Abstract
Background
Mucosal-associated invariant T (MAIT) cells play key roles in many inflammatory diseases. However, their effects on the long-term course of oral lichen planus (OLP) and recent-onset OLP remain unclear. In this study, we aimed to investigate the function of MAIT cells in the different processes of OLP and to explore the immunological background of this disease.
Methods
The frequency, phenotype, cytokine secretion, and clinical relevance of MAIT cells were investigated. MAIT cells were collected from the peripheral blood of 14 adults with recent-onset OLP (7–120 days after disease onset) and 16 adults with long-term course OLP (>2 years after diagnosis) using flow cytometry and compared with 15 healthy blood donors. Statistical analyses were performed using the GraphPad Prism software.
Results
MAIT cells from adults with recent-onset OLP exhibited an activated phenotype, as indicated by an increased frequency of CD69+ (p < 0.05) and CD38+MAIT cells (p < 0.01) and elevated production of the proinflammatory cytokine IL-17 A (p < 0.01), compared with healthy adult donors. In adults with long-term OLP, MAIT cells exhibited an activated and exhausted phenotype, characterized by high expression of CD69 (p < 0.01) and PD-1 (p < 0.001) and increased production of granzyme B released (p < 0.01). Compared with recent-onset OLP patients, long-term OLP patients showed a decreased production of CD8+, and CD4−CD8− cells, but an increase in PD-1+ production (p < 0.05).
Conclusions
Circulating MAIT cells exhibited activation in OLP patients across varying disease durations. Given that PD-1 expression is elevated in adults with long-term OLP, it is reasonable to infer that circulating MAIT cells in long-term OLP may exhibit a more exhausted state than those in recent-onset OLP.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12903-024-04959-3.
Keywords: Oral lichen planus (OLP), Mucosal-associated invariant T cells (MAIT cells), Immunoregulatory activity, Phenotypes, Functional profile
Background
Oral lichen planus (OLP) is a common T cell-mediated inflammatory disease of the oral mucosa, with a global prevalence of 0.5-4% [1, 2]. Clinically, OLP can occur at any site within the oral cavity and often manifests as inflamed ulcerations with a white lace or linear pattern in a predominantly bilateral and symmetrical distribution [3]. Due to the chronic and prolonged course of the disease, as well as the possibility of long-term erosion lesions leading to cancerous changes, the World Health Organization (WHO) lists OLP as a precancerous state [2]. OLP is related to autoimmune function; conventional T cell subsets such as CD4+ helper T (Th) and CD8+ cytotoxic T cells infiltrated by inflammation have garnered much attention [4, 5]. Th1, Th2, and Th17 cells are also associated with OLP pathogenesis [3]. However, little is known about the roles of non-conventional T cell subsets in the OLP process, particularly regarding the potential influence of innate-like lymphoid cells.
Mucosal-associated invariant T (MAIT) cells are unique T cells that express a semi-invariant T-cell receptor (TCR) α chain: Vα7.2Jα33 in humans, bridging innate and adaptive immunity [6, 7]. In addition to recognizing intermediates of the vitamin synthesis pathway presented by the major histocompatibility complex (MHC) class I-related molecule 1 (MR1), MAIT cells can also be activated by cytokines [8–10]. Activated MAIT cells proliferate and rapidly release Th1, Th17, and other pro-inflammatory cytokines and cytotoxic molecules [11–13].
Several studies have reported altered MAIT cell functions in autoimmune inflammatory diseases such as multiple sclerosis (MS), systemic lupus erythematosus (SLE), and rheumatoid arthritis (RA), and have implicated MAIT cells as potential therapeutic targets for these diseases [14]. Furthermore, MAIT cells have shown more marked changes in long-term type 1 diabetes than in recent-onset type 1 diabetes [15]. Recent evidence has shown alterations in MAIT cells in OLP [16]. The immunoregulatory role of defective MAIT cells, which produce cytokines and express functional proteins, may contribute to the pathophysiology of OLP [16]. However, OLP often presents a heterogeneous and prolonged clinical course. Immune function disorders are believed to occur according to OLP evolution [3, 17]. Although there have been some studies on the role of MAIT cells in OLP, there are no relevant studies on the role of MAIT cells in different stages of OLP.
Here, we characterized circulating MAIT cells in adults with recent-onset OLP and adults with long-term OLP, in comparison with healthy individuals, to ascertain MAIT cell profiles in accordance with OLP evolution. This will contribute to the understanding of the pathogenesis of OLP, and also provide new ideas for future studies on the predictive value of the MAIT cell ratio for the long-term prognosis of patients with OLP.
Materials and methods
Participants and sample collection
This protocol was reviewed by the Institutional Ethics Committee of the Stomatology Hospital, Southern Medical University, in accordance with the Declaration of Helsinki [approval no. (2023) 02]. Each participant signed an informed consent. We collected peripheral blood from 15 healthy adults (as healthy controls) and 30 patients with OLP. The OLP group included 14 adults with recent-onset OLP and 16 adults suffering from long-term OLP (follow-up > 2 years). The patients were recruited from the Department of Oral Medicine and had been clinically and histopathologically diagnosed with OLP under the WHO criteria [18]. The healthy controls included in this study were all from the Department of Oral Maxillofacial Surgery. Individuals with corticosteroid or immunomodulatory treatments within the previous three months were excluded from the study, as well as those with ongoing systemic disorders and infectious/inflammatory diseases. Smokers and alcoholics were also excluded from this study. The clinical characteristics of the subjects are presented in Table 1.
Table 1.
Clinical characteristics of the individuals with Oral lichen planus and healthy donors
| Characteristics | Healthy Controls | OLP patients | |
|---|---|---|---|
| RO OLP | LT OLP | ||
| n | 15 | 14 | 16 |
| Age, years | 46.20 ± 11.23(30–68) | 50.64 ± 12.73(35–68) | 47.50 ± 9.42(35–67) |
| Sex ratio M/F | 6/9 | 6/8 | 8/8 |
| OLP duration | - | 51.1 ± 39.2(7-120 days) | 3.1 ± 2.5(2–12 years) |
Data are presented mean ± SD(range)unless stated otherwise
M, male; F,female; RO, recent-onset LT, long-term; OLP, Oral lichen planus
We used the RAE (reticular, atrophic, erosive) scoring system recommended in a prior study to assess the severity of OLP (Table S1) [19]. Clinical information (age, course of disease, RAE score, etc.) of patients with recent and long-term onset was collected, and the patients’ condition was comprehensively evaluated.
Isolation of PBMCs
The following methods were referenced from a previous study [16]. Initially, the individuals’ venous blood was obtained using EDTA-K2 anticoagulant vacuum tubes. Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood using Ficoll-Hypaque density gradient centrifugation (TBD Biosciences). The PBMC layer was then washed twice with PBS and treated with an RBC lysis solution for 10 min at room temperature to eliminate erythrocyte contamination. Next, the separated PBMCs were subjected to flow cytometry, either by direct staining or in vitro stimulation.
Phenotyping using flow cytometry
After washing twice with PBS, the isolated PBMCs were directly stained for surface antigens [16]. To label the MAIT cells and detect the expression of surface markers, all the cells were resuspended in stain buffer (1 × 106 cells per 100 µL) and stained with the following antibodies: APC-Cy7-CD3, PE/Cyanine7-TCR Vα7.2, PE-CD161, FITC-CD4, PerCP-Cy5.5-CD8, BV421-CD69, APC-CD103, APC-R700-CD38, PE-CF594-HLA-DR, BV605-Tim-3, and BUV737-PD-1 at 4 °C for 30 min in the dark. Information regarding the antibodies used is listed in Table S2. All the antibodies were purchased from BD Biosciences (San Jose, CA, USA) or BioLegend (London, UK).
Analysis of intracellular cytokines using flow cytometry
For detection of intracellular cytokines, PBMCs were activated with 2 µL/mL of Leukocyte Activation Cocktail containing phorbol myristate acetate (PMA), ionomycin, and the protein transport inhibitors monensin (BD Biosciences) at 37 °C with 5% CO2 for 5 h in RMPI 1640 medium containing 10% fetal bovine serum (FBS, Procell). After incubation, the cells labelled with surface markers antibodies were resuspended in Fix/Perm buffer (Transcription Factor Buffer Set, BD Biosciences), at 4 °C for 45 min protected from the light [16]. Then, the fixed and permeabilized cells were washed with Perm/Wash buffer and stained, protected from light at 4 °C for 30 min, with the following antibodies: Alexa 647- IL-17 A, PE-CF594-IL-22, BV421-Granzyme B, Alexa Fluor 700-TNF-a, and FITC- IFN-γ. Finally, the cells were washed and resuspended in staining buffer for detection [16]. Data were acquired on a BD LSRFortessa flow cytometer (BD Biosciences) and were analyzed with Flow Jo v. 10.8.1 software (Tree Star). MAIT cells were sorted using stepwise gating and the expression of different intracellular factors was calculated.
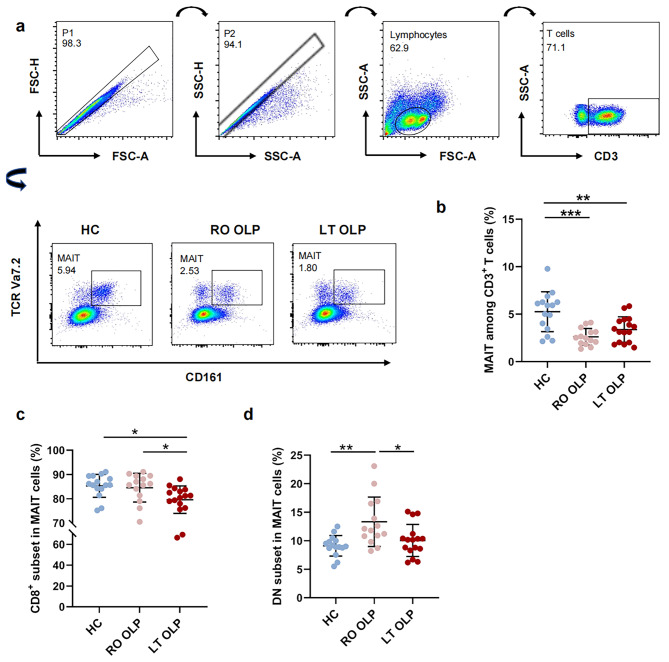
The gating strategy is shown in Fig. 1a. For the analysis of cell populations and cytokine production, the first gates, SSC-A versus SSC-H and FSC-A versus FSC-H, were used to secure single-cell clusters. Then, a second gate is designed to circle the lymphocyte cluster based on forward and side scatter characteristics, that is, the size and granularity of the cells [16]. In the next part, APC-Cy7-CD3 positive cells were identified from the lymphocytes as CD3+ T cells, and MAIT cells were identified from CD3+T cells. Finally, other surface and intracellular antigens were recognized from CD3+T cells and the final target MAIT cells. Subsequent analysis was performed on the cell populations.
Fig. 1.
Frequency alterations of circulating MAIT and its different subsets in OLP patients at different stages. Multicolor flow cytometric analyses of CD3 + TCR Vα7.2 + CD161 high T-cells in peripheral whole blood samples of patient with recent-onset OLP (n = 14), long term OLP (n = 16), and healthy controls (n = 15), which gender- and age-matched. (a) Representative flow cytometry plots of three groups’ samples showing the gating strategy to identify TCR Vα7. 2 + CD161 high T-cells (referred to here as MAIT cells). (b) Summary frequency of peripheral blood MAIT cells from patients with recent-onset(n = 14) or long-term OLP (n = 16) and healthy controls (n = 15). (c-d) Summary frequency of CD8+ (c) or CD4-CD8- (d) circulating MAIT cells from patients with recent-onset(n = 14) or long-term OLP (n = 16) and healthy controls (n = 15). *p < 0.05; **p < 0.01; ***p < 0.001; ns, not statistically significant. Correlations were calculated using the Spearman’s correlation analysis. DN, CD4-, CD8- double negative. HC, healthy controls; LT, long-term; RO, recent-onset; OLP, Oral lichen planus
Statistics
All graphs and data analyses were carried out using GraphPad Prism 9.4 and SPSS 25.0. The Shapiro-Wilk normality test was used to determine whether each dataset had a normal distribution. Depending on the situation, the non-parametric two-tailed Wilcoxon-Mann-Whitney or non-parametric Spearman’s correlation tests with precise p-values were used to compare all datasets. Differences with a p < 0.05 were considered significant.
Results
Circulating MAIT frequency and its different subsets are altered in patients with OLP at different disease states
First, we examined CD3+CD161+TCRVa7.2+ MAIT cells in peripheral blood samples from 30 patients with OLP (14 recent-onset and 16 long-term patients with OLP) and 15 healthy individuals using flow cytometry (Fig. 1a). The clinical characteristics of individuals with OLP and healthy donors are shown in Table 1. Our findings showed that the percentage of MAIT cells among CD3+T cells was markedly reduced in both the recent-onset (p < 0.001) and long-term OLP groups (p < 0.01, Fig. 1b) compared to healthy controls. However, the proportion of MAIT cells in the recent-onset OLP group was not significantly different from that in the long-term OLP group (p = 0.367, Fig. 1b). In human blood, MAIT cells can be divided into subsets based on CD4 and CD8 expression, with the majority of MAIT cells being CD8+, approximately 10% CD4−CD8− (double negative, DN), and very few CD4+ [20]. As shown in Fig. 1c–d, CD8+ MAIT cells still represented the major subset in the peripheral blood of healthy controls and patients with OLP, although the percentage of CD8+ MAIT cells in patients with long-term OLP was slightly lower than that in healthy controls (p<0.05) and in patients with recent-onset OLP (p < 0.05). The percentage of circulating DN MAIT cells was strikingly higher in patients with recent-onset OLP than in healthy controls (p < 0.01, Fig. 1d). As OLP progressed from recent-onset to long-term, the percentage of DN MAIT cells decreased slightly (p < 0.05, Fig. 1d). Collectively, these data indicated that the phenotype of MAIT cells in the recent-onset and long-term OLP groups was altered.
Circulating MAIT cell phenotype is altered in adults with OLP
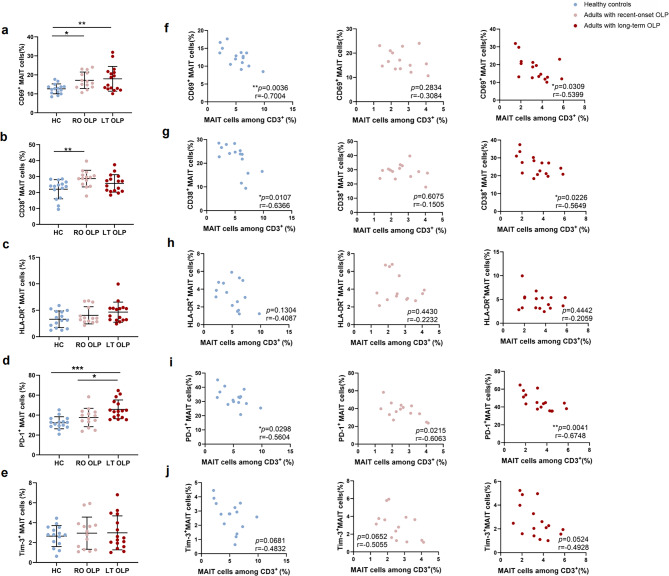
To assess the phenotypic characteristics of peripheral blood MAIT cells in patients with OLP in more detail, we probed MAIT cells from patients with OLP for the features of immune activation and exhaustion ex vivo. We then stained the isolated PBMCs with markers of activation, CD69, CD38, and HLA-DR, to determine their expression frequency. As shown in Fig. 2. a, MAIT cells from patients with recent-onset (p < 0.05) or long-term OLP (p < 0.01) showed significantly higher CD69 levels than those from healthy individuals. Compared with healthy controls, the percentage of CD38+ MAIT cells from patients with recent-onset OLP was higher (p < 0.01, Fig. 2. b). We did not observe any significant differences in the HLA-DR+ MAIT cell frequencies between control donors and adults with recent-onset or long-term OLP (Fig. 2. c). To determine whether MAIT cells undergo exhaustion in the OLP microenvironment at different disease stages, we selected two immune inhibitory receptors, PD-1 and Tim-3, for content determination. The percentage of PD-1+ MAIT cells in participants with long-term OLP was higher than that in healthy control individuals (p < 0.001, Fig. 2. d) and patients with recent-onset OLP (p < 0.05, Fig. 2. d). The frequency of Tim-3+MAIT cells was similar among the three groups (Fig. 2. e). In addition, CD69+, CD38+, and PD-1+ MAIT cell frequencies negatively correlated with MAIT cell frequency in both healthy controls and adults with long-term OLP, but not in those with recent-onset OLP (Figs. 2f, g and i and 3e). Moreover, correlation tests showed that the frequency of OLP CD69+ MAIT and CD38+ MAIT cells in adults with recent-onset or long-term OLP was positively correlated with RAE scores (Fig. 3a, b, d). This trend was also observed in the correlation between HLA-DR+ MAIT cell frequency and the RAE score in patients with recent-onset OLP.
Fig. 2.
The phenotype alterations of circulating MAIT cells in OLP patients at different stages. (a ∼ e) Summary frequency of CD69+ (a), CD38+ (b), HLA-DR+ (c), PD-1+ (d), or Tim-3+ (e) MAIT cells in healthy donors (n = 15) and participants with recent-onset (n = 14) or long-term OLP (n = 16). (f ∼ j) Correlation between frequency of circulating MAIT cells and frequency of CD69+ (f), CD38+(g), HLA-DR+ (h), PD-1+ (i) or Tim-3+ (j) MAIT cells in healthy donors and participants with recent-onset or long-term OLP. *p < 0.05; **p < 0.01; ***p < 0.001; ns, not statistically significant. Correlations were calculated using the Spearman’s correlation analysis. HC, healthy controls; LT, long-term; RO, recent-onset; OLP, Oral lichen planus
Fig. 3.
The phenotype alterations of peripheral blood MAIT cells in OLP at different stages and its clinical significance. (a–c) Correlations between the percentage of CD69+ (a), CD38+ (b), or HLA-DR+ (c) MAIT cells and RAE scores in participants with recent-onset (n = 14) or long-term (n = 16) OLP. (d) The correlation between the various phenotypes of MAIT cell in participants with recent-onset and long-term OLP and the RAE scores is displayed in a heatmap with r-values. (e) Correlograms of circulating MAIT cell frequency and marker expression in healthy donors and participants with recent-onset (n = 14) or long-term OLP(n = 16)
Taken together, these results demonstrated that circulating MAIT cells are chronically activated in patients with recent-onset OLP and are progressively exhausted in patients with long-term OLP. We speculate that the low frequency of circulating MAIT cells in patients with OLP may be associated with chronic activation and exhaustion of MAIT cells in vivo.
Functional profile of OLP MAIT cells
Immune cells participate in the immune responses by secreting various cytokines, and MAIT cells are no exception as unconventional, innate-like T lymphocytes. To clarify the functional profile of MAIT cells in the peripheral blood of patients with OLP, isolated PBMCs were activated with PMA/ionomycin and their repertoire of functions was evaluated using flow cytometry staining for TNF-a, IFN-γ, IL-22, IL-17 A, and granzyme B (GzB). As illustrated in Fig. 4, IL-17 A (p < 0.01) secreted by MAIT cells was significantly increased in patients with recent-onset OLP compared to that in controls, and GzB (p < 0.01) secreted by MAIT cells was also significantly increased in patients with long-onset OLP, indicating that the stimulant could significantly improve the immune function of MAIT cells. However, no significant difference was observed in the percentages of other cytokines released by MAIT cells, including IFN-γ, TNF-α, and IL-22, between healthy controls and patients with OLP.
Fig. 4.

Functional profile of MAIT cells in peripheral blood of patients with OLP. To assess the production of cytokines GzB, IFN-γ, TNF-α, IL-17 A, and IL-22 of OLP MAIT after activation with PMA and ionomycin. Detection and difference of MAIT cell-associated cytokines from blood of adults with recent-onset (n = 14) or long-term (n = 16) OLP and healthy donors (n = 15) after PMA/ionomycin stimulation. Data were expressed as mean ± SD. *p < 0.05; **p < 0.01. OLP, Oral lichen planus. GzB, granzyme B
Discussion
OLP is an autoimmune inflammatory disease involving multiple types of immune cells. In the present study, we evaluated the phenotype and cytokine expression profiles of MAIT cells in patients with different stages of OLP. To the best of our knowledge, this is the first report to extensively describe whether the characteristics of circulating MAIT cells in patients with OLP are affected by different periods of onset.
Several studies have shown that patients with autoimmune diseases such as MS, SLE, and RA have a lower frequency of MAIT cells. The MAIT cell frequency has also been correlated with disease severity in autoimmune liver diseases and bronchial asthma [21, 22]. MAIT cells can be divided into CD8+, CD4+, and DN phenotypes. CD8 + MAIT cells, which typically express CD8aa and markers such as CD16 and NKG2D, are highly cytotoxic and critical in the fight against bacterial and fungal infections. CD4+ MAIT cells are less common and may regulate the immune response and assist in antibody production. However, their functions require further investigation. DN MAIT cells lack CD4 and CD8 expression but may express CD161, which is thought to be important in mucosal immunity and rapid response to infection, and may exhibit tissue-resident properties for early pathogen control. In the present study, the frequency of MAIT cells in the OLP group was decreased, and no significant difference was observed between the recent-onset and long-term groups, suggesting that MAIT cell alterations in the OLP group were not affected by the disease duration. Compared with the healthy control group, the frequency of CD8+MAIT cells decreased in the long-term OLP group, and there was an increasing trend in the expression of DN MAIT cells, which is consistent with previous reports [16], indicating that the dominant cell toxicity function of MAIT cells in the onset of OLP may change as the disease progresses over time. This may have been influenced by late-stage treatment interventions.
In individuals with recent-onset OLP, MAIT cells harbored activated phenotypes (CD69 and CD38 upregulation). MAIT cells exhibited an activated and exhausted phenotype, characterized by high expression of CD69 and PD-1 in adults with long-term OLP. Moreover, the expression of CD69 and CD38 in MAIT cells was positively correlated with disease activity in both recent-onset and long-term OLP. These findings suggested that activated MAIT cells may augment the pathology of OLP. Notably, CD69+, CD38+, and PD-1+ MAIT cell frequencies negatively correlated with MAIT cell frequency in both healthy controls and adults with long-term OLP, but not in those with recent-onset OLP. This suggested that MAIT cell profiles may differ as the disease progresses.
It is well established that MAIT cells can release pro-inflammatory cytokines in Th1 or Th17 patterns in response to TCR mediation and that the pathogenesis of several disorders is influenced by IL-17 released by MAIT cells [23–25]. Previous studies have reported that MAIT cells produce high levels of IL-17; however, no significant difference was observed between the OLP and HC group [17]. In contrast, our findings showed that participants with recent-onset OLP exhibited elevated production of the proinflammatory cytokine IL-17 A, and long-term OLP also showed a similar trend, but there was no significant difference between the OLP groups. This suggested that MAIT cells may exert a pro-inflammatory role through a Th17-type immune response during OLP pathogenesis. In addition, GzB-producing MAIT cells increased in individuals with long-term OLP compared to healthy donors, indicating that GzB may be involved in the apoptosis of basal keratinocytes in OLP as the disease progresses.
Conclusions
Collectively, adults with recent-onset OLP displayed higher frequencies of CD69+ and CD38+ MAIT cells, as well as higher levels of the proinflammatory cytokine IL-17 A, indicating an activated nature of their MAIT cells. Individuals with prolonged OLP displayed an active and depleted phenotype of MAIT cells, marked by elevated GzB production and high expression of CD69 and PD-1. Our study reinforces the potential of MAIT cells as a key indicator of disease progression. However, there are still limitations to be considered, such as the relatively small sample size and lack of a longitudinal experimental design. Subsequent studies should include more clinical indicators to explore the predictive value of the long-term prognosis of patients with OLP.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the nurses and other staffs of the Stomatology Hospital, Southern Medical University for their kind supports.
Abbreviations
- MAIT
Mucosal-associated invariant T
- OLP
Oral lichen planus
- Th
Cells helper T cells
- TCR
T-cell receptor
- MHC
Major histocompatibility complex
- MR1
MHC-related molecule 1
- MS
Multiple sclerosis
- SLE
Systemic lupus erythematosus
- RA
Rheumatoid arthritis
- RAE
Reticular, atrotrophic, erosive
- PBMCs
Peripheral blood mononuclear cells
- PMA
Phorbol myristate acetate
- DN
Double negative
- GzB
Granzyme B
- WHO
World Health Organization
- SD
Standard deviation
- M
Male
- F
Female
- RO
Recent-onset
- LT
Long-term
- FBS
Fetal bovine serum
Author contributions
XW: Data curation, Formal analysis, Investigation, Software, Writing-original draft. SC: Data curation, Resources, Software, Writing-original draft. YY: Investigation, Rescources. XX: Investigation, Rescources. XX: Project administration, Investigation, Rescources. WM: Project administration, Resources, Writing-review & editing.All authors reviewed the manuscript.
Funding
This work was supported by the National Key R&D Program of China 2022YFC2402903 Science research cultivation program of stomatological hospital, Southern medical university (PY2022011) and Nonprofit Industry Research Specific Fund of National Health and Family Planning Commission of China (grant no.201502018).
Data availability
The datasets used during the present study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was reviewed and approved by the Institutional Ethics Committee of the Stomatology Hospital, Southern Medical University[approval no. (2023) 02]. All patients and healthy volunteers gave written informed consents before participation in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaoli Wu and Siting Chen contributed equally to this work.
Contributor Information
Xiaoqin Xiong, Email: 1183141018@qq.com.
Wenxia Meng, Email: mengwx2008@foxmail.com.
References
- 1.Hamour AF, Klieb H, Eskander A. Oral lichen planus. CMAJ. 2020;192(31):E892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warnakulasuriya S, Kujan O, Aguirre-Urizar JM, Bagan JV, González-Moles M, Kerr AR, Lodi G, Mello FW, Monteiro L, Ogden GR, et al. Oral potentially malignant disorders: a consensus report from an international seminar on nomenclature and classification, convened by the WHO collaborating centre for oral Cancer. Oral Dis. 2021;27(8):1862–80. [DOI] [PubMed] [Google Scholar]
- 3.Afzali S, Mohammadisoleimani E, Mansoori Y, Mohaghegh P, Bahmanyar M, Mansoori B, Pezeshki B, Nikfar G, Tavassoli A, Shahi A, et al. The potential roles of Th17 cells in the pathogenesis of oral lichen planus. Inflamm Res. 2023;72(7):1513–24. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Zhang D, Han Q, Zhao X, Zeng X, Xu Y, Sun Z, Chen Q. Role of distinct CD4(+) T helper subset in pathogenesis of oral lichen planus. J Oral Pathol Med. 2016;45(6):385–93. [DOI] [PubMed] [Google Scholar]
- 5.Ke Y, Dang E, Shen S, Zhang T, Qiao H, Chang Y, Liu Q, Wang G. Semaphorin4D drives CD8(+) T-Cell lesional trafficking in oral Lichen Planus via CXCL9/CXCL10 upregulations in oral keratinocytes. J Invest Dermatol. 2017;137(11):2396–406. [DOI] [PubMed] [Google Scholar]
- 6.Ussher JE, Willberg CB, Klenerman P. MAIT cells and viruses. Immunol Cell Biol. 2018;96(6):630–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ioannidis M, Cerundolo V, Salio M. The Immune Modulating properties of Mucosal-Associated Invariant T cells. Front Immunol. 2020;11:1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toubal A, Nel I, Lotersztajn S, Lehuen A. Mucosal-associated invariant T cells and disease. Nat Rev Immunol. 2019;19(10):643–57. [DOI] [PubMed] [Google Scholar]
- 9.Corbett AJ, Eckle SB, Birkinshaw RW, Liu L, Patel O, Mahony J, Chen Z, Reantragoon R, Meehan B, Cao H, et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature. 2014;509(7500):361–5. [DOI] [PubMed] [Google Scholar]
- 10.Ussher JE, Bilton M, Attwod E, Shadwell J, Richardson R, de Lara C, Mettke E, Kurioka A, Hansen TH, Klenerman P, et al. CD161 + + CD8 + T cells, including the MAIT cell subset, are specifically activated by IL-12 + IL-18 in a TCR-independent manner. Eur J Immunol. 2014;44(1):195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurioka A, Ussher JE, Cosgrove C, Clough C, Fergusson JR, Smith K, Kang YH, Walker LJ, Hansen TH, Willberg CB, et al. MAIT cells are licensed through granzyme exchange to kill bacterially sensitized targets. Mucosal Immunol. 2015;8(2):429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Legoux F, Salou M, Lantz O. MAIT Cell Development and functions: the microbial connection. Immunity. 2020;53(4):710–23. [DOI] [PubMed] [Google Scholar]
- 13.Zumwalde NA, Gumperz JE. Mucosal-Associated Invariant T cells in tumors of epithelial origin. Adv Exp Med Biol. 2020;1224:63–77. [DOI] [PubMed] [Google Scholar]
- 14.Chiba A, Murayama G, Miyake S. Characteristics of mucosal-associated invariant T cells and their roles in immune diseases. Int Immunol. 2021;33(12):775–80. [DOI] [PubMed] [Google Scholar]
- 15.Nel I, Beaudoin L, Gouda Z, Rousseau C, Soulard P, Rouland M, Bertrand L, Boitard C, Larger E, Lehuen A. MAIT cell alterations in adults with recent-onset and long-term type 1 diabetes. Diabetologia. 2021;64(10):2306–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang JY, Wang F, Zhou G. Characterization and function of circulating mucosal-associated invariant T cells and γδT cells in oral lichen planus. J Oral Pathol Med. 2022;51(1):74–85. [DOI] [PubMed] [Google Scholar]
- 17.El-Howati A, Thornhill MH, Colley HE, Murdoch C. Immune mechanisms in oral lichen planus. Oral Dis. 2023;29(4):1400–15. [DOI] [PubMed] [Google Scholar]
- 18.van der Meij EH, van der Waal I. Lack of clinicopathologic correlation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications. J Oral Pathol Med. 2003;32(9):507–12. [DOI] [PubMed] [Google Scholar]
- 19.Zhou G, Zhang J, Ren XW, Hu JY, Du GF, Xu XY. Increased B7-H1 expression on peripheral blood T cells in oral lichen planus correlated with disease severity. J Clin Immunol. 2012;32(4):794–801. [DOI] [PubMed] [Google Scholar]
- 20.Provine NM, Klenerman P. MAIT Cells in Health and Disease. Annu Rev Immunol. 2020;38:203–28. [DOI] [PubMed] [Google Scholar]
- 21.Böttcher K, Rombouts K, Saffioti F, Roccarina D, Rosselli M, Hall A, Luong T, Tsochatzis EA, Thorburn D, Pinzani M. MAIT cells are chronically activated in patients with autoimmune liver disease and promote profibrogenic hepatic stellate cell activation. Hepatology. 2018;68(1):172–86. [DOI] [PubMed] [Google Scholar]
- 22.Hinks TS, Zhou X, Staples KJ, Dimitrov BD, Manta A, Petrossian T, Lum PY, Smith CG, Ward JA, Howarth PH, et al. Innate and adaptive T cells in asthmatic patients: relationship to severity and disease mechanisms. J Allergy Clin Immunol. 2015;136(2):323–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haga K, Chiba A, Shibuya T, Osada T, Ishikawa D, Kodani T, Nomura O, Watanabe S, Miyake S. MAIT cells are activated and accumulated in the inflamed mucosa of ulcerative colitis. J Gastroenterol Hepatol. 2016;31(5):965–72. [DOI] [PubMed] [Google Scholar]
- 24.Heymann F, Tacke F. Immunology in the liver–from homeostasis to disease. Nat Rev Gastroenterol Hepatol. 2016;13(2):88–110. [DOI] [PubMed] [Google Scholar]
- 25.Dias J, Boulouis C, Sobkowiak MJ, Lal KG, Emgård J, Buggert M, Parrot T, Gorin JB, Leeansyah E, Sandberg JK. Factors influencing functional heterogeneity in Human Mucosa-Associated Invariant T cells. Front Immunol. 2018;9:1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used during the present study are available from the corresponding author upon reasonable request.