Abstract
Background
Radiotherapy has both immunostimulant and immunosuppressive effects, particularly in radiation-induced lymphopenia. Proton therapy has demonstrated potential in mitigating this lymphopenia, yet the mechanisms by which different types of radiation affect the immune system function are not fully characterized. The Circulating Immunes Cells, Cytokines and Brain Radiotherapy (CYRAD) trial aims to compare the effects of postoperative X-ray and proton radiotherapy on circulating leukocyte subpopulations and cytokine levels in patients with head and neck (CNS and ear nose throat) cancer.
Methods
CYRAD is a prospective, non-randomized, single-center non interventional study assessing changes in the circulating leukocyte subpopulations and cytokine levels in head and neck cancer patients receiving X-ray or proton radiotherapy following tumor resection. Dosimetry parameters, including dose deposited to organs-at-risk such as the blood and cervical lymph nodes, are computed. Participants undergo 29 to 35 radiotherapy sessions over 40 to 50 days, followed by a 3-month follow-up. Blood samples are collected before starting radiotherapy (baseline), before the 11th (D15) and 30th sessions (D40), and three months after completing radiotherapy. The study will be conducted with 40 patients, in 2 groups of 20 patients per modality of radiotherapy (proton therapy and photon therapy). Statistical analyses will assess the absolute and relative relationship between variations (depletion, recovery) in immune cells, biomarkers, dosimetry parameters and early outcomes.
Discussion
Previous research has primarily focused on radiation-induced lymphopenia, paying less attention to the specific impacts of radiation on different lymphoid and myeloid cell types. Early studies indicate that X-ray and proton irradiation may lead to divergent outcomes in leukocyte subpopulations within the bloodstream. Based on these preliminary findings, this study aims to refine our understanding of how proton therapy can better preserve immune function in postoperative (macroscopic tumor-free) head and neck cancer patients, potentially improving treatment outcomes.
Protocol version
Version 2.1 dated from January 18, 2023.
Trial registration
The CYRAD trial is registered from October 19, 2021, at the US National Library of Medicine, ClinicalTrials.gov ID NCT05082961.
Keywords: Radiotherapy, Photons, Protons, Lymphopenia, Myeloid, Lymphoid, Cytokines, Immune response
Background
Radiotherapy is a crucial component in the management of numerous cancers, utilized in about 60% of patients at some time of their treatment [1, 2]. While traditionally viewed as having localized impact only, emerging evidence suggests that radiotherapy may exert systemic influences as well. This interest has been rekindled by observations of the abscopal effect, wherein tumor regression is noted outside the immediate treatment zones [3]. However, this phenomenon is rare and appears to vary with the type of cancer tissue [4, 5]. In addition, radiotherapy is associated with immunosuppressive effects, notably a severe and persistent reduction in peripheral blood lymphocytes, i.e., radiation-induced lymphopenia (RIL) [2] that occurs even when irradiated organs contain low number lymphoid organs. Beyond lymphopenia, research from animal studies revealed that radiotherapy can selectively affect certain leukocyte subpopulations, which affects the immune anti-cancer responses [6–10].
Radiotherapy modalities—including the particles used, treatment fields, particle energy, dose rate, total dose, and fractionation—play a crucial role in its effects. The size of the irradiated volume affects circulating lymphocytes and those in primary (e.g., bone marrow, thymus) and secondary (e.g., lymph nodes) lymphoid organs, with a strong correlation between the extent of healthy tissue irradiation and the incidence of lymphopenia [11, 12]. A third component also appears, which is related to immune cell infiltration of the tumor site and corresponding irradiation.
At present, around 90% of external beam radiotherapy protocols use beams from linear accelerators [13]. Alternatives include external beams of charged particles such as protons, carbon ions, helium ions. Intensity-modulated radiotherapy (IMRT) with photons is designed to create steep dose gradients to spare adjacent organs at risk from high doses but may expose large volumes of healthy tissue to low doses [14]. A 4D physical simulation model has suggested that the ballistic properties of proton therapy might better preserve circulating lymphocytes [15]. Proton therapy has shown to lower the incidence of RIL in multiple types of cancers compared to photon radiotherapy [16–19].
Despite technological advances, the specific processes by which lymphocytes are destroyed under different types of radiation remain unclear. Preliminary data suggest that necrosis may be more prevalent than apoptosis in proton irradiation, possibly due to the higher relative biological effectiveness (RBE) of protons compared to photons [20]. A 4D physical simulation model suggests that the ballistic properties of proton therapy might better preserve circulating lymphocytes [21].
In the Circulating Immunes Cells, Cytokines and Brain Radiotherapy (CYRAD) study, we examine the circulating cell and cytokine response following X-ray or proton radiotherapy in the postoperative context of head and neck (CNS and ear nose throat) cancer.
Methods/design
The CYRAD study is a prospective, non-randomized, single-center translational non-therapeutic study with a parallel assignment interventional model, conducted at the Comprehensive Cancer Centre François Baclesse (https://www.clinicaltrials.gov/study/NCT05082961). It aims to assess the impact of postoperative proton therapy as opposed to conventional X-ray irradiation on immune lymphoid and myeloid cells (such as lymphocytes and monocytes) and immune chemical messengers (cytokines/chemokines) in patients with resected tumors.
The study and this manuscript have been written in accordance with standard protocol items, namely recommendations for interventional trials (SPIRIT).
Study objectives
The primary endpoint of this research is to assess CD8+ T-cell kinetics during and up to 3 months of radiotherapy by photons or protons in comparison with baseline pre-radiotherapy counts. Secondary endpoints include counts of other leukocyte subpopulation counts (e.g., CD4+ T cells, regulatory T cells, B cells, NK cells, neutrophils, monocytes, and myeloid-derived suppressive cells) as well as circulating cytokine/chemokine levels.
Study population
As this study is designed to exclusively focus on the effects of radiation on healthy tissues, including the brain and blood, it addresses operated head and neck / CNS patients without residual macroscopic disease. Eligibility criteria are listed in Table 1. More precisely, to isolate the effects of irradiation from other influences like the tumor itself or systemic treatments such as chemotherapy, patients without macroscopic tumors in place are eligible: patients have to have undergo surgery for head and neck, including pharyngeal, sinonasal, salivary gland and CNS tumors, which typically require postoperative irradiation to prevent locoregional recurrence. Patients candidate to a treatment with either conventional X-ray (arm 1) or protons (arm 2) to better spare healthy tissues and reduce side effects are eligible.
Table 1.
Eligibility criteria in the CYRAD study
| Inclusion criteria | Non-inclusion criteria |
|---|---|
|
• Patient aged 18 or over • Head and neck cancer (upper aerodigestive tract / pharyngeal, sinonasal, salivary gland, base of the skull, brain tumors) operated on • Complete tumor resection surgery or with microscopic tumor residue R1 • All possible histologies, such as squamous cell carcinoma, adenocarcinoma, adenoid cystic carcinoma, chordoma, chondrosarcoma, meningioma, etc. • Patient candidate for exclusive post-operative radiotherapy at the minimum total dose of 54 Gy to limit effect of heterogeneous total doses in X-ray or equivalent biological dose in proton irradiation • Patients affiliated to the national social security system • Signed informed consent before any specific procedure related to the study |
• Macroscopic postoperative tumor residue R2 • History of cancer within 5 years (except for treated basal cell carcinoma of the skin and treated cervical cancer). • History of radiotherapy (except brachytherapy of the cervix or prostate) • Chemotherapy or any other systemic oncological treatment (such as cetuximab or immunotherapy) concomitant with radiotherapy • Long-term immunosuppressive treatment or corticosteroid therapy • Patients deprived of liberty or under guardianship, protected adult • Patients unable to undergo trial monitoring for geographical, social or psychopathological reasons • Pregnant or breastfeeding women • Emergency and palliative situations |
Study experimental plan
The study will be proposed by physicians, either surgeons, medical or radiation oncologists to eligible patients. An explanation of the study and an information note will be given to them. Patients will be enrolled in the study once provided their written informed consent. An identification number will be assigned to each patient to be used throughout the study. All patients participating may object at any time, leading to the prompt disposal of their biological material, as well as the cessation of data collection. The inclusion period of the study is planned over three years. The participation of patients will last up to three months after the completion of radiotherapy.
Blood sampling and analysis
Sampling
The blood sampling procedures outlined for the study at the Comprehensive Cancer Centre François Baclesse involve collecting blood samples from patients at specific intervals during and after their radiotherapy treatment. These samples are critical for documenting the changes in circulating levels of various blood cells and biological parameters (cytokines, chemokines), and assessing the immunological impacts of radiotherapy at various stages of treatment.
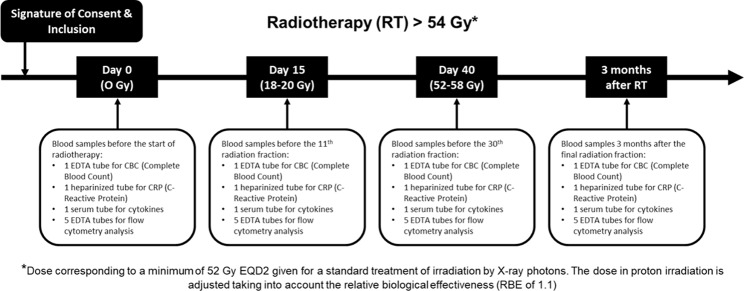
Patients are required to provide blood samples at four timepoints: before starting radiotherapy to establish a baseline (0 Gy, Day 0); before the 11th session (18 to 20 Gy, Day 15) to assess early treatment effects; before the 30th session (54 to 58 Gy, Day 40) to monitor ongoing immune responses; and three months after completing radiotherapy to evaluate longer-term effects on the immune system (Fig. 1).
Fig. 1.
Blood sampling workflow of the study
At each of these time points, the following samples are collected: a 5 mL sample in an ethylenediaminetetraacetic acid (EDTA) tube for a complete blood count (CBC), which helps to assess overall health and detect disorders like anemia and infection; a 5 mL heparinized tube sample for C-reactive protein (CRP), a marker of inflammation; an 8 mL sample in a dry tube for serum cytokine assay, important for evaluating the immune system’s signaling and response; and a 25 mL EDTA tube with Ficoll for the purification of peripheral blood mononuclear cells (PBMCs), which are critical immune cells including lymphocytes and monocytes.
Flow cytometry
Sample processing for PBMCs and cytokine levels involves different routes. These samples are sent to the Caen Basse-Normandie tumor library (TCBN), where they undergo a preparatory stage. There, PBMCs are isolated using a centrifugation technique with standard Ficoll method for separating these cells from other blood components. Both the PBMC and serum samples are then frozen to preserve their integrity until further analysis.
The analysis of the PBMCs involves advanced single-cell techniques like flow cytometry, performed on the ISOCELL cytometry platform (PLATON unit, Comprehensive Cancer Centre François Baclesse). This process is used to characterize and quantify various circulating immune cell populations, including different subtypes of lymphoid cells (CD45+CD3+ T cells, CD45+CD3+CD8+ cytotoxic T cells, CD45+CD3+CD4+ helper T cells, and CD45+CD4+CD25+FoxP3+ regulatory T cells), NK cells (CD45+CD3−CD56+), and B lymphocytes (CD45+CD3−CD19+). Additionally, detail assessments are conducted on CD45+CD33+ myeloid cells, including CD45+CD33+HLA-DRhigh myeloid-derived suppressor cells and CD45+CD33+HLA-DRlow monocytes. Further, it evaluates the expression of inhibitory receptors (PD-1, TIM-3, LAG-3, CTLA4) on these cells, and their capability to produce cytokines following ex-vivo restimulation.
Parallel to these cellular analyses, cytokines and chemokines in the serum are quantified using multiplex immunoassays, providing a broad view of the inflammatory and immune status of the patients.
Clinical outcomes assessment
At inclusion, before the 11th session, before the 30th session, and again 3 to 4 months after the end of radiotherapy, clinical examination is conducted for each participating patient. This includes assessing the patient’s physical condition and recording any side effects or complications related to the treatment. Specific aspects covered include the WHO performance status to evaluate general health and daily activity levels, weight monitoring to check for significant weight loss or gain, documentation of concomitant treatments such as anti-inflammatories and corticosteroids, and observation of symptoms like asthenia (generalized weakness) and specific radiotherapy-related side effects such as radiodermatitis and edema. As part of usual clinical practice, patient also realizes a tumoral evaluation with computed tomography (CT) or magnetic resonance imaging (MRI) at baseline and 3 months after the end of radiotherapy.
Standard radiotherapy treatment
The radiotherapy technique uses MRT, preferably using a simultaneous integrated boost, or IMPT by a pencil beam scanning. A Monte Carlo dose calculation algorithm is encouraged to account for heterogeneities at tissue interfaces. The IMPT technique preferably uses a sequential boost technique for the different clinical target volume (CTV) dose prescription levels to avoid very low fraction dose to distant tissues that might result in a (controversial) higher proton RBE [22]. In CNS and ear nose throat cancers, doses and structures (healthy tissues/organs and tumor volume) are defined per standard practice at the institution.
Dosing regimen
In brain tumors, one or two dose-risk levels are defined. In ear nose throat cancers, the dose regimen consists of high-risk PTV receiving 60–66 Gy in 30–33 fractions and low-risk PTV receiving 54.45 Gy in fractions of 1.65 Gy, depending on tumor type and whether the surgical resection is complete or leaves microscopic residue.
Organ-at-risk
The minimal data set for organs at risk that must be delineated includes a comprehensive list: brachial plexus, brainstem, cerebellum, chiasma, constrictor muscles of the pharynx, eyes, larynx, lenses, lips, mandible, middle and inner ears, optic nerves, oral cavity, parotids, pituitary gland, spinal cord, submandibular glands, temporomandibular joints, large vessels (arteries and veins of the neck and at the skull base), nodal areas, and thyroid. These organs are critical for ensuring precise and safe radiation targeting, minimizing damage to non-target tissues. Dosimetric criteria for each of these organs include assessments of the maximum dose delivered to 2% of the organ volume and the average dose. These evaluations are made using DICOM RT data and by observing tissue effects on imaging. This process involves a comparison between the initial multimodal image planning and follow-up images to track any changes or impacts from the treatment. The dose-volume relationships for these structures can be assessed with respect to trial outcomes.
Treatment planning
The planning computed tomography (CT) scan for the study is conducted with contiguous slices ranging from 1 to 2 millimeters in thickness, covering from the vertex of the head to at least the apex of the lungs. The delineation of tumor volumes is performed by a radiation oncologist, with validation by a referring ear, nose, and throat (ENT) surgeon based on operative and histological reports.
For example, in ear, nose throat tumors, the definition of target volumes for subclinical disease involvement, or CTV, follows a geo-anatomical approach. This involves using preoperative imaging and perioperative findings to identify the gross or macroscopic tumor, supplemented by information from the operative and histological reports. A high-risk CTV involves a geometric expansion of 5 mm around the macroscopic tumor fragments. The low-risk CTV is then defined by expanding an additional 5 mm around the high-risk CTV, and further extended as necessary to account for potential routes of dissemination and anatomical barriers.
For the purposes of intensity modulated radiation therapy (IMRT), isotropic margins of 5 mm from CTV to planning target volume (PTV) are applied. These margins may be adapted depending on the performance of image-guided radiation therapy (IGRT), which includes repositioning techniques and on-board accelerator imaging. In cases using intensity modulated proton therapy (IMPT), the range and setup uncertainties are considered at 3–5% and 3 mm respectively for robust optimization and a 5 mm-isotropic PTV is generated for comparisons with the photon arm.
Imaging (including CT scans and MRIs) performed on patients is centralized and stored in pseudonymized format, to ensure confidentiality and data integrity. Imaging analyses are conducted to assess the correlation between observed tissue effects on follow-up imaging and any toxicities experienced by the patients.
Dosimetric data
In the course of routine healthcare for patients, clinical data are gathered and logged into the electronic health record system during pre-radiotherapy consultations, as well as throughout the monitoring phases during and after the treatment. Dosimetric data are prospectively collected on the Raystation software [23]. This set of data includes information on retrospectively contoured, blind to referring physicians by JT/TPN, blood volumes in the neck / skull base, as well as both target and non-target lymph node areas that have been exposed to various radiation dose levels.
Statistical considerations
Sample size
The main criterion is the variation of circulating CD8+ T-cell counts between inclusion prior to radiotherapy and completion of radiotherapy by photons or protons. No power calculation was performed to determine the sample size. The study will be conducted with 40 patients, in 2 groups of 20 patients per modality of radiotherapy (proton therapy and photon therapy). Statistical analysis will be mainly descriptive, based on both quantitative and qualitative methods. In particular, the levels of various circulating biomarkers of immunity, and their evolution before, during and after radiotherapy will be described.
Dose to leukocyte-related organs
High blood perfusion organs including the brain and lungs, were identified as being related to the risk of radiation-induced lymphopenia [24]. The cervical lymph node areas are a critical site for T-cell activation and lymphocyte storage and may therefore also be related to the risk of lymphopenia. Mean dose delivered to the brain, lung, and cervical neck areas are extracted. The mean dose delivered to the blood is calculated via the effective dose to immune cells (EDIC) [25, 26], which is the sum of the blood equivalent uniform doses contributed by each blood-containing organ, assuming that these vessels are uniformly distributed throughout the body.
Statistical analysis and modeling
The initial analytical approach involves comparing results from flow cytometry with clinical and the dose to leukocyte-related organs using a linear regression model. This model aims to identify any potential correlations between the radiation dose delivered to the blood, lymph node regions, and relative organs at risk, and variations in blood cell counts. Subsequently, the model is being refined to predict changes in blood cell counts based on the doses administered to associated organs. In the final step, the efficacy of proton therapy over photon therapy in modulating the immune system will be assessed through simulations derived from the developed model (referred to as a digital twin). In addition, interplay between leukocyte subpopulations is analyzed using correlations and principal component analysis.
Data management
A Web Based Data Capture (WBDC) system is used for data collection and query handling. The investigator ensures that data are recorded on the eCRFs as specified in the study protocol and in accordance with the instructions provided.
The investigator ensures the accuracy, completeness, and timeliness of the data recorded and of the provision of answers to data queries according to the Clinical Study Agreement. The investigator will sign the completed eCRFs. A copy of the completed eCRFs will be archived at the study site.
Withdrawal from study
The reasons for why a patient may discontinue to participate to the study include the following circumstances:
Radiotherapy break lasting more than 3 open days.
Patient’s decision (the data already collected during the search can be kept and exploited unless the patient opposes it).
Investigator’s decision.
Discussion
This project aims to investigate the differential impact of proton therapy compared to traditional X-ray irradiation on circulating immune cells and on immune signaling molecules, including cytokines and chemokines, in patients with head and neck (CNS / ear nose throat) cancer.
While prior research has extensively documented radiation-induced lymphopenia, less attention has been given to the specific effects of radiation on various lymphoid and myeloid subtypes [9]. Preclinical studies have shown that X-ray and proton irradiation of the brain lead to different outcomes in leukocyte subpopulations within the bloodstream [8, 27]. Proton therapy offers the advantage of precisely targeting tumors while minimizing damage to surrounding healthy tissues [28]. This precision not only helps in preserving immune function but could also potentially enhance the effectiveness of the antitumor response by maintaining a healthy population of immune cells capable of combating cancer. In head and neck cancer, this preservation is increasingly seen as key to promoting sustained and effective immune surveillance, potentially preventing tumor recurrence and metastasis [29].
Given these insights, the study is designed to refine treatment strategies that aim not only for immediate tumor control but also for improved long-term outcomes and quality of life by protecting immune health. New modeling approaches are also developed to accurately recapitulate and predict circulating cell counts, and establish accurate predictions of patient’s outcomes. This research is integral in advancing our understanding of how different radiation therapies can be optimized to bolster the immune system while effectively combating cancer.
Acknowledgements
Technicians, Research assistants, and TCBN staff.
Abbreviations
- CBC
Complete blood count
- CRP
C-reactive protein
- CT
Computed tomography
- CTV
Clinical target volume
- EDTA
Ethylenediaminetetraacetic acid
- ENT
Ear, nose, and throat
- IGRT
Image-guided radiation therapy
- IMPT
Intensity modulated proton therapy
- IMRT
Intensity-modulated radiotherapy
- MRI
Magnetic Resonance Imaging
- MRT
Modulated radiation therapy
- PBMCs
Peripheral blood mononuclear cells
- PTV
Planning target volume
- RBE
Relative biological effectiveness
- RIL
Radiation-induced lymphopenia
- SPIRIT
Standard protocol items: Recommendations for interventional trials
- TCBN
Caen Basse-Normandie tumor library
Author contributions
JT: concept, methodology, inclusions, data collection, analyses, writing, review, TPN: methodology, data collection, analyses, writing, review; JC: methodology, data collection, analyses, review; BC: methodology, regulatory aspects, review; JMG: methodology, regulatory aspects, review; NR: data collection, analyses, review; MC: methodology, writing, review, SV: concept, methodology, data collection, analyses, writing, review.
Funding
This study (NCT05082961) has received financial support from the CNRS through the 80 Prime program and Ligue Contre le Cancer. In the context of these external grants, the study protocol has undergone peer review by the funding bodies. The funding agencies are not involved in the design or conduct of the study, nor in the collection, management, analysis, or interpretation of the data. They were not involved in the writing of the manuscript.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study has received ethical approval from the Comité de Protection des Personnes Ouest III in October 2021 (N°IDRCB 2021-A01862-39). Eligible patients will be proposed to participate by physicians (surgeons, medical or radiation oncologists), who will give them an information file. Patients will be enrolled in the study once provided their written informed consent. This study will be performed in accordance with the relevant guidelines and regulations (Declaration of Helsinki).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Trial status
Patient enrolment is underway.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Juliette Thariat and Mathieu Césaire are clinical coordinators.
References
- 1.Delaney G, Jacob S, Featherstone C, Barton M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer. 2005;104:1129–37. [DOI] [PubMed] [Google Scholar]
- 2.de Andrade Carvalho H, Villar RC. Radiotherapy and immune response: the systemic effects of a local treatment. Clinics. 2018;73:e557s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the Abscopal Effect in a patient with Melanoma. N Engl J Med. 2012;366:925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janopaul-Naylor JR, Shen Y, Qian DC, Buchwald ZS. The Abscopal Effect: a review of pre-clinical and clinical advances. IJMS. 2021;22:11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rowell NP. The abscopal effect and its implications for radiotherapy-immunotherapy combinations. Transl Cancer Res. 2023;12:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farese AM, Hankey KG, Cohen MV, MacVittie TJ. Lymphoid and myeloid recovery in Rhesus macaques following total body X-Irradiation. Health Phys. 2015;109:414–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macintyre AN, French MJ, Sanders BR, Riebe KJ, Shterev ID, Wiehe K, et al. Long-term recovery of the adaptive Immune System in Rhesus macaques after total body irradiation. Adv Radiation Oncol. 2021;6:100677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pham T-N, Coupey J, Toutain J, Candeias SM, Simonin G, Rousseau M et al. Early effects of different brain radiotherapy modalities on circulating leucocyte subpopulations in rodents. Int J Radiat Biol. 2024;:1–12. [DOI] [PubMed]
- 9.Pham T-N, Coupey J, Candeias SM, Ivanova V, Valable S, Thariat J. Beyond lymphopenia, unraveling radiation-induced leucocyte subpopulation kinetics and mechanisms through modeling approaches. J Exp Clin Cancer Res. 2023;42:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh S, Huang J, Inkman M, Zhang J, Thotala S, Tikhonova E, et al. Radiation-induced circulating myeloid-derived suppressor cells induce systemic lymphopenia after chemoradiotherapy in patients with glioblastoma. Sci Transl Med. 2023;15:eabn6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mole RH. Whole body irradiation—radiobiology or medicine? Br J Radiol. 1953;26:234–41. [DOI] [PubMed] [Google Scholar]
- 12.Ellsworth SG. Field size effects on the risk and severity of treatment-induced lymphopenia in patients undergoing radiation therapy for solid tumors. Adv Radiation Oncol. 2018;3:512–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goitein M, Cox JD. Should Randomized clinical trials be required for Proton Radiotherapy? JCO. 2008;26:175–6. [DOI] [PubMed] [Google Scholar]
- 14.Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiation Oncology*Biology*Physics. 2006;65:1–7. [DOI] [PubMed] [Google Scholar]
- 15.Hammi A, Paganetti H, Grassberger C. 4D blood flow model for dose calculation to circulating blood and lymphocytes. Phys Med Biol. 2020;65:055008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohan R, Liu AY, Brown PD, Mahajan A, Dinh J, Chung C, et al. Proton therapy reduces the likelihood of high-grade radiation-induced lymphopenia in glioblastoma patients: phase II randomized study of protons vs photons. Neurooncology. 2021;23:284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebrahimi S, Lim G, Liu A, Lin SH, Ellsworth SG, Grassberger C, et al. Radiation-Induced Lymphopenia risks of Photon Versus Proton Therapy for Esophageal Cancer patients. Int J Part Therapy. 2021;8:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang P, Shiraishi Y, Verma V, Jiang W, Song J, Hobbs BP, et al. Lymphocyte-sparing effect of Proton Therapy in patients with esophageal Cancer treated with definitive chemoradiation. Int J Part Therapy. 2017;4:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim N, Myoung Noh J, Lee W, Park B, Park H, Young Park J, et al. Proton Beam therapy reduces the risk of severe radiation-induced lymphopenia during chemoradiotherapy for locally advanced non-small cell lung cancer: a comparative analysis of proton versus photon therapy. Radiother Oncol. 2021;156:166–73. [DOI] [PubMed] [Google Scholar]
- 20.Paganetti H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol. 2014;59:R419–72. [DOI] [PubMed] [Google Scholar]
- 21.Durante M, Loeffler JS. Charged particles in radiation oncology. Nat Rev Clin Oncol. 2010;7:37–43. [DOI] [PubMed] [Google Scholar]
- 22.Carabe-Fernandez A, Dale RG, Jones B. The incorporation of the concept of minimum RBE (RBEmin) into the linear-quadratic model and the potential for improved radiobiological analysis of high-LET treatments. Int J Radiat Biol. 2007;83:27–39. [DOI] [PubMed] [Google Scholar]
- 23.Bodensteiner D. RayStation: external beam treatment planning system. Med Dosim. 2018;43:168–76. [DOI] [PubMed] [Google Scholar]
- 24.Venkatesulu B, Giridhar P, Pujari L, Chou B, Lee JH, Block AM, et al. Lymphocyte sparing normal tissue effects in the clinic (LymphoTEC): a systematic review of dose constraint considerations to mitigate radiation-related lymphopenia in the era of immunotherapy. Radiother Oncol. 2022;177:81–94. [DOI] [PubMed] [Google Scholar]
- 25.Chen F, Jin J-Y, Hui TSK, Jing H, Zhang H, Nong Y, et al. Radiation Induced Lymphopenia is Associated with the effective dose to the circulating Immune cells in breast Cancer. Front Oncol. 2022;12:768956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu C, Jin J-Y, Zhang M, Liu A, Wang J, Mohan R, et al. The impact of the effective dose to immune cells on lymphopenia and survival of esophageal cancer after chemoradiotherapy. Radiother Oncol. 2020;146:180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coupey J, Pham TN, Toutain J, Ivanova V, HUE E, Helaine C, et al. Investigating the effects of protons versus x-rays on radiation-induced lymphopenia after brain irradiation. bioRxiv. 2024. 10.1101/2024.03.02.583088. [Google Scholar]
- 28.Mohan R. A review of proton therapy – current status and future directions. Precision Radiation Oncol. 2022;6:164–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordon KB, Smyk DI, Gulidov IA. Proton Therapy in Head and Neck Cancer Treatment: state of the Problem and Development prospects (review). Sovrem Tehnol Med. 2021;13:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.