Abstract
The interference of IgM-class rheumatoid factor (RF) in the solid-phase radioimmunoassay (RIA) of rubella virus IgM antibodies was studied. Acute rubella infections did not significantly activate RF. False-positive rubella antibody results were obtained, however, when patients with raised RF levels were tested. If a low rubella IgG antibody titre was present, a high level of RF was required to cause a false-positive IgM result; conversely, in sera with high IgG titres, only a low level of RF was required for interference. Although the false-positive IgM titres obtained were generally low, thet did show a positive correlation to both RF levels and rubella IgG titres. False-positive results were successfully avoided by removing the RF by absorption with heat-aggregated human gamma globulin. The absorption procedure did not affect true rubella IgM antibody titres.
Full text
PDF

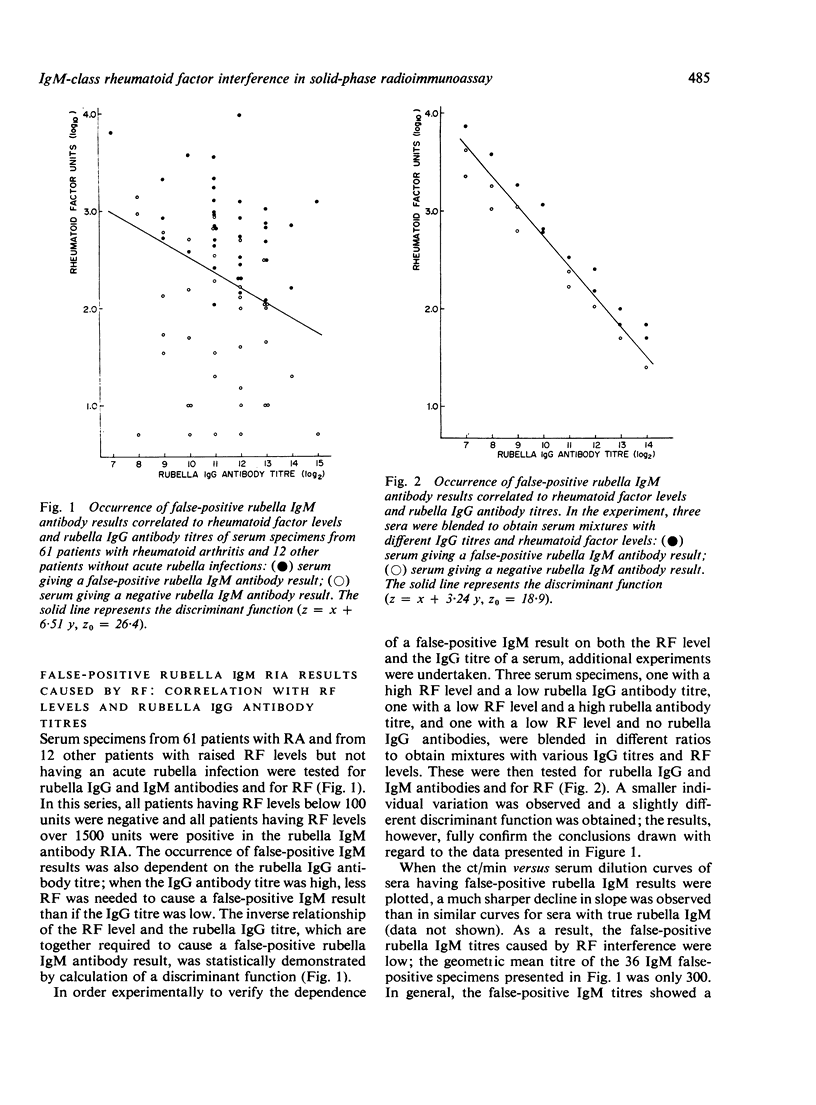
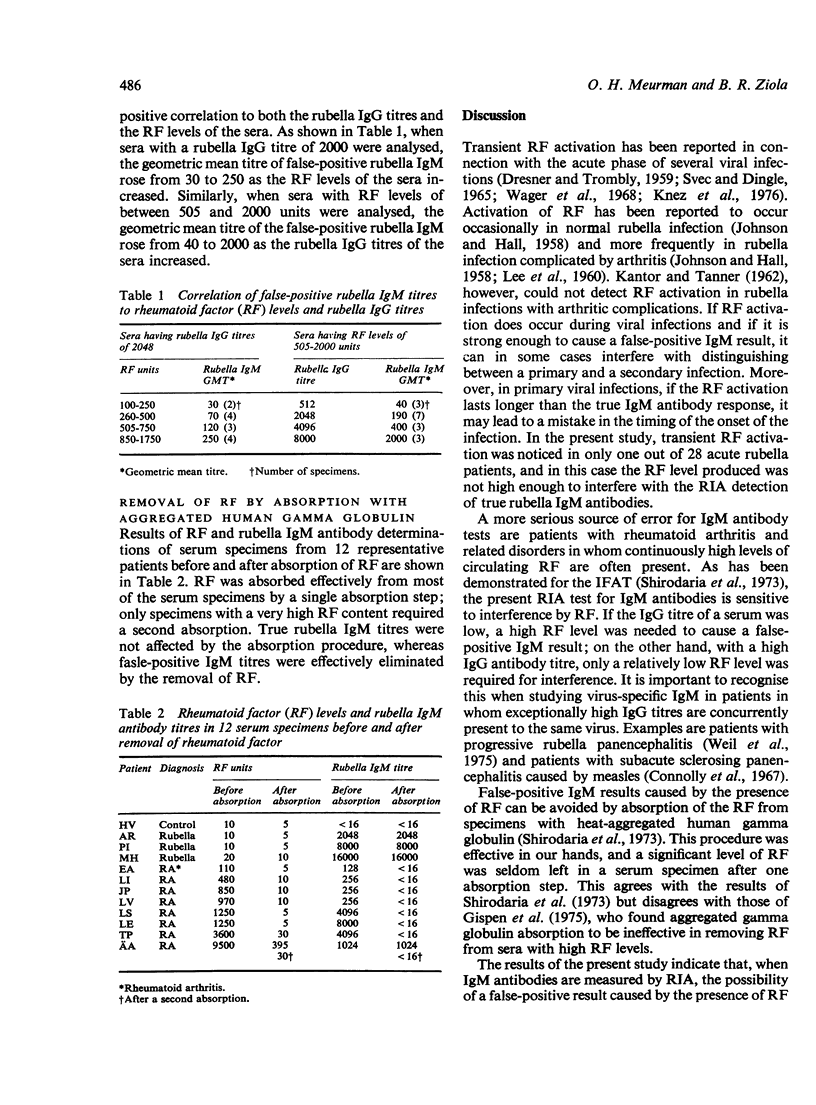

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Connolly J. H., Allen I. V., Hurwitz L. J., Millar J. H. Measles-virus antibody and antigen in subacute sclerosing panencephalitis. Lancet. 1967 Mar 11;1(7489):542–544. doi: 10.1016/s0140-6736(67)92117-4. [DOI] [PubMed] [Google Scholar]
- DRESNER E., TROMBLY P. The latex-fixation reaction in nonrheumatic diseases. N Engl J Med. 1959 Nov 12;261:981–988. doi: 10.1056/NEJM195911122612001. [DOI] [PubMed] [Google Scholar]
- Gerna G., Chambers R. W. Rubella antibody assay by the immunoperoxidase technique: comparison with the hemagglutination inhibition test for determination of immune status. J Infect Dis. 1976 Apr;133(4):469–472. doi: 10.1093/infdis/133.4.469. [DOI] [PubMed] [Google Scholar]
- Gispen R., Nagel J., Brand-Saathof B., De Graaf S. Immunofluorescence test for IgM rubella antibodies in whole serum after absorption with anti-gammaFc. Clin Exp Immunol. 1975 Dec;22(3):431–437. [PMC free article] [PubMed] [Google Scholar]
- Haire M., Hadden D. S. Immunoglobulin responses in rubella and its complications. Br Med J. 1970 Jul 18;3(5715):130–132. doi: 10.1136/bmj.3.5715.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON R. E., HALL A. P. Rubella arthritis; report of cases studied by latex tests. N Engl J Med. 1958 Apr 10;258(15):743–745. doi: 10.1056/NEJM195804102581506. [DOI] [PubMed] [Google Scholar]
- KANTOR T. G., TANNER M. Rubella arthritis and rheumatoid arthritis. Arthritis Rheum. 1962 Aug;5:378–383. doi: 10.1002/art.1780050405. [DOI] [PubMed] [Google Scholar]
- Kalimo K. O., Meurman O. H., Halonen P. E., Ziola B. R., Viljanen M. K., Granfors K., Toivanen P. Solid-phase radioimmunoassay of rubella virus immunoglobulin G and immunoglobulin M antibodies. J Clin Microbiol. 1976 Aug;4(2):117–123. doi: 10.1128/jcm.4.2.117-123.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knez V., Stewart J. A., Ziegler D. W. Cytomegalovirus specific IgM and IgG response in humans studied by radioimmunoassay. J Immunol. 1976 Nov;117(5 PT2):2006–2013. [PubMed] [Google Scholar]
- LEE P. R., BARNETT A. F., SCHOLER J. F., BRYNER S., CLARK W. H. Rubella arthritis. A study of twenty cases. Calif Med. 1960 Sep;93:125–128. [PMC free article] [PubMed] [Google Scholar]
- Liebhaber H., Pajot T., Riordan J. T. Growth of high titered rubella virus in roller bottle cultures of Vero cells. Proc Soc Exp Biol Med. 1969 Jan;130(1):12–14. doi: 10.3181/00379727-130-33477. [DOI] [PubMed] [Google Scholar]
- Meurman O. H., Viljanen M. K., Granfors K. Solid-phase radioimmunoassay of rubella virus immunoglobulin M antibodies: comparison with sucrose density gradient centrifugation test. J Clin Microbiol. 1977 Mar;5(3):257–262. doi: 10.1128/jcm.5.3.257-262.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVEC K. H., DINGLE J. H. THE OCCURENCE OF RHEUMATOID FACTOR IN ASSOCIATION WITH ANTIBODY RESPONSE TO INFLUENZA A2(ASIAN) VIRUS. Arthritis Rheum. 1965 Aug;8:524–529. doi: 10.1002/art.1780080406. [DOI] [PubMed] [Google Scholar]
- Shirodaria P. V., Fraser K. B., Stanford F. Secondary fluorescent staining of virus antigens by rheumatoid factor and fluorescein-conjugated anti-IgM. Ann Rheum Dis. 1973 Jan;32(1):53–57. doi: 10.1136/ard.32.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voller A., Bidwell D. E. A simple method for detecting antibodies to rubella. Br J Exp Pathol. 1975 Aug;56(4):338–339. [PMC free article] [PubMed] [Google Scholar]
- Wager O., Räsänen J. A., Hagman A., Klemola E. Mixed cryoimmunoglobulinaemia in infectious mononucleois and Cytomegalovirus mononucleosis. Int Arch Allergy Appl Immunol. 1968;34(4):345–361. doi: 10.1159/000230129. [DOI] [PubMed] [Google Scholar]
- Weil M. L., Itabashi H., Cremer N. E., Oshiro L., Lennette E. H., Carnay L. Chronic progressive panencephalitis due to rubella virus simulating subacute sclerosing panencephalitis. N Engl J Med. 1975 May 8;292(19):994–998. doi: 10.1056/NEJM197505082921903. [DOI] [PubMed] [Google Scholar]
- Ziola B. R., Matikainen M. T., Salmi A. Polystyrene balls as the solid-phase of a double-antibody radioimmunoassay for human serum albumin. J Immunol Methods. 1977;17(3-4):309–317. doi: 10.1016/0022-1759(77)90113-2. [DOI] [PubMed] [Google Scholar]


