microbiota: a new piece in understanding hepatocarcinogenesis. Cancer Lett. 2020;1(474):15–22. [DOI] [PubMed] [Google Scholar]
- 73.Zhou J, Tripathi M, Sinha RA, Singh BK, Yen PM. Gut microbiota and their metabolites in the progression of non-alcoholic fatty liver disease. Hepatoma Res. 2021. 10.20517/2394-5079.2020.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scarpellini E, Scarlata GG, Santori V, Scarcella M, Kobyliak N, Abenavoli L. Gut microbiota, deranged immunity, and hepatocellular carcinoma. Biomedicines. 2024;12(8):1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schneider KM, Mohs A, Gui W, Galvez EJ, Candels LS, Hoenicke L, Muthukumarasamy U, Holland CH, Elfers C, Kilic K. Imbalanced gut microbiota fuels hepatocellular carcinoma development by shaping the hepatic inflammatory microenvironment. Nat Commun. 2022. 10.1038/s41467-022-31312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dai X, Hou H, Zhang W, Liu T, Li Y, Wang S, Wang B, Cao H. Microbial metabolites: critical regulators in NAFLD. Front Microbiol. 2020;7(11): 567654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu G, Abas O, Strickland AB, Chen Y, Shi M. CXCR6+ CD4+ T cells promote mortality during trypanosoma Brucei infection. PLoS Pathog. 2021;17(10): e1009968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Culligan EP, Hill C, Sleator RD. Probiotics and gastrointestinal disease: successes, problems and future prospects. Gut pathogens. 2009;1:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Elshaer AM, El-Kharashi OA, Hamam GG, Nabih ES, Magdy YM, Abd El Samad AA. Involvement of TLR4/CXCL9/PREX-2 pathway in the development of hepatocellular carcinoma (HCC) and the promising role of early administration of lactobacillus plantarum in Wistar rats. Tissue Cell. 2019;60:38–47. [DOI] [PubMed] [Google Scholar]
- 80.Wong VW, Wong GL, Chim AM, Chu WC, Yeung DK, Li KC, Chan HL. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann Hepatol. 2013;12(2):256–62. [PubMed] [Google Scholar]
- 81.Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J Immunol. 2009;183(7):4169–75. [DOI] [PubMed] [Google Scholar]
- 82.Russo E, Nannini G, Dinu M, Pagliai G, Sofi F, Amedei A. Exploring the food–gut axis in immunotherapy response of cancer patients. World J Gastroenterol. 2020;26(33):4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fotiadis CI, Stoidis CN, Spyropoulos BG, Zografos ED. Role of probiotics, prebiotics and synbiotics in chemoprevention for colorectal cancer. World J Gastroenterol WJG. 2008;14(42):6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Larrosa M, González-Sarrías A, García-Conesa MT, Tomás-Barberán FA, Espín JC. Urolithins, ellagic acid-derived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities. J Agric Food Chem. 2006;54(5):1611–20. [DOI] [PubMed] [Google Scholar]
- 85.Rodríguez-Lara A, Rueda-Robles A, Sáez-Lara MJ, Plaza-Diaz J, Álvarez-Mercado AI. From non-alcoholic fatty liver disease to liver cancer: microbiota and inflammation as key players. Pathogens. 2023;12(7):940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.González-Sarrías A, Larrosa M, Tomás-Barberán FA, Dolara P, Espín JC. NF-κB-dependent anti-inflammatory activity of urolithins, gut microbiota ellagic acid-derived metabolites, in human colonic fibroblasts. Br J Nutr. 2010;104(4):503–12. [DOI] [PubMed] [Google Scholar]
- 87.Heebøll S, El-Houri RB, Hellberg YE, Haldrup D, Pedersen SB, Jessen N, Christensen LP, Grønbæk H. Effect of resveratrol on experimental non-alcoholic fatty liver disease depends on severity of pathology and timing of treatment. J Gastroenterol Hepatol. 2016;31(3):668–75. [DOI] [PubMed] [Google Scholar]
- 88.Carpi RZ, Barbalho SM, Sloan KP, Laurindo LF, Gonzaga HF, Grippa PC, Zutin TL, Girio RJ, Repetti CS, Detregiachi CR, Bueno PC. The effects of probiotics, prebiotics and synbiotics in non-alcoholic fat liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH): a systematic review. Int J Mol Sci. 2022;23(15):8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yeh CB, Hsieh MJ, Lin CW, Chiou HL, Lin PY, Chen TY, Yang SF. The antimetastatic effects of resveratrol on hepatocellular carcinoma through the downregulation of a metastasis-associated protease by SP-1 modulation. PLoS ONE. 2013;8(2): e56661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Athar M, Back JH, Kopelovich L, Bickers DR, Kim AL. Multiple molecular targets of resveratrol: anti-carcinogenic mechanisms. Arch Biochem Biophys. 2009;486(2):95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Malaguarnera L. Influence of resveratrol on the immune response. Nutrients. 2019;11(5):946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martínez-Flórez S, Gutiérrez-Fernández B, Sánchez-Campos S, González-Gallego J, Tuñón MJ. Quercetin attenuates nuclear factor-κB activation and nitric oxide production in interleukin-1β–activated rat hepatocytes. J Nutr. 2005;135(6):1359–65. [DOI] [PubMed] [Google Scholar]
- 93.Marcolin E, Forgiarini LF, Rodrigues G, Tieppo J, Borghetti GS, Bassani VL, Picada JN, Marroni NP. Retracted: quercetin decreases liver damage in mice with non-alcoholic steatohepatitis. Basic Clin Pharmacol Toxicol. 2013;112(6):385–91. [DOI] [PubMed] [Google Scholar]
- 94.Acharya S, Adamová D, Adhya SP, Adler A, Adolfsson J, Aggarwal MM, Rinella GA, Agnello M, Agrawal N, Ahammed Z, Ahmad S. Investigations of anisotropic flow using multiparticle azimuthal correlations in p p, p− Pb, Xe–Xe, and Pb–Pb collisions at the LHC. Phys Rev Lett. 2019;123(14): 142301. [DOI] [PubMed] [Google Scholar]
- 95.Lamuel-Raventos RM, Onge MP. Prebiotic nut compounds and human microbiota. Crit Rev Food Sci Nutr. 2017;57(14):3154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Plaz Torres MC, Bodini G, Furnari M, Marabotto E, Zentilin P, Giannini EG. Nuts and non-alcoholic fatty liver disease: are nuts safe for patients with fatty liver disease? Nutrients. 2020;12(11):3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fotschki B, Juśkiewicz J, Jurgoński A, Sójka M. Fructo-oligosaccharides and pectins enhance beneficial effects of raspberry polyphenols in rats with nonalcoholic fatty liver. Nutrients. 2021;13(3):833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Russo E, Fiorindi C, Giudici F, Amedei A. Immunomodulation by probiotics and prebiotics in hepatocellular carcinoma. World J Hepatol. 2022;14(2):372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fotschki B, Laparra JM, Sójka M. Raspberry polyphenolic extract regulates obesogenic signals in hepatocytes. Molecules. 2018;23(9):2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wongkrasant P, Pongkorpsakol P, Ariyadamrongkwan J, Meesomboon R, Satitsri S, Pichyangkura R, Barrett KE, Muanprasat C. A prebiotic fructo-oligosaccharide promotes tight junction assembly in intestinal epithelial cells via an AMPK-dependent pathway. Biomed Pharmacother. 2020;1(129): 110415. [DOI] [PubMed] [Google Scholar]
- 101.Wang J, Tian S, Yu H, Wang J, Zhu W. Response of colonic mucosa-associated microbiota composition, mucosal immune homeostasis, and barrier function to early life galactooligosaccharides intervention in suckling piglets. J Agric Food Chem. 2018;67(2):578–88. [DOI] [PubMed] [Google Scholar]
- 102.Fatima N, Akhtar T, Sheikh N. Prebiotics: a novel approach to treat hepatocellular carcinoma. Canadian J Gastroenterol Hepatol. 2017. 10.1155/2017/6238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, Honda K. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499(7456):97–101. [DOI] [PubMed] [Google Scholar]
- 104.Xiang H, Sun D, Liu X, She ZG, Chen Y. The role of the intestinal microbiota in nonalcoholic steatohepatitis. Front Endocrinol. 2022;8(13):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li K, Liu J, Qin X. Research progress of gut microbiota in hepatocellular carcinoma. J Clin Lab Anal. 2022;36(7): e24512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tarantino G, Citro V. Could adverse effects of antibiotics due to their use/misuse be linked to some mechanisms related to nonalcoholic fatty liver disease? Int J Mol Sci. 2024;25(4):1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Francino M. Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Front Microbiol. 2016;12(6): 164577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ramirez J, Guarner F, Bustos Fernandez L, Maruy A, Sdepanian VL, Cohen H. Antibiotics as major disruptors of gut microbiota. Front Cell Infect Microbiol. 2020;24(10): 572912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ribeiro CF, Silveira GG, Candido ED, Cardoso MH, Espinola Carvalho CM, Franco OL. Effects of antibiotic treatment on gut microbiota and how to overcome its negative impacts on human health. ACS Infect Dis. 2020;6(10):2544–59. [DOI] [PubMed] [Google Scholar]
- 110.Bassetti M, Villa G, Pecori D, Arzese A, Wilcox M. Epidemiology, diagnosis and treatment of clostridium difficile infection. Expert Rev Anti Infect Ther. 2012;10(12):1405–23. [DOI] [PubMed] [Google Scholar]
- 111.Sinh P, Barrett TA, Yun L. Clostridium difficile infection and inflammatory bowel disease: a review. Gastroenterol Res Pract. 2011;2011(1): 136064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cojocariu C, Stanciu C, Stoica O, Singeap AM, Sfarti C, Girleanu I, Trifan A. Clostridium difficile infection and inflammatory bowel disease. Turk J Gastroenterol. 2014;25(6):603–10. [DOI] [PubMed] [Google Scholar]
- 113.Choi HH, Cho YS. Fecal microbiota transplantation: current applications, effectiveness, and future perspectives. Clin Endosc. 2016;49(3):257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Craven L, Rahman A, Parvathy SN, Beaton M, Silverman J, Qumosani K, Hramiak I, Hegele R, Joy T, Meddings J, Urquhart B. Allogenic fecal microbiota transplantation in patients with nonalcoholic fatty liver disease improves abnormal small intestinal permeability: a randomized control trial. Off J Am Coll Gastroenterol ACG. 2020;115(7):1055–65. [DOI] [PubMed] [Google Scholar]
- 115.Gulati M, Singh SK, Corrie L, Kaur IP, Chandwani L. Delivery routes for faecal microbiota transplants: available, anticipated and aspired. Pharmacol Res. 2020;1(159): 104954. [DOI] [PubMed] [Google Scholar]
- 116.Blair HA. SER-109 (VOWST™): a review in the prevention of recurrent clostridioides difficile infection. Drugs. 2024;84(3):329–36. [DOI] [PubMed] [Google Scholar]
- 117.Wang Y, Hunt A, Danziger L, Drwiega EN. A comparison of currently available and investigational fecal microbiota transplant products for recurrent clostridioides difficile infection. Antibiotics. 2024;13(5):436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Quraishi MN, Widlak M, Bhala NA, Moore D, Price M, Sharma N, Iqbal TH. Systematic review with meta-analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment Pharmacol Ther. 2017;46(5):479–93. [DOI] [PubMed] [Google Scholar]
- 119.Vrieze A, Out C, Fuentes S, Jonker L, Reuling I, Kootte RS, Van Nood E, Holleman F, Knaapen M, Romijn JA, Soeters MR. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J Hepatol. 2014;60(4):824–31. [DOI] [PubMed] [Google Scholar]
- 120.Panda S, El Khader I, Casellas F, Lopez Vivancos J, Garcia Cors M, Santiago A, Cuenca S, Guarner F, Manichanh C. Short-term effect of antibiotics on human gut microbiota. PLoS ONE. 2014;9(4): e95476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhu J, Ding J, Li S, Jin J. Ganoderic acid A ameliorates non-alcoholic steatohepatitis (NASH) induced by high-fat high-cholesterol diet in mice. Exp Ther Med. 2022;23(4):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yen IC, Lin JC, Chen Y, Tu QW, Lee SY. Antrodia cinnamomea attenuates non-alcoholic steatohepatitis by suppressing NLRP3 inflammasome activation in vitro and in vivo. Am J Chin Med. 2020;48(08):1859–74. [DOI] [PubMed] [Google Scholar]
- 123.Chen L, Zhang L, Wang W, Qiu W, Liu L, Ning A, Cao J, Huang M, Zhong M. Polysaccharides isolated from Cordyceps Sinensis contribute to the progression of NASH by modifying the gut microbiota in mice fed a high-fat diet. PLoS ONE. 2020;15(6): e0232972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li S, Tan HY, Wang N, Cheung F, Hong M, Feng Y. The potential and action mechanism of polyphenols in the treatment of liver diseases. Oxidat Med Cell Longevity. 2018. 10.1155/2018/8394818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jeong JJ, Park HJ, Cha MG, Park E, Won SM, Ganesan R, Gupta H, Gebru YA, Sharma SP, Lee SB, Kwon GH. The lactobacillus as a probiotic: focusing on liver diseases. Microorganisms. 2022;10(2):288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.YU J, LIU Q. Advances in lactobacillus Rhamnosus GG and pediatric nonalcoholic fatty liver disease. Chin J Appl Clin Pediatr. 2022:389–92.
- 127.Zhang HL, Yu LX, Yang W, Tang L, Lin Y, Wu H, Zhai B, Tan YX, Shan L, Liu Q, Chen HY. Profound impact of gut homeostasis on chemically-induced pro-tumorigenic inflammation and hepatocarcinogenesis in rats. J Hepatol. 2012;57(4):803–12. [DOI] [PubMed] [Google Scholar]
- 128.Singh V, Yeoh BS, Abokor AA, Golonka RM, Tian Y, Patterson AD, Joe B, Heikenwalder M, Vijay-Kumar M. Vancomycin prevents fermentable fiber-induced liver cancer in mice with dysbiotic gut microbiota. Gut Microbes. 2020;11(4):1077–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Eguchi A, Mizukami S, Nakamura M, Masuda S, Murayama H, Kawashima M, Inohana M, Nagahara R, Kobayashi M, Yamashita R, Uomoto S. Metronidazole enhances steatosis-related early-stage hepatocarcinogenesis in high fat diet-fed rats through DNA double-strand breaks and modulation of autophagy. Environ Sci Pollut Res. 2022;29:779–89. [DOI] [PubMed] [Google Scholar]
- 130.Yamagishi R, Kamachi F, Nakamura M, Yamazaki S, Kamiya T, Takasugi M, Cheng Y, Nonaka Y, Yukawa-Muto Y, Thuy LT, Harada Y. Gasdermin D–mediated release of IL-33 from senescent hepatic stellate cells promotes obesity-associated hepatocellular carcinoma. Sci Immunol. 2022;7(72):eabll7209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.